Abstract
The inner cell mass (ICM) of the mammalian blastocyst contains pluripotent epiblast (EPI) and differentiating primitive endoderm (PE) cells, intermingled like salt and pepper. In mouse, the earliest known driver of EPI and PE cell fates is FGF4, but it was unclear whether cellular or molecular heterogeneity existed upstream of Fgf4. In a new study, published in the journal Nature Cell Biology, researchers in the lab of Takashi Hiiragi take us one step closer to understanding the origins of cellular heterogeneity in the ICM. Using single cell transcriptional profiling, Ohnishi and colleagues show that cellular heterogeneity is unlikely to precede the role of FGF4, and that FGF4 may help specify EPI as well as PE cell fates, providing exciting new insight into the origins of pluripotency.
See also: Y Ohnishi et al (December 2013)
Because the ICM is the source of embryonic stem (ES) cells, understanding how the ICM is established and regulated is of great interest to developmental and stem cell biologists alike. Recent work has helped clarify many of the molecular mechanisms regulating the establishment of the ICM and of the pluripotent EPI cells that reside within the ICM of the mouse blastocyst. In 2006, researchers in Janet Rossant's lab first showed that the ICM is not a homogenous group of pluripotent EPI cells, but is already subdivided into EPI and PE cell types at the 64-cell stage (Chazaud et al, 2006). Notably, EPI and PE cells appear randomly intermingled in the ICM, like salt and pepper, suggesting that intercellular signaling, rather than positional cues, establishes EPI and PE fates. Also in 2006, technology for examining whole transcriptomes of individual cells enabled resolution of two distinct transcriptional profiles within the ICM (Kurimoto et al, 2006), and allowing researchers to define EPI and PE cell types in molecular terms at the 64-cell stage.
The signaling pathway that regulates EPI and PE cell differentiation in the ICM was also discovered. Several lines of evidence indicate that EPI cells produce FGF4 ligand, which acts on neighboring cells to induce PE fate (Chazaud et al, 2006; Yamanaka et al, 2010; Kang et al, 2013; Krawchuk et al, 2013). Notably, the expression patterns of Fgf4 and its receptor, encoded by Fgfr2, are strongly complementary at the 64-cell stage, and weakly complementary as early as the 32-cell stage, with Fgf4 higher in EPI and Fgfr2 higher in PE cells (Guo et al, 2010; Frankenberg et al, 2011). Yet if Fgf4 and Fgfr2 exhibit expression differences between EPI and PE cells, then what regulates their expression? Several models can be envisioned, and two are discussed here (Fig1). In the first model, molecular differences between pre-EPI and pre-PE cells precede and cause the complementary expression of Fgf4 and Fgfr2 (an FGF-independent model). In the second, FGF-dependent model, no molecular differences precede the complementary expression of Fgf4 and Fgfr2. Rather, FGF signaling itself could create complementary Fgf4 and Fgfr2 expression, for example through a lateral inhibition-type mechanism (Perrimon et al, 2012). Yet, how the ICM is first ‘salted and peppered’ is still an open question.
Figure 1.
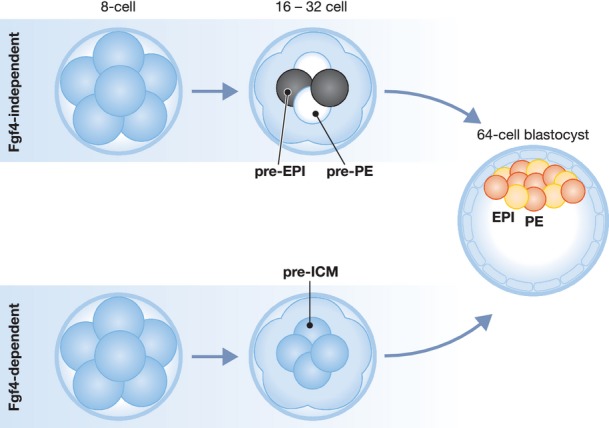
Origins of salt and pepper heterogeneity in the ICM of the mouse blastocyst
Rounds of cell division first establish inside and outside cell populations by the 16-cell stage. Expression of Fgf4 (red) and Fgfr2 (yellow) are complementary by the 32–64-cell stage, and establish EPI and PE fates by the 64-cell stage. One possibility is that transcriptional heterogeneity in the ICM (black and white) exists before or independently of Fgf4 (top row). Alternatively, transcriptional heterogeneity in the ICM is caused by Fgf4 acting on a pre-ICM (grey) precursor (bottom row). The findings of Ohnishi and colleagues are consistent with an Fgf4-dependent model of the origins of EPI and PE cell types.
A study from Takashi Hiiragi's lab tackles this intriguing question using single cell transcriptome analysis and mouse knockouts (Ohnishi et al, 2013). First, Ohnishi and colleagues examine ICM cell transcriptomes before, during, and after the 64-cell stage, to determine when EPI and PE cell types can first be distinguished transcriptionally. Strikingly, the authors report no readily distinguishable transcriptional differences among ICM cells prior to the 64-cell stage. Rather, Ohnishi and colleagues show that EPI and PE genes are often coexpressed in ICM cells prior to the 64-cell stage. Thus no major molecular differences between ICM cells precede the stage at which Fgf4 and Fgfr2 expression differences are evident. Next, Ohnishi and colleagues examine transcriptomes of ICM cells that lack Fgf4. Remarkably, bimodal transcriptional differences that are evident in normal ICM cells at the 64-cell stage are lost in the absence of Fgf4 at this stage. Thus no major molecular differences exist without Fgf4. Taken together, these observations strongly argue that EPI and PE arise in an Fgf4-dependent manner, from a common ICM precursor.
One of the most exciting findings in the study concerns what becomes of the EPI cell transcriptional signature in the absence of Fgf4. Prior work predicts that ICM cells lacking Fgf4 would adopt the EPI transcriptional signature, because disruption of FGF signaling converts the ICM to NANOG-expressing EPI cells, at the expense of PE gene expression (Chazaud et al, 2006; Nichols et al, 2009; Yamanaka et al, 2010; Kang et al, 2013; Krawchuk et al, 2013). Moreover, the efficiency with which ES cells can be derived from the ICM is increased when FGF signaling is dampened (Nichols et al, 2009), arguing that more ICM cells acquire functional properties of EPI cells in the absence of FGF signaling. However, the analysis performed by Ohnishi and colleagues suggests something new. While ICM cells remained generally more transcriptionally similar to EPI than PE in the absence of Fgf4, they did not develop a fully EPI gene expression signature. Rather, Fgf4 null ICM cells were notably more similar to each other than to either EPI or PE. This raises the interesting possibility that the manifestation of EPI gene expression signature is delayed in the absence of Fgf4. Additionally, the observations suggest a previously unsuspected role for FGF4 in the establishment of EPI, and not just PE fate. Clearly, these observations will shake up how we think about the origins of cellular heterogeneity in the ICM.
Acknowledgments
We are grateful for financial support in the form of grants from the Ellison Medical Foundation and NIH R01GM104009 to A.R., and CIRM TG2-01157 to M.A.H.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Frankenberg S, Gerbe F, Bessonnard S, Belville C, Pouchin P, Bardot O, Chazaud C. Primitive endoderm differentiates via a three-step mechanism involving nanog and RTK signaling. Dev Cell. 2011;21:1005–1013. doi: 10.1016/j.devcel.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Guo G, Huss M, Tong G, Wang C, Li Sun L, Clarke N, Robson P. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell. 2010;18:675–685. doi: 10.1016/j.devcel.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Kang M, Piliszek A, Artus J, Hadjantonakis AK. FGF4 is required for lineage restriction and salt-and-pepper distribution of primitive endoderm factors but not their initial expression in the mouse. Development. 2013;140:267–279. doi: 10.1242/dev.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawchuk D, Honma-Yamanaka N, Anani S, Yamanaka Y. FGF4 is a limiting factor controlling the proportions of primitive endoderm and epiblast in the ICM of the mouse blastocyst. Dev Biol. 2013;384:65–71. doi: 10.1016/j.ydbio.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Kurimoto K, Yabuta Y, Ohinata Y, Ono Y, Uno K, Yamada R, Ueda H, Saitou M. An improved single-cell cDNA amplification method for efficient high-density oligonucleotide microarray analysis. Nucleic Acids Res. 2006;34:e42. doi: 10.1093/nar/gkl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y, Huber W, Tsumura A, Kang M, Xenopoulos P, Kurimoto K, Oleś AK, Araúzo-Bravo MJ, Saitou M, Hadjantonakis AK, Hiiragi T. Cell-to-cell expression variability followed by signal reinforcement progressively segregates early mouse lineages. Nat Cell Biol. 2013;16:27–37. doi: 10.1038/ncb2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N, Pitsouli C, Shilo BZ. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb Perspect Biol. 2012;4:a005975. doi: 10.1101/cshperspect.a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development. 2010;137:715–724. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]


