Abstract
Early mucosal restitution occurs as a consequence of intestinal epithelial cell (IEC) migration to reseal superficial wounds, but its exact mechanism remains largely unknown. Caveolin-1 (Cav1), a major component associated with caveolar lipid rafts in the plasma membrane, is implicated in many aspects of cellular functions. This study determined if c-Src kinase (Src)-induced Cav1 phosphorylation promotes intestinal epithelial restitution after wounding by activating Cav1-mediated Ca2+ signaling. Src directly interacted with Cav1, formed Cav1-Src complexes, and phosphorylated Cav1 in IECs. Inhibition of Src activity by its chemical inhibitor PP2 or suppression of the functional caveolin scaffolding domain by caveolin-scaffolding domain peptides prevented Cav1-Src interaction, reduced Cav1 phosphorylation, decreased Ca2+ influx, and inhibited cell migration after wounding. Disruption of caveolar lipid raft microdomains by methyl-β-cyclodextrin reduced Cav1-mediated Ca2+ influx and repressed epithelial restitution. Moreover, Src silencing prevented subcellular redistribution of phosphorylated Cav1 in migrating IECs. These results indicate that Src-induced Cav1 phosphorylation stimulates epithelial restitution by increasing Cav1-mediated Ca2+ signaling after wounding, thus contributing to the maintenance of gut mucosal integrity under various pathological conditions.
Keywords: calcium influx, intracellular calcium, cyclopiazonic acid, Src activity, IEC-6 cells, cell migration, caveolin-1 scaffolding domain peptide
the gastrointestinal epithelium is exposed to a wide array of luminal noxious substances, and acute mucosal injury occurs commonly during critical pathological conditions. Early epithelial restitution, an important repair modality in the gastrointestinal mucosa, occurs as a consequence of epithelial cell migration over the damaged area after superficial injury, a process that is independent of cell proliferation (6, 27, 42, 49). Defective regulation of this process underlies various critical pathological states, such as mucosal bleeding and ulcers, disruption of epithelial integrity, and gut barrier dysfunction (10, 11, 14). This rapid reepithelialization is a complex process that is highly regulated by numerous extracellular and intracellular factors. Our previous studies (33, 34, 36, 38–41) and others (2, 14) have shown that cytosolic free Ca2+ concentration ([Ca2+]cyt) plays an important role in regulation of intestinal epithelial cell (IEC) migration after injury and that increasing [Ca2+]cyt stimulates epithelial restitution. Ca2+ entry due to store depletion, often called store-operated Ca2+ (SOC) entry (SOCE), is mediated by Ca2+-permeable channels termed SOC channels (4, 28). SOCE contributes to the sustained increase in [Ca2+]cyt and refilling of the stores with Ca2+ after wounding, but the exact mechanism underlying this process is largely unknown.
Caveolar lipid rafts are key molecules involved in the regulation of Ca2+ influx. Caveolin-1 (Cav1), a major component associated with caveolar lipid rafts in the plasma membrane (PM), has been shown to act as a potential regulator of Ca2+ influx via SOCE (29, 43). Caveolae, flask-shaped PM invaginations in different cell types, highly express a ∼22-kDa protein, Cav1 (17, 23, 26, 47). Cav1 is a multifunctional scaffolding protein, and its various binding partners associate with a diverse set of cellular processes ranging from cholesterol homeostasis, vesicular transport, and regulation of the cell cycle and cell polarity to regulation of cell transformation and signal transduction (23, 31). Ca2+ influx occurs via caveolae in response to endoplasmic reticulum (ER)-stored Ca2+ depletion in different types of cells (1, 12, 15, 16). The Cav1 scaffolding domain (CSD) of Cav1 interacts with Ca2+-permeable channels such as the transient receptor potential canonical (TRPC) type 1 (TRPC1) channel within the caveolae in certain types of mammalian cells (19, 47). Increased Cav1 expression in smooth muscle cells enhances Ca2+ entry in response to Ca2+ store depletion (1, 29). Disruption of caveolar proteins, with chemical inhibitors or silencing of the Cav1 gene in mice, revealed that loss of Cav1 expression inhibits SOCE (12, 47), whereas restoration of wild-type Cav1 in Cav1−/− cells rescues SOCE.
Cav1 copurifies with cytoplasmic signaling molecules, including G proteins (α- and βγ-subunits), H-Ras, c-Src, and other Src family tyrosine kinases (18, 22). Cav1 is a major substrate for COOH-terminal Src kinase (Src) in v-Src-transformed cell lines (7, 20), and Cav1 phosphorylated on Tyr14 through the recruitment of Src (5) plays distinct roles in various cellular functions (18, 22). The Src-interacting domain of caveolin was localized to caveolin residues 82–101, and a peptide encoding this sequence dose dependently suppresses the activation of Src (21). The aim of the current study was to determine if Src modulates Cav1 after wounding, thus activating Cav1-mediated SOCE and epithelial restitution. Our results indicate that Src physically interacts with and phosphorylates Cav1 in IECs and that activated Cav1 stimulates cell migration by increasing Ca2+ signaling.
MATERIALS AND METHODS
Chemicals and supplies.
Disposable culture ware was purchased from Corning Glass Works (Corning, NY). Tissue culture media, isopropyl-β-d-thiogalactopyranoside (IPTG), Lipofectamine 2000, and dialyzed FBS were obtained from Invitrogen (Carlsbad, CA), and biochemicals were obtained from Sigma (St. Louis, MO). Primary antibodies against Cav1, phosphorylated Cav1 (pCav1), c-Src, and phosphorylated Src (pSrc) were purchased from Cell Signaling Technology (Danvers, MA); methyl-β-cyclodextrin (MβCD) from Sigma; the Src inhibitor PP2 from EMD Millipore (Bedford, MA); CSD peptide from Enzo Life Sciences (Farmingdale, NY); and antennapedia (AP) and FITC-conjugated AP peptides from Anaspec (Fremont, CA).
Cell culture.
Stable Cdx2-transfected IEC-6 (IEC-Cdx2L1) cells were developed by Suh and Traber (46) and are characterized in previous publications (32, 35, 37–39). The expression vector, the LacSwitch system (Stratagene, La Jolla, CA), was used to direct the conditional expression of the Cdx2 gene, and IPTG served as the inducer for gene expression (45). Stock IEC-Cdx2L1 cells were grown in DMEM supplemented with 5% heat-inactivated FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate. Before experiments, IEC-Cdx2L1 cells were grown in DMEM containing 4 mM IPTG for 16 days to induce cell differentiation. The IEC-6 cell line (30) was purchased from the American Type Culture Collection at passage 13 and cultured as described in our previous publications (33, 36, 38, 51, 52). Passages 15–20 were used in this study.
RNA interference.
The small interfering RNA (siRNA) that was designed to specifically cleave Src mRNA (siSrc) was purchased from Santa Cruz Biotechnology. Scrambled control siRNA (C-siRNA) without sequence homology to any known genes was used as the control. For each 60-mm cell culture dish, 20 μl of the 5 μM stock siSrc or C-siRNA were mixed with 500 μl of Opti-MEM (Invitrogen). This mixture was gently added to a solution containing 6 μl of Lipofectamine 2000 in 500 μl of Opti-MEM. The solution was incubated for 15 min at room temperature and gently overlaid onto monolayers of cells in 3 ml of medium, and cells were harvested for various assays after 48 h of incubation.
Immunoprecipitation and immunoblot analysis.
Cell samples, dissolved in ice-cold RIPA buffer, were sonicated and centrifuged at 4°C, and the supernatants were collected for immunoprecipitation (IP). Equal amounts of proteins (500 μg) for each sample were incubated with the specific antibody against Cav1 or Src (4 μg) at 4°C for 3 h, protein A/G-PLUS-agarose was added, and the samples were incubated overnight at 4°C. The precipitates were washed five times with ice-cold Tris-buffered saline (TBS), and the beads were resuspended in SDS sample buffer. For immunoblotting, samples were subjected to electrophoresis on polyacrylamide gels, as described previously (51, 52). Briefly, the protein was transferred to nitrocellulose membranes, which were incubated for 1 h in 5% nonfat dry milk in 1× TBS-0.1% Tween 20 (TBS-T) buffer. Immunological evaluation was then performed overnight at 4°C in 5% nonfat dry milk-TBS-T buffer containing a specific antibody against Cav1, pCav1, Src, or pSrc. The membranes were subsequently washed with 1× TBS-T and incubated with the secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. The immunocomplexes on the membranes were reacted for 1 min with chemiluminiscence reagent (NEL-100, DuPont NEN).
Measurement of [Ca2+]cyt.
Detailed digital imaging methods employed for measuring [Ca2+]cyt are described in our previous publications (33, 34, 36, 39, 41). Briefly, cells were plated on 25-mm coverslips and incubated with the culture medium containing 3.3 μM fura 2-AM for 30 min under an atmosphere of 10% CO2 in air. The fura 2-AM-loaded cells were then superfused with standard bath solution for 20–30 min at 22–24°C to wash out extracellular dye and permit intracellular esterases to cleave cytosolic fura 2-AM into active fura 2. Fura 2 fluorescence from the cells and background fluorescence were imaged using a Nikon Diaphot microscope equipped for epifluorescence. Fluorescent images were obtained using a microchannel plate image intensifier (Opelco, Washington, DC) coupled by fiber optics to a Pulnix charge-coupled device video camera (Stanford Photonics, Stanford, CA). Image acquisition and analyses were performed with a Metamorph Imaging System (Universal Imaging). The final values of [Ca2+]cyt were obtained from fura 2-fluorescent emission excited at 380 and 340 nm from calibrated ranges, as described in our previous publications (33, 34, 41).
Measurement of cell migration.
Migration assays were carried out as described in our earlier publications (32–41, 52). Cells were plated at 6.25 × 104/cm2 in DMEM containing FBS on 60-mm dishes thinly coated with Matrigel according to the manufacturer's instructions (BD Biosciences, Bedford, MA) and incubated as described for stock cultures. Cells were fed on day 2, and cell migration was assayed on day 4. For initiation of migration, the cell layer was scratched with a single-edge razor blade cut to a length of ∼27 mm. The scratch was made over the diameter of the dish and extended over a 7- to 10-mm-wide area. The migrating cells in six contiguous 0.1-mm squares were counted at ×100 magnification beginning at the scratch line and extending as far as the cells had migrated. All experiments were carried out in triplicate, and the results are reported as the number of migrating cells per millimeter of scratch.
Immunofluorescence staining.
Immunofluorescence was performed as described elsewhere (48) with minor changes (35, 39). Cells were fixed using 3.7% formaldehyde, and the samples were incubated overnight at 4°C with primary antibody against Cav1 and pCav1 diluted 1:300 in blocking buffer and then incubated with secondary antibodies conjugated with Cy5 and Alexa Fluor 488 (Molecular Probes, Eugene, OR), respectively, to detect subcellular localization for 2 h at room temperature. After they were rinsed, the slides were incubated with 4′,6-diamidino-2-phenylindole (1:5,000 dilution; Molecular Probes) for 10 min to stain nuclei, rinsed again, mounted, and viewed through a confocal microscope (model LSM700, Zeiss). Images were processed using PhotoShop software (Adobe, San Jose, CA).
Statistical analysis.
Values are means ± SE from six dishes. IP and immunoblotting results are representative of data from three experiments. The significance of the difference between means was determined by analysis of variance. The level of significance was determined using Duncan's multiple-range test (13). P < 0.05 was considered significant.
RESULTS
Expression of Cav1 and Src proteins and their interactions in IECs.
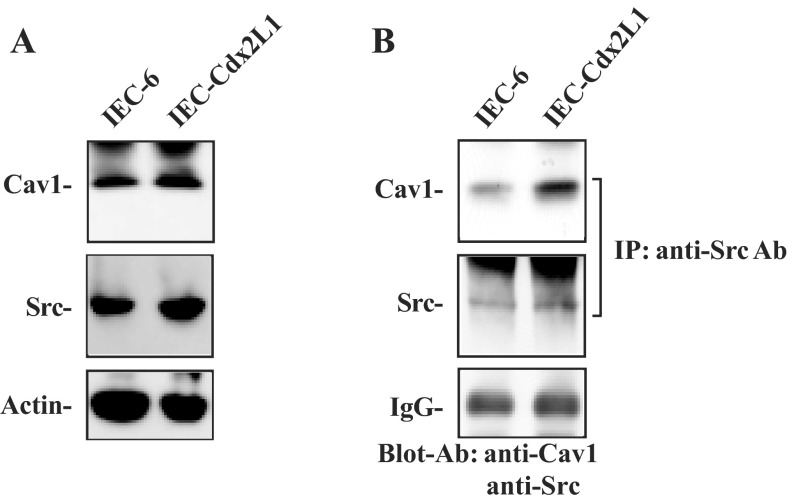
To determine if Src-mediated Cav1 phosphorylation plays a role in the regulation of intestinal epithelial restitution, basal expression of Cav1 and Src proteins and their interaction were examined in two different lines of IECs, including parental IEC-6 cells and differentiated IEC-Cdx2L1 cells. IEC-6 cells originate from intestinal crypts, are nontumorigenic, and retain undifferentiated status of intestinal crypt cells; IEC-Cdx2L1 cells represent differentiated IECs. As reported in our previous studies (32, 35), induced expression of the Cdx2 gene in IEC-Cdx2L1 cells by treatment with 4 mM IPTG for 16 days resulted in a differentiated phenotype. These differentiated IEC-Cdx2L1 cells were polarized and exhibited multiple morphological and molecular characteristics of intestinal epithelial differentiation. This line of differentiated IEC-Cdx2L1 cells has been extensively used and widely accepted as an in vitro model system for cell division-independent stage of epithelial restitution (37–39). As shown in Fig. 1A, IEC-6 and differentiated IEC-Cdx2L1 cells highly expressed total Cav1 and Src proteins as measured by Western immunoblot analysis. To examine Cav1-Src association, whole cell lysates were incubated with the antibody specifically against Src for IP, and the levels of Cav1 and Src in the pulldown materials were examined using anti-Cav1 or Src antibody. Src associated with Cav1 in both cell lines, but the level of Cav1-Src complexes was higher in differentiated IEC-Cdx2L1 than parental IEC-6 cells (Fig. 1B). We also used IgG as a negative control in IP assays and found that incubation with IgG under the same condition did not pull down Cav1 or Src protein (data not shown). These results indicate that Src physically interacts with Cav1 and forms Cav1-Src complexes in IECs.
Fig. 1.
Levels of caveolin 1 (Cav1) and Src proteins and Cav1-Src interactions in intestinal epithelial cells (IECs). A: representative immunoblots of Cav1 and Src in IEC-6 cells and differentiated IEC-Cdx2L1 cells. Levels of total Cav1 and Src were examined by Western blot analysis, and actin immunoblotting was performed as an internal control for equal loading. B: levels of Cav1 and Src in materials immunoprecipitated (IP) by the anti-Src antibody (Ab) in cells described in A. After whole cell lysates (500 μg) were immunoprecipitated by the specific antibody against Src, precipitates were separated by SDS-PAGE. Levels of Cav1 and Src were measured using Western blot analysis with specific antibodies. IgG heavy chain is presented to show equal loading. Blots represent similar results from 3 separate experiments.
Src inhibition by PP2 decreases phosphorylated Cav1-Src complexes and reduces SOCE and cell migration.
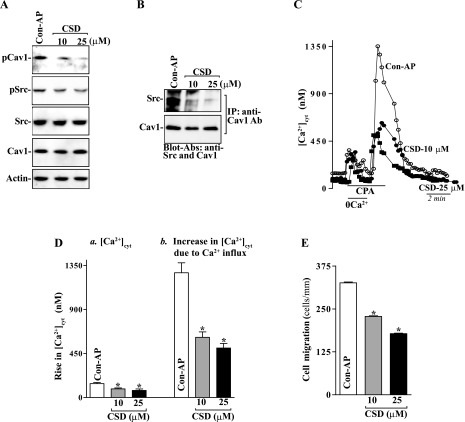
To elucidate the exact relationship between Cav1 phosphorylation by Src kinase and intestinal epithelial restitution, the following two studies were carried out. First, we examined the effects of decreased levels of pSrc on the levels of Cav1-Src complexes in differentiated IEC-Cdx2L1 cells by treatment with its specific chemical inhibitor PP2. Exposure to PP2 for 24 h dose dependently decreased the levels of pSrc and phosphorylated Cav1 (pCav1) but failed to alter the levels of total Src and total Cav1 proteins (Fig. 2A). When PP2 at different concentrations was added to the medium, levels of pSrc and pCav1 were decreased by ∼30% at 10 μM and ∼70% at 20 μM. Consistently, the levels of Cav1-Src complexes decreased significantly in cells exposed to PP2 when measured using the antibody against pCav1 or pSrc (Fig. 2B). Second, we examined if Src inhibition by PP2 affected SOCE and cell migration. As shown in Fig. 2, C and D, PP2 treatment not only decreased resting [Ca2+]cyt but also inhibited store depletion-induced Ca2+ influx induced by cyclopiazonic acid (CPA). In control cells, exposure to CPA, an inhibitor of Ca2+-Mg2+-ATPase in the ER and sarcoplasmic reticulum, resulted in an initial transient increase in [Ca2+]cyt in the absence of extracellular Ca2+, which was apparently due to Ca2+ mobilization from intracellular Ca2+ stores. Addition of extracellular Ca2+ to the cell superfusate after store depletion by CPA caused a sustained increase in [Ca2+]cyt because of the SOCE. In PP2-treated cells, however, resting [Ca2+]cyt was reduced by ∼40%, while Ca2+ influx due to SOCE was decreased by ∼60%, compared with control cells. Importantly, inhibition of Src activity and the subsequent decrease in [Ca2+]cyt by PP2 suppressed cell migration after wounding (Fig. 2, E and F). The numbers of PP2-treated cells migrating over the wounded edge were decreased by ∼65% at 10 μM and ∼75% at 20 μM. In addition, exposure to PP2 did not affect cell viability as measured by Trypan blue staining (data not shown). These results indicate that Src inhibition by PP2 reduces Cav1 phosphorylation, decreases Ca2+ influx, and represses cell migration after wounding.
Fig. 2.
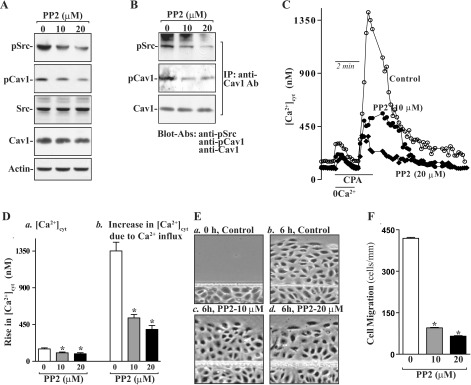
Levels of phosphorylated Src (pSrc) and phosphorylated Cav1 (pCav1), Cav1-Src interactions, Ca2+ influx, and cell migration after exposure to PP2 in differentiated IEC-Cdx2L1 cells. Cells were grown in standard DMEM for 4 days and then exposed to 0, 10, and 20 μM PP2 for 24 h. A: representative Western blots of pSrc, pCav1, total Src, and total Cav1 proteins. Actin immunoblotting was performed as an internal control for equal loading. B: levels of pSrc, pCav1, and total Cav1 proteins in materials immunoprecipitated by the anti-Cav1 Ab in cells described in A. C: representative records of resting cytosolic Ca2+ concentration ([Ca2+]cyt) and Ca2+ influx after cyclopiazonic acid (CPA)-induced Ca2+ store depletion as measured in peripheral areas in cells described in A. 0Ca2+, Ca2+-free solution. D: summary of resting [Ca2+]cyt (left) and amplitude of CPA-induced Ca2+ influx (right) data. Values are means ± SE (n = 25). *P < 0.05 compared with cells incubated with vehicle alone (0 μM). E: images of cell migration [0 h after wounding (a), control cells 6 h after wounding (b), cells treated with 10 μM PP2 6 h after wounding (c), and cells treated with 20 μM PP2 6 h after wounding (d)]. F: summary of cell migration data 6 h after removal of part of the monolayer. Values are means ± SE from 6 dishes. *P < 0.05 compared with cells incubated with vehicle (DMSO) alone.
CSD peptide inhibits Cav1-Src association and represses cell migration.
It has been shown that the Src-interacting domain of Cav1 is located within residues 82–101, a cytosolic membrane-proximal region of Cav1 (47), whereas CSD peptide derived from this region functionally suppresses the activation of Src tyrosine kinase (21). To examine the functional consequences of CSD peptide on the interaction of Cav1-Src complexes after wounding, a synthetic peptide corresponding to the Cav1 CSD sequence (DGIWKASFTTFTVTKYWFYR) was used. The CSD peptide was conjugated to an AP internalization sequence (RQIKIWFQNRRMKWKK) to improve membrane permeability, as reported earlier (1). An AP peptide was used as an experimental control in this study. First, we determined the membrane permeability of the peptide in IEC-Cdx2L1 cells. Use of the FITC-labeled AP peptide (AP-FITC) showed that >95% of cells were positive (data not shown). Neither AP nor CSD affected cell viability as measured by Trypan blue staining (data not shown). Second, we examined changes in the levels of Cav1-Src complexes after treatment with AP and CSD peptides. As shown in Fig. 3A, exposure of IEC-Cdx2L1 cells to CSD dose dependently decreased pCav1 protein expression but failed to alter the levels of pSrc, total Cav1, and total Src proteins. However, CSD treatment inhibited Src-Cav1 association as indicated by a decrease in the levels of Cav1-Src complex when the cell lysates were immunoprecipitated with anti-Cav1 (Fig. 3B). Blocking of the Src-interacting domain of Cav1 by CSD also decreased resting [Ca2+]cyt and inhibited CPA-induced Ca2+ influx (Fig. 3, C and D). Resting [Ca2+]cyt and CPA-induced Ca2+ influx were decreased by ∼30% and ∼55%, respectively, in CSD-exposed cells compared with control cells. Inhibition of Ca2+ influx by treatment with CSD peptide also impaired intestinal epithelial restitution (Fig. 3E). The numbers of CSD-treated cells migrating over the denuded area 6 h after wounding were decreased by ∼30% (10 μM) and 45% (25 μM). These results strongly suggest that Src regulates Cav1 phosphorylation and Cav1-mediated Ca2+ influx through a process involving the Src-interacting domain of Cav1.
Fig. 3.
Effect of treatment with Cav1 scaffolding domain (CSD) peptide on Cav1-Src interactions, pCav1 and pSrc proteins, Ca2+ influx, and cell migration in differentiated IEC-Cdx2L1 cells. Cells were grown in standard DMEM for 4 days and then exposed to CSD peptides (10 and 25 μM) for 4 h. Antennapedia peptide (AP) was used as control (Con-AP). A: representative immunoblots of pCav1, pSrc, total Src, and total Cav1 proteins. Actin immunoblotting was performed as an internal control for equal loading. B: levels of Src and Cav1 proteins in materials immunoprecipitated by the anti-Cav1 Ab as described in A. C: representative records showing time course of [Ca2+]cyt after exposure to CPA in the absence (0Ca2+) or presence of extracellular Ca2+ in cells described in A. D: summary of resting [Ca2+]cyt (left) and amplitude of CPA-induced Ca2+ influx (right) data from cells described in C. Values are means ± SE (n = 25). *P < 0.05 compared with cells incubated with AP (Con-AP). E: summary of cell migration data 6 h after removal of part of the monolayer. Values are means ± SE from 6 dishes. *P < 0.05 compared with cells treated with AP (Con-AP).
Disruption of caveolae by MβCD decreases Ca2+ influx and inhibits cell migration.
MβCD is a cholesterol-depleting agent that disassembles caveolae at the PM and impairs SOCE (1, 25, 29). Treatment with MβCD decreased the levels of pCav1, although it did not affect total Cav1 and Src levels (Fig. 4A). The decreased levels of pCav1 by MβCD failed to alter resting [Ca2+]cyt but inhibited Ca2+ influx after store depletion (Fig. 4B) in differentiated IEC-Cdx2L1 cells. Treatment with MβCD also inhibited cell migration after wounding (Fig. 4C). The number of MβCD-treated cells migrating over the wounded edge was decreased by ∼35%. Exposure to MβCD did not affect cell viability (data not shown). These data indicate that caveolae predominantly localized with Cav1 regulate cell migration through Cav1-mediated Ca2+ influx after wounding.
Fig. 4.
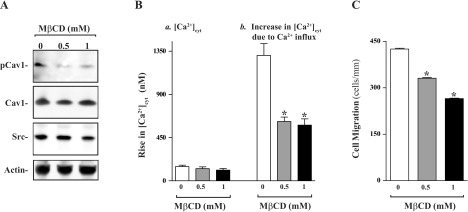
Effect of disruption of plasma membrane caveolae by methyl-β-cyclodextrin (MβCD) on levels of pCav1, Cav1, and Src proteins, Ca2+ influx, and cell migration in differentiated IEC-Cdx2L1 cells. A: levels of pCav1, total Cav1, and Src proteins in cells treated with MβCD for 2 h. B: summary of resting [Ca2+]cyt (left) and amplitude of CPA-induced Ca2+ influx (right) data. Values are means ± SE (n = 25). *P < 0.05 compared with cells incubated with vehicle alone (0 μM). C: summary of cell migration data 6 h after wounding. Values are means ± SE from 6 dishes. *P < 0.05 compared with cells incubated with vehicle alone (0 μM).
Src silencing reduces Cav1-Src complexes and prevents pCav1 subcellular redistribution after wounding.
Specific siRNA targeting Src mRNA (siSrc) was used to cleave rat Src mRNA. siSrc has been shown to have a unique combination of specificity, efficacy, and reduced toxicity (38, 52). Our initial study demonstrated that >95% of IEC-Cdx2L1 cells were positive when they were transfected with a fluorescent FITC-conjugated siSrc for 48 h (data not shown). As shown in Fig. 5A, transfection with siSrc for 48 h decreased Src levels by >80%. To determine the specificity of siSrc in this study, we reprobed the membrane with anti-Cav1 antibody and showed that levels of Cav1 protein were not affected when cells were transfected with siSrc. Src silencing by siSrc also decreased Cav1-Src complexes by ∼60% compared with control cells and cells transfected with C-siRNA (Fig. 5B). Furthermore, decreased Cav1-Src association by Src silencing also suppressed cell migration by ∼55% after wounding (Fig. 5C). Transfection with C-siRNA at the same concentrations showed no inhibitory effects on Src expression and cell migration. In addition, neither siSrc nor C-siRNA affected cell viability as measured by Trypan blue staining (data not shown).
Fig. 5.
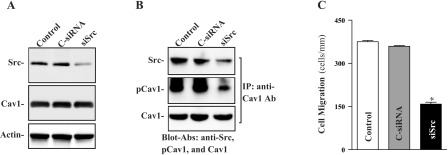
Effect of Src silencing by small interfering RNA (siRNA) targeting the Src mRNA coding region (siSrc) on levels of Src and Cav1, Cav1-Src interactions, and cell migration in differentiated IEC-Cdx2L1 cells. A: representative immunoblots of Src and Cav1. Cells were transfected with control siRNA (C-siRNA) or siSrc by Lipofectamine 2000, and whole cell lysates were harvested 48 h thereafter. Levels of Src and Cav1 proteins were analyzed by Western immunoblotting, and actin immunoblotting was performed as an internal control for equal loading. B: levels of Src, pCav1, and total Cav1 proteins in materials immunoprecipitated by the anti-Cav1 Ab from samples described in A. C: summary of cell migration data 6 h after wounding. Values are means ± SE from 6 dishes. *P < 0.05 compared with cells transfected with C-siRNA.
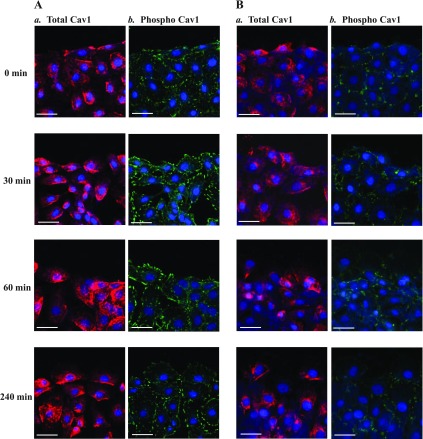
Results presented in Fig. 6 further show that Src silencing by siSrc prevented Cav1 subcellular redistribution after wounding in differentiated IEC-Cdx2L1 cells. In control cells, pCav1 was evenly distributed in the leading edge of migrating cells during restitution after wounding, although there were no significant changes in subcellular localization of total Cav1 (Fig. 6A, a and b). In an Src-silenced population of cells, however, increased pCav1 in the leading edge of migrating cells decreased dramatically (Fig. 6Bb). We examined changes in immunostaining of focal adhesion kinase under similar conditions and found no significant changes in the levels and subcellular distribution of focal adhesion kinase in migrating cells after Src silencing (data not shown). These findings suggest that increased Cav1-Src complexes are predominantly located in the leading edge of migrating cells after injury and that this specific subcellular distribution of Cavl is prevented by Src silencing.
Fig. 6.
Effect of Src silencing on subcellular distribution of total Cav1 and pCav1 (phospho Cav1) after wounding in differentiated IEC-Cdx2L1 cells. Cells were transfected with siSrc or C-siRNA for 48 h, and immunostaining was performed 0–240 min after wounding. A: representative images showing Cav1 and pCav1 localization at 0, 30, 60, and 240 min after wounding in cells transfected with C-siRNA. Scale bar, 100 μm; magnification ×400. Red, total Cav1; green, pCav1; blue, 4′,6-diamidino-2-phenylindole (nuclei). B: representative images showing Cav1 and pCav1 localization in Src-silenced cells.
DISCUSSION
Early epithelial restitution to reseal superficial wounds is crucial for the maintenance of gut mucosal integrity under physiological and pathological conditions, but the exact mechanism underlying this primary repair modality remains poorly understood. Cav1, a multifunctional scaffolding protein, is involved in many aspects of cellular functions, including cell motility (8, 9). Cav1 has been shown to associate with lipid raft domains in the PM through its multiple binding sites (29, 31). In the present study, we established the importance of Cav1 phosphorylation by Src kinase in the regulation of epithelial restitution after wounding, thereby advancing our understanding of the mechanism of early mucosal restitution. Our results further show that Src directly interacts with and causes Cav1 phosphorylation in IECs and that phosphorylated Cav1 increases cell migration by enhancing Ca2+ influx.
Results reported here clearly indicate that Src results in Cav1 phosphorylation in IECs. As shown in Fig. 1, Src and Cav1 were highly expressed in IECs, and they formed the Cav1-Src complex, whereas inhibition of Src kinase activity by PP2 reduced pCav1 levels and suppressed Ca2+ influx through SOC channels. Treatment with PP2 also repressed cell migration after wounding (Fig. 2). These results are consistent with our previous studies (36, 37, 41) and others (2, 14) showing that reduced levels of [Ca2+]cyt prevent stimulation of IEC migration during restitution. Several studies have revealed that Cav1 contributes to the assembly of SOC influx channels by regulating PM localization of TRPC1 channels (19, 47), and this process is regulated by its phosphorylation on Tyr14 (5, 21). Cav1 is predominantly localized within cholesterol-rich lipid rafts, termed caveolae microdomains, and mediates Ca2+ influx after store depletion (1, 29). Disruption of caveolar proteins by treatment with their chemical inhibitor or specific Cav1 gene silencing in vivo decreases Cav1-mediated Ca2+ influx (1), whereas restoration of wild-type Cav1 expression in Cav1−/− cells rescues SOCE, indicating its direct role in regulating Ca2+ influx. In addition, increased Cav1 in smooth muscle cells from patients with idiopathic pulmonary artery hypertension is associated with increased Ca2+ entry following Ca2+ store depletion (50).
Our results also indicate that Src associates with and phosphorylates Cav1 by interacting with the Src-interacting domain of Cav1, which is located at residues 82–101. Competitive inhibition of the binding affinity of the Src-interacting domain by CSD peptides inhibited Src-Cav1 association, decreased the resting levels of [Ca2+]cyt and CPA-induced Ca2+ influx, and repressed cell migration after wounding (Fig. 3). It has been shown that the Src-interacting domain of caveolin is localized at a cytosolic membrane-proximal region of Cav1 (21) and that the caveolin-mediated inhibition is related to tyrosine phosphorylation of caveolin (5). Sundivakkam et al. (47) reported that CSD also directly interacts with the TRPC1 channel and regulates TRPC1 channel-mediated Ca2+ entry, whereas specific deletion of CSD augments Ca2+ store release-induced Ca2+ influx. In addition, disruption of caveolar lipid rafts through cholesterol depletion by MβCD also decreased pCav1, reduced Ca2+ influx, and suppressed cell migration after wounding (Fig. 4). MβCD disassembles PM caveolae due to cholesterol depletion, thus reducing pCav1 and inhibiting the inositol 1,4,5-trisphosphate (IP3)-sensitive signaling pathway (1, 47). In this regard, IP3 has been shown to trigger the release of Ca2+ from intracellular Ca2+ stores through binding to IP3 receptors and TRPC3 channels (1, 17). Cav1 also augments Ca2+ entry through a process involving G protein-coupled receptors or by interaction with type 1 IP3 receptors or TRPC3 channels (1, 17). IECs do not express TRPC3 channels (data not shown), but they highly express TRPC1 channels, which function as SOC channels, mediating Ca2+ influx after store depletion (36).
Results reported here also show that Src-mediated Cav1 phosphorylation regulates pCav1 cellular localization in IECs after wounding. pCav1 was evenly distributed in the leading edge of migrating cells in the control group, but this specific subcellular redistribution of pCav1 after wounding was prevented by Src silencing. Consistent with our current observations, Beardsley et al. (3) reported that Cav1 knockdown in human primary endothelial cells inhibits cell polarization and impedes cell directional movement. Other studies conducted in mouse embryonic fibroblasts show that Src also regulates cell polarity by modulation of Rho GTPases such as Rac1 and Cdc42 (8). Together, these findings provide evidence indicating that Src-mediated Cav1 phosphorylation is critical for the activation of Cav1-mediated Ca2+ signaling and subsequent stimulation of IEC migration after wounding.
In summary, these results indicate that Src phosphorylates Cav1 and promotes intestinal epithelial restitution after wounding. Src directly interacts with Cav1 and forms the Cav1-Src complex, which is essential for activation of Cav1-mediated Ca2+ signaling. Inactivation of Src by its chemical inhibitor PP2 or blockade of the Src-interacting domain by CSD peptides decreases Cav1-mediated Ca2+ influx and represses epithelial restitution. Furthermore, Src silencing prevents pCav1 subcellular redistribution on the edge of migrating cells after wounding. These findings suggest that Src functions as an upstream regulator of Cav1 and plays an important role in control of Cav1-mediated Ca2+ signaling after wounding, thus contributing to the maintenance of intestinal epithelial integrity under biological conditions.
GRANTS
This work was supported by Merit Review Awards from the Department of Veterans Affairs (J. N. Rao, D. J. Turner, J. M. Donahue, and J.-Y. Wang) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-57819, DK-61972, and DK-68491 (J.-Y. Wang). J.-Y. Wang is a Senior Research Career Scientist, Medical Research Service, US Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.R. and R.Z. performed the experiments; N.R., R.Z., J.M.D., D.J.T., and J.N.R. analyzed the data; N.R., J.-Y.W., J.M.D., D.J.T., and J.N.R. interpreted the results of the experiments; J.-Y.W. and J.N.R. are responsible for conception and design of the research; J.-Y.W. edited and revised the manuscript; J.-Y.W. and J.N.R. approved the final version of the manuscript; J.N.R. prepared the figures; J.N.R. drafted the manuscript.
REFERENCES
- 1.Adebiyi A, Narayanan D, Jaggar JH. Caveolin-1 assembles type 1 inositol 1,4,5-trisphosphate receptors and canonical transient receptor potential 3 channels into a functional signaling complex in arterial smooth muscle cells. J Biol Chem 286: 4341–4348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agle KA, Vongsa RA, Dwinell MB. Calcium mobilization triggered by the chemokine CXCL12 regulates migration in wounded intestinal epithelial monolayers. J Biol Chem 285: 16066–16075, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beardsley A, Fang K, Mertz H, Castranova V, Friend S, Liu J. Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J Biol Chem 280: 3541–3547, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bootman MD, Lipp P, Berridge MJ. The organization and functions of local Ca2+ signals. J Cell Sci 114: 2213–2222, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14 recruitment of C-terminal Src kinase. J Biol Chem 277: 8771–8774, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Dignass AU, Tsunekawa S, Podolsky DK. Fibroblast growth factors modulate intestinal epithelial cell growth and migration. Gastroenterology 106: 1254–1262, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Glenney JR., Jr. Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem 264: 20163–20166, 1989 [PubMed] [Google Scholar]
- 8.Grande-García A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol 177: 683–694, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grande-Garcia A, del Pozo MA. Caveolin-1 in cell polarization and directional cell migration. Eur J Cell Biol 87: 641–647, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Guo X, Rao JN, Liu L, Rizvi M, Turner DJ, Wang JY. Polyamines regulate β-catenin tyrosine phosphorylation via Ca2+ during intestinal epithelial cell migration. Am J Physiol Cell Physiol 283: C722–C734, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Guo X, Rao JN, Liu L, Zuo TT, Turner DJ, Bass BL, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: novel function for polyamines. Am J Physiol Cell Physiol 285: C1174–C1187, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Hardin CD, Vallejo J. Dissecting the functions of protein-protein interactions: caveolin as a promiscuous partner. Focus on “Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells.” Am J Physiol Cell Physiol 296: C387–C389, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Harter JL. Critical values for Duncan's new multiple range test. Biometrics 16: 671–685, 1960 [Google Scholar]
- 14.Hines OJ, Ryder N, Chu J, McFadden D. Lysophosphatidic acid stimulates intestinal restitution via cytoskeletal activation and remodeling. J Surg Res 92: 23–28, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Isshiki M, Ando J, Korenaga R, Kogo H, Fujimoto T, Fujita T, Kamiya A. Endothelial Ca2+ waves preferentially originate at specific loci in caveolin-rich cell edges. Proc Natl Acad Sci USA 95: 5009–5014, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isshiki M, Ying YS, Fujita T, Anderson RG. A molecular sensor detects signal transduction from caveolae in living cells. J Biol Chem 277: 43389–43398, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Kamishima T, Burdyga T, Gallagher JA, Quayle JM. Caveolin-1 and caveolin-3 regulate Ca2+ homeostasis of single smooth muscle cells from rat cerebral resistance arteries. Am J Physiol Heart Circ Physiol 293: H204–H214, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA 92: 1381–1385, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol 70: 1174–1183, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol 14: 1750–1775, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. J Biol Chem 271: 29182–29190, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem 277: 41295–41298, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem 275: 11934–11942, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Loza-Coll MA, Perera S, Shi W, Filmus J. A transient increase in the activity of Src-family kinases induced by cell detachment delays anoikis of intestinal epithelial cells. Oncogene 24: 1727–1737, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Maniatis NA, Brovkovych V, Allen SE, John TA, Shajahan AN, Tiruppathi C, Vogel SM, Skidgel RA, Malik AB, Minshall RD. Novel mechanism of endothelial nitric oxide synthase activation mediated by caveolae internalization in endothelial cells. Circ Res 99: 870–877, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Mercier I, Jasmin JF, Pavlides S, Minetti C, Flomenberg N, Pestell RG, Frank PG, Sotgia F, Lisanti MP. Clinical and translational implications of the caveolin gene family: lessons from mouse models and human genetic disorders. Lab Invest 89: 614–623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution: characterization of cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest 89: 1501–1511, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1118–L11126, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Quaroni A, Wands J, Trelstat RL, Isselbacher KJ. Epithelial cell cultures from rat small intestine. J Cell Biol 80: 248–265, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quest AF, Lobos-González L, Nuñez S, Sanhueza C, Fernández JG, Aguirre A, Rodríguez D, Leyton L, Torres V. The caveolin-1 connection to cell death and survival. Curr Mol Med 13: 266–281, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Rao JN, Li J, Li L, Bass BL, Wang JY. Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin II. Am J Physiol Gastrointest Liver Physiol 277: G1149–G1158, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Rao JN, Li L, Golovina VA, Platoshyn O, Strauch ED, Yuan JX, Wang JY. Ca2+-RhoA signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol 280: C993–C1007, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Rao JN, Platoshyn O, Li L, Guo X, Golovina VA, Yuan JX, Wang JY. Activation of K+ channels and increased migration of differentiated intestinal epithelial cells after wounding. Am J Physiol Cell Physiol 282: C885–C898, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Rao JN, Guo X, Liu L, Zou T, Murthy KS, Yuan JX, Wang JY. Polyamines regulate Rho-kinase and myosin phosphorylation during intestinal epithelial restitution. Am J Physiol Cell Physiol 284: C848–C859, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Rao JN, Platoshyn O, Golovina VA, Liu L, Zou T, Marasa BS, Turner DJ, Yuan JX, Wang JY. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am J Physiol Gastrointest Liver Physiol 290: G782–G792, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Rao JN, Liu L, Zou T, Marasa BS, Boneva D, Wang SR, Malone DL, Turner DJ, Wang JY. Polyamines are required for phospholipase C-γ1 expression promoting intestinal epithelial cell restitution after wounding. Am J Physiol Gastrointest Liver Physiol 292: G335–G343, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Rao JN, Liu SV, Zou T, Liu L, Xiao L, Zhang X, Bellevance E, Yuan JX, Wang JY. Rac1 promotes intestinal epithelial restitution by increasing Ca2+ influx through interaction with phospholipase C-γ1 after wounding. Am J Physiol Cell Physiol 295: C1499–C1509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao JN, Rathor N, Zou T, Liu L, Xiao L, Yu TX, Cui YH, Wang JY. STIM1 translocation to the plasma membrane enhances intestinal epithelial restitution by inducing TRPC1-medidated Ca2+ signaling after wounding. Am J Physiol Cell Physiol 299: C579–C588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao JN, Wang JY. Regulation of gastrointestinal mucosal growth. In: Colliquium Series on Integrated Systems Physiology: From Molecule to Function, edited by Granger ND, Granger J. San Francisco, CA: Morgan and Claypool, 2011, p. 1–114 [Google Scholar]
- 41.Rao JN, Rathor N, Zhuang R, Zou T, Liu L, Xiao L, Turner DJ, Wang JY. Polyamines regulate intestinal epithelial restitution through TRPC1-mediated Ca2+ signaling by differentially modulating STIM1 and STIM2. Am J Physiol Cell Physiol 303: C308–C317, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutten MJ, Ito S. Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol Gastrointest Liver Physiol 244: G171–G182, 1983 [DOI] [PubMed] [Google Scholar]
- 43.Sathish V, Abcejo AJ, Thompson MA, Sieck GC, Prakash YS, Pabelick CM. Caveolin-1 regulation of store-operated Ca2+ influx in human airway smooth muscle. Eur Respir J 40: 470–478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silen W, Ito S. Mechanism for rapid-epithelialization of the gastric mucosal surface. Annu Rev Physiol 47: 217–229, 1985 [DOI] [PubMed] [Google Scholar]
- 45.Studier FW. Stable expression clones and auto-induction for protein production in E. coli. Methods Mol Biol 1091: 17–32, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 16: 619–625, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sundivakkam PC, Kwiatek AM, Sharma TT, Minshall RD, Malik AB, Tiruppathi C. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol 296: C403–C413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vielkind U, Swierenga SH. A simple fixation procedure for immunofluorescent detection of different cytoskeletal components within the same cell. Histochemistry 91: 81–88, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Wang JY, Johnson LR. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol Gastrointest Liver Physiol 259: G584–G592, 1990 [DOI] [PubMed] [Google Scholar]
- 50.Xia H, Khalil W, Kahm J, Jessurun J, Kleidon J, Henke CA. Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am J Pathol 176: 2626–2637, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao L, Rao JN, Zou T, Liu L, Cao S, Martindale JL, Su W, Chung HK, Gorospe M, Wang JY. miR-29b represses intestinal mucosal growth by inhibiting translation of cyclin-dependent kinase 2. Mol Biol Cell 24: 3038–3046, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhuang R, Rao JN, Zou T, Liu L, Xiao L, Cao S, Hansraj NZ, Gorospe M, Wang JY. miR-195 competes with HuR to modulate stim1 mRNA stability and regulate cell migration. Nucleic Acids Res 41: 7905–7919, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]