Abstract
Deficiency of ABCB4 is associated with several forms of cholestasis in humans. Abcb4−/− mice also develop cholestasis, but it remains uncertain what role other canalicular transporters play in the development of this disease. We examined the expression of these transporters in Abcb4−/− mice compared with their wild-type littermate controls at ages of 10 days and 3, 6, and 12 wk. Elevated plasma bile acid levels were already detected at 10 days and at all ages thereafter in Abcb4−/− mice. The expression of Bsep, Mrp2, Atp8b1, Abcg5, and Abcg8 liver proteins did not change at 10 days, but Bsep, Mrp2, and Atp8b1 were reduced, whereas Abcg5 and Abcg8 expression were increased in Abcb4−/− mice at all later ages. Lower bile acid concentrations were also detected in the bile of 6-wk-old Abcb4−/− mice. Immunofluorescence labeling revealed distorted canalicular architecture in the liver tissue by 12 wk in Abcb4−/− mice. Whereas Bsep and Mrp2 remained associated with the apical membrane, Atp8b1 was now localized in discrete punctuate structures adjacent to the canalicular membrane in these mice. Expression of Bsep mRNA was increased in the livers of 10-day-old Abcb4−/− mice, whereas Ost-α was decreased. By 12 wk, Bsep, Mrp2, and Abcg5 mRNA were all increased, whereas Ost-α and Ntcp were reduced. These findings indicate that canalicular transporters that determine the formation of bile are altered early in the development of cholestasis in Abcb4−/− mice and may contribute to the pathogenesis of cholestasis in this disorder.
Keywords: Abcb4, phospholipid, bile, cholestasis, canalicular transporter
phosphatidylcholine (PC) is an important biliary lipid. It not only solubilizes cholesterol in bile but also protects biliary epithelium from bile salt cytotoxicity by combining with bile salts to form micelles (12, 25). Although the concentration of PC in bile varies greatly among different vertebrate species (17), it is a crucial constituent in human bile (6, 7). PC is transported into the bile lumen by the multidrug resistance protein 3 (MDR3/ABCB4) in humans or Mdr2 (Abcb4) in mouse (6, 22). MDR3/Mdr2 is a floppase that maintains PC on the outer bilayer of the apical canalicular membrane, where it is subsequently transported into bile (22, 23). Deficiencies of ABCB4 are associated with several forms of cholestatic liver disease in humans, including progressive familial intrahepatic cholestasis type 3 (PFIC3) in infants, intrahepatic cholestasis of pregnancy, low phospholipid cholelithiasis, and certain types of drug and idiopathic chronic cholestasis in adults (5, 8, 14). Patients with PFIC3 are homozygously deficient for ABCB4, and PC is absent in bile (13). These patients have a persistent high-serum γ-glutamyltransferase activity, which distinguishes them from PFIC1 and 2, and moderately elevated levels of serum bile salts and bilirubin (5). Liver histology in patients with PFIC3 demonstrates portal fibrosis, bile ductular proliferation, and inflammation. A genetic animal model for PFIC3 is the Abcb4−/− (Mdr2−/−) mouse (22). These mice also develop cholestasis, with elevated serum levels of bile salt, bilirubin, and liver enzymes. In addition, bile duct proliferation, liver fibrosis, and reduced biliary excretion of bile salts, bilirubin, and cholesterol are characteristics of this mouse (9, 10, 15, 16, 20, 22). The mechanism of liver damage in patients with PFIC3 and mice with Abcb4−/− is thought to result from bile salt cytotoxicity to the biliary epithelium attributable to the absence of biliary PC in bile to form micelles. Subsequently, the detergent effects of bile salt result in progressive injury to the bile canaliculi and cholangiocytes.
Previous investigators have reported the molecular and cellular events that are associated with ductular proliferation, inflammation, and fibrosis in these Abcb4−/− mice and have substantially advanced our understanding of the pathogenesis of liver injury in ABCB4/Abcb4-deficient livers (1, 18, 19, 21). However, it remains unclear whether hepatic bile salt transporters play a role in the pathogenesis of cholestasis and liver injury in ABCB4/Abcb4 deficiency. In this report, we assessed the hepatic expression of bile salt transporters during the early stages of development of cholestatic liver injury beginning within 10 days of birth in Abcb4−/− mice. Our results demonstrated that, despite elevated mRNA expression of Bsep and Mrp2, their protein expression is greatly reduced early on before the development of liver injury in Abcb4−/− mice. These findings indicate that the defects in the biliary excretion of PC that classically characterize ABCB4/Abcb4 deficiency may also result in an alteration in the expression and/or function of other canalicular membrane transporters and that these may contribute to the development of liver injury in this disease.
MATERIALS AND METHODS
Animals.
Heterozygous breeding pairs of FVB/129P2-Abcb4tm1Bor/J [abcb4 (+/−)] mice were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were kept in a pathogen-free environment on a controlled 12-h:12-h light/dark regime in the animal facility of Yale University School of Medicine. After breeding (heterozygotes to heterozygotes), Abcb4 knockout mice and their wild-type (WT) littermates were killed at 10 days and 3, 6, and 12 wk after birth. Of note, for 6- and 12-wk mice, only males were used. They were also fasted overnight before death. Plasma, bile, and liver were collected. All tissues were snap frozen in liquid N2 and stored in a −80°C freezer.
Plasma biochemistry and liver histology.
Plasma levels of the liver enzymes alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were analyzed by the Analytical Core in the Mouse Metabolic Phenotyping Center at Yale University. 3α-Hydroxy bile salt concentrations were determined in plasma, gallbladder bile, and liver tissue using a kit from Diazyme Laboratories (Poway, CA). Specifically, liver tissue was homogenized in 70% ethanol. For 100 mg liver, 1 ml 70% ethanol was added. The extract was analyzed for bile acid concentrations. Formalin-fixed tissue was embedded in paraffin, and sections were stained with Sirius Red or hematoxylin and eosin (H&E).
Immunohistochemistry and immunofluorescence.
Immunohistochemistry was performed using an antibody to cytokeratin 19 (Troma-III) developed by R. Kemler and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA, using a diaminobenzidine peroxidase kit (Vector Laboratories, Burlingame, CA). Images were acquired using an Olympus BX51 microscope. Immunofluorescence was performed on cryostat sections of frozen liver tissue as described previously (2). Fluorescent images were taken using a Zeiss LSM 510 confocal microscope and processed with PhotoShop.
Mouse hepatocyte preparation and treatment.
Hepatocytes from 3-mo-old WT and Abcb4−/− mice were isolated using collagenase perfusion as previously described (3). Cells were maintained in collagen sandwich culture and treated with 25 μM and 50 μM tauroursodeoxycholic acid (TUDCA) or taurodeoxycholic acid (TDCA) for up to 48 h. Total cell lysate was collected using RIPA buffer and subjected for Western blot analysis.
Quantitative real-time PCR and Western blot analysis.
As described previously (4), gene mRNA expression was detected using TaqMan real-time PCR in an ABI7500 system, and protein was analyzed by Western blotting. GAPDH gene was used as reference to normalize data. The TaqMan primer/probes and antibodies are as described in Ref. 24.
Statistical analysis.
Data are presented as means ± SD. Differences between experimental groups were assessed for significance using one-way ANOVA. Two-tailed Student's t-test was used to calculate the P value. A P value of <0.05 was considered statistically significant.
RESULTS
Plasma bile acid levels were significantly higher in 10-day Abcb4−/− mice.
Elevated plasma bile acid levels were detected as early as 10 days and at all later ages in the Abcb4-null homozygotes, but not the Abcb4+/− heterozygotes, compared with the WT mice (Table 1). These findings indicate that the homozygote-deficient mice are cholestatic within 10 days after birth. However, plasma ALT and ALP levels were unchanged among the three groups at 10 days and 3 wk of age and did not increase in the Abcb4−/− group until 6 and 12 wk of age (Table 1), indicating that signs of hepatic injury did not begin until the mice were at least 3 wk old.
Table 1.
Plasma ALT, ALP, and bile salt levels in mice 10 days or 3, 6, or 12 wk old
| Genotype | 10 Days | 3 Wk | 6 Wk | 12 Wk | |
|---|---|---|---|---|---|
| ALT, IU/l | +/+ | 9.8 ± 4.3 | 14.2 ± 7.3 | 42.4 ± 7.1 | 41.8 ± 6.8 |
| +/− | 7.2 ± 1.9 | 22.5 ± 9.5 | 52.3 ± 9.3 | 52.1 ± 17.0 | |
| −/− | 13.4 ± 5.1 | 27.7 ± 21.5 | 301.7 ± 90.1* | 292.1 ± 89.3* | |
| ALP, IU/l | +/+ | 339.0 ± 35.1 | 291.2 ± 93.7 | 172.9 ± 13.2 | 71.5 ± 5.7 |
| +/− | 300.9 ± 34.8 | 258.9 ± 35.2 | 168.8 ± 13.0 | 76.4 ± 8.2 | |
| −/− | 326.3 ± 33.0 | 244.5 ± 41.8 | 408.0 ± 164.3* | 199.6 ± 29.6* | |
| Bile salts, μM | +/+ | 14.3 ± 2.1 | 4.9 ± 0.9 | 1.6 ± 0.9 | 16.9 ± 15.2 |
| +/− | 14.9 ± 6.5 | 11.2 ± 8.3 | 1.9 ± 0.8 | 12.0 ± 8.9 | |
| −/− | 49.7 ± 25.2* | 85.6 ± 17.4* | 47.6 ± 25.4* | 46.9 ± 16.3* |
Values are means ± SD. Plasma alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were elevated in 6- and 12-wk but not 10-day and 3-wk Abcb4−/− mice compared with wild-type (WT) and heterozygote controls. In contrast, plasma bile acids were elevated by 10 days after birth and at all ages thereafter in Abcb4−/− mice. n = 5–7 for each group;
P < 0.01 to WT.
Bile duct proliferation was most marked at 6 wk in Abcb4−/− livers.
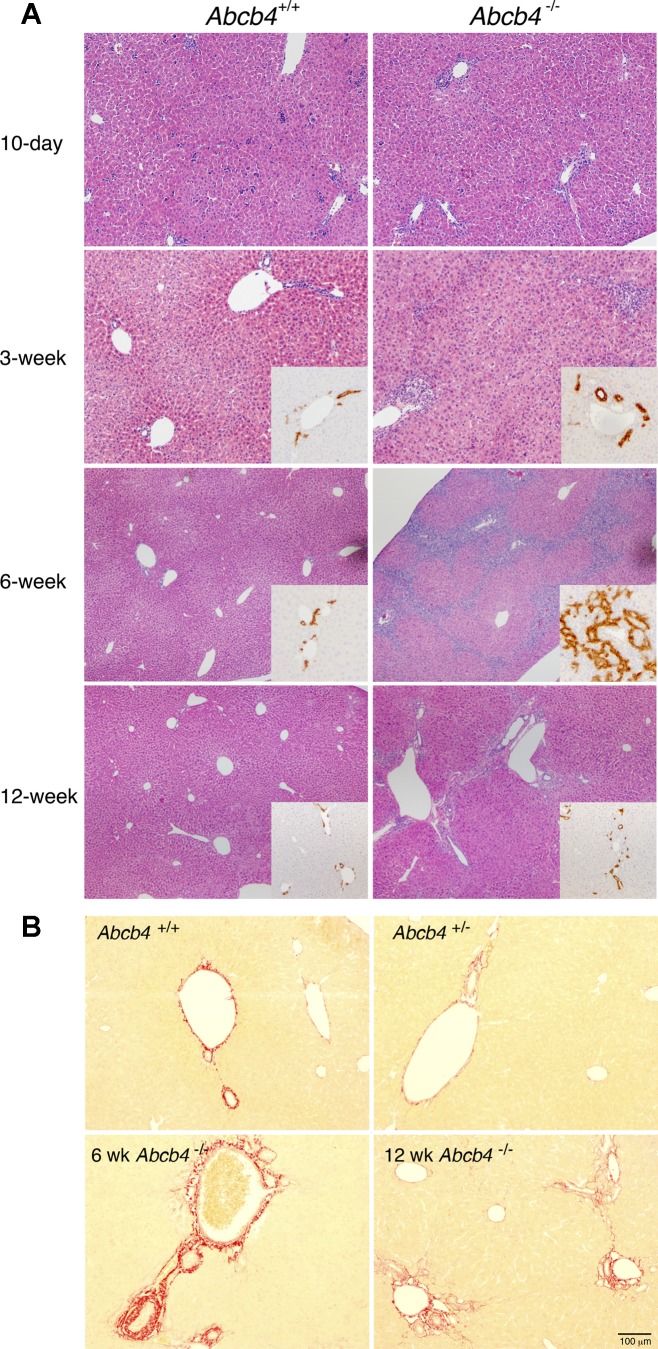
Assessment of liver histology H&E showed no significant differences among the three genotypes at 10 days and 3 wk of age (Fig. 1 and data not shown). In contrast, bile duct proliferation and inflammatory infiltrates became pronounced in the livers of the homozygous knockout mice by 6 wk of age. The degree of bile duct proliferation was confirmed by CK19 immunohistochemistry staining (Fig. 1A, inset). In addition, periductal fibrosis was also noted by Sirius Red staining in these 6-wk Abcb4−/−-deficient livers (Fig. 1B). Interestingly, this massive proliferation of the bile duct epithelium and periductal fibrosis in the homozygous knockout livers became less prominent by the time these animals reached 12 wk of age (Fig. 1B). Throughout this time, the liver histology of both the Abcb4+/− heterozygotes and WT remained within normal limits.
Fig. 1.
A: liver histology with hematoxylin and eosin (H&E) from Abcb4 wild-type (WT) and knockout mice (10 day to 12 wk). Note marked bile duct proliferation in 6-wk-old Abcb4−/− mice (inset, CK19). B: Sirius Red staining indicates less periductal fibrosis in the liver of 12-wk-old Abcb4−/− mouse compared with the liver of 6-wk-old Abcb4−/− mouse.
Altered protein expression of canalicular bile transporters.
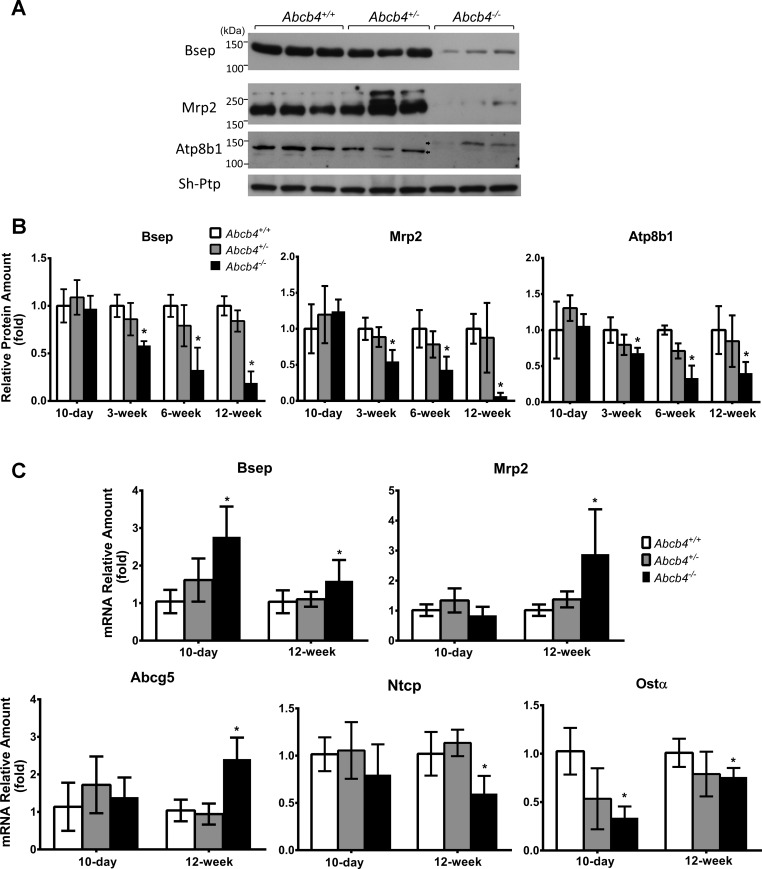
Expression of liver transporter proteins including Bsep, Mrp2, Atp8b1, Abcg5, and Abcg8 were unchanged at 10 days. However, at all later ages the levels of Bsep, Mrp2, and Atp8b1, proteins were reduced and Abcg5 and Abcg8 expression levels were increased in Abcb4−/− mice (Fig. 2 and data not shown). Western blots of Atp8b1 protein demonstrated a higher molecular weight in 12-wk-old Abcb4−/− mice (Fig. 2), suggesting altered posttranslational changes.
Fig. 2.
mRNA and protein expression of bile salt transporters were altered in Abcb4−/− liver. A: Western blot of Bsep, Mrp2, and Atp8b1 in the liver of 12-wk-old mice. B: quantitative analysis of liver protein expression of Bsep, Mrp2, and Atp8b1 at different ages. C: liver mRNA expression of Bsep, Mrp2, Abcg5, Ntcp, and Ost-α in 10-day-old and 12-wk-old mice. Means ± SD, n = 5–7 for each group. *P < 0.05 to WT.
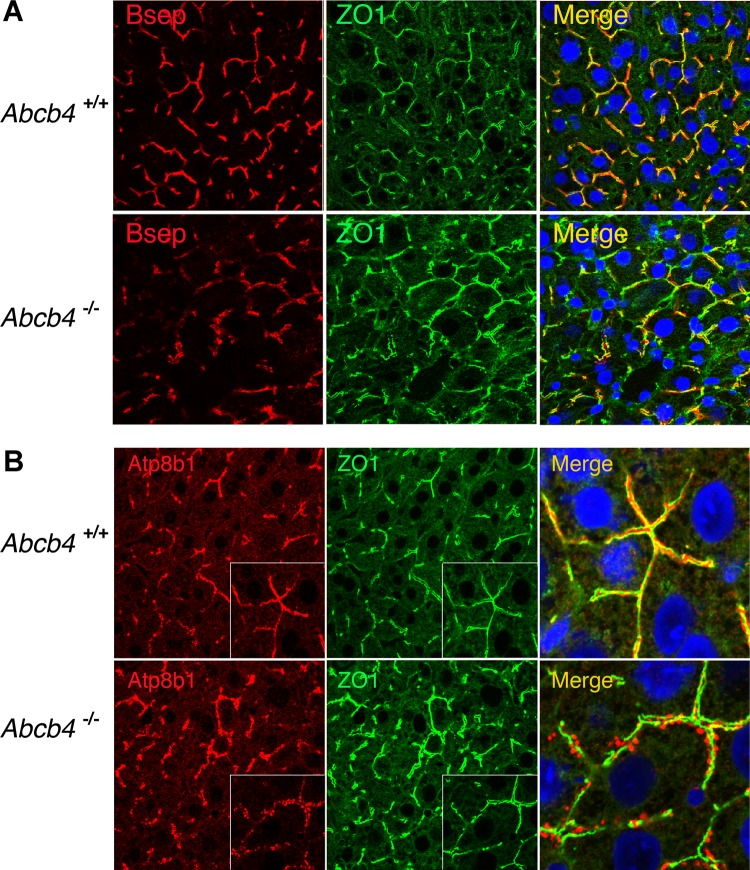
Examination of liver histology in these mice revealed that canalicular architecture was altered in the liver of 12-wk-old Abcb4−/− mice as reflected by immunofluorescent labeling of canalicular markers and the tight junction protein, zonnula occludin (ZO1) (Fig. 3). Although canalicular membrane transporters remained associated with the apical membrane, the density of labeling of Bsep and Mrp2 also appeared to be decreased, whereas the membrane-labeling intensity of Abcg5 was higher in Abcb4−/− liver, in agreement with Western blot detection (Fig. 3A and data not shown). In contrast, Atp8b1 was relocated to discrete punctuate structures that appear to be associated with the canalicular membrane in the Abcb4−/− mice (Fig. 3B). These structures were not noted by immunofluorescence when other canalicular proteins were labeled.
Fig. 3.
Immunofluorescent labeling of hepatic Bsep and Atp8b1 membrane expression in 12-wk-old Abcb4 −/− mice. A: although reduced, Bsep was localized on canalicular membrane of hepatocytes. B: in contrast, Atp8b1 formed discrete punctuate structures adjacent to the canalicular membrane in Abcb4−/− mice. Of note, decreased hepatic Mrp2 and Mdr1 membrane expression was also seen in these mice (data not shown). ZO1, zonnula occludens 1.
Bile salt concentrations are increased in liver and gallbladder bile in Abcb4−/− mice.
To test whether the reduced expression of Bsep protein would alter bile salt concentrations in liver tissue and gallbladder bile, we analyzed bile salt levels in 3-, 6-, and 12-wk-old animals. As shown in Table 2, significantly higher bile salts were detected in the livers of 6- and 12-wk-old Abcb4−/− mice, whereas no differences in bile salt levels were noted in livers from 3-wk-old animals. We also analyzed bile salt concentrations in gallbladder bile from 6-wk-old animals and found that bile salt concentrations in gallbladder bile were significantly lower in Abcb4−/− mice despite elevations of bile salts in the livers (WT, 79.0 ± 7.2 mM; heterozygotes, 78.2 ± 8.6 mM; homozygotes, 39.2 ± 20.0 mM*; mean ± SD, n = 6 for each group; *P < 0.001 to WT). These findings are consistent with impaired canalicular excretion of bile acids as a result of the downregulation of Bsep protein expression.
Table 2.
Increased liver bile salt levels in 6-wk- and 12-wk-old Abcb4−/− mice
| Genotype | 10 Days | 3 Wk | 6 Wk | 12 Wk | |
|---|---|---|---|---|---|
| Bile acids, nmol/g liver | +/+ | ND | 225.1 ± 22.2 | 101.4 ± 41.6 | 118.4 ± 93.8 |
| +/− | ND | 201.3 ± 44.0 | 91.7 ± 25.8 | 102.0 ± 71.5 | |
| −/− | ND | 179.9 ± 33.5 | 273.6 ± 74.8* | 328.7 ± 46.9* |
Values are means ± SD. Liver bile salt levels were increased in Abcb4−/− mice 6 and 12 wk after birth. n = 4–6 for each group;
P < 0.01 to WT;
ND, not determined.
mRNA expression levels of Bsep are increased by 10 days of age.
To determine whether the reduced expression of bile salt transporter proteins were due to transcriptional or translational regulation, we analyzed hepatic mRNA expression using real-time quantitative PCR. Liver expression of Bsep mRNA was increased in 10-day Abcb4−/− mice, whereas Mrp2, Abcg5, Abcg8, and Ntcp were not changed, and Ost-α was decreased in these mice compared with their WT controls. By 12 wk of age, mRNA of Bsep, Mrp2, and Abcg5 were all increased, whereas Atp8b1 and Abcg8 were unchanged, and expression of Ost-α and Ntcp were reduced (Fig. 2C and data not shown). The alterations in mRNA expression of hepatic bile salt transporters are similar to well described adaptive responses of these transporters that have been observed in other cholestatic models.
TDCA treatment did not reduce Bsep protein expression in cultured Abcb4−/− hepatocytes.
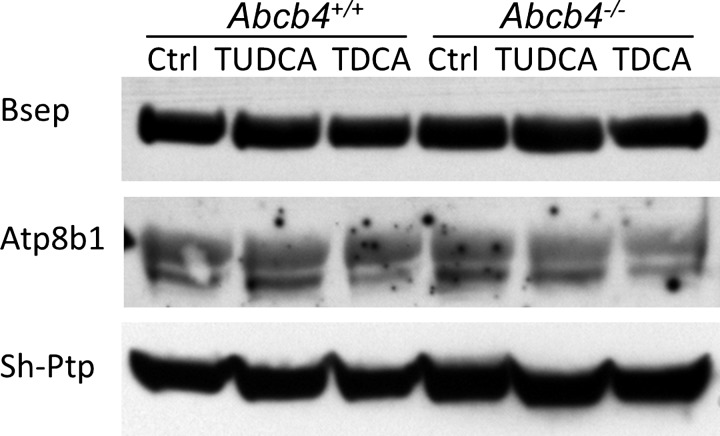
To gain insights into the decreased protein expression of bile salt transporters observed in Abcb4−/− livers, we tested whether hydrophobic bile acid overload can reduce Bsep expression in isolated hepatocytes. In this experiment, hepatocytes from WT and Abcb4−/− mice were maintained in collagen sandwich culture system and treated with TUDCA and TDCA for up to 48 h. To our surprise, no difference was detected between WT cells and knockout cells in both Bsep and Atp8b1 protein expression (Fig. 4). Both preparations formed nice canaliculi visible by light microscopy although there were fewer canaliculi after 25 μM TDCA treatment in both. Immunofluorescent labeling of ZO1 and Bsep did not reveal marked differences as seen in the liver tissue from 12-wk-old mice.
Fig. 4.
Western blot demonstrated that bile acid treatment did not alter Bsep and Atp8b1 protein expression in cultured hepatocytes. Hepatocytes from WT and Abcb4−/− mice were maintained in collagen sandwich culture and treated with 25 μM tauroursodeoxycholic acid (TUDCA) or 25 μM taurodeoxycholic acid (TDCA) for 24 h. Total cell lysate was analyzed.
DISCUSSION
ABCB4/Abcb4 deficiency in humans and mice leads to cholestatic liver injury, associated with altered serum levels of bile salt, bilirubin, and liver enzymes. We hypothesized that the expression and/or function of bile transporters may be altered in the livers of ABCB4/Abcb4 deficiency, which would contribute to the development and progression of this disease. In this report, we assessed the expression of bile transporters in Abcb4−/− mouse livers. We found that Abcb4−/− mice were cholestatic even at 10 days of age (Table 1) but that the protein expression of bile transporters were not changed compared with WT and Abcb4+/− mice, including Bsep, Mrp2, Atp8b1, Abcg5, and Abcg8 (Fig. 2). As their age increased, the protein expression of Bsep, Mrp2, and Atp8b1 in Abcb4−/− livers gradually decreased, in parallel with the progression of liver injury (Fig. 1). By 6 wk of age, hepatic Bsep and Mrp2 protein expression in Abcb4−/− livers was substantially reduced to 33% and 43% of the WT controls, respectively. Significantly lower bile salt concentration (only 50% of the WT control) was also detected in the gallbladder bile from these mice consistent with predicted reductions in the function of the bile salt export pump. The reduction in expression of Bsep, Mrp2, and Atp8b1 proteins was further confirmed by immunofluorescent labeling (Fig. 3). Paradoxically, Abcg5 and 8 protein expression was increased in these mice although prior studies indicate that cholesterol excretion is also impaired in Abcb4-deficient mice (15). Together, these findings indicate that altered expression of bile transporters in Abcb4−/− mice may contribute to the development of liver injury.
In contrast to changes in protein expression, the expression of Bsep mRNA was significantly increased even by 10 days of age in Abcb4−/− livers. Raised hepatic mRNA levels of Bsep and Mrp2 were also detected in 12-wk-old Abcb4−/− livers. The elevated mRNA expression of these transporters is likely due to the well described adaptive responses to cholestasis, as they are targets of the bile acid nuclear receptor Fxr (Nr1h4). When bile acid levels rise, Fxr is activated, which in turn stimulates Bsep and Mrp2 mRNA expression. However, this increase in mRNA expression was associated with lower, not higher, levels of protein expression, suggesting that impairment in posttranscriptional and/or posttranslational regulation may account for the reduction in protein expression of these two ABC transporters. It is conceivable that alterations in the lipid composition of the canalicular membrane that result from the absence of Mdr2 have impaired the canalicular membrane structure and caused or contributed to the instability of Bsep and Mrp2 in the Abcb4−/− livers. However, Abcg5 and 8 are also localized to the canalicular membrane, yet their protein expression was increased instead. One could argue that Bsep and Mrp2 protein, but not Abcg5 and 8 protein, are more sensitive to alterations in membrane lipid composition and/or are more susceptible to the detergent effects of bile salts in the absence of PC. The latter seems unlikely because Bsep protein expression was not changed in isolated Abcb4−/− hepatocyte sandwich cultures when treated with TDCA (Fig. 4), albeit the time of exposure was considerably less than in the intact mutant mouse. Previous studies indicate that inflammatory cytokines can reduce Bsep and Mrp2 protein expression. Inflammatory infiltrates were also observed at early ages of the Abcb4−/− livers, so that decreased Bsep and Mrp2 protein expression could result from an immune response secondary to the effects of cholestasis. Irrespective of the cause, reduced Bsep and Mrp2 protein expression and function would be expected to further aggravate the liver injury.
A previous study indicates that the PC floppase ABCB4 and the phosphatidylserine flippase ATP8B1 play complementary functional roles in maintaining canalicular membrane lipid integrity, as liver injury is actually reduced in the Abcb4 and Atp8b1 double knockout mice compared with that seen in the liver from either gene knockout alone (11). In the present study, Western blots revealed that the molecular mass of Atp8b1 in 12-wk-old Abcb4−/− livers was larger than their WT controls (Fig. 2A). Immunofluorescence imaging demonstrated that Atp8b1 formed granular structures along the canalicular membrane but in submembranous areas rather than within the canalicular membrane in these mice, strikingly different from the localization of Atp8b1 seen in control livers. These observations suggest that Atp8b1 underwent posttranslation modification, leading to relocalization from or inability to traffic to the apical membrane. The inability of Atp81 to localize at the apical membrane would impair its ability to maintain (flip) phosphatidylserine to the cytosolic inner leaflet of the bilayer. This might be viewed as a beneficial adaptive response in an Abcb4-deficient mouse, where PC is absent from the outer leaflet of the canalicular membrane bilayer, exactly analogous to the improvement in liver injury seen in the Atp8b1 and Abcb4 double knockout mice. This is a novel, not previously described compensatory finding in the Abcb4−/− mouse model. Although we did not directly assess Atp8b1 function in 12-wk-old Acb4−/− mice, a more detailed study of possible Atp8b1 dysfunction in the Abcb4−/− mice is warranted.
In summary, by examining the progression of liver injury and the associated changes in bile transporters over time in the Abcb4−/− mouse, we have discovered that significant changes occur in bile salt transport and Bsep and Mrp2 expression early on in the development of liver injury that results from Abcb4−/− deficiency. Unlike the current hypothesis that relates the progression of the liver injury to the inability of bile acids to form micelles with PC in bile and subsequent detergent effects of hydrophobic bile acids on the biliary epithelium, the current study supports the additional concept that changes in the lipid composition of the canalicular membrane impair the function of Bsep and Mrp2 early in the course of the development of the disease, thereby impairing the major determinants of bile formation and thus contributing significantly to the pathogenesis of cholestasis that results from the absence of PC in the canalicular membrane.
GRANTS
This work was supported by NIH grants DK25636 (J. Boyer), DK34989 (Yale Liver Center), and a PSC Partners for Cure grant (S.-Y. Cai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.-Y.C. and J.L.B. conception and design of research; S.-Y.C., A.M., and C.J.S. performed experiments; S.-Y.C. and C.J.S. analyzed data; S.-Y.C. and J.L.B. interpreted results of experiments; S.-Y.C. prepared figures; S.-Y.C. drafted manuscript; S.-Y.C., C.J.S., and J.L.B. edited and revised manuscript; S.-Y.C., A.M., C.J.S., and J.L.B. approved final version of manuscript.
REFERENCES
- 1.Baghdasaryan A, Fickert P, Fuchsbichler A, Silbert D, Gumhold J, Horl G, Langner C, Moustafa T, Halilbasic E, Claudel T, Trauner M. Role of hepatic phospholipids in development of liver injury in Mdr2 (Abcb4) knockout mice. Liver Int 28: 948–958, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Cai SY, Gautam S, Nguyen T, Soroka CJ, Rahner C, Boyer JL. ATP8B1 deficiency disrupts the bile canalicular membrane bilayer structure in hepatocytes, but FXR expression and activity are maintained. Gastroenterology 136: 1060–1069, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai SY, He H, Nguyen T, Mennone A, Boyer JL. Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J Lipid Res 51: 2265–2274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis 30: 134–146, 2010 [DOI] [PubMed] [Google Scholar]
- 6.de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, Oude Elferink RP, Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA 95: 282–287, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deleuze JF, Jacquemin E, Dubuisson C, Cresteil D, Dumont M, Erlinger S, Bernard O, Hadchouel M. Defect of multidrug-resistance 3 gene expression in a subtype of progressive familial intrahepatic cholestasis. Hepatology 23: 904–908, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Denk GU, Bikker H, Lekanne Dit Deprez RH, Terpstra V, van der Loos C, Beuers U, Rust C, Pusl T. ABCB4 deficiency: A family saga of early onset cholelithiasis, sclerosing cholangitis and cirrhosis and a novel mutation in the ABCB4 gene. Hepatol Res 40: 937–941, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Elamiri A, Perwaiz S, Tuchweber B, Yousef IM. Effect of mdr2 mutation with combined tandem disruption of canalicular glycoprotein transporters by cyclosporine A on bile formation in mice. Pharmacol Res 48: 467–472, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, Marschall HU, Denk H, Trauner M. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 127: 261–274, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Groen A, Romero MR, Kunne C, Hoosdally SJ, Dixon PH, Wooding C, Williamson C, Seppen J, Van den Oever K, Mok KS, Paulusma CC, Linton KJ, Oude Elferink RP. Complementary functions of the flippase ATP8B1 and the floppase ABCB4 in maintaining canalicular membrane integrity. Gastroenterology 141: 1927–1937, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, therapeutics. Cell Mol Life Sci 65: 2461–2483, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquemin E, de Vree JM, Cresteil D, Sokal EM, Sturm E, Dumont M, Scheffer GL, Paul M, Burdelski M, Bosma PJ, Bernard O, Hadchouel M, Elferink RP. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology 120: 1448–1458, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Kubitz R, Bode J, Erhardt A, Graf D, Kircheis G, Muller-Stover I, Reinehr R, Reuter S, Richter J, Sagir A, Schmitt M, Donner M. Cholestatic liver diseases from child to adult: the diversity of MDR3 disease. Z Gastroenterol 49: 728–736, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Langheim S, Yu L, von BK, Lutjohann D, Xu F, Hobbs HH, Cohen JC. ABCG5 and ABCG8 require MDR2 for secretion of cholesterol into bile. J Lipid Res 46: 1732–1738, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol 145: 1237–1245, 1994 [PMC free article] [PubMed] [Google Scholar]
- 17.Moschetta A, Xu F, Hagey LR, Berge-Henegouwen GP, van Erpecum KJ, Brouwers JF, Cohen JC, Bierman M, Hobbs HH, Steinbach JH, Hofmann AF. A phylogenetic survey of biliary lipids in vertebrates. J Lipid Res 46: 2221–2232, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Nakken KE, Nygard S, Haaland T, Berge KE, Arnkvaern K, Odegaard A, Labori KJ, Raeder MG. Multiple inflammatory-, tissue remodelling- and fibrosis genes are differentially transcribed in the livers of Abcb4 (−/−) mice harbouring chronic cholangitis. Scand J Gastroenterol 42: 1245–1255, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Nakken KE, Nygard S, Haaland TK, Berge KE, Odegaard A, Labori KJ, Raeder MG. Gene expression profiles reflect sclerosing cholangitis activity in abcb4 (−/−) mice. Scand J Gastroenterol 44: 211–218, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Oude Elferink RP, Ottenhoff R, van Wijland M, Smit JJ, Schinkel AH, Groen AK. Regulation of biliary lipid secretion by mdr2 P-glycoprotein in the mouse. J Clin Invest 95: 31–38, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol 43: 1045–1054, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75: 451–462, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Smith AJ, Timmermans-Hereijgers JL, Roelofsen B, Wirtz KW, van Blitterswijk WJ, Smit JJ, Schinkel AH, Borst P. The human MDR3 P-glycoprotein promotes translocation of phosphatidylcholine through the plasma membrane of fibroblasts from transgenic mice. FEBS Lett 354: 263–266, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology 51: 181–190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res 50 Suppl: S406–S411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]