Defining a drug is an easy task: It is a molecule delivered to the body to produce a biological effect. Its mode of action is to alter one or more biochemical pathways, for instance, by binding to a receptor or by modifying the activity of an enzyme. Defining a placebo is a bit more complicated. A placebo is usually defined in pharmacological terms as an inert substance with no pharmacological action. However, this definition is superficial, as the effectiveness of a placebo comprises many things, including the words, rituals, symbols and meanings that accompany its use. Thus, the placebo is not the substance alone, but its administration together with a concomitant set of sensory and social stimuli that tell the patient that he or she is being treated. Indeed, a placebo is the entire ritual of the therapeutic act.
Most of the confusion about the placebo effect comes from the different usage and meaning that clinicians who conduct a clinical study and neuroscientists assign to the word. Clinicians are generally interested in any positive effects to be seen within a control group of patients, regardless of the cause of those effects. In the absence of a drug, improvements can result from many factors including the spontaneous remission of a disease, statistical regression to the mean, the patient or doctor's bias, or the patient's expectation of improvement. By contrast, neuroscientists are only interested in those improvements that derive from active processes in the patient's brain, such as expectations of benefit and learning mechanisms. Clinical trials are only aimed at establishing whether patients who take the true treatment, be it pharmacological or not, are better off than those who take the placebo. Although this pragmatic approach yields fruitful results in a clinical trial setting, it is virtually useless to neuroscientists who want to understand what is going on in the brain when a placebo is given, that is, when a therapeutic ritual is performed without the actual administration of any therapy.
… a placebo is the entire ritual of the therapeutic act.
Taking these considerations into account, the placebo effect acquires an important biological meaning and represents an excellent model for the neuroscientist to understand how the human brain works. Indeed, the vast expansion of placebo research over the past decades has taught us that many mechanisms are involved, ranging from modulation of anxiety to activation of reward mechanisms, from classical associative conditioning to social learning and from genetics to different personality traits. There is not one single placebo effect, but many, each with different mechanisms across a variety of medical conditions and therapeutic interventions. Similar to our better understanding of cancer, in which different mechanisms are responsible for different types of the disease, we have learned over the past few years that the question “What is the mechanism of the placebo effect?” is wrong. A better question is: “What are the mechanisms across different conditions?”
… humans are endowed with endogenous systems that can be activated by verbally induced positive expectations, therapeutic rituals, healing symbols, and, more generally, by social interactions
One of the most interesting aspects of placebo research is related to the newly emerging concept that placebos activate the same biochemical pathways that are activated by the drugs we administer in routine medical practice. This view is an interesting challenge from both an evolutionary and a neurobiological point of view. In other words, humans are endowed with endogenous systems that can be activated by verbally induced positive expectations, therapeutic rituals and healing symbols and, more generally, by social interactions. For example, there is now compelling experimental evidence that placebo analgesia can be mediated by at least two systems: the endogenous opioid system and the endocannabinoid system 1, 2. In addition, the cholecystokinergic system can modulate the opioid system so as to produce placebo responses of different magnitudes 3. Likewise, administration of placebo to Parkinson patients induces the release of dopamine in the striatum 4. These observations represent an epochal transition from general concepts, such as suggestibility and power of mind, to a true physiology of the placebo effect.
This new perspective may have profound implications both in routine medical practice and in clinical trials. For example, when morphine is administered, it binds to opioid receptors and inhibits pain transmission, but at the same time, the ritual of its administration induces the activation of the same opioid receptors. Similarly, when an anti-Parkinson dopaminergic drug is given, it stimulates dopamine receptors, but at the same time, the ritual of its administration activates the same dopamine receptors. Considering that drugs and placebos share common receptors and biochemical pathways, one of the main challenges is therefore to understand the similarities and differences between the actions of drugs and those of placebos. Despite these common pathways, clear differences do exist in some conditions including pain and Parkinson's disease: duration of action, variability of the effect and magnitude of the effect.
In general, the duration of the effect of a drug is longer than that of a placebo. As far as we know, this holds true for painkillers and anti-Parkinson agents, whereas much less is known about other therapeutic interventions. For example, the effect of the powerful anti-Parkinson drug apomorphine lasts much longer on average than that of a placebo. The variability of the effectiveness of a drug is also much lower than that of a placebo; the effectiveness of drugs is fairly consistent, while the effectiveness of placebo ranges widely across patients.
However, when a placebo is effective, the magnitude of that effect matches that of a drug. For example, some good placebo responders may show a reduction in the UPDRS (Unified Parkinson's Disease Rating Scale) by up to 50%, similar to drugs 5. The placebo effect can be even larger in pain reduction up to 5–6 points on a scale ranging from 0 (no pain) to 10 (unbearable pain); drug companies go to great lengths to produce drugs that reduce pain by 2–3 points. In irritable bowel syndrome, the analgesic response to a placebo can be even larger than to lidocaine 6. However, it is important to point out that only a small percentage of placebo responders show such huge effects. Owing to the response variability, the average magnitude is significantly larger for drugs compared with placebos.
Duration, variability and magnitude are related to efficacy, and we can also make several considerations about toxicity. In fact, placebos may produce so-called nocebo or negative effects, which represent the evil twin of the placebo effect. Patients who receive placebo in analgesic clinical trials for migraine, for example, often report a high frequency of adverse events. These negative effects correspond to those expected of the anti-migraine medication against which the placebo is compared. For example, anorexia and memory difficulties, which are typical adverse events of anti-convulsants, are present only in the placebo group of such trials, which suggests that the adverse events in placebo arms of clinical trials of anti-migraine medications depend on the adverse events of the active medication against which the placebo is compared. These findings are in keeping with the important role of expectation in the placebo/nocebo phenomenon, such that sometimes patients get what they expect, perhaps from reading the side effect information of the real drug. The number of dropouts in clinical trials owing to nocebo effects is a crucial aspect that may confound the interpretation of many clinical trials.
Much less is known about the biological mechanisms of nocebos, mainly because of the ethical limitations of giving negative information to patients. Today we know that anticipatory anxiety plays a key role, and anxiety triggers the activation of cholecystokinin, which, in turn, facilitates pain transmission 7. A deactivation of endogenous opioids and dopamine has also been found to take part in the nocebo phenomenon 8.
Similarities and differences between drugs and placebos are not confined to the classical clinical setting: Placebos can reproduce some effects of recreational drugs and cognitive performance-boosting drugs. Moreover, placebos may also show effects similar to those of the ergogenic drugs used in sport to increase physical performance. This raises important ethical and legal issues for anti-doping agencies, since placebos—which are not detectable in blood or urine—have been found to increase performance in some conditions 9. The question is thus whether it is ethical to use placebo procedures in sport to mimic the ergogenic action of drugs.
Several important questions also arise as to how to exploit the placebo effect in routine clinical practice. However, two opposite questions can be posed, depending on the setting in which placebos are administered. In routine clinical practice, one wants to maximize the placebo effect, so the main question in which physicians are interested is: “How can we decrease the variability and increase the duration and magnitude of placebo effects?” By contrast, in clinical trials, we want to minimize the placebo response in order to better emphasize the drug effect; thus, those conducting clinical trials would like to answer the question: “How can we decrease variability, duration and magnitude of placebo effects?”.
There is a growing tendency to justify any bizarre procedure and healing practice by claiming that they may induce positive expectations and outcomes via the placebo effect
Today, we are in a good position to partially resolve these two challenges. Indeed, it is possible to manipulate, at least in part, the placebo response in both directions. First, by using a learning procedure, we can decrease variability and increase duration and magnitude. To do this, pharmacological pre-conditioning is carried out whereby a real drug is administered for several days before it is replaced with a placebo. Most patients show huge placebo responses, which indicates that learning plays a key role in the placebo effect. This is desirable in routine medical practice, as it is then possible to reduce drug intake in the long run. Second, by using a negative conditioning procedure—which creates a mismatch between what the patient expects and what he or she gets—it is possible to decrease the magnitude of the placebo effect. Doing this is necessary for clinical trials, as subjects with low placebo responses can be better compared with subjects who take the active treatment. Unfortunately, this negative conditioning procedure can only control for the learning component of the placebo effect, but it has no effect on spontaneous remission and regression to the mean.
A better understanding of the similarities and differences between drugs and placebos represents an important challenge for future research, which will lead to better medical practice and better interpretation of clinical trials. The crucial starting point is the understanding of the biological underpinnings of placebos and their relationship to drug action. As far as we know, at least two possibilities can be envisaged: Drugs and placebos can act either on the same receptors or, otherwise, on the same type of receptor but in different regions of the central nervous system, for example in pain (Fig 1). There is some experimental evidence that the second mechanism is more likely. For example, narcotics bind to the mu-opioid receptors in one region of the brain, whereas placebos act, through the activation of endogenous ligands, on mu-opioid receptors in a different region, with an overall additive effect 10.
Figure 1.
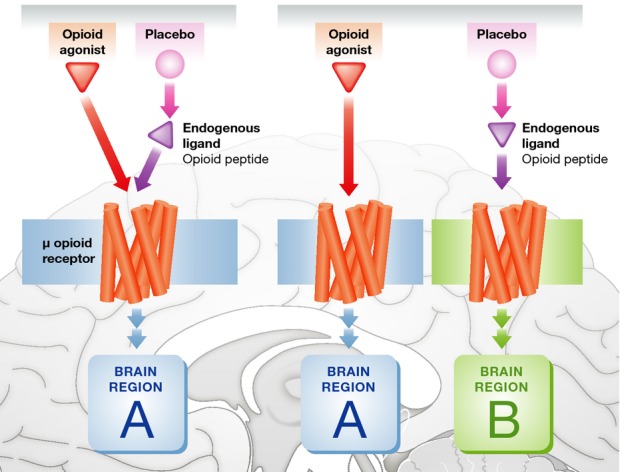
Two possible mechanisms of action of opioid drugs and placebos.
Opioids and placebos (through the activation of endogenous ligands) may act on the very same μ receptors located in the same region of the brain or, otherwise, they may act on the same type of opioid receptors but in different regions.
Despite these recent insights into the neurobiology of placebo effects, there is a paradox in placebo research: The more we know, the more difficult our correct communication to the general public seems to become. Indeed, there is danger lurking around the corner. As placebo responses can be triggered by the very ritual of the therapeutic act, any therapeutic ritual can, in principle, activate the same biochemical mechanisms. If fake pills prescribed by doctors can generate positive expectations and outcomes, so talismans handed out by quacks and bizarre rituals performed by shamans may also induce positive expectations.
There is a growing tendency to justify any bizarre procedure and healing practice by claiming that they may induce positive expectations and outcomes via the placebo effect. After the discovery that endocannabinoids are activated by placebos and positive expectations 2, I was contacted by many people with weird and eccentric proposals aimed at enhancing expectations, beliefs, trust and hope. These individuals often claim that any procedure that increases expectations and beliefs is justified, no matter where it comes from. This is a worrisome future perspective that we should avoid by improving good communication between science, ethics and the media. The results of placebo research should be better explained to both journalists and the general public, because misuse could have a devastating social impact and undermine the credibility of modern medicine itself. The future ethical and biological debate promises to be exciting and stimulating, for we are dealing with the foibles and vulnerable aspects of human beings: expectation, belief, trust, hope and suggestibility. Understanding their underlying biology is exciting, but it may turn out to be dangerous and alarming if badly exploited.
Conflict of interest
The author declares that he has no conflict of interest.
References
- 1.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human (micro)-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedetti F, Amanzio M, Rosato R, Blanchard C. Non-opioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 2011;17:1228–1230. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti F, Amanzio M, Thoen W. Disruption of opioid-induced placebo responses by activation of cholecystokinin type-2 receptors. Psychopharmacology. 2011;213:791–797. doi: 10.1007/s00213-010-2037-y. [DOI] [PubMed] [Google Scholar]
- 4.de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B, Lopiano L. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7:587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- 6.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, expectancy and desire to placebo effect in irritable bowel syndrome patients. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 9.Benedetti F, Pollo A, Colloca L. Opioid-mediated placebo responses boost pain endurance and physical performance – Is it doping in sport competitions? J Neurosci. 2007;27:11934–11939. doi: 10.1523/JNEUROSCI.3330-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable effects of opiates and expectations on pain. J Neurosci. 2012;32:8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


