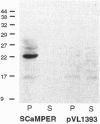
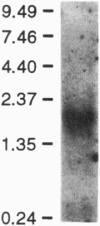
Abstract
Release of Ca2+ stored in endoplasmic reticulum is a ubiquitous mechanism involved in cellular signal transduction, proliferation, and apoptosis. Recently, sphingolipid metabolites have been recognized as mediators of intracellular Ca2+ release, through their action at a previously undescribed intracellular Ca2+ channel. Here we describe the molecular cloning and characterization of a protein that causes the expression of sphingosyl-phosphocholine-mediated Ca2+ release when its complementary RNA is injected into Xenopus oocytes. SCaMPER (for sphingolipid Ca2+ release-mediating protein of endoplasmic reticulum) is an 181 amino acid protein with two putative membrane-spanning domains. SCaMPER is incorporated into microsomes upon expression in SO cells or after translation in vitro. It mediates Ca2+ release at 4 degrees C as well as 22 degrees C, consistent with having ion channel function. The EC50 for Ca2+ release from Xenopus oocytes is 40 microM, similar to sphingosyl-phosphocholine-mediated Ca2+ release from permeabilized mammalian cells. Because Ca2+ release is not blocked by ryanodine or La3+, the activity described here is distinct from the Ca2+ release activity of the ryanodine receptor and the inositol 1,4,5-trisphosphate receptor. The properties of SCaMPER are identical to those of the sphingolipid-gated Ca2+ channel that we have previously described. These findings suggest that SCaMPER is a sphingolipid-gated Ca2+-permeable channel and support its role as a mediator of this pathway for intracellular Ca2+ signal transduction.
Full text
PDF

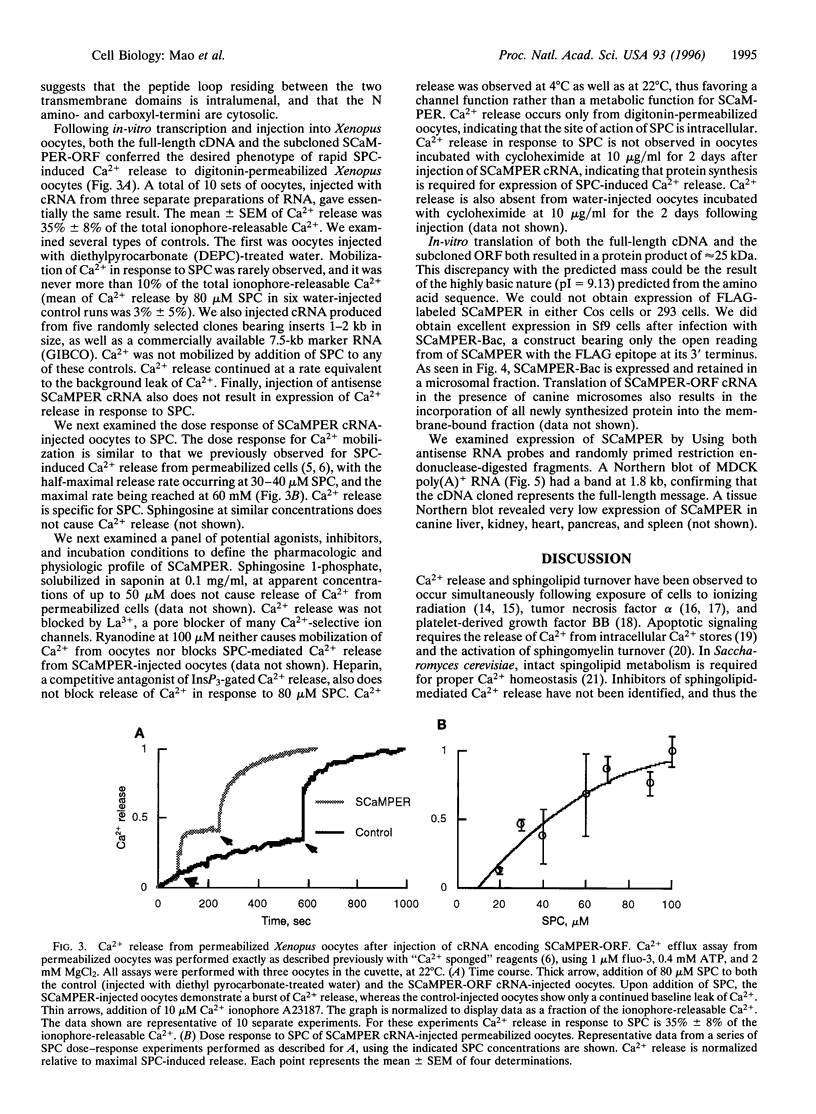

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Finbow M. E., Harrison M., Jones P. Ductin--a proton pump component, a gap junction channel and a neurotransmitter release channel. Bioessays. 1995 Mar;17(3):247–255. doi: 10.1002/bies.950170311. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J., Gill D. L. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990 Jun 29;248(4963):1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A., Kan C. C., Ehleiter D., Persaud R. S., McLoughlin M., Fuks Z., Kolesnick R. N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994 Aug 1;180(2):525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan D. E., Bleakman D., Virudachalam S., Lee D., Grdina D., Kufe D. W., Weichselbaum R. R. The role of intracellular calcium in the cellular response to ionizing radiation. Radiat Res. 1994 Jun;138(3):392–400. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kim S., Lakhani V., Costa D. J., Sharara A. I., Fitz J. G., Huang L. W., Peters K. G., Kindman L. A. Sphingolipid-gated Ca2+ release from intracellular stores of endothelial cells is mediated by a novel Ca(2+)-permeable channel. J Biol Chem. 1995 Mar 10;270(10):5266–5269. doi: 10.1074/jbc.270.10.5266. [DOI] [PubMed] [Google Scholar]
- Kindman L. A., Kim S., McDonald T. V., Gardner P. Characterization of a novel intracellular sphingolipid-gated Ca(2+)-permeable channel from rat basophilic leukemia cells. J Biol Chem. 1994 May 6;269(18):13088–13091. [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Kukuljan M., Labarca P., Latorre R. Molecular determinants of ion conduction and inactivation in K+ channels. Am J Physiol. 1995 Mar;268(3 Pt 1):C535–C556. doi: 10.1152/ajpcell.1995.268.3.C535. [DOI] [PubMed] [Google Scholar]
- Marks A. R., Tempst P., Hwang K. S., Taubman M. B., Inui M., Chadwick C., Fleischer S., Nadal-Ginard B. Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8683–8687. doi: 10.1073/pnas.86.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989 Nov 9;342(6246):192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Ohta H., Yatomi Y., Sweeney E. A., Hakomori S., Igarashi Y. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 1994 Dec 5;355(3):267–270. doi: 10.1016/0014-5793(94)01218-0. [DOI] [PubMed] [Google Scholar]
- Olivera A., Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993 Oct 7;365(6446):557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Nicotera P. Role of calcium in toxic and programmed cell death. Adv Exp Med Biol. 1991;283:419–425. doi: 10.1007/978-1-4684-5877-0_57. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Li Y., Wickman K., Ackerman M., Peralta E., Clapham D. New mammalian chloride channel identified by expression cloning. Nature. 1992 Mar 19;356(6366):238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- Renard S., Lingueglia E., Voilley N., Lazdunski M., Barbry P. Biochemical analysis of the membrane topology of the amiloride-sensitive Na+ channel. J Biol Chem. 1994 Apr 29;269(17):12981–12986. [PubMed] [Google Scholar]
- Roth D. A., Rehemtulla A., Kaufman R. J., Walsh C. T., Furie B., Furie B. C. Expression of bovine vitamin K-dependent carboxylase activity in baculovirus-infected insect cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8372–8376. doi: 10.1073/pnas.90.18.8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann M. A., Gardner P., Raffin T. A. Recombinant human tumor necrosis factor alpha induces calcium oscillation and calcium-activated chloride current in human neutrophils. The role of calcium/calmodulin-dependent protein kinase. J Biol Chem. 1993 Jan 25;268(3):2134–2140. [PubMed] [Google Scholar]
- Suzuki M., Takahashi K., Ikeda M., Hayakawa H., Ogawa A., Kawaguchi Y., Sakai O. Cloning of a pH-sensitive K+ channel possessing two transmembrane segments. Nature. 1994 Feb 17;367(6464):642–645. doi: 10.1038/367642a0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Desai N. N., Olivera A., Seki T., Brooker G., Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991 Jul;114(1):155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Beeler T., Dunn T. Suppressors of the Ca(2+)-sensitive yeast mutant (csg2) identify genes involved in sphingolipid biosynthesis. Cloning and characterization of SCS1, a gene required for serine palmitoyltransferase activity. J Biol Chem. 1994 Aug 26;269(34):21480–21488. [PubMed] [Google Scholar]