The correct balance between self-renewal and expansion of stem cells versus differentiation is essential to maintain tissue homeostasis and prevent neoplastic transformation. The MYC proto-oncogene family (comprising MYC, MYCN and MYCL) is a family of proteins that plays a crucial role in the decision of stem and progenitor cells to expand or undergo differentiation. MYC proteins are transcription factors that act through activation and repression of a wide variety of target genes and, when deregulated, promote oncogenic transformation of most cell types 1. There is overwhelming support to the notion that MYC is essential to preserve the stem cell state and inhibit differentiation. This is also true in the developing nervous system where MYC ablation leads to premature neuronal differentiation, whereas MYC accumulation locks cells in an undifferentiated and abnormally proliferative state 2, 3. In this issue of EMBO reports, Zinin et al 4 present, however, an unexpected pro-neurogenic function of MYC in neural progenitors that diverges from the established mitogenic and anti-differentiation functions of this family of proteins.
Through loss- and gain-of-function experiments in the developing chick neural tube, the authors identify a novel function of MYC in neuronal differentiation of radial glial precursors (RGPs), the multipotent progenitors in the neurogenic areas of the brain. Concurrent silencing of the two MYC family members that are expressed in the developing neural tube (MYC and MYCN) led to inhibition of neuronal differentiation. Rather than what might have been surmised from the established activities of MYC transcription factors, the inefficient neuronal differentiation of RGPs depleted of MYC was not a consequence of reduced proliferation, therefore suggesting that MYC operates in the developing neural tube as a bona fide neuronal differentiation factor.
The idea that MYC can promote differentiation was reinforced by gain-of-function experiments in which the ectopic expression of either MYC or MYCN stimulated neuronal differentiation and simultaneously depleted the progenitor cell population. Interestingly, the positive effects of MYC expression on neuronal differentiation required the DNA-binding property, and presumably the transcriptional activity, of MYC. In contrast, RGPs were resistant to MYC-induced apoptosis. What might be the downstream mechanisms by which MYC promotes differentiation of neural progenitors? A clue came from the analysis of cells dissociated from the neural tube and cultured in vitro. RGPs that had been ectopically transduced with MYC in vivo and allowed to grow in culture displayed the “canonical” enhancement of proliferation that has commonly been associated with MYC activation in multiple systems. The markedly divergent consequences of activating MYC in the context of a multicellular organism when compared to a defined culture system suggested that the organ architecture could be instrumental to funnel the signaling events emanating from MYC transcription factors. Because a primary regulator of the proliferation versus differentiation decision in neural progenitors in the tissue context is NOTCH signaling, the authors inquired whether MYC-induced neuronal differentiation involved NOTCH signaling. Indeed, RGPs that expressed MYC displayed reduced levels of NOTCH1. Consistently, they exhibited downregulation of the NOTCH target genes HES1 and HES5 and inhibition of a NOTCH-responsive reporter. The mechanistic implication of NOTCH1 in MYC-triggered neuronal differentiation was confirmed by the observation that expression of the constitutively active, intracellular domain of NOTCH1 (NICD1) prevented MYC-induced neurogenesis. These data are compatible with a role of NOTCH signaling in maintaining the progenitor pool. While these experiments indicate that the differentiation function of MYC in RGPs requires repression of NOTCH signaling in vivo, it remains to be determined whether the downregulation of NOTCH signaling by MYC is direct or involves one or more additional factors (Fig 1). The importance of organotypic multicellular polarity for the differentiation effect of MYC in RGPs is further supported by the marked increase in apico-basal cell divisions induced by MYC at the expense of planar divisions. While planar orientation of cell divisions indicates symmetric stem cell division, apico-basal divisions are associated with differentiation of the basal daughter cell, and asymmetric distribution of NOTCH that is depleted from basal daughter cells. The study by Zinin et al does not specifically address what the fate of the apical cell in the apico-basal cell division upon MYC overexpression is, or whether the endogenous MYC protein is differentially expressed in apical and basal daughter cells in apico-basal divisions. However, it is plausible that the basal daughter cell maintains the highest MYC protein levels.
Figure 1.
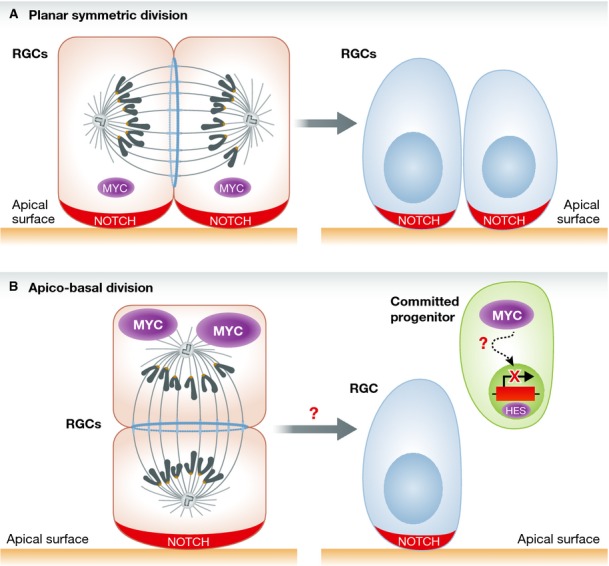
A model for MYC-induced differentiation in the chick neural tube: facts and concepts.
(A) Lower levels of MYC, indicated by the smaller symbol, in RGCs promote symmetric planar cell division, whereby active NOTCH signaling maintains the RGC pool.(B) With signaling that promotes neurogenesis, MYC is induced (larger symbol) leading to asymmetric apico-basal cell division. This causes the acquisition of neuronal differentiation features in daughter cells, probably in one of the daughter cells, in association with NOTCH downregulation and inhibition of the NOTCH target genes HES1 and HES5. Dashed arrow indicates the unclear mechanisms of NOTCH inhibition by MYC. Undefined are also the signaling pathways that regulate MYC expression in RGCs and committed progenitors. RGC: radial glial cells.
The main conclusions of this study are apparently at odds with the widely accepted notion that MYC inhibits differentiation and promotes proliferation and self-renewal of stem and progenitor cells 1. The inclusion of MYC in the cocktail of transcription factors that are necessary to reprogram somatic cell types into induced pluripotent stem cells is one of the best examples of MYC's ability to maintain the expansion of stem and progenitor cells 5.
However, the notion that MYC may drive specific pathways of differentiation is not unprecedented, as it has been shown that MYC can stimulate differentiation of keratinocytes and hematopoietic cells 6–8. Interestingly, these findings were obtained in cell systems with multicellular organotypic interactions. Although studies utilizing cultured systems have been invaluable in identifying MYC activities, it is obvious that they lack the critical components that in vivo regulate the balance between self-renewal and differentiation and the impact of the interactions between different cell types on this balance. The new function of MYC as driver of neurogenesis in the chick neural tube as documented in this study adds an additional layer to the broad spectrum of MYC activities 9. The study also opens many questions. Future studies will have to determine whether the pro-differentiation function of MYC can also be identified in the mammalian nervous system and elucidate the direct molecular events engaged by MYC to promote neurogenesis. Probably the most critical question is what controls the opposing functions of MYC (unrestrained proliferation and differentiation) in neural cells, or how MYC activity switches from sustaining proliferation to promoting differentiation. It can be speculated that different levels, duration, or the timing of MYC expression determine the cellular responses. The differentiation fate induced by high levels of MYC could represent a safeguard mechanism to limit the oncogenic potential of MYC proteins. For now, it is reasonable to conclude that the study from Zinin et al strengthens the case that MYC can exhibit opposite cellular functions that are likely context-dependent. The conclusions from this study also should have an impact on scientists who are skeptical about the idea that an oncogene can promote differentiation.
Acknowledgments
Work in the author's laboratory is supported by R01-CA101644 and R01-CA131126 (A.L.), R01-CA085628 and R01-CA127643 (A.I.) from the National Institute grants and National Institute of Health and the Institute of Neurological Disorders and Stroke R01NS061776 (A.I.).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Eilers M, Eisenman RN. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knoepfler PS, Cheng PF, Eisenman RN. Genes Dev. 2002;2002:2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X, Heng JI, Guardavaccaro D, et al. Nat Cell Biol. 2008;2008:643–653. doi: 10.1038/ncb1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinin N, Adameyko I, Wilhelm M, et al. EMBO Rep. 2014 doi: 10.1002/embr.201337424. DOI 10.1002/embr.201337424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappell J, Dalton S. Cold Spring Harb Perspect Med. 2013;3:a014381. doi: 10.1101/cshperspect.a014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurlin PJ. Cold Spring Harb Perspect Med. 2013;3:a014332. doi: 10.1101/cshperspect.a014332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson A, Murphy MJ, Oskarsson T, et al. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandarillas A, Watt FM. Genes Dev. 1997;11:2869–2882. doi: 10.1101/gad.11.21.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt FM, Frye M, Benitah SA. Nat Rev Cancer. 2008;8:234–242. doi: 10.1038/nrc2328. [DOI] [PMC free article] [PubMed] [Google Scholar]


