Abstract
Chronic cardiorespiratory disease is associated with low birthweight suggesting the importance of the developmental environment. Prenatal factors affecting fetal growth are believed important, but the underlying mechanisms are unknown. The influence of developmental programming on bronchial hyperreactivity is investigated in an animal model and evidence for comparable associations is sought in humans. Pregnant Wistar rats were fed either control or protein-restricted diets throughout pregnancy. Bronchoconstrictor responses were recorded from offspring bronchial segments. Morphometric analysis of paraffin-embedded lung sections was conducted. In a human mother-child cohort ultrasound measurements of fetal growth were related to bronchial hyperreactivity, measured at age six years using methacholine. Protein-restricted rats' offspring demonstrated greater bronchoconstriction than controls. Airway structure was not altered. Children with lesser abdominal circumference growth during 11–19 weeks' gestation had greater bronchial hyperreactivity than those with more rapid abdominal growth. Imbalanced maternal nutrition during pregnancy results in offspring bronchial hyperreactivity. Prenatal environmental influences might play a comparable role in humans.
Under the ‘developmental origins of health and disease’ hypothesis physiological and anatomical changes invoked by early environmental factors are able to influence later health. This is sometimes referred to as ‘programming’ or ‘developmental induction’1. Factors capable of invoking developmental induction before birth include maternal diet2, body composition3 and endocrine status4. Epidemiological evidence suggests that faltering fetal growth is associated with adverse respiratory outcomes. Following from early observations of increased chronic obstructive pulmonary disease in adults who were of low birthweight5, studies linking faltering fetal growth to wheeze in childhood have provided further evidence that early environmental factors can influence respiratory development6,7. These epidemiological observations do not provide information about underlying pathophysiological mechanisms; to understand these, animal models of fetal growth restriction are required8. Moreover, these models should reflect that whilst the majority of of human infants in westernized countries are of normal weight at birth, adverse consequences may occur as a result of growth faltering during a critical window of development.
Animal data demonstrate that maternal protein restriction in rats results in hypertension in the offspring9. This is linked to clinical evidence that aortic compliance is lower in adults born at low birthweight10 and that low birthweight individuals have an increased likelihood in adulthood of cardiovascular disease, including hypertension11. In part, the association between early growth restriction and hypertension may reflect adaptive changes affecting vascular smooth muscle. Animal and epidemiological studies suggest that bronchial smooth muscle might be similarly sensitive to environmental influences. Hyperreactivity of bronchial smooth muscle has been demonstrated in individuals born at low birthweight (a surrogate for restricted fetal growth)12,13. Animal models also show bronchial hyperreactivity (BHR) to be present in mice exposed to adverse environmental factors which are likely to restrict fetal growth, for example maternal stress14. We hypothesize that an adverse in utero environment, in this case imbalanced nutrition, is associated with BHR in the offspring. The primary objective of the animal work included in this study was to investigate this using a model which has already demonstrated a number of the cardiovascular risk factors associated with poor fetal growth (including hypertension and endothelial dysfunction)15,16, Since Rho A has been implicated in bronchial hyper-responsiveness in mouse models17, rat models18 and humans19,20 a secondary objective was to use the animal model to explore whether Rho A, and associated kinases ROCK1 and ROCK2 may be sensitive to developmental stress and hence serve as a link between adverse factors in the fetal environment and later BHR. Finally, to test the relevance of factors affecting fetal growth to human respiratory development, we analysed data from an epidemiological cohort where both detailed prenatal ultrasound measurements and childhood BHR measurements are available.
Results
Animal model
Birthweight and growth
There were no between group differences in litter size or birthweight. This was true for both male (C, 7.60 g ± 0.16, n = 7; PR, 7.74 g ± 0.40, n = 6; P > 0.05) and female (C, 7.41 g ± 0.19, n = 7; PR, 7.46 g ± 0.34, n = 6; P > 0.05) pups. Similarly growth of the offspring was not different between the groups (data not shown) and there was no significant difference between the rats at 75 days of age (C, 320.6 g; PR, 280.4 g; p > 0.05).
Bronchoconstriction
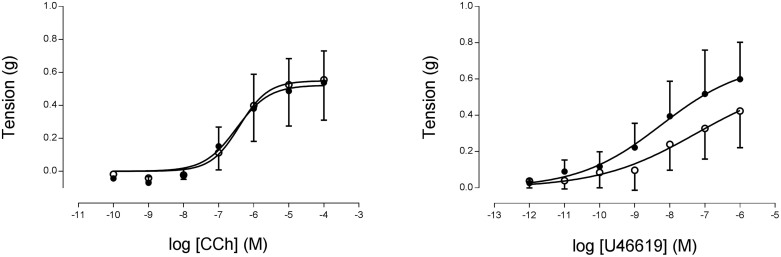
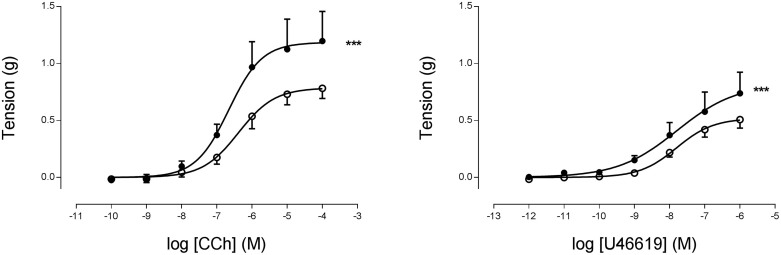
In isolated bronchi from 35-day-old rats, both CCh and U46619 produced a concentration-dependent constriction that was similar between groups (p > 0.05; Figure 1). By 75 days of age maximal response to both agonists was significantly increased in the PR bronchi compared to controls (p < 0.001; Figure 2). In the presence of the Rho kinase inhibitor Y27632, differences in response to carbachol were abolished (Figure S1). Responses to the depolarising KPSS wash were similar between the groups at both time points (data not shown).
Figure 1. Cumulative addition of (A) CCh or (B) the thromboxane mimetic U46619 to isolated bronchi from 35 day old male offspring from C (o, n = 6) or PR (•, n = 5) dams.
Figure 2. Cumulative addition of (A) CCh or (B) U46619 to isolated bronchi from 75 day old male offspring from C (o, n = 6) or PR (•, n = 4) dams.
*** indicates p < 0.001% max response C vs. PR.
RhoA, ROCK1 and ROCK2 mRNA expression levels
In isolated bronchi from 75-day-old male offspring, mRNA levels of Rho A were similar between the groups (C, 0.59 ± 0.03, n = 5; PR, 0.60 ± 0.02, n = 4; P > 0.05). Equally, mRNA levels of ROCK1 (C, 0.62 ± 0.11, n = 5; PR, 0.65 ± 0.05, n = 4; P > 0.05) and ROCK2 (C, 0.78 ± 0.04, n = 5; PR, 0.94 ± 0.13, n = 4; P > 0.05) were not different between the two groups.
Morphometry
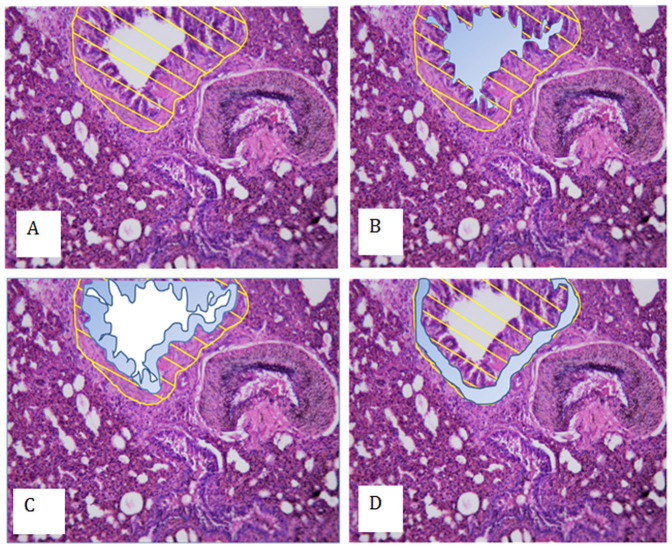
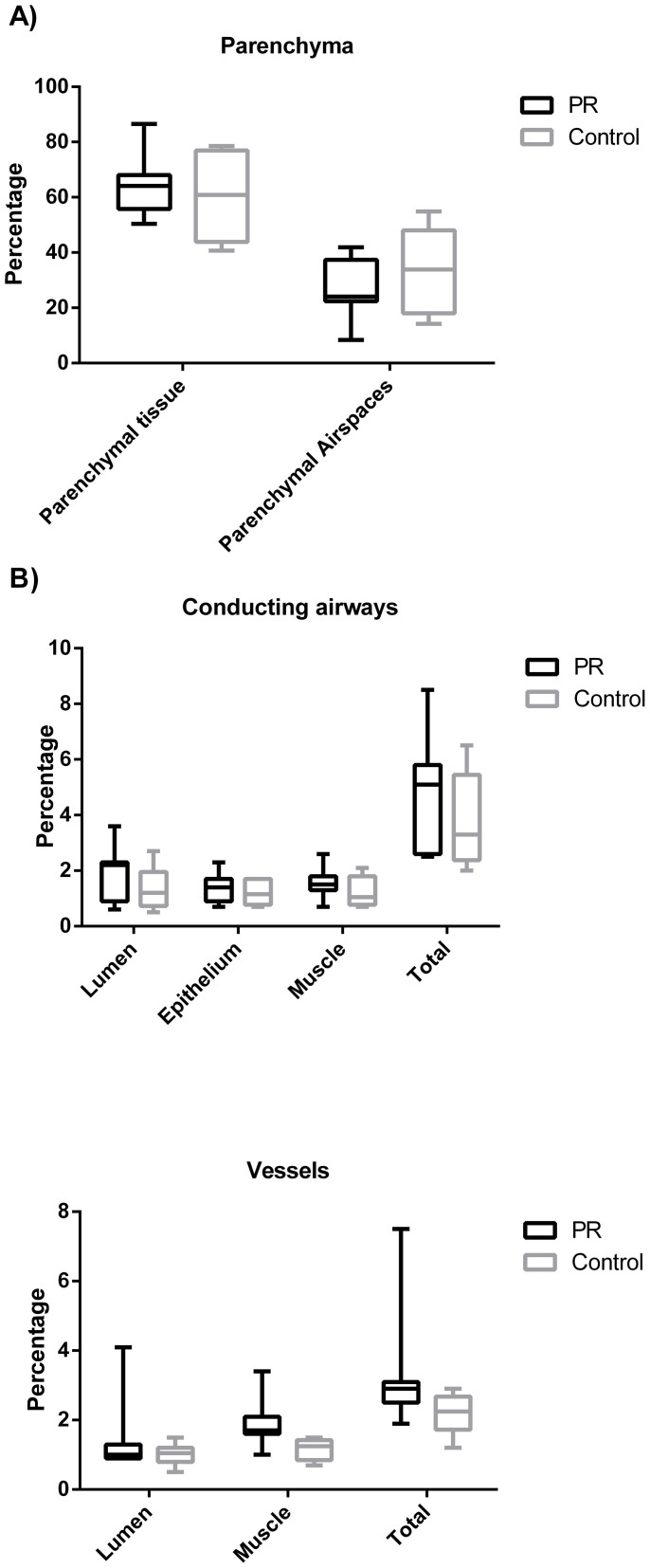
There was no between group difference in percentage airway smooth muscle at day 225. Equally, no between group differences were seen for percentage of any other airway component (lumen or epithelium), percentage parenchymal tissue or airspaces, or vessel lumen. The volume fraction of vascular muscle was significantly greater in the protein restricted group compared to control (median 1.70 vs. 1.25, p = 0.01) (Figures 3 and 4).
Figure 3. Comparison of airway wall histology in offspring of control and protein restricted mothers.
Figure 4. Volume factionation of lung components as percentage of entire lung for both PR and C groups at 225 days of age (C = 6, PR = 6).
Fetal growth and BHR in childhood
Mothers and children followed up to 6 years were broadly similar in terms of asthma, atopy and allergic disorders to those who were not followed up. Participant mothers were, however, of lower parity and were slightly older, taller, less likely to smoke in pregnancy and were of higher educational attainment than non-participants (Table S2). Of the participating mother-child pairs, those who provided methacholine provocation challenge data had a significantly higher number of siblings than those who did not complete a methacholine challenge but were otherwise comparable (Table S3). Maternal smoking during pregnancy was not found to be a confounder of the relationship between fetal growth and BHR.
Methacholine provocation challenge
Higher inverse log values of the dose response slope, (lower BHR), were significantly associated with increasing conditional abdominal circumference growth between 11 and 19 weeks' gestation (p = 0.037). That is to say, greater faltering of abdominal growth was associated with greater BHR. There were no significant associations between birthweight and BHR. Moreover, no association was found between abdominal circumference growth between 19 and 34 weeks' gestation, or birth head or abdominal circumference and BHR (Table 1).
Table 1. Linear regression for fetal and infant growth variables and BHR.
| Unadjusted analysis | Adjusted analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P-value | n | Beta | 95% CI | P-value | n | |
| Birthweight | −0.040 | −0.400 to 0.319 | 0.83 | 225 | −0.051 | −0.408 to 0.307 | 0.78 | 224 |
| Head circumference at birth | −0.001 | −0.362 to 0.359 | 0.99 | 225 | −0.017 | −0.376 to 0.341 | 0.92 | 224 |
| Abdominal circumference at birth | 0.108 | −0.261 to 0.478 | 0.56 | 225 | 0.134 | −0.239 to 0.506 | 0.48 | 221 |
| Head circumference growth between 11 and 19 weeks' gestation | −0.152 | −0.678 to 0.374 | 0.57 | 114 | −0.224 | −0.726 to 0.279 | 0.38 | 113 |
| Abdominal circumference growth between 11 and 19 weeks' gestation | 0.490 | −0.138 to 1.119 | 0.13 | 110 | 0.643 | 0.038 to 1.247 | 0.037 | 109 |
| Head circumference growth between 19 and 34 weeks' gestation | −0.066 | −0.410 to 0.278 | 0.71 | 216 | −0.050 | −0.393 to 0.293 | 0.77 | 213 |
| Abdominal circumference growth between 19 and 34 weeks' gestation | 0.131 | −0.199 to 0.461 | 0.43 | 224 | 0.246 | −0.095 to 0.584 | 0.15 | 221 |
Measurements of circumferential growth were calculated as the conditional change in the relevant circumference between either 11 and 19 or 19 and 34 weeks' gestation conditional on either 11 or 19 week circumference respectively. Measurements were corrected for regression to the mean and exact age at measurement Not all women were scanned at each time point and positioning of the fetus occasionally precluded some measurements. If identified as a likely confounder in the multivariate model, the following were adjusted for: maternal history of asthma, eczema, rhinitis or atopy; paternal history of asthma, eczema or rhinitis; maternal age, body mass index, height, smoking in pregnancy, educational achievement and parity; child's gender and parental social class.
Discussion
This paper demonstrates, for the first time, BHR following maternal dietary restriction in an animal model. This is supported by observations in a human population birth-cohort. In the offspring of protein-restricted rats, bronchoconstriction to both CCh and U46619 was significantly enhanced compared to control animals; this phenotype was not due to altered airway structure. We also demonstrated an inverse association between abdominal circumference growth between 11 and 19 weeks' gestation and BHR measured in 6-year-old children. These findings suggest that unfavourable in utero conditions might adversely affect respiratory development and perhaps predispose to subsequent respiratory disease.
This study shows that restricted fetal growth is associated with BHR in both rats and humans. Our data also suggest that airway smooth muscle changes may underlie this association. These are novel animal model findings that are supported by the Southampton Women's Survey, one of few epidemiological cohorts with detailed longitudinal ultrasound scan measurements of fetal growth. Reduced abdominal growth is recognized as an important fetal adaptation to in utero adversity, which is believed to protect brain growth27. Notably there was no association between infant birthweight and BHR in childhood; the association found between reduced abdominal growth in early pregnancy and BHR shows similarities to the findings in the animal model and suggests common ‘programming’ mechanisms, possibly mediated via subtly altered fetal growth patterns acting in both animals and humans subjected to developmental stress. The significant relationship between abdominal circumference growth in early pregnancy and BHR may reflect a period of particular importance in bronchial smooth muscle development. Recently, Zaina-Taieb et al have used a very similar protocol to ours in order to study the development of bronchopulmonary dysplasia. Their study looked at early morphometry with differences in alveolarisation, whereas our study found no difference at 28 or 225 d. Interestingly, Zana-Taieb et al showed significant differences in birthweight, such that the protein restricted group could be considered IUGR. Previous studies have also linked IUGR to changes in lung structure whilst our study produced normal sized offspring with hyper-reactive airways. It is not clear how their protocol produced IUGR offspring whilst ours did not.
The significance of early growth restriction in relation to respiratory health has been appreciated since the report of associations between infant weight and death in adulthood from respiratory disease5. Moreover, impaired lung function has been found in individuals who were of low birthweight28,29,30. Reduced forced expiratory flows have been demonstrated in lower birthweight individuals within a group of term babies of normal average birthweight; the lowest lung function was found in infants who gained weight rapidly after birth31. This pattern of growth may indicate mismatched pre- and postnatal nutrient supply and thus identify infants subjected to fetal growth restriction. Within the same cohort lower infant lung function has been shown to be associated with wheezing illnesses in early childhood32. Taken together with epidemiological evidence showing lower birthweight to be associated with later respiratory morbidity, including asthma33 and chronic obstructive pulmonary disease5, this suggests lung function may be persistently impaired following fetal growth faltering and supports the concept of lung function ‘tracking’ throughout life34.
An association between low birthweight and BHR has been recognized for some time35 but studies addressing the effect of fetal growth restriction upon this specific aspect of lung function have been confounded by the effects of respiratory complications of prematurity and their treatment36,37. Although there is a wealth of evidence on low birthweight and respiratory health38,39, there is a relative paucity of data relating these outcomes to fetal growth within those who are not classified as growth restricted. Offspring from our animal study are of normal birthweight but were exposed to nutrient stress during prenatal development, similarly our human data include infants whose birth weights were within the normal range but whose growth, possibly due to adverse environmental influences, faltered during a critical developmental window. This may be highly relevant to understanding the prenatal origins of respiratory disease in the developed world where most children are born well grown but some have suffered a stress during a critical developmental window. Four studies have explored the relationship between ultrasound measures of fetal size and wheeze; of these, three found an association between restricted fetal growth and childhood wheeze6,7,40. Recently a fourth study failed to find any such association41, although this study did not assess equivalent dimensions at each gestation. Twin studies, which study the effects of fetal growth restriction independently from shared gestation, have demonstrated greater BHR to cold air challenge in the smaller twin of twin pairs discordant for birthweight13. In the present study fetal growth was directly assessed by longitudinal ultrasound of equivalent dimensions and confounding by neonatal respiratory disease or treatment is unlikely as participants were term subjects of normal birthweight.
Animal data suggest that adverse prenatal factors which are likely to affect fetal growth are associated with increased BHR. For example, increased BHR has been found in offspring of mice exposed to noise stress during pregnancy14. Similarly, we demonstrate that isocaloric reduction of maternal protein intake in rats leads to BHR in the offspring. BHR is thought to arise from an interplay between airway remodelling, inflammatory processes and altered smooth muscle18,42,43,44, however the exaggerated bronchial responsiveness in our model was not associated with gross airway remodelling. This suggests that BHR precedes bronchial remodelling, that remodelling is not a prerequisite for BHR development and that developmental factors may alter intrinsic smooth muscle function. Recently it has been shown that chronic airway smooth muscle stimulation can lead to muscle shortening and greater constriction to subsequent challenges45, while airway remodelling has been reported in response to a methacholine challenge alone46. Taken together this suggests repeated constrictions may predispose to remodelling in their own right.
A possible explanation for the exaggerated constriction in the present study is altered signalling in the bronchial smooth muscle contractile apparatus. Maternal nutrient restriction in sheep leads to excessive vasoconstriction in specific arterial beds of the offspring47,48,49 which is associated with an increase in myosin light chain kinase mRNA levels47,48. An increase in contractile apparatus could explain our results, but such increases would also have manifested in an increased contraction to a depolarising K+ wash. This did not occur, and enhanced constriction was only seen in response to agonists, suggesting a role for Rho A and the Ca2+-independent pathway. Rho A and its downstream target Rho-associated kinase enhance constriction by inactivating myosin light chain phosphatase50. Previous reports have demonstrated that inhibition of Rho associated kinase leads to decreased bronchial contraction51, a finding which we have confirmed in the present study. Crucially, however, we have shown that the exaggerated response to CCh seen in the PR group is no longer seen in the presence of the Rho kinase inhibitor, suggesting a key role for Rho A in the BHR induced by maternal protein restriction. At present it is unclear why increased bronchial responsiveness was seen in the 75-day-old but not the 35-day-old rats, although potentially an age-dependent change in Rho A or Ca2+-dependent signalling may explain this. Other studies have specifically implicated translocation of Rho A to the membrane in the development of BHR18. The importance of this post-translational modification might also explain why we detected no difference between experimental groups in Rho A mRNA. Further investigation within this animal model would support exploration of the mechanisms believed to underlie increased BHR, whilst in vivo animal model testing would confirm the relevance of the model to human disease.
In summary, in utero stress conferred by protein restriction during pregnancy increases bronchial reactivity in rats and there are epidemiological data that suggest a similar effect might exist in humans. Taken together these findings suggest adverse in utero conditions are associated with a specific impairment of respiratory development.
Methods
Ethics statement
All animal procedures carried out in this study were in accordance with the regulations of the British Home Office Animals (Scientific Procedures) Act, 1986 (under licence number PPL 30/2884) and this study was approved by the local ethical review committee. Animals were sacrificed by cervical dislocation. For human participants written informed parental consent was obtained and ethical approval for this and all other human aspects of the study was granted by the Southampton and South West Hampshire Research Ethics Committee (276/97, 307/97, 089/99, 06/Q1702/104).
Animal model
Virgin female Wistar rats (Harlan, UK) were mated with stud males. After confirming conception mothers were fed either a control (C; 18% casein) or a protein-restricted diet (PR; 9% casein)21. Diets were isocaloric and of comparable vitamin and mineral content16. Mothers and pups were fed standard laboratory chow postpartum.
Pups were weighed at 48 hours and litters culled to eight. Weaning was at 21 days. At 35, 75 or 225 days of age male offspring were sacrificed and lung tissue harvested.
Assessment of bronchoconstriction
Lungs were excised and placed in 4°C physiological salt solution (PSS), (NaCl, 119; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.17; NaHCO3, 25; KH2PO4, 1.18; EDTA, 0.026; and D-glucose, 5.5 mM). Third generation bronchi were dissected into 2 mm segments and mounted on a wire myograph (Danish Myo Technology A/S, DK). Segments were maintained in 37°C PSS, gassed with 95% O2 and 5% CO2.
Segments were stretched to optimal resting tension (1.5 g) and allowed one hour equilibration. Functional integrity was tested using 125 mM KPSS solution (PSS with equimolar KCl for NaCl substitution). Cumulative concentration-response curves were constructed for the acetylcholine mimetic, carbachol (Sigma-Aldrich®) (CCh, 1 nM–10 μM) and the thromboxane mimetic, U46619 (Tocris Bioscience) (1 pM–1 μM). In a subgroup, CCh responses were repeated in the presence of the Rho kinase inhibitor Y27632 (Sigma-Aldrich; 10 μM).
Molecular biology
mRNA expression in isolated bronchi was determined using real time PCR relative to β-actin (TaqMan, Applied Biosystems, Warrington, U.K.). Primers and probes are given in Table S3.
Morphometry
Left lungs were formalin fixed for 24 hours and embedded in Paraffin wax. An unbiased stereological analysis approach was employed22. Starting at a random point a 5 um section was cut 500 μm intervals throughout the entire lung. Sections were H&E stained, 30–70 photographs taken of each section and 1 in 10 analysed to determine the volume fraction of airway tissue, airway lumen, epithelium, smooth muscle using a point counting system (Figure 4).
Human participants
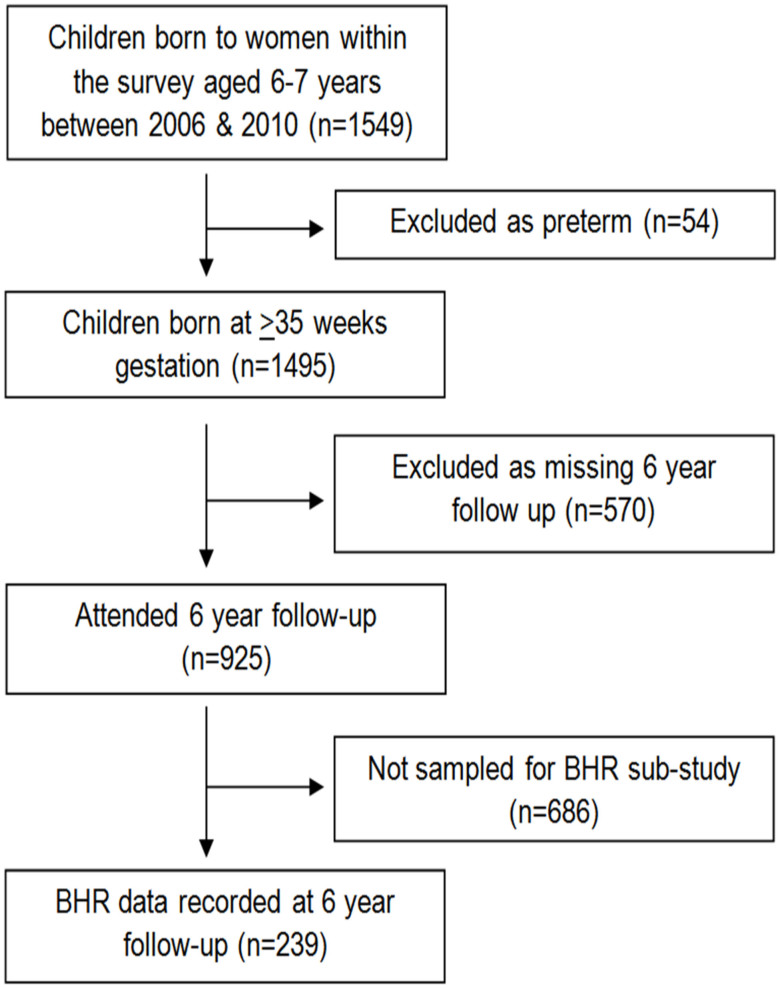
Participants were mother-child pairs from the Southampton Women's Survey23. Six-year-old children were invited for respiratory follow-up; 246 underwent methacholine challenge (Figure 5). Seven children born < 35 weeks' gestation were excluded to remove the effects of prematurity.
Figure 5. Follow-up within the Southampton Women's Survey.
Fetal growth
Gestational age was determined from last menstrual period and early ultrasound data. Fetal head and abdominal circumferences at 11, 19 and 34 weeks' gestation were measured according to standardized landmarks using Acuson 128 XP, Aspen and Sequoia ultrasound machines calibrated to 1540 m/s. Head and abdominal circumferences and weight were measured at birth. Conditional velocities of prenatal head and abdominal circumference growth were calculated, correcting for age at measurement and regression to the mean24.
Bronchial hyperreactivity
Bronchial hyperreactivity was measured by bronchial provocation challenge, according to ATS/ERS guidelines25. Incremental methacholine concentrations (0.06 mg/ml to 16 mg/ml) were delivered using a dosimeter (Koko; PDS Instrumentation; Louisville, USA) and a compressed air driven nebulizer (Sidestream®; Respironics, UK). Challenges were terminated following a 20% fall in the FEV1 or, if this did not occur, following the 16 mg/ml dose. BHR was expressed as the inverse of the slope of the regression line through FEV1 drop and logged methacholine concentration such that lower inverse log slope values indicate increased BHR.
Log slope = 100/[regression slope of FEV1 drop and log10(cumulative methacholine dose) + 10].
A constant removes negative values and an inverse transformation ensures the variable is normally distributed26.
Statistical analysis
Animal data are mean ± (S.E.M) and constrictor responses are change in raw tension (g) or percentage KPSS-induced tone. Cumulative response curves were analysed by fitting a four-parameter logistic equation using non-linear regression to obtain pEC50 (effective concentration equal to 50% of maximum) and maximal response which were compared by Student's t test (Prism 5.0, GraphPAD software Inc., San Diego, CA, U.S.A.). Significance was accepted if p < 0.05. The investigator was blinded to dietary group. Lung structures were expressed as percentage of total lung and compared using the Mann Whitney rank sum test.
Childhood BHR was expressed as the inverse slope of the regression line through FEV1 fall and logged methacholine concentration, lower inverse log slope indicating increased BHR26. Using this continuous outcome it was possible to use data from all participants and to maximize power to assess the relationship between fetal growth and BHR using linear regression. The following potential confounders were identified a priori: maternal age, body mass index, height, smoking in pregnancy, education, parity, history of asthma, eczema, rhinitis or atopy; paternal history of asthma, eczema or rhinitis; child's gender and parental social class. A forward stepwise multivariate model was built including all variables associated at p ≤ 0.1. Size and growth velocity measures were standardized and outcomes were expressed as change in BHR per SD change in predictor. Stata®11 (Stata Corp., College Station, TX) was used for all analyses. An online supplement provides additional detail.
Author Contributions
J.W.H., C.T., J.S.A.L., K.M.G., H.M.I., C.C., S.M.R. and G.R. designed research; K.C.P., J.S.A.L., C.T., E.R.T., S.A.C., H.A.W., S.W., S.A.C., P.H.M.K. and S.A.D. conducted research; H.M.I., S.A.D., C.T. and K.C.P. analysed data; K.C.P., C.T., S.A.C. and J.W.H. wrote the paper; J.W.H. and G.R. had primary responsibility for final content. All authors read and approved the final manuscript.
Additional information
Funding This work within the Southampton Women's Survey has been funded by the Medical Research Council, University of Southampton, The Gerald Kerkut Charitable Trust, British Heart Foundation, and the Food Standards Agency (contract no N05071). The research is supported by infrastructure provided by the NIHR Southampton Respiratory Biomedical Research Unit, the NIHR Southampton Biomedical Research Centre and the NIHR Wellcome Trust Clinical Research Facility. Dr Katharine Pike was supported by a grant from the British Lung Foundation. Dr Shelley Davis was supported by a Medical Research Council Respiratory Capacity Building PhD Studentship.
Supplementary Material
supplementary data
Acknowledgments
We are grateful to the Southampton Women's Survey Study Group and the nurses of the Southampton Wellcome Trust Clinical Research Facility for their assistance in collecting human data for this study.
Footnotes
K.M.G. has acted as a consultant to Abbott Nutrition and Nestle Nutrition, and has received reimbursement for speaking at an Abbott Nutrition Conference on Pregnancy Nutrition and Later Health Outcomes and at a Nestle Nutrition Institute Workshop. He is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. PHMK has received PhD funding from the Gerald Kerkut Charitable Trust and travel support from the Physiological society. H.M.I. has received funding from Bayer plc for a statistical consultancy and also has grants pending with the Heart Foundation and Asthma Research UK. John Holloway has received support for a PhD studentship for SAD from the Medical Research Council. The remaining authors have no conflicts of interests to declare.
References
- Pike K. C., Hanson M. A. & Godfrey K. M. Developmental mismatch: consequences for later cardiorespiratory health. BJOG 115, 149–157 (2008). [DOI] [PubMed] [Google Scholar]
- Seaton A. From nurture to Nature--the story of the Aberdeen asthma dietary hypothesis. QJM 101, 237–239 (2008). [DOI] [PubMed] [Google Scholar]
- Weiss S. T. Obesity: insight into the origins of asthma. Nat Immunol 6, 537–539 (2005). [DOI] [PubMed] [Google Scholar]
- von Hertzen L. C. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J Allergy Clin Immunol 109, 923–928 (2002). [DOI] [PubMed] [Google Scholar]
- Barker D. J. et al. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 303, 671–675 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike K. C. et al. Patterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 years. Thorax 65, 1099–1106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S. et al. First- and second-trimester fetal size and asthma outcomes at age 10 years. Am J Respir Crit Care Med 184, 407–413 (2011). [DOI] [PubMed] [Google Scholar]
- Krauss-Etschmann S. et al. Of flies, mice and men: a systematic approach to understanding the early life origins of chronic lung disease. Thorax 68, 380–384 (2013). [DOI] [PubMed] [Google Scholar]
- Langley S. C. & Jackson A. A. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 86, 217–222 (1994). [DOI] [PubMed] [Google Scholar]
- Martyn C. N. et al. Growth in utero, adult blood pressure, and arterial compliance. Br Heart J 73, 116–121 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J., Bull A. R., Osmond C. & Simmonds S. J. Fetal and placental size and risk of hypertension in adult life. BMJ 301, 259–262 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. N., Elliman A., Bryan E. & Silverman M. Clinical significance of airway responsiveness in children of low birthweight. Pediatr Pulmonol 7, 251–258 (1989). [DOI] [PubMed] [Google Scholar]
- Nikolajev K., Koskela H. & Korppi M. Birth weight and adult lung function: a within-pair analysis of twins followed up from birth. World J Pediatr 4, 222–226 (2008). [DOI] [PubMed] [Google Scholar]
- Pincus-Knackstedt M. K. et al. Prenatal stress enhances susceptibility of murine adult offspring toward airway inflammation. J Immunol 177, 8484–8492 (2006). [DOI] [PubMed] [Google Scholar]
- Brawley L. et al. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res 54, 83–90 (2003). [DOI] [PubMed] [Google Scholar]
- Rodford J. L. et al. Endothelial dysfunction and reduced antioxidant protection in an animal model of the developmental origins of cardiovascular disease. J Physiol 586, 4709–4720 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenrath M. et al. Rho-kinase and contractile apparatus proteins in murine airway hyperresponsiveness. Exp Toxicol Pathol 60, 9–15 (2008). [DOI] [PubMed] [Google Scholar]
- Chiba Y., Sakai H. & Misawa M. Augmented acetylcholine-induced translocation of RhoA in bronchial smooth muscle from antigen-induced airway hyperresponsive rats. Br J Pharmacol 133, 886–890 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y., Matsusue K. & Misawa M. RhoA, a possible target for treatment of airway hyperresponsiveness in bronchial asthma. J Pharmacol Sci 114, 239–47 (2010). [DOI] [PubMed] [Google Scholar]
- Schaafsma D., Gosens R., Zaagsma J., Halayko A. J. & Meurs H. Rho kinase inhibitors: A novel therapeutical intervention in asthma? Eur J Pharmacol 585, 398–406 (2008). [DOI] [PubMed] [Google Scholar]
- Torrens C., Poston L. & Hanson M. A. Transmission of raised blood pressure and endothelial dysfunction to the F2 generation induced by maternal protein restriction in the F0, in the absence of dietary challenge in the F1 generation. Br J Nutr 100, 760–766 (2008). [DOI] [PubMed] [Google Scholar]
- Howard C. V. & Reed M. G. Unbiased stereology: Three-dimensional measurement in microscopy. (Bios Scientific Publishers, Oxford, 1998). [Google Scholar]
- Inskip H. M. et al. Cohort profile: The Southampton Women's Survey. Int J Epidemiol 35, 42–48 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P. Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med 14, 1417–1436 (1995). [DOI] [PubMed] [Google Scholar]
- Crapo R. O. et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 161, 309–329 (2000). [DOI] [PubMed] [Google Scholar]
- Chinn S., Arossa W. A., Jarvis D. L., Luczynska C. M. & Burney P. G. Variation in nebulizer aerosol output and weight output from the Mefar dosimeter: implications for multicentre studies. Eur Respir J 10, 452–456 (1997). [DOI] [PubMed] [Google Scholar]
- Campbell S. & Thoms A. Ultrasound measurement of the fetal head to abdomen circumference ratio in the assessment of growth retardation. Br J Obstet Gynaecol 84, 165–174 (1977). [DOI] [PubMed] [Google Scholar]
- Nikolajev K., Heinonen K., Hakulinen A. & Lansimies E. Effects of intrauterine growth retardation and prematurity on spirometric flow values and lung volumes at school age in twin pairs. Pediatr Pulmonol 25, 367–370 (1998). [DOI] [PubMed] [Google Scholar]
- Rona R. J., Gulliford M. C. & Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ 306, 817–820 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D. A., Ebrahim S. & Davey Smith G. Association of birth weight with adult lung function: findings from the British Women's Heart and Health Study and a meta-analysis. Thorax 60, 851–858 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. S. et al. Small size at birth and greater postnatal weight gain: relationships to diminished infant lung function. Am J Respir Crit Care Med 170, 534–540 (2004). [DOI] [PubMed] [Google Scholar]
- Pike K. C. et al. The relationship between infant lung function and the risk of wheeze in the preschool years. Pediatr Pulmonol 46, 75–82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen S. O., Sterne J. A., Montgomery S. M. & Azima H. Birth weight, body mass index and asthma in young adults. Thorax 54, 396–402 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen S. The beginnings of chronic airflow obstruction. Br Med Bull 53, 58–70 (1997). [DOI] [PubMed] [Google Scholar]
- Chan K. N. et al. Airway responsiveness in low birthweight children and their mothers. Arch Dis Child 63, 905–910 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J. M., Riley S. P., Popkin J. & Coates A. L. The long-term pulmonary sequelae of prematurity: the role of familial airway hyperreactivity and the respiratory distress syndrome. N Engl J Med 312, 742–745 (1985). [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Ohki Y., Nako Y. & Morikawa A. Transcutaneous oxygen tension measurements during methacholine challenge of prematurity in infants with chronic lung disease. Pediatr Pulmonol 25, 338–342 (1998). [DOI] [PubMed] [Google Scholar]
- Tedner S. G., Örtqvist A. K. & Almqvist C. Fetal growth and risk of childhood asthma and allergic disease. Clin Exp Allergy 42, 1430–1447 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike K., Pillow J. J. & Lucas J. S. Long term respiratory consequences of intrauterine growth restriction. Sem Fetal Neonatal Med 17, 92–98 (2012). [DOI] [PubMed] [Google Scholar]
- Turner S. W. et al. Associations between fetal size, maternal α-tocopherol and childhood asthma. Thorax 65, 391–397 (2010). [DOI] [PubMed] [Google Scholar]
- Sonnenschein-van der Voort A. M. et al. Fetal and infant growth and asthma symptoms in preschool children: the Generation R Study. Am J Respir Crit Care Med 185, 731–737 (2012). [DOI] [PubMed] [Google Scholar]
- Oliver M. N. et al. Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason? Am J Respir Cell Mol Biol 37, 264–272 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermode J. A. et al. The effect of airway remodelling on airway hyper-responsiveness in asthma. Respir Med 105, 1798–1804 (2011). [DOI] [PubMed] [Google Scholar]
- Lei Y., Cao Y., Zhang Y., Edvinsson L. & Xu C. B. Enhanced airway smooth muscle cell thromboxane receptor signaling via activation of JNK MAPK and extracellular calcium influx. Eur J Pharmacol 650, 629–638 (2011). [DOI] [PubMed] [Google Scholar]
- Bosse Y., Chin L. Y., Pare P. D. & Seow C. Y. Chronic activation in shortened airway smooth muscle: a synergistic combination underlying airway hyperresponsiveness? Am J Respir Cell Mol Biol 42, 341–348 (2010). [DOI] [PubMed] [Google Scholar]
- Grainge C. L. et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med 364, 2006–2015 (2011). [DOI] [PubMed] [Google Scholar]
- Khan O. A. et al. Effects of pre-natal and early post-natal undernutrition on adult internal thoracic artery function. Eur J Cardiothorac Surg 28, 811–815 (2005). [DOI] [PubMed] [Google Scholar]
- Cleal J. K. et al. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci U S A 104, 9529–9533 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens C. et al. Effects of pre- and periconceptional undernutrition on arterial function in adult female sheep are vascular bed dependent. Exp Physiol 94, 1024–1033 (2009). [DOI] [PubMed] [Google Scholar]
- Kimura K. et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245–248 (1996). [DOI] [PubMed] [Google Scholar]
- Yamagata S. et al. Effect of a calcium sensitization modulator, Y-27632, on isolated human bronchus and pulmonary artery. Pulm Pharmacol Ther 13, 25–29 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary data