Abstract
Stem cells ensure the maintenance of tissue homeostasis throughout life by tightly regulating their self-renewal and differentiation. In a recent study published in Nature, Nakada et al, 2014 unveil an unexpected endocrine mechanism that regulates hematopoietic stem cell (HSC) self-renewal.
See also: D Nakada et al (January 2014)
In the hematopoietic system, stem cells are largely quiescent and divide only rarely to protect genomic integrity of the hematopoietic stem cell (HSC) pool; however, this behavior can be altered to adapt to physiological needs and changes in the microenvironment (Wilson et al, 2008). The stem cell niche has been largely viewed as a support system that protects the stem cell pool via cell–cell contact and paracrine signals. There has been a significant progress in defining the niche cells and molecular cues that constitute the immediate HSC niche in the bone marrow (Morrison & Scadden, 2014). However, little has been known about possible long-range signals that govern HSC behavior.
Nakada et al (2014) discovered that the replicative activity of the HSC pool is augmented in female versus male mice, an observation that led them to study the role of sex hormones in HSC regulation. They showed that estradiol (E2) promotes cycling of HSC and multipotent progenitors (MPP) and increases their differentiation into megakaryocyte–erythroid progenitors (MEP). As the number of HSC did not alter upon stimulation by estradiol despite increased HSC proliferation and red cell and platelet production, the authors propose that estradiol largely promotes asymmetric rather than symmetric self-renewal in the bone marrow.
Estradiol function is mediated by nuclear hormone receptors, estrogen receptor-α and-β (encoded by Esr1 and Esr2, respectively), which are transcription factors that dimerize and bind to regulatory sequences of target genes in response to the ligand (Heldring et al, 2007). The authors narrowed down the observed effect of estradiol on HSC self-renewal to estrogen receptor-α (ER-α) and activation of cell cycle-related genes to increase the proliferation of HSC. They further showed that HSCs in male mice also express ER-α and are capable of increasing their cycling in response to estradiol, identifying estradiol production as the defining factor that underlies the sex specificity of this HSC regulatory mechanism (Fig 1). This work thus uncovers an unanticipated role for a female sex hormone in a trait that has not been previously considered as sexually dimorphic, such as HSC self-renewal.
Figure 1.
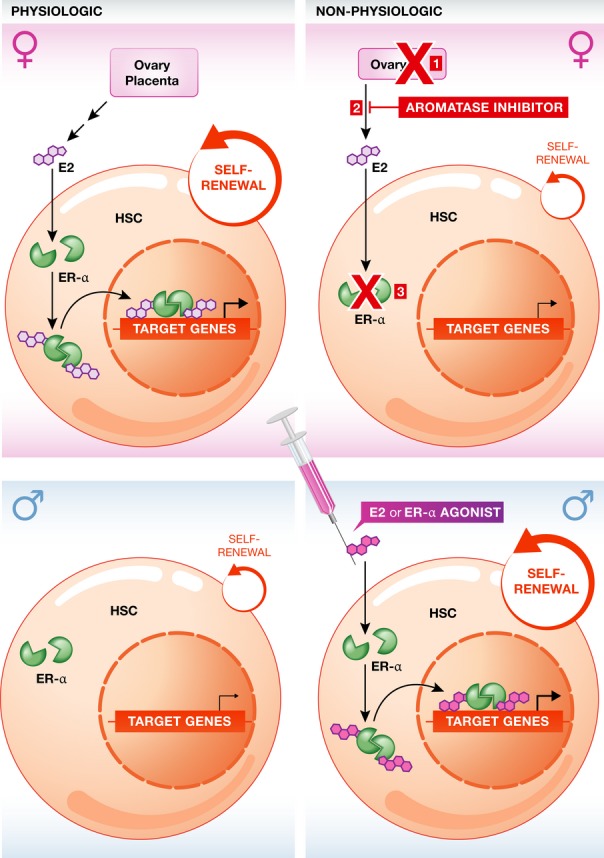
Estradiol promotes hematopoietic stem cell (HSC) self-renewal.
In female mice, HSC proliferation is augmented by circulating estradiol (E2), which is produced from the ovaries during reproductive years, and at higher levels from the placenta during pregnancy. E2 binds to the estrogen receptor-α (ER-α, green), which upon dimerization is translocated to the nucleus and enhances the expression of target genes involved in HSC proliferation (top left panel). Interrupting this pathway by ovariectomy (1), by the administration of an aromatase inhibitor that blocks the production of estradiol from its precursors (2), or by genetic disruption of Esr1, the gene encoding for ER-α (3), leads to reduced basal proliferation rate of HSC in females (top right panel). In males, despite the expression of ER-α in HSC, paucity of estradiol determines a lower basal HSC proliferation rate (bottom right panel). However, exogenous administration of E2 or ER-α agonists can induce an increase in HSC self-renewal also in males as well (bottom right panel).
These findings raise the question as to why a female-specific mechanism to increase HSC proliferation and blood cell production would have evolved. From menarche to the onset of menopause, estradiol is cyclically secreted from the ovary. During pregnancy, its level increases 1,000-fold as its production is overtaken by placental trophoblasts (Gruber et al, 2002). Connecting these observations, one can hypothesize that such estrogen-mediated mechanism of HSC asymmetric self-renewal would enable the maternal adaptation to the increased blood cell demand associated with physiological blood loss during childbirth. On the other hand, during gestation, HSCs in both female and male fetuses are equally exposed to estrogens as the placental trophoblasts release them also into the fetal circulation (Kaludjerovic & Ward, 2012). Further studies are needed to define whether estrogens also play a role in the rapid cycling and symmetric self-renewal of the fetal HSC, which is critical for establishing the HSC pool during development.
For decades, exogenous estrogen has been used in the clinic due to the observed effects on breast epithelium, uterine endometrium, vasculature, and bone (Gruber et al, 2002; Practice Committee of ASRM, 2008). Furthermore, selective ER modulators have proven useful in the treatment for specific subsets of breast and ovarian cancers (Riggs & Hartmann, 2003). Nakada et al (2014) show that the estrogen-mediated HSC proliferative effect can be modulated in vivo by the administration of pharmacologic agonists and antagonists of the pathway. Hence, this work identifies HSC as yet another target tissue to be considered when assessing the therapeutic or secondary effects of estrogen or anti-estrogen treatments.
One tempting clinical application of estrogens might be to stimulate ER-α signaling to accelerate the recovery from chemotherapy and HSC transplantation by increasing HSC self-renewal and platelet production. On the other hand, potential implications of the increased cell cycle activity of HSC exposed to higher level of estrogens could include higher risk for mutations during cell divisions, susceptibility to genotoxic agents, and/or accelerated aging. Moreover, as leukemic stem cells (LSC) exploit many of the same mechanisms as HSC for sustaining self-renewal (Heidel et al, 2011), future studies investigating ER-α signaling as a potential mechanism that promotes LSC self-renewal, or as a therapeutic target to increase the cycling and drug sensitivity of otherwise quiescent LSC, will be of clinical interest. Thus, in addition to challenging the way we think of the extent of the HSC microenvironment and the endocrine system as a modulator of stem cell activity, this work opens new unexplored avenues to assess the physiological, pathological, and developmental functions of sex hormones.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. New Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- Heidel FH, Mar BG, Armstrong SA. Self-renewal related signaling in myeloid leukemia stem cells. Int J Hematol. 2011;94:109–117. doi: 10.1007/s12185-011-0901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Kaludjerovic J, Ward WE. The interplay between estrogen and fetal adrenal cortex. J Nutr Metab. 2012;2012:837901. doi: 10.1155/2012/837901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ. Oestrogen increases haematopoietic stem cell self-renewal in females and during pregnancy. Nature. 2014;505:555–558. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committee of ASRM. Estrogen and progestogen therapy in postmenopausal women. Fertil Steril. 2008;90:S88–S102. doi: 10.1016/j.fertnstert.2008.08.091. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Hartmann LC. Selective estrogen-receptor modulators – mechanisms of action and application to clinical practice. New Engl J Med. 2003;348:618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, Wath van der RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]


