Abstract
Albuminuria is associated with metabolic syndrome and diabetes. It correlates with the progression of chronic kidney disease, particularly with tubular atrophy. The fatty acid load on albumin significantly increases in obesity, presenting a proinflammatory environment to the proximal tubules. However, little is known about changes in the redox milieu during fatty acid overload and how redox-sensitive mechanisms mediate cell death. Here, we show that albumin with fatty acid impurities or conjugated with palmitate but not albumin itself compromised mitochondrial and cell viability, membrane potential and respiration. Fatty acid overload led to a redox imbalance which deactivated the antioxidant protein peroxiredoxin 2 and caused a peroxide-mediated apoptosis through the redox-sensitive pJNK/caspase-3 pathway. Transfection of tubular cells with peroxiredoxin 2 was protective and mitigated apoptosis. Mitochondrial fatty acid entry and ceramide synthesis modulators suggested that mitochondrial β oxidation but not ceramide synthesis may modulate lipotoxic effects on tubular cell survival. These results suggest that albumin overloaded with fatty acids but not albumin itself changes the redox environment in the tubules, inducing a peroxide-mediated redox-sensitive apoptosis. Thus, mitigating circulating fatty acid levels may be an important factor in both preserving redox balance and preventing tubular cell damage in proteinuric diseases.
Keywords: tubular cell, albuminuria, peroxiredoxin, apoptosis, lipotoxicity
one of the most devastating complications of diabetes mellitus is diabetic nephropathy. In association with type 2 diabetes and metabolic syndrome, obesity has been shown to be an independent risk factor for chronic kidney diseases (14, 18, 27). In relation to obesity and glomerular diseases, proteinuria is considered a reliable marker that correlates with the progression of renal disease, particularly with tubulointerstitial fibrosis (3, 9, 19). In patients, increased proteinuria translates to a poorer prognosis compared with those with lower or minimal protenuria.
The pathomechanisms regarding how proteinuria aggravates the progression of tubular damage are not yet clearly understood. Albuminuria is a complex issue and the exact role of albumin as a mediator of pathological changes in the tubules has been a matter of constant debate. This includes the origin of albuminuria (glomerular protein leakage or dysfunctional tubular reabsorption) (5, 15) and the toxicity of delipidated albumin vs. albumin with fatty acid (FA) ligands. Novel studies demonstrate that even in physiological situations, nephrotic amounts of albumin are reabsorbed normally in the tubules (6, 20). This would indicate that albumin itself is not toxic to the proximal tubule. Lately it has been suggested that perhaps not albumin itself but various FAs carried on albumin may be more important in inducing pathological changes, primarily through indirect mechanisms. Indeed, circulating albumin can effectively bind and carry these FAs to the proximal tubules (25). In diseases associated with some degree of proteinuria such as metabolic syndrome or diabetes, albumin becomes saturated with FAs (28). It is therefore logical to postulate that these FAs can exacerbate tubulointerstitial injury (1, 24). Hence, in metabolic syndrome-related proteinuric diseases, the lipid moiety of albumin (but not necessarily albumin itself) may play a role in the associated cell death and aggravation of tubulointerstitial damage.
Mitochondria play a central role in cell energetics and homeostasis, and mitochondrial redox imbalance can also mediate tubular cell apoptosis through redox-sensitive signaling. Mitochondria have previously been shown to be a target of albumin-induced apoptosis (10) but the potential role of mitochondrial energy failure and redox imbalance and whether this is related to albumin or the bound lipid moiety have not been investigated. The role of redox signaling in cell homeostasis is now widely accepted but poorly understood in the renal environment, including that of the tubules. Superoxide or hydrogen peroxide (H2O2)—emitted either from mitochondrial or cellular sources—can regulate various events. Evidence of the participation of redox-sensitive protein thiols in these mechanisms is emerging. Peroxiredoxins, which are highly abundant ubiquitous antioxidant enzymes (13), can effectively control not only H2O2 but other organic peroxide and peroxynitrite levels as well (17, 26). They also participate in several peroxide-driven redox sensor mechanisms through their easily oxidized cysteine residues (4).
In this study, we exposed NRK-52E rat tubular epithelial cells to FA-free albumin, nondelipidated albumin, and palmitate (bound to albumin) to investigate the possibility of a redox imbalance and consequent redox-sensitive signaling in tubular apoptosis. We show that nondelipidated albumin and palmitate but not FA-free albumin itself induced a dose-dependent decline in mitochondrial and cell viability and function through a peroxide-mediated redox-sensitive signaling pathway. Through the participation of peroxiredoxin 2 (Prdx2), we suggest a new mechanism to link FAs to a redox imbalance-induced tubular cell apoptosis, where overexpression of Prdx2 rescued cells from apoptosis-related caspase-3 cleavage.
MATERIALS AND METHODS
Materials.
Cell culture media and JC-1 were from Life Technologies/Invitrogen (Grand Island, NY). Oligomycin, antimycin A, FCCP, etomoxir, 96% grade nondelipidated albumin (A7906), purified albumin (FA free, A8806), and sodium palmitate were all from Sigma (St. Louis, MO). Both albumin products were endotoxin-free. Palmitate was conjugated to albumin in a molar ratio of 6:1. Myriocin was from Cayman Chemical (Ann Arbor, MI).
Cell culture.
Rat tubular epithelial cells (NRK-52E, CRL-1571, ATCC, Manassas, VA) were maintained in DMEM with 5% fetal bovine serum in a cell culture incubator under 5% CO2 atmosphere. Cells were grown to 70% confluency and then split onto six-well plates, glass bottom confocal dishes, or special SeaHorse 24-well plates. All treatments were carried out in serum-free DMEM medium. Cells used were between passages 5–20.
For the overexpression of Prdx2, cells were transfected with a Prdx2 cDNA plasmid (MC200303, OriGene, Rockville, MD) according to the manufacturer's protocol. Optimal time of overexpression was confirmed by Western blotting.
Treatments were 1) nondelipidated albumin [BSA(FA) at 20 and 30 mg/ml for 6 and 24 h], 2) FA-free (pure) albumin at 20 and 30 mg/ml for 6 and 24 h, 3) FA-free BSA conjugated and saturated with palmitate at 150 and 300 μM for 6 and 24 h (with albumin concentrations of ∼1 and ∼3 mg/ml, respectively). In the FA entry/ceramide production modulating experiments, etomoxir was used at 100 μM together with 1 mM carnitine and myriocin was applied at 1 μM.
Mitochondrial viability.
Mitochondrial viability was assessed by the MTT assay where viable mitochondria convert the yellow tetrazolium salt 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) to purple formazan. After treatments, cells were washed and then incubated with 0.2 mg/ml MTT in PBS for 1 h at 37°C. MTT was removed, cells were solubilized in DMSO, and the conversion of MTT to formazan was measured at 550 nm in a spectrophotometer. Viability was expressed as percentage of control.
Mitochondrial membrane potential.
Changes in mitochondrial membrane potential (ΔΨ) upon treatments were assessed using the cationic probe JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimdazol carbocyanine iodide). At the end of the treatments, cells were washed and incubated with 10 μM JC-1 at 37°C for 10 min. Red and green fluorescence were determined qualitatively using confocal microscopy (Zeiss LSM510) as well as quantitatively in a Molecular Devices M5 spectrofluorometer with plate reader (green: excitation 485 nm, emission 535 nm; red: excitation 560 nm, emission 595 nm). Relative membrane potential was calculated as red/green ratio from three parallel readings.
Mitochondrial bioenergetics.
To study mitochondrial bioenergetics in NRK-52E cells, we utilized a SeaHorse XF24 Extracellular Flux Analyzer (SeaHorse Bioscience, Billerica, MA). Here, we adopted a protocol described previously by Darley-Usmar et al. (8) to determine the long-standing effects of tubular epithelial stressors on the bioenergetic parameters of the tubules. Seventy-five thousand cells/well were selected as optimal and seeded onto the SeaHorse XF24 V7 plates. We used sequential injections of oligomycin (2 μM), FCCP (4 μM), and antimycin A (10 μM) to determine basal oxygen consumption rate (OCR), ATP-linked respiration, maximal and reserve respiratory capacity. In each experiment, cells were either kept untreated or were divided into treatment groups described above in serum-free DMEM media. After treatments, cells were washed twice with DMEM and then 625 μl/well low-glucose serum-free DMEM, containing 5 mM pyruvate, was loaded onto the plates. Plates were equilibrated in a non-CO2 incubator at 37°C for 1 h before bioenergetic profiles were obtained.
Apoptosis/necrosis flow cytometry.
For early/late apoptosis and necrosis measurements in control, treated, or transfected cells, a commercially available Annexin V kit was used (Invitrogen) with FITC-Annexin V and propidium iodide labelings. After treatments and incubating cells according to instructions, a flow cytometer was used to analyze cells (10,000 cells were used in each reading).
Western blotting.
For Western blot studies, cells were washed twice in sterile PBS after treatments and harvested in lysis buffer containing a mixture of protease and phosphatase inhibitors, 2% Triton X-100, 300 mM NaCl, 20 mM Tris, 2 mM EDTA, and 1% NP-40. Samples were centrifuged at 10,000 rpm for 5 min at 4°C. In the cytochrome c release experiments, mitochondrial and cellular fractions were separated using a Cell Fractionation kit (Abcam, Cambridge, MA) according to the manufacturer's instructions. Protein concentrations were determined using a BCA protein assay kit (Sigma). Equal amounts of protein were mixed with SDS sample buffer including 2% β-mercaptoethanol as a reducing agent, boiled for 10 min, then loaded and separated on reducing gels, and transferred to a nitrocellulose membrane. For cytochrome c, membranes were probed with a cyt c antibody (Biolegend, San Diego, CA), while the purity of mitochondria was checked with a complex V (ATP5A) antibody (MitoSciences, Eugene, OR). For Prdx2 and the oxidized forms, membranes were probed with anti-Prdx2 and Prdx-SO3 antibodies (Abcam). Hyperoxidized forms of Prdx2 were also detected on a gel under nonreducing conditions. For catalase, an anti-catalase primary antibody was used (Cell Signaling, Danvers, MA). For apoptosis, the membrane was probed with anti-phospho JNK and cleaved caspase-3 primary antibodies (Cell Signaling). After washes, this was followed by the appropriate horseradish peroxidase-conjugated secondary antibody (1:10,000) and ECL chemiluminescent substrate (Pierce, Rockford, IL) and bands were visualized on film. Alternatively, we used fluorescent conjugated secondary antibodies (1:5,000) and visualized the blots with a LiCor Odyssey scan system.
Statistical analysis.
Data were expressed as means ± SD. Statistical significance between groups was determined by ANOVA and Student's t-test as appropriate. In the ANOVA analyses, Bonferroni's post hoc test was used to compare groups with the help of the Graph Pad Prism program. P < 0.05 was considered as the minimum level of statistical significance.
RESULTS
Nondelipidated albumin and palmitate but not FA-free albumin itself alter tubular mitochondrial viability and membrane potential and lead to cytochrome c release.
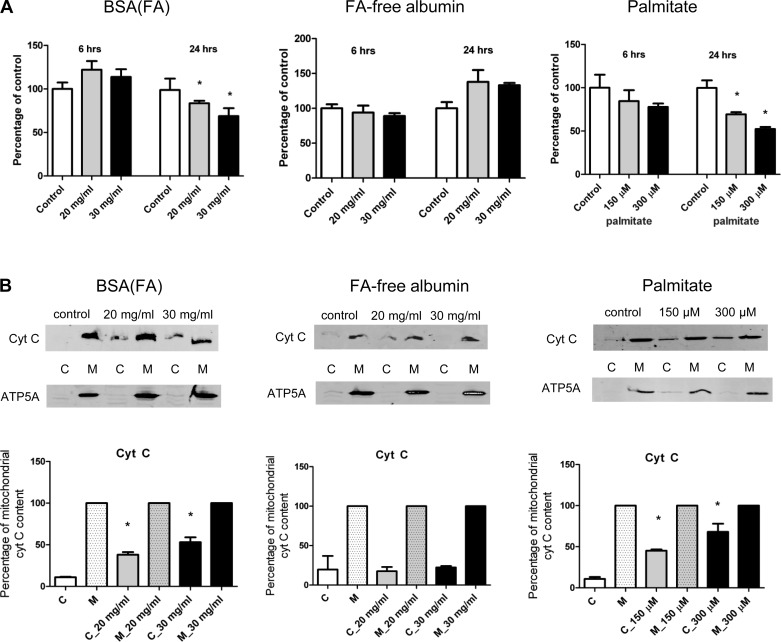
Treatment of NRK-52E cells with nondelipidated albumin or palmitate but not FA-free albumin led to time- and dose-dependent decreases in mitochondrial viability (Fig. 1A). Consistently, palmitate and nondelipidated albumin led to the release of cytochrome c from mitochondria in a dose-dependent manner (Fig. 1B) and to the loss of ΔΨ (Fig. 2).
Fig. 1.
Mitochondrial viability and cytochrome c release in NRK-52E cells. Proximal tubular cells were exposed to different concentrations of nondelipidated albumin, fatty acid (FA)-free albumin, or palmitate at different time points and analyzed by the MTT assay. A: nondelipidated albumin [BSA(FA)] caused a time- and dose-dependent decline of mitochondrial viability, while FA-free albumin had no effect. Cells exposed to albumin-conjugated palmitate showed a time- and dose-dependent decline in their mitochondrial viability. B: representative Western blot analysis showing dose-dependent mitochondrial cytochrome c release from tubular cells treated with BSA(FA) or palmitate. *P < 0.05 vs. control, means ± SD; experiments were done in triplicate and absorbances were expressed as percentage of control. Mitochondrial cytochrome c content was considered as 100% in each group, and cytochrome c release was expressed as percentage of the corresponding mitochondrial content.
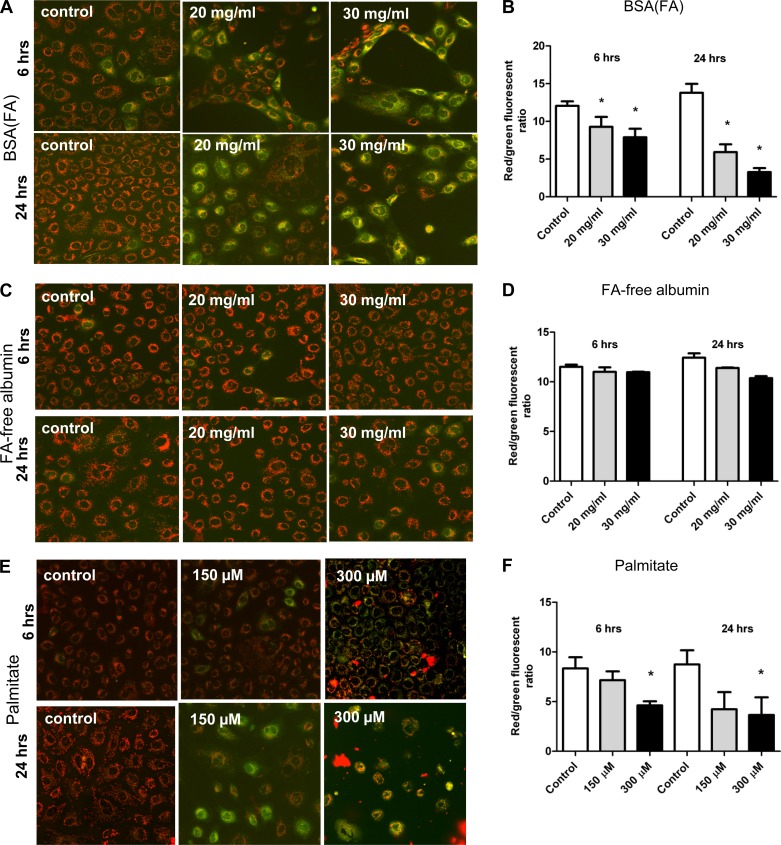
Fig. 2.
Changes in mitochondrial membrane potential (ΔΨ) in NRK-52E cells. Cells were loaded with the cationic JC-1 dye after various exposures to detect changes in ΔΨ by 2 different methods—imaging by confocal microscopy and spectrofluorometric readings by a plate reader. A–B: exposure to BSA(FA) caused loss of ΔΨ as shown by increased diffuse cytosolic green staining and decreased red/green ratios. C–D: FA-free albumin had no effect on ΔΨ as cells maintained dotted red staining of JC-1 and unchanged red/green ratios. E–F: similarly to BSA(FA), palmitate caused a significant loss of potential. *P < 0.05 vs. control, means ± SD; experiments were done a minimum of 3 times.
Cellular bioenergetic defects are linked to the lipid moiety of albumin.
To determine whether exposure of tubular epithelial cells to albumin, albumin-bound FA, or palmitate affects mitochondrial respiration and cellular bioenergetics, we utilized a SeaHorse XF24 Extracellular Flux Analyzer to measure mitochondrial respiration in intact NRK-52E cells. A 6-h exposure to nondelipidated BSA led to only mild changes in bioenergetics (data not shown). A 24-h treatment caused a dose-dependent impairment in basal respiration, ATP turnover, maximal and reserve respiratory capacity, consistently in both nondelipidated BSA and palmitate exposures (Fig. 3, A–B, E–F). ATP-linked respiration was calculated as the difference between the OCR value before oligomycin addition and the lowest OCR value (State IV respiration) after oligomycin injection. Reserve respiratory capacity was calculated as the maximal respiration OCR value after FCCP injection minus basal respiration. In contrast, a 24-h treatment with FA-free albumin did not significantly alter bioenergetic parameters (Fig. 3, C–D). These results particularly highlight that albumin-bound FA, but not albumin itself, alters mitochondrial bioenergetics in the proximal tubule and that the bioenergetic defects seen are clearly linked to the lipid moiety.
Fig. 3.
Extracellular flux analysis of mitochondrial function in NRK-52E cells. Cells were grown on XF24 cell culture plates at 75,000 per well and after various exposures, they were analyzed in a SeaHorse XF24 Extracellular Flux Analyzer. A–B: NRK-52E cells exposed to BSA(FA) for 24 h exhibited reduced basal respiration, ATP turnover, maximal and reserve respiratory capacities. C–D: exposure to FA-free albumin did not have any significant effect on mitochondrial respiratory parameters and energetics. E–F: exposure to palmitate led to an even more pronounced decline in energetic parameters. The XF24 graphs are representative for 3–4 different experiments, with 6–7 wells of control or treated groups per plate. *P < 0.05 vs. control, means ± SD. O, oligomycin; F, FCCP; A, antimycin A.
Nondelipidated albumin and palmitate induce a redox-sensitive early apoptosis in NRK-52E cells.
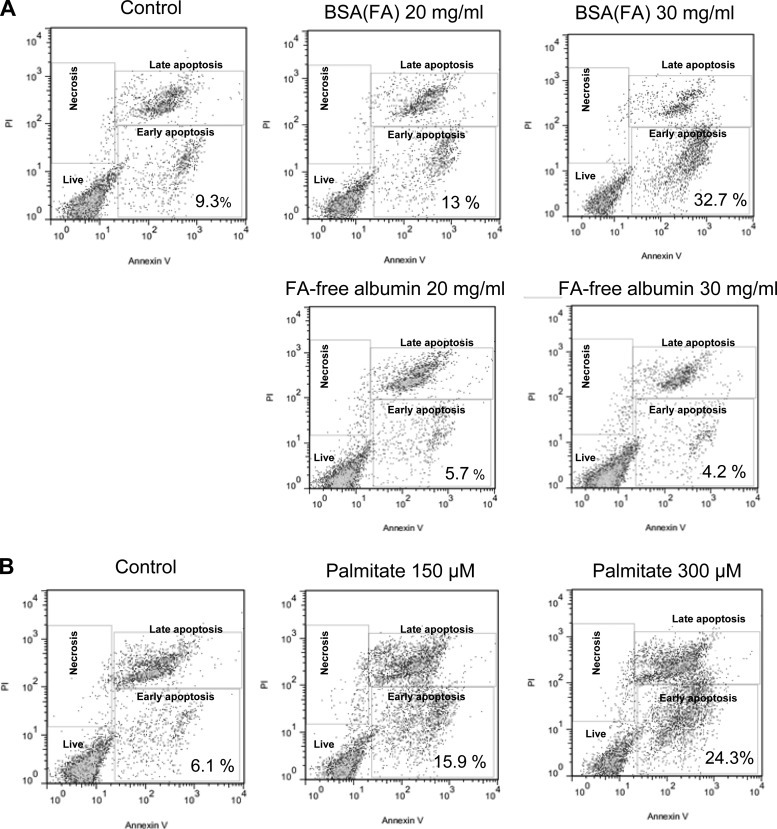
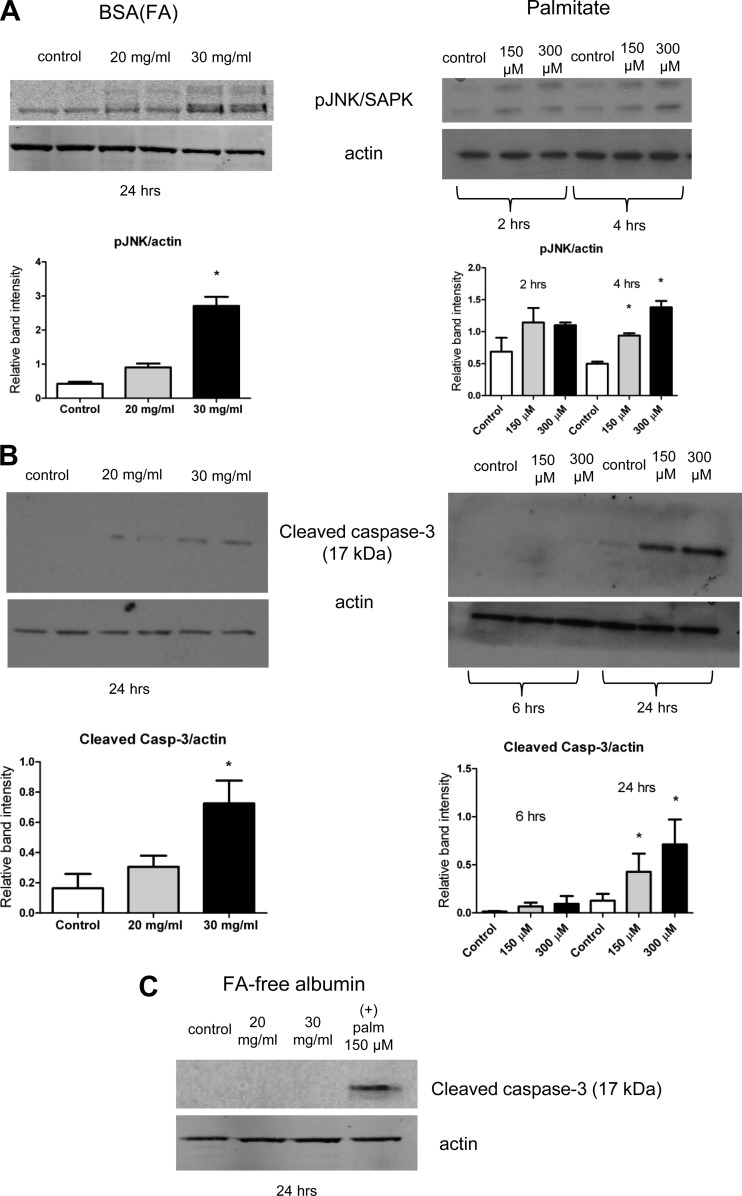
Next, to determine tubular cell death and the underlying cellular and molecular mechanisms, we examined whether the albumin-bound FA-induced mitochondrial energy decline was associated with cell death and whether this occured through a redox-sensitive apoptosis signaling. Here, we first investigated the nature of tubular cell death with various treatments (early/late apoptosis vs. necrosis) and then examined the possibility of the activation of a redox-sensitive apoptosis pathway. Figure 4 displays the flow cytometry results after treatments, where cells were labeled with FITC-Annexin V and propidium iodide. Only FA-bearing albumin and palmitate, but not pure albumin, significantly increased early apoptosis in the 24-h groups in the doses examined (Fig. 4, A–B). Late apoptosis also started to occur more frequently at higher doses. Oxidative stress and the accumulation of peroxides and other reactive species from mitochondrial and cytosolic sources are widely accepted as important modulators in apoptosis. These species can induce redox-sensitive apoptotic pathways. When we looked at a potential redox-sensitive mechanism, we found JNK phosphorylation, followed by caspase-3 cleavage at 24 h (Fig. 5, A–B). Again, FA-free albumin did not promote apoptosis or cause redox imbalance (Fig. 5C). This corroborates the idea of a lipid moiety-induced early apoptotic redox signaling, followed by caspase-3 cleavage leading into late apoptosis 24 h later.
Fig. 4.
Apoptosis of tubular epithelial cells exposed to albumin and FAs. Cells were treated with BSA(FA), FA-free BSA, or palmitate and the population of apoptotic/necrotic cells was analyzed by flow cytometry after an Annexin V/PI assay. A: only BSA(FA) but not FA-free albumin caused an increased ratio of early apoptotic cells after 24 h. B: palmitate treatment also caused a significant increase of the early and late apoptotic populations after 24 h. The flow cytometry results are representative of 3 different experiments, repeated on cells with similar passage numbers.
Fig. 5.
Activation of redox-sensitive apoptotic pathways in tubular cells. Activation of pJNK/SAPK after BSA(FA) or palmitate exposure was detected with a pJNK antibody and Western blotting (A), while cleaved caspase-3 detection further confirmed apoptosis at 24 h (B). C: FA-free BSA did not induce caspase cleavage. Palmitate at 150 μM dose served as positive control. Western blots are representative of at least 3 different experiments. β-Actin served as a loading control. *P < 0.05 vs. control, means ± SD.
Albumin-bound FAs cause dimerization and inactivation of the peroxide-sensing antioxidant enzyme Prdx2 and induction of catalase.
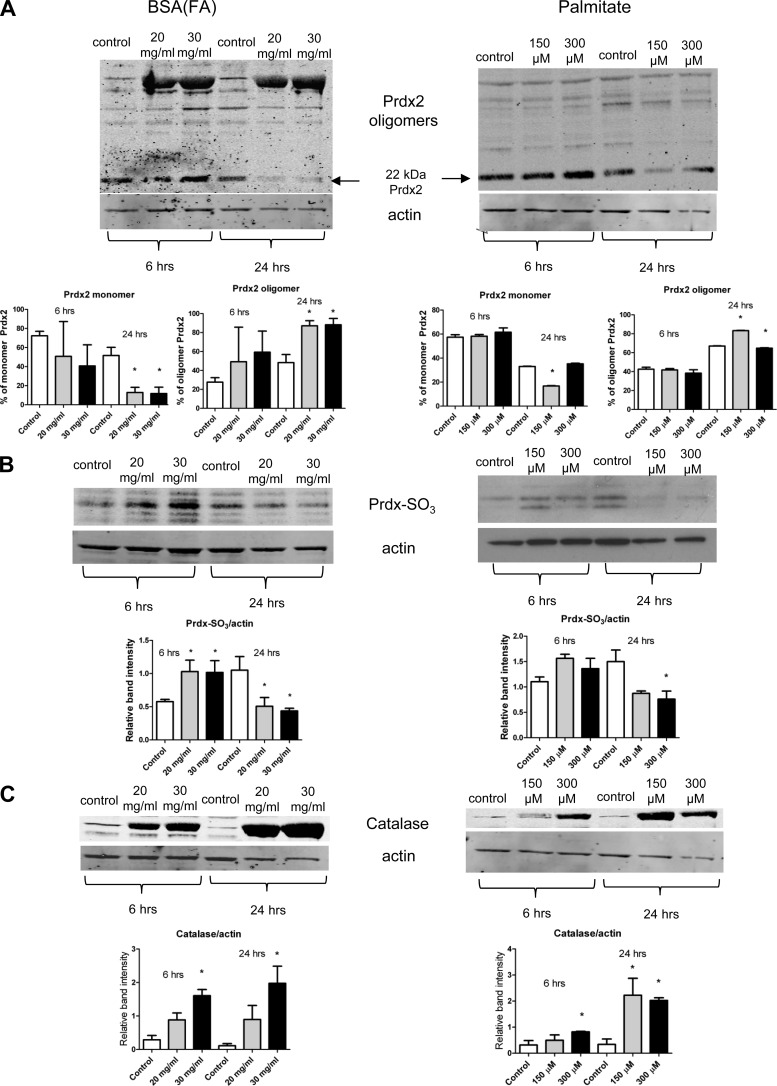
Redox-sensitive apoptotic pathways are generally superoxide- or peroxide-mediated, and cellular/mitochondrial redox imbalance is often associated with the accumulation of H2O2 or other peroxides. These products can derive from both mitochondrial and cytosolic sources. Therefore, we asked whether important antioxidant enzymes such as peroxiredoxins and catalase, which can serve as lines of defense against peroxides, may be involved in FA exposure-mediated tubular cell damage. Under nonreducing conditions, we observed an increase in the oligomer/monomer ratio of Prdx2 at 6 and 24 h in both treatments (Fig. 6A), indicating overoxidation of Prdx2, which was also accompanied by the degradation of the monomer form at 24 h (Fig. 6A at 22 kDa). This was also consistent with the dynamics of the hyperoxidized sulfenic acid form Prdx-SO3, indicating increased oxidation of the Cys sites to sulfenic acid at 6 h possibly followed by degradation at 24 h (Fig. 6B). Catalase expression was increased in both treatments at 6 and 24 h (Fig. 6C), providing further evidence for the presence of peroxides and H2O2.
Fig. 6.
Overoxidation of peroxiredoxin 2 (Prdx2) and catalase activation in tubular cells. To confirm a peroxide-mediated redox cascade, tubular epithelial cell lysates were checked for Prdx2 and catalase expression after treatments. A: both BSA(FA) and palmitate treatment increased the oligomer/monomer ratio of Prdx2, together with a reduction and degradation of the monomer form at 24 h. The Western blot was run under nonreducing conditions. B: BSA(FA) and palmitate treatments first increased (6 h) the sulfenic acid form Prdx-SO3, followed by a reduction (24 h; possibly because of degradation) as shown by Western blot analysis. C: both BSA(FA) and palmitate increased catalase expression as a line of defense against peroxides, in a dose-dependent manner at 6 and 24 h in tubular epithelial cells. Western blots are representative of at least 2–3 different experiments. *P < 0.05 vs. control, means ± SD.
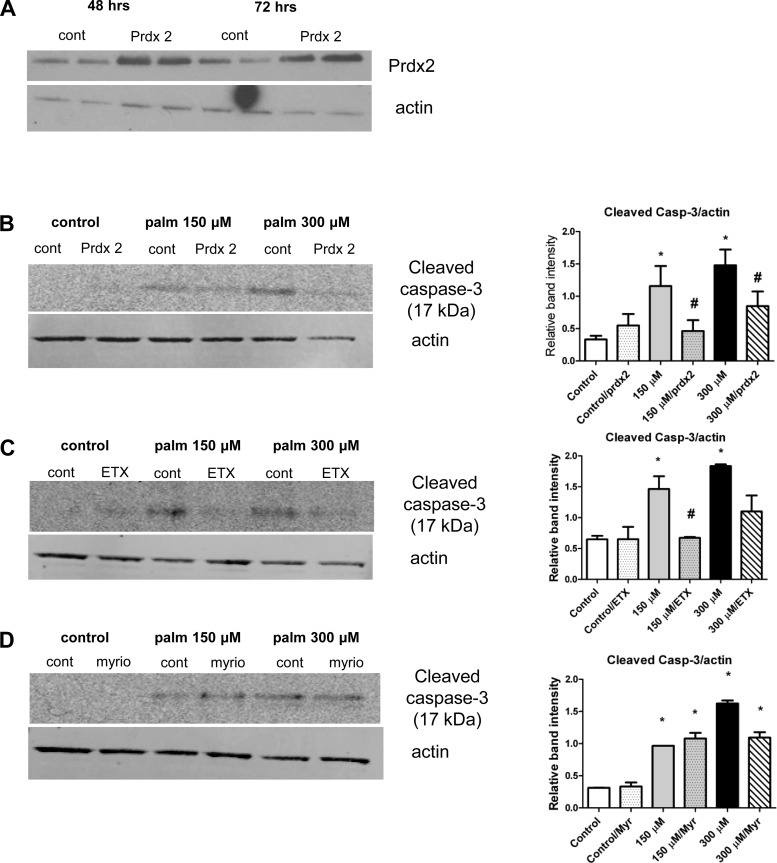
Lipotoxic effects are peroxide-dependent, partially through mitochondrial β oxidation but not ceramide synthesis-dependent: blocking mitochondrial FA entry or transfection with Prdx2 attenuates apoptosis in tubular epithelial cells.
To decipher the mechanistic details of FA-induced, peroxide-driven, redox-regulated apoptosis, we transfected NRK-52E cells with a Prdx2 plasmid as this enzyme handles not only H2O2 but large amounts of peroxides as well. Cells were transfected at 70% confluency and were treated with palmitate (as this treatment caused the strongest effects) at doses similar to those used in the nontransfected cell experiments. The optimal transfection period was determined before experiments were conducted (Fig. 7A). We also pretreated cells with the inhibitor etomoxir in the presence of carnitine or the de novo ceramide synthesis inhibitor myriocin. Apoptosis-related redox signaling (caspase-3 cleavage) was then examined in all experiments. In Prdx2-transfected tubular cells, caspase-3 cleavage was almost completely prevented in both lower and higher dose palmitate groups (Fig. 7B). Similar results were obtained with etomoxir pretreatment but myriocin did not stop the cleavage of caspase-3, indicating that the apoptosis is mostly peroxide-driven, where the peroxide partially derives from mitochondrial β oxidation but is not ceramide synthesis-dependent (Fig. 7, C–D).
Fig. 7.
Transfection of NRK-52E cells with Prdx2 or blocking mitochondrial FA entry is protective against apoptosis while ceramide synthesis does not play a role. NRK-52E tubular epithelial cells were transfected with Prdx2. A: confirmation of the transfection and optimal times; 48 h was chosen for further experiments. B: Prdx2 transfection abolishes caspase-3 cleavage induced by palmitate. Coincubation with etomoxir and carnitine prevents (C) but myriocin does not prevent caspase-3 cleavage (D). Western blots are representative of at least 3 different experiments. *P < 0.05 vs. control; #P < 0.05 vs. corresponding nontransfected or nontreated group, means ± SD.
DISCUSSION
Albumin is the most abundant protein in the tubular fluid, yet its origin and role remain controversial. A large group of investigators provided a significant body of evidence that the lipid environment and albumin-bound FA may be the culprits in tubular damage instead of albumin itself (1, 20, 24). The detailed aspects of these mechanisms and how FA overload may trigger a mitochondrial and cellular decline through redox-sensitive pathways are largely unknown. Our current studies are aimed to elucidate these pathways in tubular epithelial cells using various exposures.
First, our data suggest that mitochondria are targets as we observed impairments in mitochondrial function of the tubular epithelial cells upon nondelipidated BSA or palmitate load. This is reflected in the gradual decreases in ΔΨ and viability and is strongly corroborated by the release of cytochrome c and the respiratory measurements with the XF24 analyzer in intact cells. A decline in baseline respiration was accompanied by a loss in ATP turnover. More importantly, the marked decrease in spare respiratory capacity (maximal-basal) suggests an impaired ability of defense as this parameter is an important measure of the extra mitochondrial reserve that cells can use under redox stress (12).
Next, we showed that, in concert with the decline of mitochondrial parameters, tubular cells undergo apoptosis, probably due to the subsequent generation of H2O2 and other peroxides. This seems to be cytosolic in nature with the cytosolic lipid stress ultimately impacting cellular and mitochondrial survival. For the first time, our studies reveal a concerted role for Prdx2 and catalase in tubular epithelial cell protection.
It has recently been suggested that the oxidation of Prdx may be necessary as a front line defense in a peroxide attack to protect the cell from further damage (7). Yet, if regeneration of the sulfenic acid form (Prdx2-SO3) fails, and degradation follows (as the 24-h experiments suggest), apoptosis can occur. This phenomenon can explain our observations in our lipid overload experiments. One interpretation of the redox signaling data is that cytosolic Prdx2 provides the most abundant and first line of defense against the peroxide overproduction. As this event escalates, Prdx2 gets hyperoxidized as our nonreducing conditions and the Prdx-SO3 accumulation suggests, followed by degradation and/or formation of dimer(s) (Fig. 6, A–B). While Prdx2 can get deactivated this way, catalase cannot, and its increased expression indicates a secondary defense against accumulating peroxides (Fig. 6C). Another interpretation is that at lower concentrations of stressors, Prdx2 serves as a defense enzyme and when this is surpassed, catalase plays a role. Interestingly, in the case of palmitate, Prdx2 seems to both lose its peroxide-removing potential and undergo degradation more readily; this may explain the weaker oligomer and the almost complete lack of Prdx-SO3 formation by 24 h on Fig. 6, A–B. To ultimately offer a route for the potential mechanism of protection, we elected to overexpress Prdx2 in NRK-52E cells. This enzyme provides an abundant defense against a variety of peroxides that may mediate the apoptosis observed in our experiments. Cells overexpressing Prdx2 were protected from palmitate-induced caspase-3 cleavage and therefore apoptosis, confirming the role of a largely peroxide-mediated redox imbalance in the tubules (Fig. 7B). It is to be determined whether the peroxides are largely of mitochondrial or cytosolic origin, or whether the mitochondrial decline and cytosolic Prdx2 hyperoxidation are parallel mechanisms. Interestingly, etomoxir (in combination with carnitine to ensure proper FA entry) but not myriocin treatment also protected against caspase-3 cleavage (Fig. 7, C–D), indicating that excess peroxides from mitochondrial β oxidation but not de novo ceramide synthesis play a role. We surmise this may indicate a combination of direct cytosolic lipotoxic effects from long-chain FA themselves and surplus peroxide generation from the mitochondria due to FA overload. It is known that, in other cell types, palmitate can also disrupt the mitochondrial membrane and affect cardiolipin (11). This would certainly explain our observation on mitochondrial membrane depolarization and may contribute to mitochondrial H2O2 release. Upregulation of peroxisomal FA oxidation in the cytosol is also a likely candidate for peroxide generation. Due to the abundant nature of Prdx, we believe this is a more specific and sensitive approach to verify the presence of peroxides/H2O2 than the widely used but rather nonspecific fluorescent dyes.
Concurrently with accumulation of Prdx-SO3 at early time points, phosphorylation of JNK was increased by albumin with bound FA. This was followed by an increase in early apoptosis. At later timepoints, we observed a mixture of early and late apoptosis, with cleavage of caspase-3 (Figs. 4–5). In stark contrast, when treated with FA-free albumin there were no changes to mitochondrial viability, cytochrome c content, or bioenergetics. Similarly, apoptotic pathways were not activated by FA-free albumin. All of our observations indicate that tubular cells are designed to deal with high-albumin concentrations, but that increased FA moieties, like those that occur in obesity, metabolic syndrome, and diabetes, challenge mitochondrial viability and predispose to apoptosis. In these diseases, the nonesterified FA load of albumin significantly changes (21). It is likely that the increased amount of FA presented to the tubular cells creates a proinflammatory state (25).
It is noteworthy that the albumin concentrations used in our albumin exposure studies are higher than those to be found in the proximal tubules in nephrotic cases (∼3 mg/ml). This is because with the use of nondelipidated albumin, we aimed to mimic a scenario where FAs carried on albumin will reach pathophysiologically relevant concentrations in the tubules. To avoid mistaken interpretations, palmitate concentrations were kept in the pathologically relevant range (150–300 μM) and complexed to albumin in a 6:1 ratio to provide an almost fully saturated albumin. Furthermore, in these experiments, due to the nature of the conjugation ratio and the dilution factors, albumin concentrations were kept close to a real case scenario (∼1 mg/ml when palmitate is 150 μM and ∼3 mg/ml when palmitate is used at 300 μM). Our findings corroborate with a body of literature suggesting a role for FA carried on albumin into the tubules (1, 2, 23, 24). Here, we reveal a novel redox signaling mechanism that, for the first time to our knowledge, may contribute to tubular apoptosis in proteinuric cases. This redox-sensitive pathway provides a link to better understand the relationship between increased FA levels and activation of apoptotic signals.
It is important to mention that an alternative mechanism has been recently offered emphasizing the role and dysfunction of tubular retrieval pathways of albumin vs. the traditional view of protein leakage from the glomeruli (6, 20). Moreover, tubular damage has also been detected in analbuminemic rats (16). However, we believe these discrepancies do not detract from the significance of our findings, regardless of the origin of the albumin. If according to the alternative mechanism the glomerulus is freely permeable and a large amount of albumin is retrieved in the tubules, our data argue that indeed in a healthy case even large amounts of albumin carrying low amounts of FA are not proapoptotic to the tubules. On the other hand, an increased and sustained FA load could be pathogenic in nature by activating secondary mechanisms such as redox-sensitive pathways. We believe our data suggest that a persistent exposure to higher FA levels, together with pathologically relevant albumin concentrations where albumin is saturated with the FA, may play a role in the apoptosis of tubular cells. In contrast, we recognize that our observations do not provide an explanation for tubular damage observed in analbuminemic cases (16). An interesting question that may warrant further studies is whether the megalin-cubilin receptor complex has different affinities to different types of albumin, which may also partially explain the different results. This has been tested in tubular cells before in vitro, where endocytosis and uptake of various albumins were not significantly different (22).
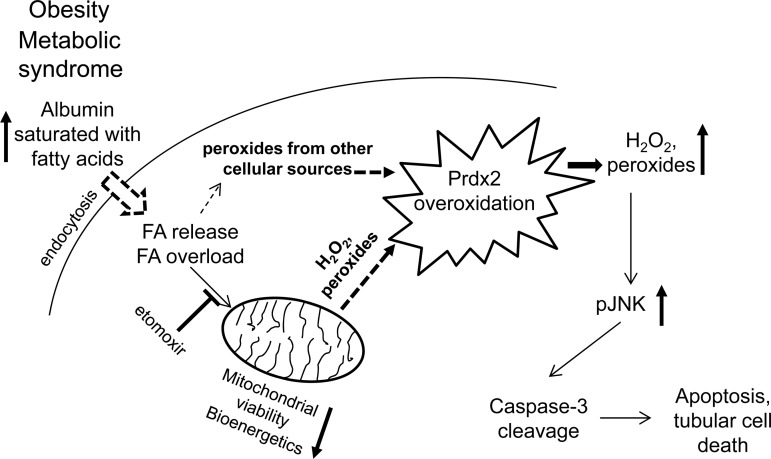
In summary, our studies highlight a new redox-sensitive apoptotic mechanism in tubular epithelial cells through hyperoxidation of Prdx2. Increased FA load leads to a general, mostly peroxide-mediated redox imbalance. Ultimately, the cells undergo redox-sensitive apoptosis, where overexpression of Prdx2 protects the cells from programmed cell death (Fig. 8). Our findings may bear broader relevance as they confirm that lowering free FA levels and/or a combination of therapies positively affecting redox balance could have beneficial impacts in preserving tubular function in metabolic syndrome patients with proteinuria.
Fig. 8.
Summary of the proposed mechanism in renal tubular epithelial cells in obesity and metabolic syndrome. An increased FA load on albumin presents increased FA load to the mitochondrial β-oxidation cycle after endocytosis. This, together with other cellular sources, will increase peroxide production and exhaust cytosolic Prdx2 defense by overoxidation. Increased peroxide levels will activate the redox-sensitive pJNK/caspase-3 apoptotic pathway leading to cell death. Blocking mitochondrial FA entry with etomoxir or overexpressing Prdx2 will provide defense against the activation of the redox-sensitive apoptotic pathway.
GRANTS
Research in this laboratory is supported partially by National Institutes of Health (NIH) Grant DK083615 to K. Stadler, a pilot and feasibility grant from the Nutrition and Obesity Research Center (NORC; through P30DK072476 from National Institute of Diabetes and Digestive and Kidney Diseases), and the Pennington Foundation. The work utilized the facilities of the Cell Biology and Bioimaging Core that is supported in part by COBRE (NIH 8 P20GM103528) and NORC (NIH 1P30DK072476) center grants from the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.R., C.K., E.C., and K.A. performed experiments; C.R., C.K., E.C., K.A., and K.S. analyzed data; C.M.E., C.K., R.C.N., and K.S. interpreted results of experiments; C.M.E. drafted manuscript; C.M.E., C.K., and R.C.N. edited and revised manuscript; C.K., R.C.N., and K.S. prepared figures; R.C.N. and K.S. conception and design of research; K.S. approved final version of manuscript.
REFERENCES
- 1.Arici M, Brown J, Williams M, Harris KP, Walls J, Brunskill NJ. Fatty acids carried on albumin modulate proximal tubular cell fibronectin production: a role for protein kinase C. Nephrol Dial Transplant 17: 1751–1757, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Baines RJ, Brunskill NJ. Tubular toxicity of proteinuria. Nat Rev Nephrol 7: 177–180, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Burton C, Harris KP. The role of proteinuria in the progression of chronic renal failure. Am J Kidney Dis 27: 765–775, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Chang TS, Jeong W, Woo HA, Lee SM, Park S, Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem 279: 50994–51001, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM. Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol 295: F1589–F1600, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Comper WD, Russo LM. The glomerular filter: an imperfect barrier is required for perfect renal function. Curr Opin Nephrol Hypertens 18: 336–342, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Day AM, Brown JD, Taylor SR, Rand JD, Morgan BA, Veal EA. Inactivation of a peroxiredoxin by hydrogen peroxide is critical for thioredoxin-mediated repair of oxidized proteins and cell survival. Mol Cell 45: 398–408, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, Landar A, Darley-Usmar VM. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med 51: 1621–1635, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis 12: 353–365, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Erkan E, Devarajan P, Schwartz GJ. Mitochondria are the major targets in albumin-induced apoptosis in proximal tubule cells. J Am Soc Nephrol 18: 1199–1208, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem 278: 31861–31870, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J 424: 99–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med 11: 571–578, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kincaid-Smith P. Hypothesis: obesity and the insulin resistance syndrome play a major role in end-stage renal failure attributed to hypertension and labelled ‘hypertensive nephrosclerosis’. J Hypertens 22: 1051–1055, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Osicka TM, Strong KJ, Nikolic-Paterson DJ, Atkins RC, Jerums G, Comper WD. Renal processing of serum proteins in an albumin-deficient environment: an in vivo study of glomerulonephritis in the Nagase analbuminaemic rat. Nephrol Dial Transplant 19: 320–328, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J Biol Chem 282: 11885–11892, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Praga M, Morales E. Obesity, proteinuria and progression of renal failure. Curr Opin Nephrol Hypertens 15: 481–486, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Remuzzi G. Nephropathic nature of proteinuria. Curr Opin Nephrol Hypertens 8: 655–663, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Shafrir E. Partition of unesterified fatty acids in normal and nephrotic syndrome serum and its effect on serum electrophoretic pattern. J Clin Invest 37: 1775–1782, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalamanova L, McArdle F, Amara AB, Jackson MJ, Rustom R. Albumin overload induces adaptive responses in human proximal tubular cells through oxidative stress but not via angiotensin II type 1 receptor. Am J Physiol Renal Physiol 292: F1846–F1857, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Soumura M, Kume S, Isshiki K, Takeda N, Araki S, Tanaka Y, Sugimoto T, Chin-Kanasaki M, Nishio Y, Haneda M, Koya D, Kashiwagi A, Maegawa H, Uzu T. Oleate and eicosapentaenoic acid attenuate palmitate-induced inflammation and apoptosis in renal proximal tubular cell. Biochem Biophys Res Commun 402: 265–271, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Thomas ME, Harris KP, Walls J, Furness PN, Brunskill NJ. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol Renal Physiol 283: F640–F647, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Thomas ME, Schreiner GF. Contribution of proteinuria to progressive renal injury: consequences of tubular uptake of fatty acid bearing albumin. Am J Nephrol 13: 385–398, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Trujillo M, Ferrer-Sueta G, Thomson L, Flohe L, Radi R. Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite. Subcell Biochem 44: 83–113, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 73: 19–33, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Weinberg JM. Lipotoxicity. Kidney Int 70: 1560–1566, 2006 [DOI] [PubMed] [Google Scholar]