Abstract
Lymphatic fluid is a plasma filtrate that can be viewed as having biological activity through the passive accumulation of molecules from the interstitial fluid. The possibility that lymphatic fluid is part of an active self-contained signaling process that parallels the endocrine system, through the activation of G-protein coupled receptors (GPCR), has remained unexplored. We show that the GPCR lysophosphatidic acid 5 (LPA5) is found in sensory nerve fibers expressing calcitonin gene-related peptide (CGRP) that innervate the lumen of lymphatic lacteals and enteric nerves. Using LPA5 as a model for nutrient-responsive GPCRs present on sensory nerves, we demonstrate that dietary protein hydrolysate (peptone) can induce c-Fos expression in enterocytes and nerves that express LPA5. Mesenteric lymphatic fluid (MLF) mobilizes intracellular calcium in cell models expressing LPA5 upon feeding in a time- and dose-dependent manner. Primary cultured neurons of the dorsal root ganglia expressing CGRP are activated by MLF, which is enhanced upon LPA5 overexpression. Activation is independent of the known LPA5 agonists, lysophosphatidic acid and farnesyl pyrophosphate. These data bring forth a pathway for the direct stimulation of sensory nerves by luminal contents and interstitial fluid. Thus, by activating LPA5 on sensory nerves, MLF provides a means for known and yet to be identified constituents of the interstitial fluid to act as signals to comprise a “neurolymphocrine” system.
Keywords: G protein-coupled receptor, intestine, lacteal, lysophosphatidic acid 5, nutrient sensing
nutrient sensing is increasingly being recognized as affecting the etiology of diseases resulting from obesity, inflammation, dysregulation of intracellular metabolism, and modification of behavior such as food intake. The basis of current models for organic nutrient sensing begins with the studies performed during early 1900s on peripheral nerves by Pavlov and with the discovery of the first hormone, secretin, by Bayliss and Starling (8). The demonstration of the release of peptide YY (PYY) by dietary oleic acid from isolated mucosal L-cells established that populations of enteroendocrine cells (EECs) could directly respond as nutrient-sensor cells (6). Current models of the gastrointestinal nutrient sensory system are centered on the indirect activation of extrinsic nerves through the binding of their receptors by peptides, such as PYY, glucagon like peptide-1 (GLP-1), cholecystokinin (CCK), ghrelin, and somatostatin, which are secreted as hormones from EECs in response to organic nutrients (9). Part of this indirect activation of sensory nerves was used to explain the increase in expression of c-Fos proto-oncogene and c-Fos protein in neurons of the central nervous system (CNS) in response to nutrient stimuli, such as lipids and carbohydrates. Because c-Fos activity is increased in neurons containing receptors for hormones released from the gut in response to nutrients, such as CCK, it has been speculated that c-Fos activity is part of satiety-inducing mechanisms in areas of the CNS, such as the nucleus of the solitary tract (71), through gut peptide receptors in response to sensory stimulation (26). Increase in c-Fos activity by direct nutrient stimulation of sensory neurons has not been examined.
Mesenteric lymphatic fluid (MLF) contains molecules derived from the intestinal lumen and the surrounding extracellular fluid of the mucosal cells. MLF is enriched with a variety of molecules including peptide hormones (22), nutrients, neurotransmitters, and potentially biologically active lymph-specific proteins that change in concentration in response to feeding (22, 41, 54). Lymphatic vessels and endothelial cells that comprise lymphatic lacteals are innervated by peptidergic nerves (35–37, 39, 40), suggesting that these nerves may mediate potential biological activity of lymphatic fluid on the CNS. However, the function of those peptidergic nerves, which are close to the lumen of lymphatic vessels, remains unknown.
The activation of G protein-coupled receptors (GPCRs) is an important process in the sequence of events that can comprise a GI chemosensory system (23). GPCR activation can be regulated by a range of multiple ligands, with downstream responses that can be attenuated or enhanced in a gradient-like manner (57, 66). Such characteristics make GPCRs ideal nutrient signal transducers in EECs or neuronal tissues. However, the potential for lymphatic fluid to act as part of a chemosensory system that can activate GPCRs has remained unexplored. A number of orphan GPCRs have recently shown to be activated by molecules that can be found in the lumen following a meal, such as long-chain fatty acids, short-chain fatty acids, and bile acids (14, 23, 42). Because several of these GPCRs are expressed in EECs, it has been assumed that these would function as chemosensors of the enteroendocrine system in general (17, 20).
The GPCR lysophosphatidic acid 5 (LPA5) (also called GPR93 or GPR92) is expressed in mucosal cells (16) and neuronal tissues, such as the spinal cord dorsal horns (50) and dorsal root ganglia (DRG) (59). The closest structurally related GPCRs to LPA5 are P2Y5 and P2Y9, which are also activated by LPA (16, 46). However, we found that only LPA5 is activated by partially digested dietary protein (peptone). LPA5 responds to both LPA and peptone (with EC50 of 8 nM and 10 mg/ml, respectively), yielding distinct effector pathways (17). Activation of LPA5 by peptone in the EEC STC1 cell line induces a calcium-dependent CCK release and synthesis, whereas LPA activation does not result in the release of CCK (16). This allows for a regulation of CCK release, by the presence of partially digested dietary protein that is independent of the presence of LPA.
Here we show the activation of GPCRs, such as LPA5, expressed in peptidergic neurons by peptone and protein components in the mesenteric lymph. This provides the means by which luminal molecules, such as organic nutrients, and unknown interstitial molecules could act as chemical signals to the CNS through primary afferent neurons that have cell bodies in sensory ganglia (such as the DRG, vagal sensory ganglia) that are close to the brain or spinal cord and in the brain stem (28).
MATERIALS AND METHODS
Animals.
Unless otherwise indicated, Sprague Dawley rats (male, 200–250 g) and C57BL/6 mice (6–8 wk) were from Charles River Laboratories and Jackson Laboratory. Procedures conform to the guidelines of the National Institutes of Health Animal Research and were approved by Institutional Animal Care and Use Committees of the University of California at Berkeley, at San Francisco, and by the University of Cincinnati Institutional Animal Care and Use Committee.
Human tissue.
All procedures were approved by the Human Research Ethics Committee of Austin Hospital, Melbourne Australia (approval H2011/04231). Jejunal tissue was derived from patients aged between 38 and 81 yr (mean 67.6 yr) that had undergone removal of 6–8-cm-long jejunum segments, for surgical reasons, using procedures as previously described (32).
Materials.
All reagents used in this study were purchased from Sigma-Aldrich unless indicated differently. Oleoyl LPA farnesyl pyrophosphate was purchased from Cayman Chemical. The LPA used in this study was dissolved in PBS (pH 7.4) with 0.1% fatty acid-free BSA (ffBSA) as a carrier.
Plasmid construction.
The mitochondria-targeted aequorin (mtAEQ) and LPA5 expression vectors were constructed as previously described (16). The vector base for the reporter constructs, pBV-luc, was a generous gift from Dr. Bert Vogelstein (The Johns Hopkins Kimmel Cancer Center). The cAMP response element (CRE; TGACGTCA)-linked luciferase reporter contained eight tandem repeats of the respective response element. The c-Fos promoter-linked luciferase reporter plasmid was constructed by isolating the rat c-Fos promoter region that includes regulatory elements for c-Fos expression [from −471 to −113 bases relative to the transcription-starting site (13)] with PCR (forward primer, 5′-GCCTCGAGCTGTTCCCGTCAATCCCTCCCTCCTTTAC-3′, and the reverse primer, 5′- GCGAAGCTTAGCTGCAATCGCGGTTGGAGTAGTAGGCG-3′) and subcloning it into pGL3-basic (Promega). All constructs were verified by DNA sequencing (DNA Sequencing Facility, University of California, Berkeley).
Primary hippocampal neuron and DRG isolation.
Cells from adult mouse hippocampus were isolated as described by Fabel et al. (24). Briefly, adult male mice were anesthetized and decapitated, and whole brains were removed. The brain was first bisected longitudinally, and the hippocampal lobe was separated along the natural separating line between the cortical white matter and alveus hippocampus. The white matter of the fimbria and subiculum was also removed. The hippocampal tissues were minced, digested with DNase (250 U/ml), neutral protease (1 U/ml Dispase), and papain (2.5 U/ml) in Hanks' balanced salt solution (HBSS), and washed three times with Dulbecco's modified Eagle's medium (DMEM)/10% fetal bovine serum (FBS; Hyclone Laboratories). The dislodged cells were suspended in DMEM/10% FBS, filtered through a sterile 107-μm nylon mesh, and mixed at equal volume with 90% Percoll (GE Healthcare) solution in PBS. The mixture was fractionated by centrifugation for 30 min, 18°C, at 20,000 g. The pellet from the centrifugation was washed using DMEM/10% FBS for three times before cells were seeded down in DMEM/F-12 (1:1)/10% FBS on polyornithine/laminin-coated dishes for 24 h. After 24 h, medium was then changed to DMEM/F12 (1:1) supplemented with Glutamine N2 (Invitrogen) and 20 ng/ml fibroblast growth factor 2 until transfection.
DRG from thoracic and lumbar spinal cord of mice were minced in cold HBSS and incubated for 60–90 min at 37°C in DMEM containing (in mg/ml): 0.5 trypsin, 1 collagenase type IA, and 0.1 DNase type IV. Soybean trypsin inhibitor (Sigma) was added to neutralize trypsin. Neurons were pelleted, suspended in DMEM containing 10% FBS, 10% horse serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 2 mM glutamine, plated on glass coverslips coated with poly-l-lysine and laminin, and cultured for 2–3 days before use in the studies as previously described (1).
Cell culture conditions and transfection.
STC-1 cells (passage 17) were kindly provided by Dr. Douglas Hanahan (University of California, San Francisco). Rat pheochromocytoma cell line, PC12, was obtained from American Type Culture Collection. The hybrid Berkeley rat intestinal epithelial 380i (hBRIE 380i) cell line is nontumorigenic and expresses enterocyte phenotypes and intestine-specific protein markers (4). The hBRIE 380i cells used in the experiments were from passage 11 to 18. Cell culture conditions for hBRIE 380i/Chinese hamster ovary (CHO) cells and the electroporation protocol were as previously described (16). STC-1 cells (passage 25 to 35) were maintained in DMEM with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin as additional supplements; at 37°C in 5% CO2-air. PC12 cells were maintained in Iscove's Modified Dulbecco's Medium (IMDM; Invitrogen) with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin as additional supplements; at 37°C in 5% CO2-air. Rat LPA5 or the empty vector stable transfectants were developed as follows; 36 h after electroporation (3 μg plasmid DNA/106 cells), resistant clones were selected and maintained in the presence of 800 μg/ml G418 (Invitrogen). The overexpression of LPA5 was verified by PCR and the calcium mobilization assay. To induce neuronal differentiation in PC12 cells, 1 × 106 PC12 cells were laid down in IMDM/10% FBS in 24-well plates for 24 h followed by one PBS wash and changed to IMDM/1% FBS containing 100 ng/ml NGF for 7 days. Fifty percent of the culture medium was replaced every other day, including fresh NGF. NGF-differentiated PC12 cells, STC-1 cells, and primary hippocampal neuron cells were transfected with the LPA5 plasmid or the empty vector (pCI-neo; Promega) at 3 μg/106 cells (for CRE reporter experiment in PC12, additional 1 μg CRE reporter plasmid/106 cells was cotransfected with the receptor), using the Superfect reagent (Qiagen) according to the manufacturer's protocol, and cells were allowed to recover for 24 h under the normal culture conditions.
Bacterial-derived protein activation of LPA5.
The activation of LPA5 was determined by exposing hBRIE380i cells that constitutively express LPA5 to increasing concentrations of peptidoglycan (PGN), muramylpeptides, desmuramylpeptides (DAP, γ-DAP), flagellin, and Pam 3 (Cys-Ser-Lys) (PAM). No induced receptor activation was observed compared with the activation by 1 μM LPA.
Determination of LPA and FPP in MLF.
The concentrations of LPA and farnesyl pyrophosphate (FPP) were measured in MLF taken from fasted rats and 60 min after rats were infused with a bolus of Ensure. Lipid measurements were determined as previously described (15). Briefly, cell lipids were extracted with chloroform:methanol:Tris buffer (2:1:1). The organic layer was extracted, followed by acidification of the aqueous phase and reextraction with chloroform. The organic phases were combined, dried under nitrogen, and resuspended in chloroform for analysis by LC-mass spectrometry.
RNA isolation.
To determine the change of c-Fos gene expression, both NGF-differentiated PC12 cells and primary hippocampal neuron cells were starved in serum-free media for 2 h after recovering from transfection, followed by 3-h treatments of peptone, LPA, or FPP in serum-free media. RNA was prepared as previously described (16).
Semi-quantitative and single-cell RT-PCR.
Reverse transcription was performed as previously described (47). The PCR primers for LPA5 were designed to match the LPA5 sequence of rat (accession number: XM_575667) and mouse (accession number: BC117528). The LPA5 forward primer sequence was 5′-GCTCTGCCTGGGCGTGTGGGCTCTCATCCTGC-3′, and the reverse primer sequence was 5′-GCGTCGGGCCTCGCCAGTGTCCAGAAGAC-3′. The PCR primers for c-Fos were designed to match the c-Fos sequence of rat (accession number: NM_022197) and mouse (accession number: NM_010234). The c-Fos forward primer sequence was 5′- CAGATGTGGACCTGTCTGGTTCCTTCTATG-3′, and the reverse primer sequence was 5′- AGGGAAGACGTGTTTCTCCTCTCTGTAATG-3′. The PCR parameters were: 20 s at 94°C, 15 s at 58°C, and 30 s at 72°C, for 30 cycles.
Single neurons were processed to obtain cDNA using Superscript III Cells Direct cDNA Synthesis Kit (Invitrogen) according to the manufacturer's instructions. Water was added to one-third of the sample for the negative reverse transcriptase (RT) control. The remaining two-thirds of the sample was reverse transcribed using Superscript III RT. PCR reactions used the following intron-spanning mouse primers: LPAR5 forward 5′ to 3′ GCTCTGCCTGGGCGTGTGGGCTCTCATCCTGC, reverse 5′ to 3′ GCGTCGGGCCTCGCCAGTGTCCAGAAGACC; transient receptor potential vanilloid 1 (TRPV1) forward 5′-tcaccgtcagctctgttgtc-3′, reverse 5′-gggtctttgaactcgctgtc-3′; β-actin forward 5′-ctggtcgtcgacaacggctcc-3′, 5′-reverse gccagatcttctccatg-3′. The PCR reaction contained primers, 0.5 units of HotStar Taq polymerase, 2.5 mM MgCl2, 10 mM dNTP, and 10× PCR buffer (Qiagen) (20 μl final volume). The PCR reaction conditions were 50 cycles of initial activation at 95°C for 15 min, denaturation at 94°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 1 min, and final extension at 72°C for 10 min. As a positive control, RNA was isolated from whole DRG, spinal cord, or gall bladder and was reverse transcribed using Omniscript RT Kit (Qiagen). As negative controls, fluid from the vicinity of the collected cells was amplified, or RT was omitted. Products were separated by electrophoresis (2% agarose), stained using ethidium bromide, and sequenced to confirm identity.
Calcium mobilization assay.
For the AEQ-based [Ca2+]i mobilization assay CHO, hBRIE 380i, or STC-1 cells were electroporated with the mtAEQ expression plasmid (2 μg/106 cells) and the LPA5 expression plasmid or empty vector (4 μg/106 cells). Cells were allowed to recover for 20 h in IMDM/10% BCS, and then the [Ca2+]i mobilization assay was performed as previously described (16). Luminescence (as relative light units, RLU) was recorded continuously. Fractional RLU was defined as the increased RLU due to a stimulus normalized to the total RLU. Total RLU was the integrated RLU value for 30 s after injection of the stimulus plus 20 s after the addition of the lysis buffer.
For Fura-2 measurement of [Ca2+]i, hBRIE 380i cells and DRG neurons were incubated in HBSS, 0.1% BSA, and 20 mM HEPES, pH 7.4, containing 2.5 to 5 μM Fura-2 AM (Molecular Probes) for 30–45 min at 37°C. Coverslips were mounted in an open chamber at 37°C. Fluorescence of individual cells was measured at 340 and 380 nm excitation and 510 nm emission using a Zeiss Axiovert microscope, an intensified CCD video camera (Stanford Photonics), and a video microscopy acquisition program (Axon Instruments). Test substances were directly added to the chamber (50 μl injection). Each coverslip received only one treatment with protease-activated receptor 2-activating protein (PAR2-AP) or the inactive reverse peptide followed by capsaicin. DRG preparations were challenged with KCl (50 mM) at the end of each experiment (2). PAR2-AP (SLIGRL-NH2) was synthesized and purified as previously described (62).
Luciferase reporter assay.
The hBRIE 380i cells were electroporated with the reporter plasmid DNA (2 μg/106 cells) and LPA5 expression construct or empty vector (6 μg/106 cells) and placed onto 24-well plates (2.5 × 105 cells/well). PC12 cells were transfected using Superfect. After 24 h in IMDM/10% BCS (IMDM/10% FBS with NGF was used for PC12 cells), the cells were serum starved for 2 h in IMDM/0.1% ffBSA. The treatments with peptone, LPA, or FPP were for 6 h, or in the case of c-Fos promoter reporter, 16 h. Luciferase activities from the samples were determined as previously described (16).
Immunocytochemistry.
Using computer sequence analysis tools, we selected the LPA5/GPR93 ECL2 domain as a target for the production of rabbit polyclonal anti-peptide antibodies. The full-length sequence was retrieved from the National Center for Biotechnology Information (NCBI) protein database and analyzed using MacVector (MacVector) and NCBI BlastP tools as well as the criteria described by Nelson et al. (56). The peptide (LCFESFSDELWKGR) was selected based on high indices of antigenicity, surface probability, and surface topology and has 100% homology with human (174-187), mouse, and rat GPR93 (178-191). Cysteine was added to the COOH terminus as a cross-linking site for the preparation of peptide-protein immunogens and affinity media. The pMAL Protein Fusion and Purification System (New England Biolaboratory) was used to generate and purify the peptide fragment following the manufacturer's protocol. The amino acid content was verified by HPLC. Rabbits were injected with rat GPR93 (acetyl-178-191-Cys-amide) that is conjugated to a keyhole limpet hemocyanin once per month for 4 mo. Nonspecificity was assessed by analysis of staining with preimmune sera, by omission of primary antisera, and by analysis of wild-type hBRIE cells and hBRIE cells transfected with the rat LPA5.
Gastrointestinal tissues were from adult Sprague Dawley rats (male, 200 g; Harlan) and C57Bl6 mice (male, 6–8 wk old, 200–250 g, Charles River Laboratories). DRG from thoracic and lumbar spinal cord were taken from C57Bl6 mice. Tissues were immersion fixed for 1–2 days at 4°C. For cryostat sections, tissues were incubated in 20–25% sucrose in PBS for 24 h at 4°C, embedded in optimal cutting temperature compound (Miles), and sectioned at 10 μm for gastrointestinal tissues or 25 μm for DRG and spinal cord. Sections were processed after being mounted on slides (gut) or as floating sections (DRG, spinal cord). Whole mounts were prepared by dissection of the longitudinal muscle with attached myenteric plexus, circular muscle, and sub-mucosa from the terminal ileum and were processed as floating preparations. Sections and whole mounts were washed in PBS containing 1–10% normal goat serum, 1% BSA, and 0.3–0.5% Triton X-100. Tissues were incubated with the following primary antibodies: LPAR5, rabbit 93ab2, 1:700; substance P, rat, 1:800 (see Cuello et. al. Ref. 21); calcitonin gene-related peptide, sheep, 1:1,000 (see Gibbins et. al. Ref. 30); antineuronal nuclear autoantibody, human, 1:10,000 (see Lucchinetti et. al. Ref. 53); nitric oxide synthase, sheep, 1:2,000 (Williamson et al. Ref. 67); isolectin-B4 (FITC conjugate), 1:400, Vector Laboratories; Calretinin, goat, 1:500, SWANT at 4°C or room temperature overnight for sections or at 4°C for 48 h for whole mount. Tissues were washed and incubated with secondary antibodies conjugated to goat or donkey anti-rabbit, rat, mouse, or guinea pig IgG conjugated to FITC, Rhodamine, or Red X, Texas Red (Jackson ImmunoResearch, 1:200) or conjugated to Alexa-488 or Alexa-568 (Molecular Probes, 1:200–1:1,500) at room temperature for 2 h. For simultaneous detection of two antigens, specimens were incubated with both primary antibodies and then secondary antibodies labeled with contrasting fluorophores. Sections and whole mounts were then washed and mounted in Prolong (Molecular Probes).
Microscopy.
For epifluorescence microscopy, specimens were observed using a Zeiss Axioplan microscope with Axiocam or Spot digital cameras. For confocal microscopy, specimens were observed using a Zeiss Axiovert microscope, a Zeiss 510 laser-scanning confocal microscope, or a Leica TCS-SP confocal microscope. The following objectives were used: Zeiss Fluor 20 (NA 1.0); Plan Apo 40 (NA 1.4), 100 (NA 1.3); Leica 10 (NA 0.4), 20 (NA 0.7), 100 (NA 1.4). For confocal microscopy, images were collected at a zoom of 1–2, and typically 10–20 optical sections were taken at intervals of 0.5–1.0 μm as previously described (19) (images were processed to adjust contrast and brightness using Adobe PhotoShop 7.0). Images of stained and control slides were collected and processed identically. Confocal images were digitally colored to represent the appropriate fluorophores. Images were viewed and analyzed using Imaris 7.6.5 from Bitplane Scientific Software.
Mesenteric lymph fluid.
Male Sprague-Dawley rats weighing 280–320 g (Harlan) were individually housed in a temperature-controlled (21 ± 1°C) vivarium on a 12-h:12-h light/dark cycle (lights on at 0600). Standard chow (LM-485 Mouse/Rat Sterilizable Diet, Harlan Laboratories) and water were provided ad libitum (except where noted). All animal procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All surgery and experiments were conducted 1–2 wk after rats were received as previously described (52).
In brief, rats were fasted for 24 h before surgery but retained free access to water. Rats were anesthetized with isoflurane anesthesia, and then the superior mesenteric lymphatic duct was cannulated 2 cm beyond the pylorus into the duodenum via a fundal incision of the stomach. The lymph cannula and the intraduodenal feeding tube were exteriorized through the right flank. After surgery, the animals were placed in Bollman restraint cages (12) and allowed to recover overnight; the animals were kept in a temperature-regulated chamber (28°C) to prevent hypothermia and received a continuous intraduodenal infusion of 5% glucose-saline solution (145 mM NaCl, 4 mM KCl, and 0.28 M glucose) at 3 ml/h until 5:00 pm on the surgery day followed by 0.9% saline infusion at 3 ml/h until the next morning. The next morning, fasting lymph was collected for 1 h while 0.9% saline was infused at 3 ml/h. A bolus of 3 ml of either dextrin or Lyposyn II (Hospira) or Ensure (Abbott Laboratories) solution/emulsion was infused into rat duodenum while the 0.9% saline infusion was paused for 30 min. Lymph samples were collected at 0 (for fasting values) 30, 60, 120, and 180 min. Rats then received continuous infusion of saline (0.15 M NaCl) at 3 ml/h overnight before Ensure or Liposyn infusion to compensate for fluid and electrolyte loss due to lymphatic drainage. Lymph samples collected from dextrin (hydrolyzed starch)-infused rats were used as a control because duodenal starch infusion does not alter the basal phospholipid composition in rat mesenteric lymph (43). We used the mesenteric lymph flow rate as an indicator to determine whether our experimental conditions and techniques of lymph collection were adequate. The solution used for dextrin treatment was made by dissolving 1.11 g dextrin (starch hydrolysate; MW = 15,000; 4 kcal/g caloric content) in 3 ml PBS at pH 6.4. The emulsion used for Liposyn treatment was made by mixing 2.2 ml of 20% Liposyn II (containing 5% safflower oil, 5% soybean oil, up to 1.2% egg phosphatides, 2.5% glycerin, and water; 2 kcal/ml caloric content; Abbott Laboratories) with 0.8 ml 0.9% saline. Ensure (Abbott Laboratories) was used as a mixed-nutrient liquid that contains 14 kcal% protein, 21 kcal% fat, and 64 kcal% carbohydrate with a caloric content of 1.48 kcal/ml. Thus dextrin, Liposyn, and Ensure treatments used in this study had a caloric content of 4.43 kcal/3 ml. All rats received a 3-ml bolus dose of either dextrin saline Liposyn or Ensure through the intraduodenal feeding tube (time 0). At 30 min after bolus infusion, the saline infusion was resumed at 3 ml/h, and lymph was collected on ice at 10–30-min intervals for 120 min after bolus infusion. Each lymph sample was treated with 10% per volume antiproteolytic cocktail (0.25 M EDTA, 0.80 mg/ml aprotinin). At the end of the lymph-collection period, rats were euthanized.
Statistical analysis.
Where applicable, data were expressed as means ± SD. Statistical difference between multiple groups was determined by one-way ANOVA with Duncan's post hoc test performed using SPSS version 11. Significance was accepted at P < 0.05.
RESULTS
LPA5 has been identified in gastrin G cells, somatostatin D cells of the gastric mucosa (33), and a CCK cell line (17). LPA5 could be a transducing GPCR that enables these cells to release their respective gut peptides in response to dietary protein. We determined LPA5 expression in the intestine in situ, in cells that could be responsive to luminal-derived signals, using an antibody (93ab2) against an LPA5 peptide fragment conserved in rat, mouse, and human. In this study, we show that LPA5-like immunoreactivity (LPA5-LI) in the small intestine (mouse, rat, and human) and distal colon (mouse) was prominently localized to submucosal ganglia and to EECs of the basal crypts and villi (Figs. 1 and 2), which demonstrates evolutionary conservation in enteric expression.
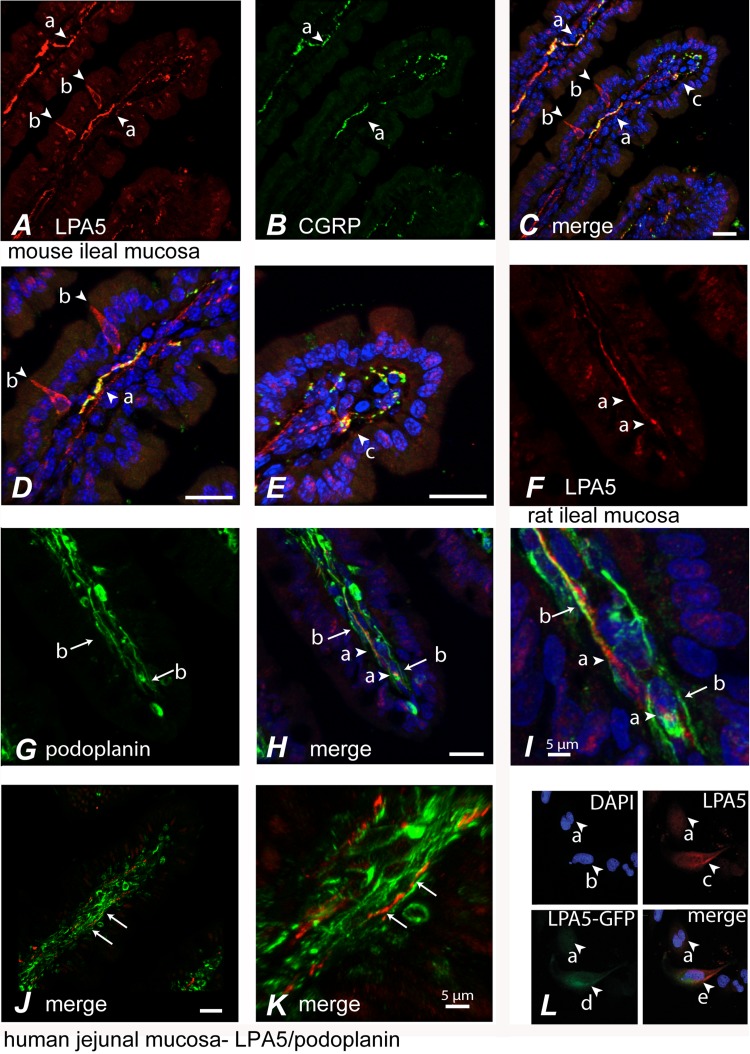
Fig. 1.
Immunocytochemical localization of lysophosphatidic acid 5 (LPA5) in sensory nerves of the mucosal lymphatic lacteals and in enteric neurons. A: LPA5-like immunoreactivity (LPA5-LI) was detected in nerve fibers (arrows, a) and enteroendocrine-like cells (arrows, b) (red) in mouse small intestinal mucosa. B and C: nerve fibers positive for calcitonin gene-related peptide (CGRP) (arrows, a) were also immunoreactive for LPA5. Higher magnifications of the mid-villus (D) and villar tip (E) from C reveal regions of colocalization, represented in yellow (arrows, a and c). LPA5-positive nerve fibers (F) (arrows, a) innervating villi were associated with the luminal-facing podoplanin-immunoreactive lymphatic endothelium (G) (arrows, b) within the lacteals of rat (G–I) and human (J–K) small intestinal mucosa. I is a higher-field magnification of micrograph from H demonstrating the expression of LPA5 arrow present on the inner face of podoplanin-expressing endothelium (arrows, b). Arrows in K indicate a region of tissue in J that displays LPA5-LI on the face of endothelial lacteal. Scale = 20 μm unless otherwise indicated. Rabbit anti LPA5 antibody, 93ab2, that was used for immunocytochemistry is immunoreactive to cells expressing LPA5-green fluorescent protein (GFP) fusion protein (L). LPA5 tagged at the COOH terminus with the enhanced GFP (LPA5-GFP fusion) was transfected into nondifferentiated hBRIE380i cells. Total cells (nuclei) were visualized as blue using a DAPI stain (arrows, a and b). Arrow, c is a cell immunoreactive to 93ab2, which colocalizes with LPA5-GFP (arrows, d and e).
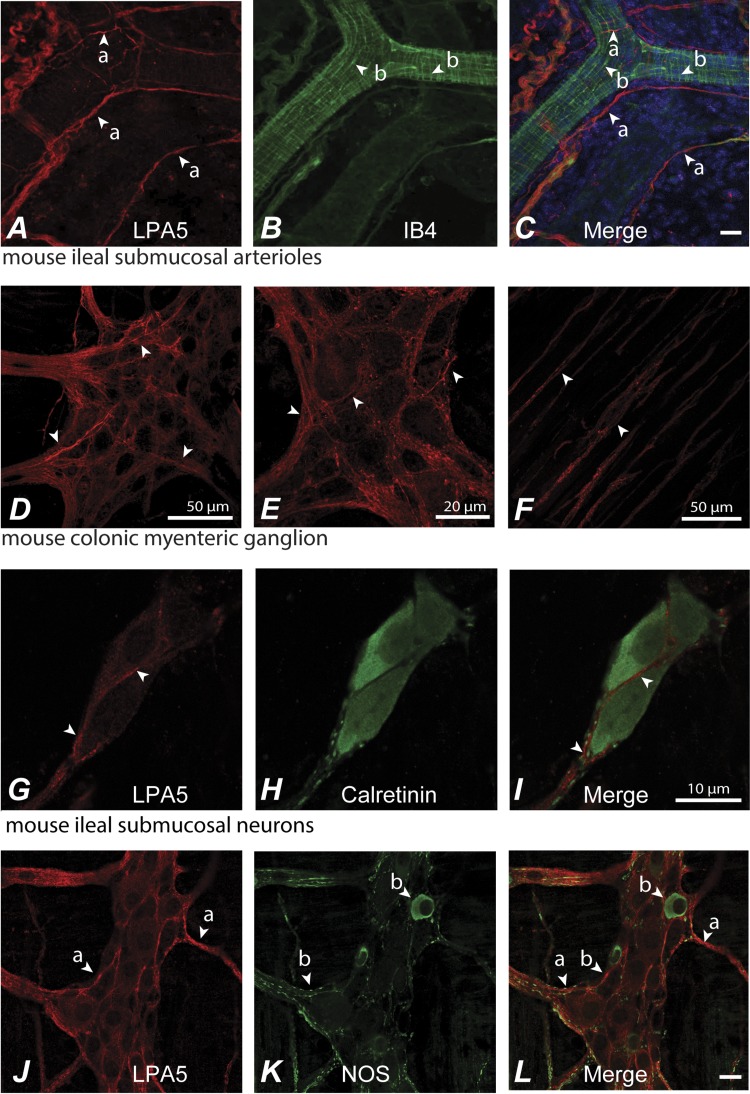
Fig. 2.
Whole mounts of the submucosal and myenteric plexuses displayed LPA5-LI immunoreactivity. A–C: in the submucosal plexus, LPA5-LI (arrows, a) was present in a subset of small-diameter isolectin B4 (IB4)-positive neuronal fibers or endothelial-like cells (arrows, b) associated with submucosal arterioles of the mouse ileum (C) (IB4-FITC labeled). LPA5-positive nerve fibers did not appear predominately on the outer surface of the arterioles. LPA5-LI was detected in myenteric ganglia of the proximal and distal colon (D), where it was localized to nerve fibers (D and its higher magnification, E). LPA5-LI was expressed by a subset of nerve fibers (arrows) and was most prominent in nerve fibers associated with myenteric ganglia (arrows). LPA5-LI was also present in nonvaricose nerve fibers within the deep muscular plexus (F). LPA5-LI was expressed by a subset of submucosal neurons, shown here in the mouse ileum. Immunoreactivity was localized to the cell surface and to intracellular vesicles (arrows) (G–I). J and K: LPA5 was expressed by a subset of nerve fibers of the mouse ileum (arrows, a and b) including those positive for nitric oxide synthase (NOS) (b) and large non-NOS-positive neurons (a). Scale = 20 μm unless otherwise indicated.
LPA5-LI was also prominent in nerve fibers innervating villus tips and in enteroendocrine-like cells of the mucosa (Fig. 1A). LPA5-LI was extensively colocalized with calcitonin gene-related peptide (CGRP) (Fig. 1, A–E) nerve fibers. The colocalization of LPA5 with CGRP associated with the inner lacteal vessel lining in the lumen of the lacteals can be visualized in the Imaris three-dimensional reconstruction of the confocal images used in Fig. 1C (Supplemental Movie S1; supplemental material for this article can be found online at the American Journal of Physiology Gastrointestinal and Liver Physiology website). Here, grouped and single immunoreactive nerve fibers are seen to be located within the lumen of the lacteal and immediately beneath the endothelial cells (of the lymphatic lacteals), where CGRP and LPA5 were colocalized. This colocalization was closely associated with luminal-facing podoplanin, a marker of lymphatic lacteal endothelium, clearly demonstrating the presence of LPA5 in nerve fibers at the lumen of the lacteals (Fig. 1, F–I). An Imaris three-dimensional reconstruction of the confocal image used in Fig. 1I is provided as Supplemental Movie S2. Human jejunal mucosa also showed LPA5 associated with podoplanin that appeared to run along the luminal face of the lacteal. This is exemplified in the Imaris three-dimensional reconstruction of the confocal images used in Fig. 1K (Supplemental Movie S3). Nonspecificity was assessed by analysis of staining with preimmune sera, by omission of primary antisera, and by analysis of wild-type hBRIE cells and hBRIE cells transfected with the rat LPA5 (Fig. 1L).
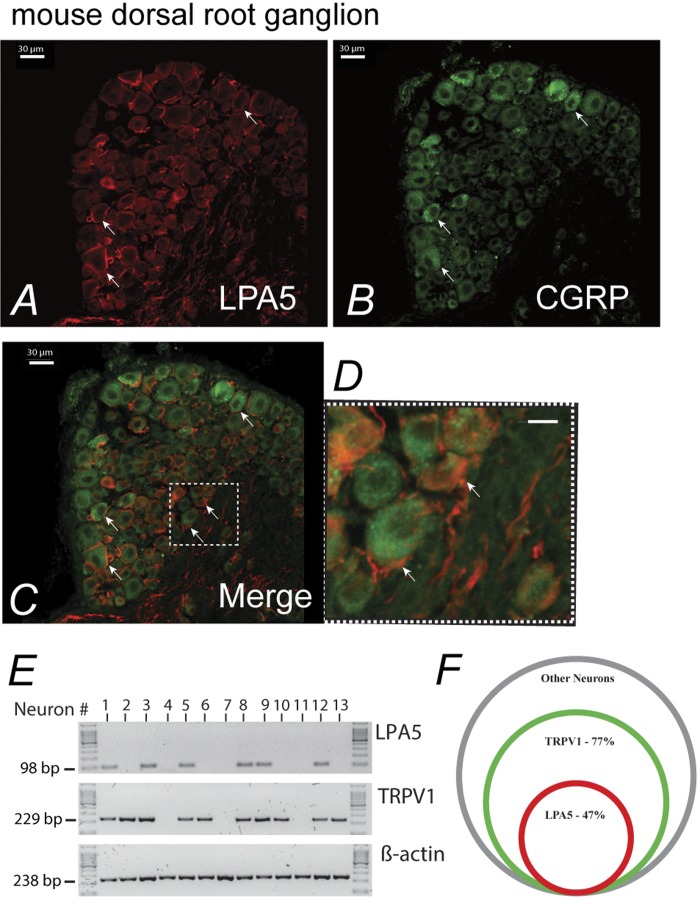
In contrast, LPA5-positive afferent nerve fibers surrounding arterioles of the submucosal plexus did not appear to innervate the lumen of these vessels (Fig. 2, A–C). Additionally, many of these fibers were positive for isolectin B4, suggesting nonpeptidergic small-diameter sensory fibers (Fig. 2C) (63). LPA5-LI was detected in whole mounts of myenteric ganglia of the proximal and distal colon, where it was localized to nerve fibers with little evidence for positive staining of neuronal cell bodies or of myenteric ICC (Fig. 2, D and E). Labeling was present in both myenteric and submucosal ganglia and in nerve fibers innervating the circular and longitudinal smooth muscle layers (Fig. 2F). There was no evidence for LPA5-LI in smooth muscle of the muscularis externa or of the muscularis mucosa. Examination of the distribution of LPA5-LI in whole mount preparations demonstrated that there was little to no labeling of neuronal cell bodies as exemplified by neurons labeled for the calcium-binding protein calretinin (Fig. 2, G–I). Immunoreactivity was largely restricted to nerve fibers running throughout myenteric ganglia (Fig. 2D) and within the deep muscular plexus (Fig. 2F). In whole mounts of the myenteric plexus, LPA5-LI was present in a subset of neurons, including nitric oxide synthase (NOS)-immunoreactive and large NOS-negative neurons indicating that the action of LPA5 might include but was not restricted to secretomotor function (Fig. 2, J–L). Because the central lacteals in the intestinal villi are not equipped with any smooth muscle cells (38), the lacteal-associated nerves are not likely to be vasomotor but rather sensory in nature. This was further supported by coexpression of LPA5 with CGRP in mucosal nerve fibers and peptidergic sensory neurons of DRG (Fig. 3, A–D). Thus observations by others for the presence of LPA5 sensory nerves of the DRG (59) were extended by the identification of LPA5 expression on subpopulation of nerve fibers expressing CGRP. Neuronal LPA5 was localized to the cell surface and intracellular vesicles. RT-PCR 47% of 78 single neurons confirmed the immunocytochemical data that LPA5 was expressed in nerve fibers, which include small-diameter TRPV1 sensory neurons (Fig. 3, E and F).
Fig. 3.
LPA5 expression in CGRP and the transient receptor potential cation channel subfamily V member 1 (TrpV1) expressing neurons of the mouse dorsal root ganglia (DRG). A and B: LPA5-LI was predominantly detected on the surface plasma lemma of the CGRP-positive DRG neurons (D is the enlarged inset in C). E: single-cell RT-PCR analysis of DRG neurons from C57BL/6J mice. The mRNA-encoding actin, TRPV1, and LPA5 from small-diameter neurons were amplified. Results from 13 neurons (of 78 neurons from 7 mice) are shown. Neurons 1, 3, 5, 8, 9, and 12 coexpressed LPA5 and TRPV1. F: Venn diagram illustrates the proportion of small-diameter neurons expressing LPA5 and TRPV1. A 47% subset of LPA5-positive neurons coexpressed TRPV1. No transcripts were amplified from bath fluid.
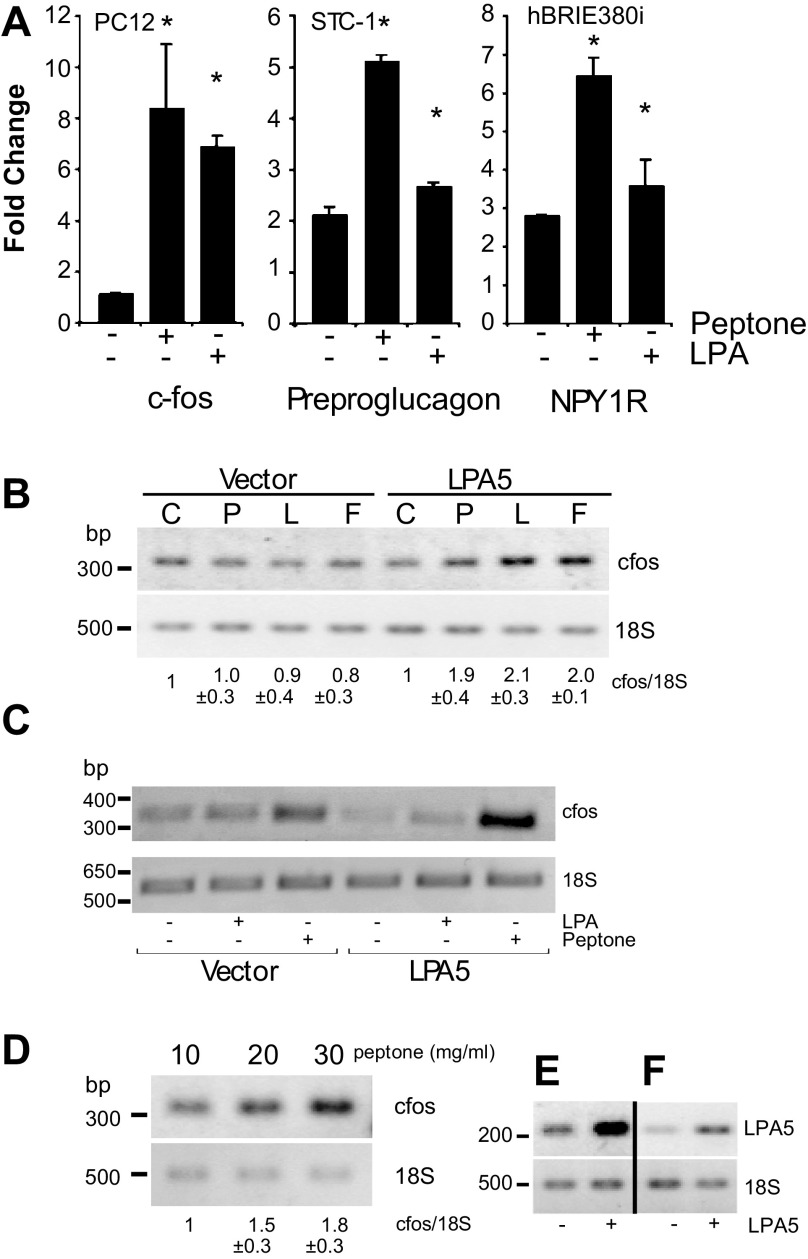
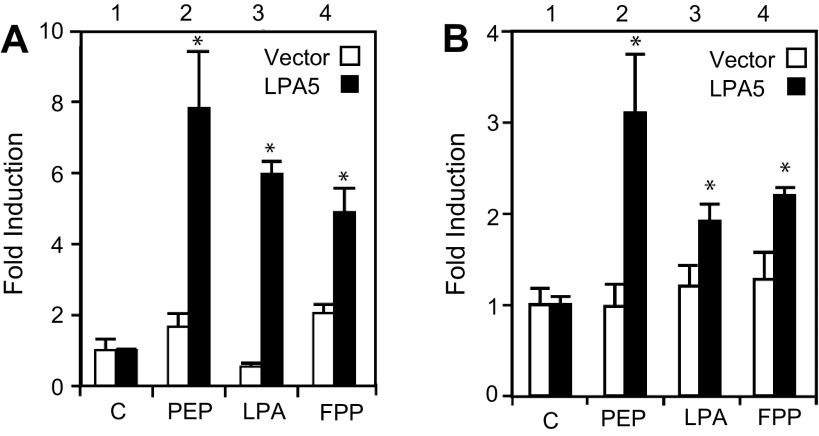
Given the expression pattern of LPA5 in the neuronal fibers within the lacteals and mucosal cells, we examined the possibility that dietary content of the lumen could induce diet-responsive genes by receptors such as LPA5. We tested the activation by peptone (peptone-LPA5) on the expression of three diet-responsive genes in LPA5-expressing cells of the mucosa. Treatments with peptone increased c-Fos promoter activity in neuroendocrine-like PC12 cells, preproglucagon promoter activity in enterochromaffin-like STC1 cells, and neuropeptide Y (NPY) 1R receptor promoter activity in enterocyte-like hBRIE380i cells (Fig. 4A). The effects of peptone, LPA, and FPP on nerve activities were further investigated using NGF-differentiated PC12 cells (a commonly used nerve cell line model) transfected with vector and LPA5 (Fig. 4B). The expression of c-Fos is commonly used as an early marker for neuronal activities (44). LPA5 activated by 25 mg/ml peptone, 1 μM LPA, and 1 μM FPP caused a twofold increase in the c-Fos expression in NGF-differentiated PC12 cells (Fig. 4C).
Fig. 4.
Increase in c-Fos promoter activity in NGF-differentiated PC12 cells, preproglucagon promoter activity in STC-1 cells, neuropeptide Y 1 receptor (NPY1R) promoter activity in hBRIE380i cells, and c-Fos expression in hippocampal cells by peptone-activated LPA5. The respective cells were transfected with the luciferase reporter construct and LPA5 cDNA or the empty vector. A: c-Fos promoter is from −341 to +16 bp relative to the transcription start site; preproglucagon promoter is from −1,054 to −35; NPY1R promoter is from −1,000 to +1. B: RT-PCR of c-Fos expression was greater in response to peptone (P) than to LPA (L) or FPP (F), compared with the empty vector control (C) in mucosal hBRIE380i cells. Cells were serum starved for 12 h and treated with 10 μM LPA or 50 mg/ml peptone for 6 h. Bars are means (relative to vector) ± SD (n = 3). C: PC12 cells differentiated by NGF. D: primary mouse hippocampal neurons were transfected with 6 μg of either LPA5 cDNA or the empty vector. Cells were allowed to recover for 24 h, and, after being serum starved for 12 h, the cells were treated with either 50 mg/ml peptone or 10 μM LPA in 50% Iscove's Modified Dulbecco's Medium/0.1% fatty acid-free bovine serum albumin for additional 6 h. The expression of c-Fos was determined by semi-quantitative RT-PCR. LPA5 expression levels (E) in PC12 cells and hippocampal neurons (F) were determined by semiquantitative RT-PCR. Representative images of ethidium bromide-stained agarose gels of the amplified fragments are depicted. Bars are means ± SD (n = 3). *P < 0.05 relative to bar 1 of the corresponding (solid and open).
We sought to confirm the effects of LPA5 activation on nerve activities using primary neuronal cultures. We used primary hippocampal neurons, a well-studied primary nerve cell culture model, that were overexpressing LPA5 (Fig. 4D). Only peptone was used as a stimulus because peptone induced the highest induction of c-Fos promoter activity in NGF-differentiated PC12 cells. Peptone at both 10 and 20 mg/ml induced a significant increase in the c-Fos expression in the hippocampal neurons transfected with LPA5 (Fig. 4, E and F), which provided more evidence suggesting that the activation of LPA5 could increase nerve activities. This was consistent with the increase in c-Fos promoter activity upon LPA5 activation in differentiated PC12 cells. (Fig. 5A).
Fig. 5.
Increase in c-Fos and cAMP response element (CRE) promoter activity upon LPA5 activation in differentiated PC12 cells. NGF differentiated PC12 cells were transfected with a c-Fos promoter-linked luciferase (A) or a CRE promoter-linked luciferase (B) reporter plasmid plus either the empty vector or LPA5 cDNA. Cells, other than controls (C), were then treated with 25 mg/ml peptone (PEP), 1 μM LPA, or 1 μM FPP. The fold induction is the ratio of the value from each sample divided by the value from the control (no treatment), which was arbitrarily designated as 1. Bars are means ± SD (n = 3). *P < 0.05 relative to bar 1 of the corresponding color.
The c-Fos promoter fragment used in this study also contains CRE, and the activation of the cAMP-related signal pathways is involved in the regulation of neurotransmitter release from PC12 cells. Therefore, we examined CRE reporter activity in response to LPA5 activation in PC12 cells. In response to 25 mg/ml peptone, 1 μM LPA, and 1 μM FPP, CRE reporter activity was increased significantly in PC12 cells transfected with LPA5 (Fig. 5B, bar 1 vs. bars 2–4). These results suggest that activated LPA5 induced c-Fos expression partially through increased activity on CRE and that peptone-like dietary components that activated LPA5 in nerves could lead to the release of neurotransmitters (17).
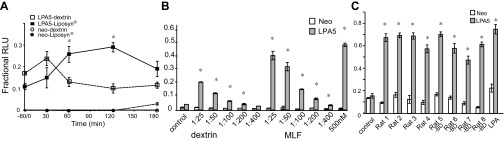
The expression pattern of LPA5 in lacteals and peptone induction of c-Fos expression led us to speculate that MLF provided a vehicle by which luminal dietary peptone-like molecules could activate LPA5 in sensory nerves. Additionally, the observed peptone-induced c-Fos expression suggests a pathway for sensory nerve activation by MLF that is independent of chylomicrons. Therefore, this would provide a means for nutrients to directly activate enteric nerves without these nerves requiring direct contact with mucosal cells of the lumen. Using an AEQ-based calcium ([Ca2+]) assay, we first tested the activation of heterologously expressed LPA5 in CHO cells by MLF from rats taken at various time points after lipid-rich Liposyn was infused into the duodenum. Before the infusion, rats that were previously cannulated at the superior mesenteric lymphatic duct were fasted overnight. Sixty minutes before the bolus infusion, MLF was collected for fasted values. Liposyn was infused in a single 3-ml bolus. MLF was then collected on ice. MLF (at 1:50 dilution) activated LPA5, as indicated by an increase in [Ca2+]i mobilization, which peaked at 120 min after the nutrient bolus (Fig. 6A). This time frame corresponds to the physiological rate of absorption of a mixed meal in the small intestine. LPA5 activation by lymph from animals given a nonabsorbable dextrin bolus decreased to basal activity after 60 min. This initial response could result from activation by nonabsorbable factors relapsed into the lymph initiated by the luminal instillation. Overall, a dose response of MLF from the Liposyn-fed animals indicated significantly enhanced activation over the dextrin group at dilutions greater than 1:400 (Fig. 6B). Intrinsic primary afferent neurons are not thought to respond directly to organic nutrients because the afferent nerve endings are separated from the luminal environment by the mucosal epithelium (10). However, our study shows that nerve endings in mesenteric lacteals containing mesenteric lymph-responsive GPCRs, such as LPA5, provide a means for primary sensory afferent neurons to be nutrient-sensing neurons similar to the EEC.
Fig. 6.
Time- and dose-dependent activation of LPA5 by mesenteric lymphatic fluid (MLF) that is a function of feeding. Lymphatic fluid was collected from rats with cannulated mesenteric lymphatic ducts after presentation of an intestinal 3-ml bolus of either dextrin as a control (1.1 g in 3 ml 0.9% NaCl) (n = 3), Liposyn, or Ensure, which is a mixed meal consisting of fat, carbohydrate, and protein. The MLF was used to determine LPA5 activation in an aequorin (AEQ)-based [Ca2+] assay. A: Chinese hamster ovary cells heterologously expressing LPA5 were treated with MLF (1:50 dilution) from dextrin-infused rats (n = 3) and Liposyn-infused (n = 3) rats. The activation of LPA5 by Liposyn-derived MLF showed significant enhancement over time, which peaked at 120 min. B: LPA5 activation displays a dose dependency on MLF from mix meal-treated animals. MLF was collected from rats with cannulated mesenteric lymphatic ducts 120 min after the presentation of an intestinal 3-ml bolus of Ensure. Changes in [Ca2+]i were determined by AEQ-based [Ca2+] assay (RLU, relative light units). Bars are means ± SD (n = 3). C: hBRIE380i cells heterologously expressing LPA5 were treated with MLF from rats with intact mesenteric lymphatic ducts (n = 4) or rats with diverted bile (-BD) (n = 4). MLF (1:5 dilution) significantly induced the mobilization of [Ca2+]i independent of the presence of bile. Bars are means ± SD. Values for each figure are significantly different than their respective controls at P < 0.05.
Because bile acids could be constituents of MLF that could contribute to the responses observed with both dextrin and diet, we tested whether the MLF taken from rats with diverted bile could activate LPA5 in mucosal hBRIE380i cells. For these studies, animals were given a single 3-ml bolus of Ensure (representing a mixed meal; 4.43 kcal; Abbott Laboratories). The absence of bile acids had no effect on LPA5 activation (Fig. 6C), confirming previous studies showing that LPA5 differs in mode of action from membrane-type bile acid receptor/TGR5.
Similarly, to determine whether bacterial-derived proteins contributed to the activation of LPA5, we tested the activation of LPA5 with increasing concentrations of PGN, muramylpeptides and desmuramylpeptides, flagellin, bacterial lipopolysaccharide, and Pam 3 [Cys-Ser-(Lys)(PAM)]. However, we found no change in [Ca2+]i mobilization. Values ranged from 0.14 ± 0.03 to 0.23 ± 0.08 RLU with an LPA response of 0.61 ± 0.1 RLU (n = 3). We conclude that both the time course and dose response of MLF are diet dependent in the intestinal lumen.
We next tested whether P2Y5 and P2Y9 were responsive to MLF. However, we found that only LPA5 was activated by MLF. One possibility that cannot be excluded was the presence of cellular products of the infused lipid from the enterocytes or molecules such as lysophosphatidic acid because the phospholipid content of mesenteric lymph was reported to increase 1 h after lipid infusion (45). However, mass spectroscopy of MLF taken from fasted and Ensure-infused animals revealed no detectable changes in the concentration of phospholipid agonists of LPA5, LPA, and FPP. No significant differences were found in the concentrations of LPA as 16:0, 18:0, and 18:1 and FPP between fasted and fed animals (n = 4 for each group).
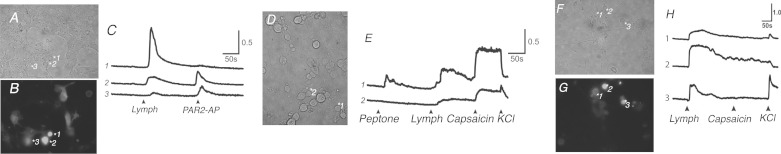
It was determined, by qRT-PCR, that DRG LPA5 mRNA was at a significantly elevated level relative to that from mouse ileal and colonic tissues. Compared with mRNA from the liver (as a control relative the mRNA) differences were as follows: DRG 37 ± 2.5, duodenum 33 ± 1, ileum 28 ± 5, colon 14 ± 0.7, and liver 1 ± 0.1 (n = 3; *P < 0.05 ± SE). Given the expression of LPA5 both in the mucosa and sensory nerve fibers of the DRG, we tested whether there was tissue specificity in receptor activation by MLF. We used Ensure-derived MLF to examine the change in transience [Ca2+]i in stable hBRIE380i cells expressing LPA5 tagged with green fluorescent protein (GFP) (LPA5-GFP) by Fura-2 AM in a [Ca2+]-imaging assay (2) (Fig. 7). We observed LPA5 activation by a rise in [Ca2+]i. MLF-LPA5-responsive cells were also responsive to PAR2-AP (Fig. 7, A–C). Because PAR2 is proalgesic and proinflammatory, we tested whether MLF could induce [Ca2+]i mobilization in primary cultured mouse DRG. Treatments with MLF at 1:10 dilution increased [Ca2+]i mobilization in neurons that were also capsaicin responsive although not all cells were responsive to peptone (Fig. 7, D and E). Importantly, endogenous receptor activity in response to peptone and MLF was also observed (Fig. 7E). This suggests that there are multiple receptors that might respond to a variety of ligands present in MLF. Primary DRG cultures heterologously expressing LPA5-GFP displayed an enhanced increase in [Ca2+]i when exposed to MLF (Fig. 7, F–H), which was independent of TRPV1 activity. Collectively, these data demonstrate that lymphatic fluid constituents activate receptor-mediated sensory nerve activity through LPA5 and possibly other unidentified receptors. The feeding-dependent activation of LPA5 by MLF provides a mechanism through which nerves outside of the submucosal and myenteric plexus could be activated by organic nutrients present in the lumen.
Fig. 7.
Mesenteric lymphatic fluid (MLF) induces a [Ca2+] response in a small intestinal epithelial cell line transfected with LPA5 and activates primary cultured mouse DRG. We examined the activation of LPA5 by Ensure-derived MLF in mucosal hBRIE380i cells and mouse primary-cultured DRG heterologously expressing LPA5 tagged with GFP (LPA5-GFP) by using Fura-2 AM (Molecular Probes) in a [Ca2+]-imaging assay. A–C: MLF activated LPA5 in hBRIE380i cells of more than one phenotype. D and E: nontransfected cultured DRG were responsive to MLF. (E2) MLF/TRPV1-responsive neurons (capsaicin) showed low sensitivity to peptone. F–H: LPA5 activation by Ensure-derived MLF was greatly enhanced over the endogenous response in LPA5-expressing DRG, further indicating that lymph-responsive somas of the DRG could be from more than one neuronal origin. Y-axis is 340/380 nm emission ratio, which is proportional to the relative changes in [Ca2+]i. [Lymph was at 1:10 dilution, protease-activated receptor 2-activating protein (PAR2-AP, SLIGRL-NH2) 100 μM, capsaicin 10 nM.]
DISCUSSION
The composition of lymphatic fluid reflects its role in maintaining fluid homeostasis (25) and blood pressure (70) by providing a means of returning interstitial fluid to the systemic circulation. Molecules can enter the mesenteric lymph through paracellular routes, through gut-associated lymphoid tissues, and transcellularly via the intestinal lipid transport system (55, 69). Together, these routes allow entry of molecules from mucosal secretions, the luminal surface, and serum capillaries. Similar to serum, mesenteric lymph also transports a range of molecules (34, 58); however, in contrast to blood capillaries, the intercellular junctions between endothelial cells in lymphatic capillaries are more open (200–800 nm) and the flow rate is far slower (1/500). Therefore, peptides and other macromolecules are sequestered in the lymphatic system before entering the circulation (58). Additionally, bioactive peptides sustain a higher half-life given that proteolytic enzymes, such as dipeptidyl peptidase IV, are found at lower concentrations than in sera (65). Using rats with mesenteric lymph fistulas, it was demonstrated that the mesenteric lymph contains gastric inhibitory polypeptide, secreted from EECs, at significantly higher concentrations and of a longer half-life than that found in the circulation (52). Similarly, GLP-1, an important incretin released from mucosal l-cells, has been reported to be five times higher in concentration in mesenteric lymph than in portal blood (22). Lymphatic concentration of GLP-1 varies in response to feeding (51). Because PYY [which is copackaged with GLP-1 in l-cell secretory vesicles (7)] is secreted in response to dietary free fatty acid (5) and CCK is secreted in response to dietary protein and fat (49), it is expected that these peptide concentrations also increase in lymphatic fluid upon feeding.
It has been shown that neuropeptides, such as vasoactive intestinal peptide and CCK that are released into the mesenteric lymph (11, 29, 31), can act on their receptors in the sympathetic and sensory nerves innervating the mesenteric lymph nodes and lymphatic vessels (61) through vagal afferents (31). Our data suggest that the nerve fibers of the lacteal are part of a visceral sensory system that could function separately from those of the vagus, perhaps through primary spinal afferent nerves. Therefore, sensory nerves within the interior of lymphatic vessels or that express the receptors for these neuropeptides (CCKAR, NPY1R, GLP-1R) could respond to luminal signals through these peptides as well as to lipid and protein components from the serosa or mucosa via LPA5. Additionally other molecules found in MLF such as phospholipids, which increase with dietary lipids (45), can become biologically active by acting as ligands to GPCRs (similar to LPA5) that are expressed on sensory nerves. Although we did not find that LPA5 was activated by bacterial peptides such as desmuramyl peptides and PAM 3, the possibility for expression of GPCRs on sensory nerves of the mesenteric lacteal that are responsive to components of the gut microbiome remains to be explored.
Most of the nerve cells express one or more subtypes of LPA receptors, and LPA regulates many important functions in nerve cells such as differentiation, myelination, and adhesion (27). Peptone can also induce the activities of the peptidergic and cholinergic neurons in the gastrointestinal tract (48). Through LPA5, FPP increases nerve activities in cultured DRG neurons, as indicated by the elevation of [Ca2+]i (59). Consistent with other reports, we showed that the activation of LPA5 (in response to peptone, LPA, and FPP) induced the expression of c-Fos (an early marker of neuronal activity) both in a nerve cell line and primary nerve cells. The expression of c-Fos is often used as an indicator for neuronal activities and differentiation in PC12 cells (18). In addition to the expression of c-Fos, activated LPA5 also induced the transactivation of CRE reporter activities in PC12 cells, which can be induced by increases in [cAMP]i and [Ca2+]i (3). The activation of LPA5 might potentially be involved in neurotransmitter release as well because changes in the [Ca2+]i and [cAMP]i regulate the release of neurotransmitters (e.g., norepinephrine and dopamine) in PC12 cells (18). Similarly, it has been demonstrated that LPA5 activation by peptone induces an increase in [Ca2+]i and cAMP levels and the secretion of CCK in STC1 cells (17). Therefore, the activation of LPA5-like GPCRs on CGRP or Substance P-expressing sensory nerves likely results in the release of these peptides. Importantly, GPCRs such as LPA5 could be considered to be ligand-directed multifunctional GPCRs. LPA5 activation by LPA mobilizes [Ca2+]i and activates ERK1/2 through both Gαi- and Gq-mediated pathways but does not result in the release of CCK. This would allow for changes in phospholipid composition of MLF following a meal as LPA or FPP (60) to activate sensory LPA5-like sensory neurons to activate downstream effectors, such as the Na+/H+ exchanger 3 (68), without affecting the neurosecretory response. It remains to be explored whether other ligand-directed multifunctional GPCRs, such as β2-adrenergic receptor or the histamine H1 receptor (H1R), can respond to changes in MLF composition and whether receptor activation results in ERK1/2 phosphorylation-coupled [cAMP]i effector responses or [cAMP]i effector responses that are independent of ERK1/2 phosphorylation.
The current studies present a mechanism for the direct activation of luminal components such as dietary nutrients of sensory nerves. Chylomicrons are a major component of mesenteric lymph fluid after a lipid-containing meal. We established LPA5 activation in enterocytes and neuronal cells occurring independently of the presence of chylomicrons. C-Fos promoter activity in PC-12 cells and c-Fos expression in cultured hippocampal cells occurred in the presence of peptone, which was devoid of chylomicrons. Additionally, the concentrations of the potent LPA5 agonists, LPA, or FPP were not found to change in the lymphatic fluid harvested from fasted or fed rats, which would indicate other nutrient-dependent secretagogues present in the MLF. The sensory neurons from primary cultured DRG exhibited changes in [Ca2+]i in responses to both peptone and to MLF in nontransfected cells. This endogenous response to peptone was not seen in all cells, which is consistent with the observed coexpression of LPA5 and TRPV1 in 47% of these neurons. Although LPA5 activation was not dependent on chylomicrons, it is likely that a number of GPCRs expressed on lacteal nerves will be discovered and found to respond to a variety of MLF constituents, including lipoproteins and chylomicrons.
The expression of LPA5 in sensory nerves and nociceptive regions of the CNS (50) brings forth a potential new mechanism for events occurring at the lumen-mucosa interface to enhance or attenuate sensory signals to the CNS through chemical signals in the lymphatic fluid. GPCRs, like LPA5, expressed in sensory nerves found in peripheral lymphatic vessels could also perform a similar function in response to molecules in the lymphatic fluid derived from the interstitium. Similar to the mucosal lacteals, the media layer of peripheral lymphatic vessel walls is also richly innervated with CGRP and other peptide-containing nerves (35, 36). Therefore, neuropeptide hormones secreted from EECs in response to luminal nutrients could also act on sensory nerves from peripheral lymphatic vessels, independently from the endocrine system. This would result from the direct contact of sensory nerves with lymphatic fluid. Thus the molecules in the MLF via responsive GPCRs could convey information with minimal dilution to the CNS by primary afferent neurons with cell bodies in sensory ganglia (such as the DRG, vagal sensory ganglia) that are close to the brain or spinal cord and in the brain stem (28). Future studies involving anterograde tracing of spinal nerves to determine whether it is possible that spinal afferent neurons could be part of the mucosal plexus that innervates the lacteals would be an important step in resolving this question.
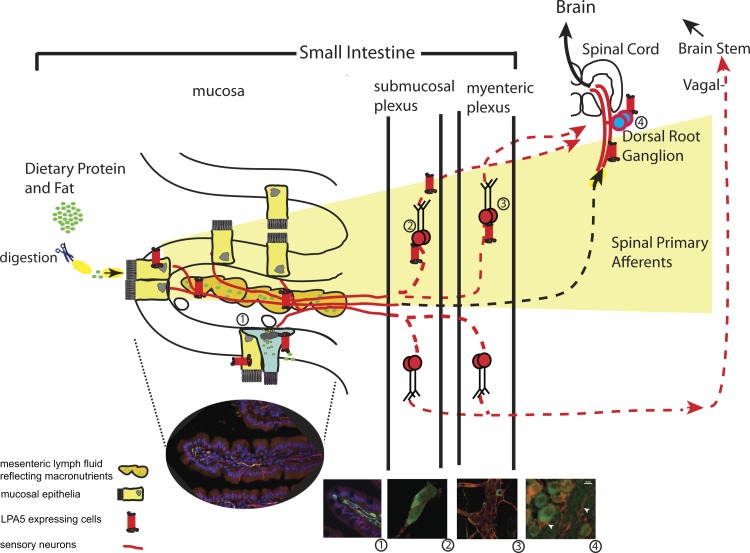
Our study is the first to demonstrate that lymphatic fluid activates sensory neurons. The direct activation of GPCRs expressed in sensory afferent neuronal fibers within lymphatic vessels by lymphatic fluid allows the intestinal contents and interstitial paracrine agents to act like hormones. Additionally, given the anorectic effects of central and circulating sensory neuropeptides such as CGRP (64), our data point to a new pathway for the modulation of behavior, such as food intake, by premetabolized dietary nutrients via MLF-GPCR activation of sensory nerves, which represents a neurolymphocrine system (Fig. 8).
Fig. 8.
Feeding-dependent activation of enteric sensory nerves through mesenteric lymphatic fluid. Partially digested protein or dietary lipid products that are present in the intestinal lumen comprise part of the lymphatic fluid derived from the intestinal lumen or from extracellular leakage. The accumulation of these molecules activates receptors such as LPA5 present on sensory nerves, which are in contact with the fluid within the mesenteric lacteal, such as CGRP (seen as green fluorescence) (1), which innervate the mucosa (1), the submucosal (2) and myenteric plexuses (3), and primary afferent neurons of the DRG (4). This newly described macronutrient chemosensory system (shaded area in yellow) would allow the central nervous system to monitor changes in the mucosa or interstitial fluid, in response to diet through intrinsic and/or extrinsic ganglia. The solid red lines and receptor represent data shown in the present study.
GRANTS
This work was supported by the National Institutes of Health (DK092138, DK059630 to P. Tso, and DK83591 to J.-C. Wang), the Searle Scholar Award (to D. Nomura), National Health and Medical Research Council of Australia (NHMRC 63303 to N. Bunnett, NHMRC 454858 to D. Poole), and U.S. Dept. of Agriculture (CA-B-NTS-0010-H to G Aponte).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.P.P., M.L., P.T., N.W.B., S.J.Y., T.L., A.S., J.-C.W., D.K.N., and G.W.A. performed experiments; D.P.P., P.T., S.J.Y., T.L., and G.W.A. analyzed data; D.P.P., M.L., P.T., N.W.B., S.J.Y., J.-C.W., D.K.N., and G.W.A. interpreted results of experiments; D.P.P., M.L., T.L., and G.W.A. prepared figures; D.P.P., M.L., P.T., N.W.B., S.J.Y., T.L., A.S., J.-C.W., D.K.N., and G.W.A. approved final version of manuscript; M.L. and G.W.A. drafted manuscript; S.J.Y., A.S., and G.W.A. edited and revised manuscript; G.W.A. conception and design of research.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Adam Testro (Department of Gastroenterology/Liver Transplant Unit, Austin Health) and Dr. Mehrdad Nikfarjam (Department of Surgery, The University of Melbourne) for patient samples used in this study. We also thank Drs. Sung Choi and Denise Schichnes for technical assistance.
REFERENCES
- 1.Amadesi S, Grant AD, Cottrell GS, Vaksman N, Poole DP, Rozengurt E, Bunnett NW. Protein kinase D isoforms are expressed in rat and mouse primary sensory neurons and are activated by agonists of protease-activated receptor 2. J Comp Neurol 516: 141–156, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci 24: 4300–4312, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrisani OM. CREB-mediated transcriptional control. Crit Rev Eukaryot Gene Expr 9: 19–32, 1999. [PubMed] [Google Scholar]
- 4.Aponte GW, Keddie A, Hallden G, Hess R, Link P. Polarized intestinal hybrid cell lines derived from primary culture: establishment and characterization. Proc Natl Acad Sci USA 88: 5282–5286, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aponte GW, Park K, Hess R, Garcia R, Taylor IL. Meal-induced peptide tyrosine tyrosine inhibition of pancreatic secretion in the rat. FASEB J 3: 1949–1955, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Aponte GW, Taylor IL, Soll AH. Primary culture of PYY cells from canine colon. Am J Physiol Gastrointest Liver Physiol 254: G829–G836, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Barbosa AJ, Nogueira JC, Penna FJ, Polak JM. Distribution of enteroglucagon- and polypeptide YY-immunoreactive cells in the gastrointestinal tract of the white-belly opossum (Didelphis albiventris). Histochemistry 88: 37–40, 1987. [DOI] [PubMed] [Google Scholar]
- 8.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol 28: 325–353, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 16, Suppl 1: 28–33, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol Gastrointest Liver Physiol 273: G422–G435, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Bloom SR, Edwards AV. Effects of autonomic stimulation on the release of vasoactive intestinal peptide from the gastrointestinal tract in the calf. J Physiol 299: 437–452, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med 33: 1349–1352, 1948. [PubMed] [Google Scholar]
- 13.Boutillier AL, Barthel F, Roberts JL, Loeffler JP. Beta-adrenergic stimulation of cFOS via protein kinase A is mediated by cAMP regulatory element binding protein (CREB)-dependent and tissue-specific CREB-independent mechanisms in corticotrope cells. J Biol Chem 267: 23520–23526, 1992. [PubMed] [Google Scholar]
- 14.Brown AJ, Jupe S, Briscoe CP. A family of fatty acid binding receptors. DNA Cell Biol 24: 54–61, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Chang JW, Nomura DK, Cravatt BF. A potent and selective inhibitor of KIAA1363/AADACL1 that impairs prostate cancer pathogenesis. Chem Biol 18: 476–484, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S, Lee M, Shiu AL, Yo SJ, Aponte GW. Identification of a protein hydrolysate responsive G protein-coupled receptor in enterocytes. Am J Physiol Gastrointest Liver Physiol 292: G98–G112, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Choi S, Lee M, Shiu AL, Yo SJ, Hallden G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol 292: G1366–G1375, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Chu E, Chu J, Socci RR, Chu TC. 7-OH-DPAT-induced inhibition of norepinephrine release in PC12 cells. Pharmacology 70: 130–139, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnett NW, Grady EF. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol 490: 239–255, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Covington DK, Briscoe CA, Brown AJ, Jayawickreme CK. The G-protein-coupled receptor 40 family (GPR40-GPR43) and its role in nutrient sensing. Biochem Soc Trans 34: 770–773, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Cuello AC, Galfre G, Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA 76: 3532–3536, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol 293: R2163–R2169, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab 8: 447–449, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18: 2803–2812, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Fanous MY, Phillips AJ, Windsor JA. Mesenteric lymph: the bridge to future management of critical illness. JOP 8: 374–399, 2007. [PubMed] [Google Scholar]
- 26.Fraser KA, Davison JS. Cholecystokinin-induced c-fos expression in the rat brain stem is influenced by vagal nerve integrity. Exp Physiol 77: 225–228, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima N, Chun J. The LPA receptors. Prostaglandins Other Lipid Mediat 64: 21–32, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Furness JB. Novel gut afferents: Intrinsic afferent neurons and intestinofugal neurons. Auton Neurosci 125: 81–85, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg 186: 253–258, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett 57: 125–130, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Glatzle J, Wang Y, Adelson DW, Kalogeris TJ, Zittel TT, Tso P, Wei JY, Raybould HE. Chylomicron components activate duodenal vagal afferents via a cholecystokinin A receptor-mediated pathway to inhibit gastric motor function in the rat. J Physiol 550: 657–664, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grootjans J, Thuijls G, Derikx JP, van Dam RM, Dejong CH, Buurman WA. Rapid lamina propria retraction and zipper-like constriction of the epithelium preserves the epithelial lining in human small intestine exposed to ischaemia-reperfusion. J Pathol 224: 411–419, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Haid D, Widmayer P, Voigt A, Chaudhari N, Boehm U, Breer H. Gustatory sensory cells express a receptor responsive to protein breakdown products (GPR92). Histochem Cell Biol 140: 137–145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauss DJ, Fogal SE, Ficorilli JV, Price CA, Roy T, Jayaraj AA, Keirns JJ. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci 87: 164–169, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Hukkanen M, Konttinen YT, Terenghi G, Polak JM. Peptide-containing innervation of rat femoral lymphatic vessels. Microvasc Res 43: 7–19, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Ichikawa S, Kasahara D, Iwanaga T, Uchino S, Fujita T. Peptidergic nerve terminals associated with the central lacteal lymphatics in the ileal villi of dogs. Arch Histol Cytol 54: 311–320, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa S, Kyoda K, Iwanaga T, Fujita T, Uchino S. Nerve terminals associated with the central lacteal lymphatics in the duodenal and ileal villi of the monkey. Acta Anat (Basel) 146: 14–21, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Ichikawa S, Shiozawa M, Iwanaga T, Uchino S. Immunohistochemical demonstration of peptidergic nerve fibers associated with the central lacteal lymphatics in the duodenal villi of dogs. Arch Histol Cytol 54: 241–248, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Ichikawa S, Uchino S, Hirata Y. Nerve terminals associated with the central lacteal endothelial cells. Okajimas Folia Anat Jpn 66: 57–59, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Ito Y, Magari S, Sakanaka M. Immunoelectron-microscopic localization of peptidergic nerve fibers around lymphatic capillaries in the rat liver. Arch Histol Cytol 53 Suppl: 199–208, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan K, Dwivedi P, Davidson S, Yang Q, Tso P, Siems W, Hill HH., Jr. Monitoring dynamic changes in lymph metabolome of fasting and fed rats by electrospray ionization-ion mobility mass spectrometry (ESI-IMMS). Anal Chem 81: 7944–7953, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329: 386–390, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Keim NL, Marlett JA. Intestinal secretion of lipids and lipoproteins during carbohydrate absorption in the rat. J Nutr 110: 1354–1364, 1980. [DOI] [PubMed] [Google Scholar]
- 44.Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci 12: 235–248, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai HC, Ney DM. Gastric digestion modifies absorption of butterfat into lymph chylomicrons in rats. J Nutr 128: 2403–2410, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Lee M, Choi S, Hallden G, Yo SJ, Schichnes D, Aponte GW. P2Y5 is a Gαi, Gα12/13 G protein coupled receptor activated by lysophosphatidic acid that reduces intestinal cell adhesion. Am J Physiol Gastrointest Liver Physiol 297: G641–G654, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee M, Hadi M, Hallden G, Aponte GW. Peptide YY and neuropeptide Y induce villin expression, reduce adhesion, and enhance migration in small intestinal cells through the regulation of CD63, matrix metalloproteinase-3, and Cdc42 activity. J Biol Chem 280: 125–136, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Li Y. Sensory signal transduction in the vagal primary afferent neurons. Curr Med Chem 14: 2554–2563, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Liddle RA. Regulation of cholecystokinin secretion by intraluminal releasing factors. Am J Physiol Gastrointest Liver Physiol 269: G319–G327, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Lin ME, Rivera RR, Chun J. Targeted deletion of LPA5 identifies novel roles for lysophosphatidic acid signaling in development of neuropathic pain. J Biol Chem 287: 17608–17617, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol 293: G963–G971, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. Using the lymph fistula rat model to study the potentiation of GIP secretion by the ingestion of fat and glucose. Am J Physiol Gastrointest Liver Physiol 294: G1130–G1138, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology 50: 652–657, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Mittal A, Middleditch M, Ruggiero K, Buchanan CM, Jullig M, Loveday B, Cooper GJ, Windsor JA, Phillips AR. The proteome of rodent mesenteric lymph. Am J Physiol Gastrointest Liver Physiol 295: G895–G903, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Muranishi S, Fujita T, Murakami M, Yamamoto A. Lymphatic transfer of macromolecules after intrapulmonary administration in the presence or absence of various absorption enhancers in rats. Pharmazie 51: 331–336, 1996. [PubMed] [Google Scholar]
- 56.Nelson DW, Sharp JW, Brownfield MS, Raybould HE, Ney DM. Localization and activation of glucagon-like peptide-2 receptors on vagal afferents in the rat. Endocrinology 148: 1954–1962, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Nygaard R, Frimurer TM, Holst B, Rosenkilde MM, Schwartz TW. Ligand binding and micro-switches in 7TM receptor structures. Trends Pharmacol Sci 30: 249–259, 2009. [DOI] [PubMed] [Google Scholar]
- 58.O'Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci 15: 405–415, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Oh DY, Yoon JM, Moon MJ, Hwang JI, Choe H, Lee JY, Kim JI, Kim S, Rhim H, O'Dell DK, Walker JM, Na HS, Lee MG, Kwon HB, Kim K, Seong JY. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem 283: 21054–21064, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saisho Y, Morimoto A, Umeda T. Determination of farnesyl pyrophosphate in dog and human plasma by high-performance liquid chromatography with fluorescence detection. Anal Biochem 252: 89–95, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Shimoda H, Kato S, Kudo T, Usui T. Lymphatic network and nerve plexus in the myenteric layer of the monkey jejunum: a topographic study using an enzyme-histochemical method. Arch Histol Cytol 61: 65–73, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med 6: 151–158, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci 19: 6497–6505, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun JY, Jing MY, Wang JF, Weng XY. The approach to the mechanism of calcitonin gene-related peptide-inducing inhibition of food intake. J Anim Physiol Anim Nutr (Berl) 94: 552–560, 2010. [DOI] [PubMed] [Google Scholar]
- 65.Toyozaki M, Osaka M, Kondo K, Yoshida M. High fat and high cholesterol diet induces DPP-IV activity in intestinal lymph. J Oleo Sci 62: 201–205, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Wess J. Molecular basis of receptor/G-protein-coupling selectivity. Pharmacol Ther 80: 231–264, 1998. [DOI] [PubMed] [Google Scholar]
- 67.Williamson S, Pompolo S, Furness JB. GABA and nitric oxide synthase immunoreactivities are colocalized in a subset of inhibitory motor neurons of the guinea-pig small intestine. Cell Tissue Res 284: 29–37, 1996. [DOI] [PubMed] [Google Scholar]
- 68.Yoo BK, He P, Lee SJ, Yun CC. Lysophosphatidic acid 5 receptor induces activation of Na+/H+ exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol 301: C1008–C1016, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshikawa H, Takada K, Muranishi S. Requirement of macromolecular complex formation for selective lymphatic transfer of bleomycin from large intestine by bifunctional delivery system. Chem Pharm Bull (Tokyo) 31: 4070–4076, 1983. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, Liu YK, Jiang H, Ge L, Shi MJ, Zhang LM. [Effect of intestinal lymph on blood pressure of rat]. Sheng Li Xue Bao 49: 433–438, 1997. [PubMed] [Google Scholar]
- 71.Zittel TT, Glatzle J, Kreis ME, Starlinger M, Eichner M, Raybould HE, Becker HD, Jehle EC. C-fos protein expression in the nucleus of the solitary tract correlates with cholecystokinin dose injected and food intake in rats. Brain Res 846: 1–11, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.