Abstract
Introduction
Postoperative morbidities, such as anastomotic leaks, are common after trimodality therapy (chemoradiation followed by surgery) for esophageal cancer. We investigated for factors associated with an increased incidence of anastomotic leaks.
Methods
Data from 285 esophageal cancer patients treated from 2000–2011 with trimodality therapy was analyzed. Anastomotic location relative to preoperative radiation field was assessed using postoperative computed tomographic imaging. Logistic regression was used to evaluate for factors associated with any or clinically relevant (CR) (≥ grade 2) leaks.
Results
Overall anastomotic leak rate was 11% (31/285), and CR leak rate was 6% (17/285). Multivariable analysis identified body mass index (BMI) (OR 1.09, 95%CI 1.00–1.17; OR 1.11, 95%CI 1.01–1.22), three-field surgery (OR 10.01, 95%CI 3.83–26.21; OR 4.83, 95%CI 1.39–16.71), and within radiation field (“in-field”) anastomosis (OR 5.37, 95%CI 2.21–13.04; OR 8.63, 95%CI 2.90–25.65) as independent predictors of both all grade and CR leaks, respectively. While patients with distal esophageal tumors and Ivor-Lewis surgery had the lowest incidence of all grade (6.5%) and CR leaks (4.2%), most of the leaks were associated with the anastomosis constructed within the field of radiation (in-field: 39% and 30% versus out-of-field: 2.6% and 1.0%, respectively, for total and CR leaks, p<0.0001, Fisher’s Exact test).
Conclusions
Esophagogastric anastomosis placed within the preoperative radiation field was a very strong predictor for anastomotic leaks in esophageal cancer patients treated with trimodality therapy, among other factors. Surgical planning should include a critical evaluation of the preoperative radiation fields to ensure proper anastomotic placement after chemoradiation therapy.
Keywords: Anastomotic leaks, Esophagectomy, Radiation, Esophageal Cancer, Trimodality Therapy
Introduction
Surgical resection alone remains a worldwide standard for the management of esophageal cancer, but the 5-year survival usually does not exceed 20 percent(1). Neoadjuvant chemoradiation prior to surgical resection (trimodality therapy) allows for disease downstaging and increases tumor resectability, with increased cure rates. Older randomized trials demonstrated probable survival benefit of preoperative chemoradiation, although a number of negative studies made the indication controversial. A meta-analysis of the trials showed a two year overall survival benefit of 13% for patients treated with neoadjuvant chemoradiation followed by surgery compared patients treated with surgery alone(2). Recently, a large randomized trial demonstrated significant improvement in overall survival and disease-free survival with the use of neoadjuvant chemoradiation compared to surgery alone(3). Chemoradiation before surgery improved median overall survival to 49.4 months compared to 24.0 months in patients treated with surgery alone.
However, preoperative chemoradiation increases the chance for toxicity and postoperative morbidity compared to surgery alone. There are several nonrandomized studies in the literature that showed an increase in surgical morbidity in patients undergoing neoadjuvant chemoradiation(4–7). Postoperative pulmonary complications have been well studied and have been shown to be related to radiation dose to the lungs(8).
The effects of neoadjuvant radiation on postoperative anastomotic leaks have been less extensively studied. In an older study, anastomotic leaks were found in 17% of patients who underwent esophagectomy with cervical anastomosis; however the use of preoperative radiotherapy was not associated with the incidence of leaks(9). A systematic review showed that reports in the literature for anastomotic leak rates vary between 0% and 26% and that the leak rate is not influenced by method of anastomosis, either stapled or hand-sewn(10). A recent Belgian study with 54 patients treated with neoadjuvant radiation followed by Ivor-Lewis esophagectomy showed that the dose to the gastric fundus was a significant predictor for anastomotic complications (leakage, ischemia, stenosis)(11).
The aim of the present study was to determine the clinical and dosimetric factors that can influence the risk of developing any grade leaks or the more clinically relevant leaks of ≥ grade 2 (or what we will term as “CR leaks” throughout the manuscript) in patients undergoing trimodality therapy. Dose to the whole stomach and associated gastric substructures were studied as well as the impact of the positioning of radiation field and the location of the anastomotic site.
Materials and Methods
Patient Data
This investigation was approved by the institutional review board and was conducted in compliance with the Health Insurance Portability and Accountability Act. This was a retrospective analysis of esophageal cancer patients treated at MD Anderson Cancer Center with neoadjuvant chemoradiation followed by surgery between 2000 and 2011. Since we wanted to evaluate the radiation dosimetry to the stomach, only patients with full dose-volume histogram (DVH) data were included. Patients who had gastrectomy were excluded. We also only included patients treated with photon-based therapy (3–dimensional conformal radiation therapy (3D-CRT) or Intensity Modulated Radiation Therapy (IMRT)).
A thorough chart review was done to document the clinical and treatment-related factors for this cohort of patients. Following surgery, follow-up monitoring included interval history and physical examination at the discretion of the treating physicians. Incidence of perioperative anastomotic leaks was recorded by grade for each patient, defined as radiographic leak only (grade 1), minimal intervention/stent placement (grade 2), major intervention/reoperation (grade 3), and conduit loss (grade 4). CR leaks were defined as leaks ≥ grade 2. Post-surgical CT scans were examined to determine if surgical anastomosis was in or out of the radiation field. Contouring of the whole stomach, fundus, antrum, and lateral body was done by one person using the Pinnacle planning software. Associated 3D and IMRT treatment plans were used to generate DVHs for each of the contoured gastric regions of interest.
Treatment Approach
Patients in this study cohort were treated with neoadjuvant chemoradiation to 50.4 Gy at 1.8 Gy per fraction. Combinations of 5-flurouracil and taxane, or with platinum-based compounds were administered concurrently with radiotherapy. Several weeks after completion of chemoradiation, most patients were restaged using CT, PET/CT, and/or EGD with biopsy of the primary disease site, and evaluated for surgical management. The most common esophagectomy procedure was Ivor Lewis, while a few patients also received transhiatal, left thoracotomy, radical (en block) resection, or minimally invasive esophagectomy.
The technique of three-dimensional conformal radiotherapy (3DCRT) or intensity modulated radiation therapy (IMRT) was used for this patient cohort. The internal gross tumor volume (iGTV) was delineated based on the 4-dimensional CT simulation images to account for tumor motion relative to diaphragmatic motion, FDG-PET/CT, and endoscopy results. The clinical target volume (CTV) included the iGTV with a radial margin of 0.5–1cm and a proximal and distal margin of 3–4 cm. Elective nodal regions were not covered, unless in the proximal locations where the supraclavicular fossa bilaterally were included in the target volume, and in the distal esophagus where the celiac axis was covered if it was involved. The nodal CTV was defined by 0.5–1 cm expansion from the nodal GTV. The planning target volume (PTV) was the CTV plus a uniform 0.5 cm expansion margin.
Statistical Methods
Logistic modeling was used to assess associations between leak incidence and various continuous and categorical variables. The continuous variables studied were age, BMI, tumor length, PTV, prescribed dose, and mean dose to whole stomach, lateral body, antrum, and fundus. Categorical variables studied were KPS, coronary artery disease (CAD) history, diabetes history, smoking history, tumor location, presence of in-field anastomosis, radiation modality, use of induction chemotherapy, salvage surgery (defined as ≥90 days after chemoradiation), surgical margin status (R0 vs R1–2), and type of surgery (Ivor-Lewis, transhiatal, three-field, or hybrid). Logistic regression analysis was then used to perform multivariable analysis of factors that were significant (p≤0.05) on univariable analysis. The two-tailed Fisher’s exact test was used to test the significance of proportions.
Results
Patient Cohort
A total of 285 patients diagnosed with esophageal cancer and treated with neoadjuvant chemoradiation followed by esophagectomy were included in our analysis; 158 patients were treated with 3D-CRT and 127 were treated with IMRT. Concurrent chemotherapy was given with all patients during chemoradiation, and 151 patients were treated with induction chemotherapy before chemoradiation. Following radiation, the most common surgical procedure was Ivor-Lewis surgery (n=222) followed by three-field and transhiatal surgery (n=31 and 29 respectively). Three patients had hybrid open thoracotomy/laparoscopy resections. Nearly all of the patients had creation of a gastric conduit (97.9%, 279/285) with only 5 cases of jejunal interposition (for one case the origin of the conduit is not known). There was no association between leaks and jejunal interposition (3 of 5 had no leaks).
Factors associated with Anastomotic Leaks
Overall there were 14 Grade 1, 8 grade 2, 8 grade 3, and 1 grade 4 leaks. Anastomotic leaks of any grade occurred in 31 patients for an overall incidence rate of 11%, and 17 patients (6%) had grade 2 or higher leaks. Table 1 shows patient and treatment related characteristics that were associated with the occurrence of any or CR anastomotic leak based on univariable analysis. For any grade leaks, tumor location, in-field anastomosis, and type of surgery (three-field vs. others) were factors associated with anastomotic leaks. In addition, lower mean doses to stomach and substructures were associated with increased incidence of anastomotic leaks. For CR leaks, BMI, diabetes, tumor location, in-field anastomosis, and the type of surgery were significant factors associated with the occurrence of leaks.
Multivariable analysis
Stepwise forward and backward multivariable analyses were performed using candidate factors that were significant at p<0.05 on univariable analysis. Subsequently, factors that were not included as candidate factors in the initial multivariable analysis due to the lack of univariable significance were tested for their ability to improve the model fit. For both all grade and CR leaks, the factors selected as independent predictors were BMI, three-field surgery, and in-field anastomosis (Table 2).
Incidence of leaks associated with the predictive factors
Patients with anastomosis done inside the radiation field had a significantly higher incidence of leaks of all grades when compared to anastomoses that were placed outside of the radiation field (31.8% vs. 7%, p<0.0001). Findings were similar for high-grade leaks as well (15.9% vs. 3.7%, p<0.0001). We evaluated the incidence of CR anastomotic leaks in relation to the significant factors that were discovered in the multivariable analysis. For BMI, we analyzed the incidence of CR leaks using the median BMI (28 kg/m2) as a cutoff. The incidence of any grade leaks only trended for but was not significantly lower in patients with a BMI < 28 kg/m2 (12/143, 8.4%) than in patients with BMI ≥ 28 kg/m2 (19/142, 13.4%, p=0.189). However the incidence of CR leaks was statistically significantly lower in patients with a BMI < 28 kg/m2 (4/143, 2.8%) than patients with BMI ≥ 28 kg/m2 (13/142, 9.2%, p=0.025).
While upper/middle tumor location was a significant risk factor for the development of anastomotic leak on univariable analysis, this factor was no longer significant on multivariable analysis. The reason is that tumor location was strongly associated with the probability of in-field anastomosis: 14 of 21 patients with upper/middle tumors had in-field anastomosis (67%), compared to 30/263 patients with lower tumors (11%) (p<0.001). To take this further, we evaluated the incidence of CR leaks based on tumor location and anastomotic location relative to the radiation field (Table 3). The large majority (92%) of patients in our cohort had tumors located in the lower third of the esophagus. When compared to patients with lower esophageal cancers, the overall leak rate was significantly higher for patients with upper esophageal tumors (33% vs. 9%, p=0.004). This was also true for CR leaks (19% vs. 5%, p=0.028). However, leaks rates were nearly equal regardless of tumor location if the anastomosis was in-field. For all grades of leaks, patients with both in-field anastomosis and an upper or middle esophagus tumor location had an anastomotic leak rate at 36% (5/14), which was not different from the patients with distal tumors and in-field anastomosis (9/30, 30%, p=1.00). This was also true for CR leaks (21% vs 23% for upper/middle vs. distal location, respectively). For patients with out-of-field anastomosis, the leak rate seems to differ based on tumor location, with higher leak rates for upper/middle location compared to distal locations, but these were not statistically significant. On the contrary, patients with distal esophageal tumors and an out-of-field anastomosis had a total leak rate of 6.4%, which was statistically significant compared to in-field anastomosis of 30% (p=0.0004). For CR leaks, distal locations had only a 2.5% risk if the anastomosis was out of field, which was significantly lower than the risk of in-field anastomosis (23%, p=0.0001). To remove the potential confounding factor of the different surgery techniques used for tumors in the distal location, we confined our analysis to only the patients who had Ivor-Lewis esophagectomies (n=215). The overall leak rate was 6.5% and the CR leak rate was 4.2%. We found that the rate of all grade and CR leaks were 39% (9/23) and 30% (7/23), respectively, when the anastomosis was placed in-field versus 2.6% (5/192) and 1.0% (2/192), respectively, if the anastomosis was placed out-of-field. The differences were highly significant (p<0.0001).
The differences in incidence of all grade and CR leaks were also significantly influenced by the type of surgery. The observed incidence rates for all grades and CR leaks were 16/222 (7.2%) and 10/222 (4.5%) for Ivor-Lewis, 13/31 (41.9%) and 6/31 (19.4%) for three-field surgery, 2/29 (7%) and 1/29 (3.4%) for transhiatal surgery, and 0/3 and 0/3 for patients who had hybrid surgery, respectively. To determine if three-field surgery was associated with an even higher rate of complications, we examined grade 3 and higher leaks and association with the type of surgery. Only 2 of 31 (6.4%) with three-field surgery had grade 3–4 leaks, as compared to 7 of 254 (2.8%) who received other types of surgery, the difference of which was not statistically significant (OR 2.43, p=0.281).
Discussion
Our findings in this study demonstrated that the anastomotic location relative to the field of radiation is an important factor influencing the occurrence of postoperative leaks after esophagectomy. While there are reports to suggest that neoadjuvant therapy, either chemotherapy or radiation, does not substantially increase the postoperative morbidity for patients who undergo esophagectomies(12), there is substantial evidence in the literature to suggest that neoadjuvant chemoradiation increases the rate of postoperative morbidity and mortality. A meta-analysis of randomized trials of patients with resectable esophageal cancer showed that chemoradiation was a risk factor for postoperative mortality (odds ratio 1.18–3.73, p= 0.01)(13). A Japanese retrospective analysis of 686 esophageal cancer patients also showed that neoadjuvant chemoradiation was an independent predictor for postoperative complications with an anastomotic leak rate of 28% in patients who received neoadjuvant radiation compared to 16.5% in patients who had surgery alone (p<0.05)(14). Our observed total leak rate of 11% was comparatively lower, but this could be due in part to the surgical approaches used in the different studies, since in the published studies the rates were only about 7% for both transhiatal and Ivor-Lewis patients, which is about what we see for our patient cohort who received Ivor-Lewis esophagectomy (6.5%). We found three-field esophagectomy was a significant risk factor for anastomotic leak on multivariable analysis with a leak rate of 42%.
Walle et al. reported a 5.6% anastomotic leak rate in their study showing the significance of D50 to the gastric fundus (the total dose delivered to 50% of the volume) as a risk factor for anastomotic complications(11). To our knowledge, this was the only published evidence that showed radiation dose to the stomach as a risk factor for anastomotic complications. Although we did find an association of the mean dose to the stomach and substructures to the incidence of all grade leaks, the higher mean dose was associated with a protective effect rather than a detrimental effect. We believe this paradoxical effect was attributed to the mean dose being inversely related to both the incidence of in-field anastomosis and three-field surgery (supplementary figure 2). Patients with upper and middle esophagus tumors were significantly more likely to have anastomotic leaks because more in-field anastomosis occurs in this location, and consequently have lower dose to the gastric structures. Three-field surgery was also more likely to be in upper-esophageal locations (12/21, 57%) compared to the lower esophageal location (19/263, 7.2%) (p<0.0001). While patients with lower esophageal tumors were associated with increased dose to the stomach, this did not increase the rate of anastomotic breakdown. Surgeons at our institution often take special care to exclude the irradiated stomach when creating the esophagogastric anastomosis.
There have been other factors that are associated with anastomotic leaks, regardless of the use of neoadjuvant chemoradiation. An earlier study from India found that low albumin, tumor involvement of the anastomotic cut margin, and cervical anastomosis were predisposing factors for leaks in patients who had upfront surgery(15). A second study, also in primary resected patients for both benign and malignant disease, found active smoking history, postoperative arrhythmias, and manually sewn vs. side-to-side stapled anastomosis as risk factors for leaks(16). While we were able to identify some patient factors, such as diabetes and higher BMI, as risk factors for anastomotic leaks, most of what we identified as critical risk factors were related to the tumor location, the type of surgery, and where the anastomosis was placed. We believe the different findings between our study and the aforementioned ones were primarily due to the use of chemoradiation in all the patients included in our study, which likely overshadowed other potential risk factors. A major finding of our study was that esophagogastric anastomosis placed in the previously irradiated field was significantly associated with increased leak rate. For these cases, irradiated normal tissues in proximity to the normal esophagus were included in the proximal portion of the esophagogastric anastomosis. Patients with upper or middle esophageal tumor location have their radiation treatment portals at or near the sites of thoracic or cervical anastomosis and therefore are more likely to have high doses of radiation targeted to normal tissue in this region. Upper/middle tumor location was a significant predictor of anastomotic leak on univariable analysis but was not included in the multivariable model, when the anastomotic site relative to the radiation field was taken into account. The leaks in the distal location were nearly fully accounted for by whether the anastomosis was placed in or out-of-field. However, the incidence of leaks in the upper/middle esophageal location was high regardless of whether the anastomosis was done in-field or out-of-field. Perhaps the number of cases was too small in this location to determine a statistically significant relationship with the field of anastomosis, but there could be other anatomic or clinical factors that were not accounted for that place the upper/middle tumor location at higher risk of developing leaks, independent of the type of surgery or the field of anastomosis.
To our knowledge, there was only one previous study that had evaluated the relationship of radiation field with anastomotic leak rates. This was in a cohort of 38 patients treated with radiation and esophagogastrectomy. They found that there was no increased leaks observed when the anastomosis was located in the preoperative radiation field(17). The reason for the discrepancy between their study and ours is unclear, but it is likely that the number of patients in their study was too small to make a significant association. It is well established that radiation leads to damage to the microvasculature and the resulting ischemia can slow tissue healing in irradiated fields(18–20). Diabetic patients often have issues with microvasculature and wound healing as well. Our analysis also showed a higher leak rate for patients with diabetes, though it was not statistically significant on multivariable analysis (19% vs. 9.5%, p = 0.101, table 1).
Our study is limited by the retrospective nature of the analysis, which cannot account for all potential factors that could explain some of our findings. While the surgical procedures at our institution have been standardized to a certain extent, multiple surgeons were involved over the time span of the study so some variation inevitably exists. Furthermore, anatomic configuration and blood supply to both the stomach and the esophagus are varied across the patient population, posing a potential confounding factor that couldn’t be accounted for in a retrospective analysis.
In conclusion, the present study demonstrates that the placement of the esophagogastric anastomosis within the preoperative radiation field is a strong, independent predictor for anastomotic leaks in esophageal cancer patients treated with trimodality therapy. These results have important implications in careful preoperative planning in order to minimize postoperative leak complications.
Supplementary Material
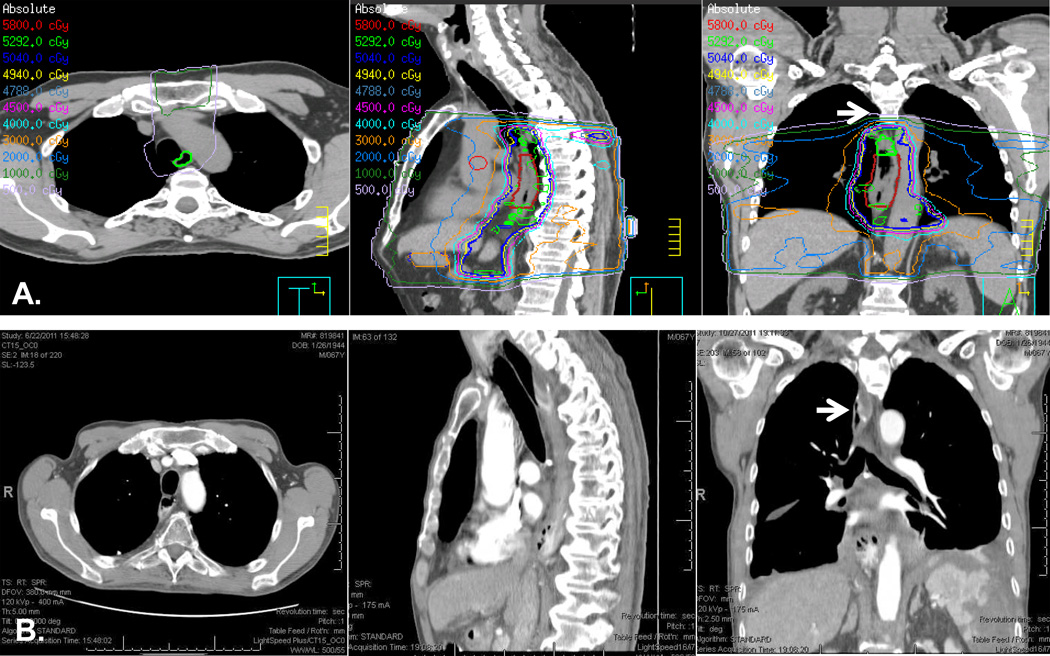
Figure 1. Illustration of a case with out-of-field anastomosis.
A. Simulation CT imaging of a mid-esophageal tumor with treatment plan encompassing areas below the aortic arch. B. Post-Ivor-Lewis esophagectomy CT imaging showing postoperative anatomy. White arrows point to the area of anastomosis above the aortic arch. This patient did not develop anastomotic leak.
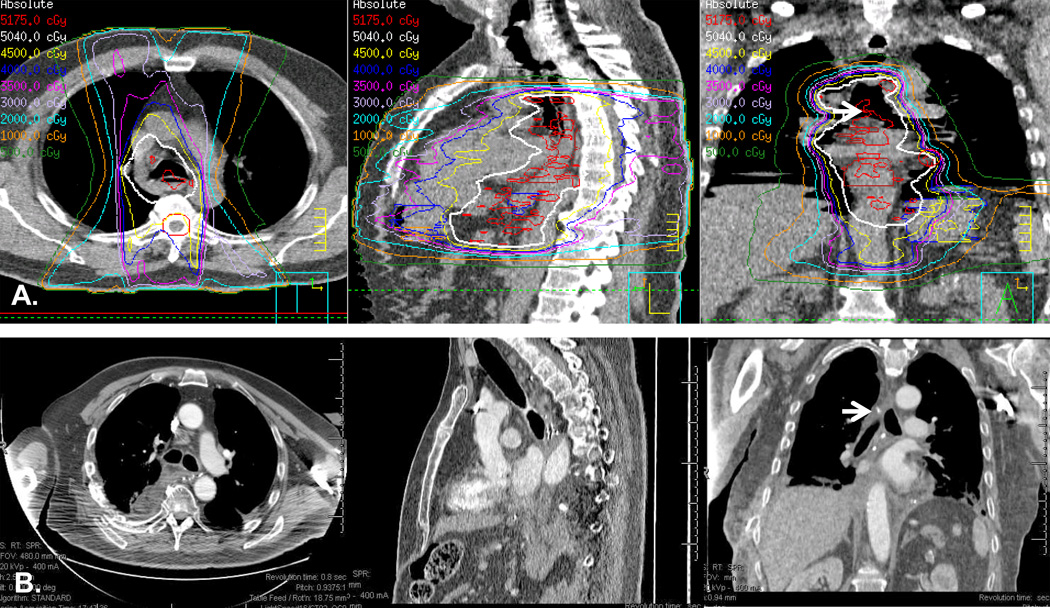
Figure 2. Illustration of a case with in-field anastomosis.
A. Simulation CT imaging of a mid-esophageal tumor with treatment plan encompassing areas below the aortic arch. B. Post-Ivor-Lewis esophagectomy CT imaging showing postoperative anatomy. White arrows point to the area of anastomosis at the level of carina within the radiation treatment field. This patient developed grade 3 anastomotic leak.
Acknowledgments
Source of Funding:
Funding was provided in part by The University of Texas MD Anderson Cancer Center and by the National Cancer Institute Cancer Center Support Grant CA016672. SHL has received funding under contract with STCube Pharmaceuticals; SGS is a consultant for GlaxoSmithKline. WLH is consultant for Ethicon.
Footnotes
Conflict of Interest
For the remaining authors none were declared.
References
- 1.Gockel I, Sultanov FS, Domeyer M, Goenner U, Junginger T. Developments in esophageal surgery for adenocarcinoma: a comparison of two decades. BMC Cancer. 2007;7:114. doi: 10.1186/1471-2407-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. The lancet oncology. 2007 Mar;8(3):226–334. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012 May 31;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 4.Hagry O, Coosemans W, De Leyn P, Nafteux P, Van Raemdonck D, Van Cutsem E, et al. Effects of preoperative chemoradiotherapy on postsurgical morbidity and mortality in cT3-4 +/- cM1lymph cancer of the oesophagus and gastro-oesophageal junction. Eur J Cardiothorac Surg. 2003 Aug 24;2:179–86. doi: 10.1016/s1010-7940(03)00274-4. discussion 86. [DOI] [PubMed] [Google Scholar]
- 5.Law S, Wong KH, Kwok KF, Chu KM, Wong J. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg. 2004 Nov;240(5):791–800. doi: 10.1097/01.sla.0000143123.24556.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds JV, Ravi N, Hollywood D, Kennedy MJ, Rowley S, Ryan A, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J Thorac Cardiovasc Surg. 2006 Sep;132(3):549–555. doi: 10.1016/j.jtcvs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Steyerberg EW, Neville BA, Koppert LB, Lemmens VE, Tilanus HW, Coebergh JW, et al. Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. J Clin Oncol. 2006 Sep 10;24(26):4277–4284. doi: 10.1200/JCO.2005.05.0658. [DOI] [PubMed] [Google Scholar]
- 8.Wang SL, Liao Z, Vaporciyan AA, Tucker SL, Liu H, Wei X, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys. 2006 Mar 1;64(3):692–699. doi: 10.1016/j.ijrobp.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Dewar L, Gelfand G, Finley RJ, Evans K, Inculet R, Nelems B. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg. 1992 May;163(5):484–489. doi: 10.1016/0002-9610(92)90393-6. [DOI] [PubMed] [Google Scholar]
- 10.Kim RH, Takabe K. Methods of esophagogastric anastomoses following esophagectomy for cancer: A systematic review. J Surg Oncol. 2010 May 1;101(6):527–533. doi: 10.1002/jso.21510. [DOI] [PubMed] [Google Scholar]
- 11.Vande Walle C, Ceelen WP, Boterberg T, Vande Putte D, Van Nieuwenhove Y, Varin O, et al. Anastomotic complications after Ivor Lewis esophagectomy in patients treated with neoadjuvant chemoradiation are related to radiation dose to the gastric fundus. Int J Radiat Oncol Biol Phys. 2012 Mar 1;82(3):e513–e519. doi: 10.1016/j.ijrobp.2011.05.071. [DOI] [PubMed] [Google Scholar]
- 12.Lin FC, Durkin AE, Ferguson MK. Induction therapy does not increase surgical morbidity after esophagectomy for cancer. Ann Thorac Surg. 2004;78(5):1783–1789. doi: 10.1016/j.athoracsur.2004.04.081. [DOI] [PubMed] [Google Scholar]
- 13.Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004 Jul;53(7):925–930. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita M, Masuda T, Okada S, Yoshinaga K, Saeki H, Tokunaga E, et al. Preoperative chemoradiotherapy for esophageal cancer: factors associated with clinical response and postoperative complications. Anticancer Res. 2009 Jul;29(7):2555–2562. [PubMed] [Google Scholar]
- 15.Patil PK, Patel SG, Mistry RC, Deshpande RK, Desai PB. Cancer of the esophagus: esophagogastric anastomotic leak--a retrospective study of predisposing factors. J Surg Oncol. 1992;49(3):163–167. doi: 10.1002/jso.2930490307. [DOI] [PubMed] [Google Scholar]
- 16.Cooke DT, Lin GC, Lau CL, Zhang L, Si MS, Lee J, et al. Analysis of cervical esophagogastric anastomotic leaks after transhiatal esophagectomy: risk factors, presentation, and detection. Ann Thorac Surg. 2009;88(1):177–184. doi: 10.1016/j.athoracsur.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Parsons A.M FDB, Detterbeck FC, Morris DE. Esophagogastric anastomosis inside or outside of the preoperative radiation field: Does it matter? Int J Radiat Oncol Biol Phys. 2005;63:S278–S279. [Google Scholar]
- 18.Schultze-Mosgau S, Grabenbauer GG, Wehrhan F, Radespiel-Troger M, Wiltfang J, Sauer R, et al. [Histomorphological structural changes of head and neck blood vessels after pre- or postoperative radiotherapy] Strahlenther Onkol. 2002 Jun;178(6):299–306. [PubMed] [Google Scholar]
- 19.Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol. 1997 Feb;42(2):99–106. doi: 10.1016/s0167-8140(96)01880-4. [DOI] [PubMed] [Google Scholar]
- 20.Tokarek R, Bernstein EF, Sullivan F, Uitto J, Mitchell JB. Effect of therapeutic radiation on wound healing. Clin Dermatol. 1994 Jan-Mar;12(1):57–70. doi: 10.1016/0738-081x(94)90257-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.