Abstract
Diabetes-induced cardiomyopathy is characterized by cardiac remodeling, fibrosis, and endothelial dysfunction, with no treatment options currently available. Hyperglycemic memory by endothelial cells may play the key role in microvascular complications in diabetes, providing a potential target for therapeutic approaches. This study tested the hypothesis that a proangiogenic environment can augment diabetes-induced deficiencies in endothelial cell angiogenic and biomechanical responses. Endothelial responses were quantified for two models of diabetic conditions: 1) an in vitro acute and chronic hyperglycemia where normal cardiac endothelial cells were exposed to high-glucose media, and 2) an in vivo chronic diabetes model where the cells were isolated from rats with type I streptozotocin-induced diabetes. Capillary morphogenesis, VEGF and nitric oxide expression, cell morphology, orientation, proliferation, and apoptosis were determined for cells cultured on Matrigel or proangiogenic nanofiber hydrogel. The effects of biomechanical stimulation were assessed following cell exposure to uniaxial strain. The results demonstrate that diabetes alters cardiac endothelium angiogenic response, with differential effects of acute and chronic exposure to high-glucose conditions, consistent with the concept that endothelial cells may have a long-term “hyperglycemic memory” of the physiological environment in the body. Furthermore, endothelial cell exposure to strain significantly diminishes their angiogenic potential following strain application. Both diabetes and strain-associated deficiencies can be augmented in the proangiogenic nanofiber microenvironment. These findings may contribute to the development of novel approaches to reverse hyperglycemic memory of endothelium and enhance vascularization of the diabetic heart, where improved angiogenic and biomechanical responses can be the key factor to successful therapy.
Keywords: diabetes, cardiomyopathy, endothelial cell, angiogenesis, nanofibers, biomechanical response
diabetes is a significant health concern in the United States, afflicting 25.8 million people in the US, which accounts for 8.3% of the population (2). Diabetes is a major predictor of heart failure and has become a major cause of disability and mortality in diabetic patients (17), with heart disease death rates about two to four times higher than patients without diabetes (2). Diabetic cardiomyopathy (DCM) is defined as myocardial dysfunction in the absence of coronary artery disease, hypertension, or significant valvular diseases (1). DCM is characterized by structural changes in the myocardium of diabetic patients, which eventually lead to left ventricular hypertrophy, diastolic and systolic dysfunction, or a combination of these conditions (25). These heterogeneous features of DCM are mainly due to cardiac cell apoptosis (15, 54) and the associated extensive myocardial fibrosis, microvascular pathology, and capillary rarefaction (48, 61). Diabetes-induced cardiac remodeling is associated with in vivo microvascular pathologies such as reduced expression of angiogenic growth factors and decreased endothelial cell infiltration and neovascularization (16, 47). In vitro cell responses such as cytokine expression, cell proliferation, and migration, as well as Ca2+ regulation are also altered under hyperglycemic conditions (8, 13, 16, 33, 53, 60, 65). However, there is a paucity of information regarding the differential effects of short-term hyperglycemic spikes vs. long-term exposure on vascular endothelial cells (14), and more studies are needed regarding the comprehensive effect of diabetic conditions on the angiogenic responses of cardiac endothelial cells at molecular and cellular levels.
It has been recently suggested that oxidative stress caused by mitochondrial superoxide overproduction in endothelial cells is the major mechanism in the development of vascular complications in diabetes (reviewed in Ref. 20). This stress results in sustained activation of antiangiogenic, proinflammatory pathways even after glycemia is normalized, a concept called “hyperglycemic memory” (20), with the molecular mechanisms for this effect still being elucidated (46). The important implication of these findings is that control of glycemia in diabetes may not prevent subsequent development of complications and there is an urgent need for novel therapies that can reverse hyperglycemic memory of cardiac endothelium.
Diabetes can also affect cell biomechanical responses, which may have important implications for cardiac function. The biomechanical environment influences endothelial cell function and regulates key functions of blood vessels including vascular permeability and dilation (18, 24, 44). Mechanical forces are considered to be the triggers that induce a growth response in the overloaded myocardium (22, 50). Endothelial cells convert mechanical signals into intracellular signals that affect gene expression and cellular function including cell proliferation, apoptosis, permeability, and alignment (9). In vitro studies have shown that cyclic strain influences the angiogenic responses of endothelial cells, including the effects on vascular network formation, cell apoptosis, migration and proliferation, and various growth factors, enzymes, genes, and proteins related to angiogenesis (19, 21, 28, 36, 38, 57, 58, 63, 64). In addition, the angiogenic responses of cells subjected to strain depend on the substrate used to culture the cells (19, 38, 57, 58, 64). A summary of the results from these studies is organized by substrate type in Table 1 and includes the cell types, strain parameters, and angiogenic responses. Several other studies investigated the simultaneous effects of mechanical stimulation and high-glucose conditions. It was shown that the glucose- and mechanical force-induced increase in extracellular matrix deposition by mesangial cells is mediated by TGF-β receptors (49). Shear-stress-induced actin alignment in aortic endothelial cells in normoglycemic conditions was abolished in high-glucose media (30). Similarly, mechanical stress potentiated glucose uptake in podocytes and this effect was enhanced by high ambient glucose (34). While these studies provide information regarding the effect of mechanical strain on different cell types, the angiogenic responses of cardiac endothelial cells under diabetic conditions in conjunction with a mechanically strained microenvironment have yet to be elucidated.
Table 1.
Effects of strain and substrate on endothelial cell responses
| Substrate/Cell Type | Strain magnitude, % | Strain Frequency, Hz | Strain Duration | Response | References |
|---|---|---|---|---|---|
| Matrigel | |||||
| 2D | |||||
| HMEC-1 | 2.5–20 | 1 | 24 h | ↓Network length; ↑ VEGF | Wilson et al. (58) |
| Collagen | |||||
| 2D | |||||
| HUVEC | 2–16 | 1 | 1–24 h | ↓Nox 4, ROS; nitric oxide release | Goettsch et al. (21) |
| BAEC | 5 | 1 | 24 h | ↑Network formation | Von Offenberg Sweeney et al. (57) |
| RCMEC | 10 | 0–0.5 | 1–24 h | ↑VEGF-R1, VEGF-R2 | Zheng et al. (64) |
| BAEC | 6–20 | 1 | 24 h | Strain-dependent inhibition of apoptosis | Liu et al. (36) |
| 3D | |||||
| RCMEC | 10 | 0, 1 | 18 h | ↑Network length, number of network tubes | Zheng et al. (64) |
| Gelatin | |||||
| 2D | |||||
| HUVEC, HAEC | 0–20 | 0.5–1 | 0–48 h | ↑Ang-2, VEGF, C-Myc, HSP60, proliferation; alignment perpendicular to strain direction | Korff et al. (32a) Hurley et al. (27), Matsumoto et al. (38) |
| Fibrin | |||||
| 3D | |||||
| BAEC | 10 | 1 | 7 days | ↓Network length, number, and branching; ↑network tubule diameter | Gassman et al. (19) |
| HUVEC | 7 | 1 | 2 days | ↑Network formation, directional migration; migration perpendicular to strain direction | Yung et al. (62) |
| HUVEC | 8 | 0.5 | 5 days | ↓Network branching; networks aligned perpendicular to strain direction | Matsumoto et al. (38) |
| Fibronectin/pronectin | |||||
| 2D | |||||
| BAEC, HUVEC | 5–7 | 1 | 24, 48 h | ↑Occludin, ZO-1 protein, MMP-2, pro-MMP-2, and directional migration | Collins et al. (11a), von Offenberg Sweeney et al. (56), Yung et al. (62) |
| Silicone elastomers | |||||
| 2D | |||||
| HUVEC | 7–10 | 1 | 3 h, 5 days | ↑Ang-2, PDGF-ββ, Tie-2, ICAM-1; ↓VCAM-1; cell elongation and alignment oblique to strain direction | Yung et al. (62), Barron et al. (5a) |
| RCMEC | 10 | 0, 0.5 | 1–24 h | ↑Cell proliferation, migration | Zheng et al. (64) |
HMEC-1, human microvascular endothelial cell-1; HUVEC, human umbilical vein endothelial cell; BAEC, bovine aortic endothelial cell; HAEC, human aortic endothelial cell; RCMEC, rat coronary microvascular endothelial cells; ROS, reacitive oxygen species; HSP60, heat shock protein 60; MMP, matrix metalloproteinase; PDGF-ββ, platelet-dervied growth factor-ββ.
Despite significant research efforts directed towards therapies for cardiovascular complications, there is still a lack of successful treatment options that focus on endothelial deficiencies and insufficient neovascularization in the diabetic environment. A novel tissue engineering approach, which includes the use of a nanoscaffold composed of self-assembling peptide RAD16-II nanofibers, has been shown to promote angiogenesis in nondiabetic conditions (12, 26, 41) and in a mouse model of diabetic wound healing in vivo (5). Studies by our group and others have demonstrated that these nanofibers support cellular function in multiple cardiac cell types including cardiomyocytes, endothelial cells, and fibroblasts (26, 42) and enhance angiogenic responses through engagement of specific cell surface integrins, namely αvβ3 (10, 52).
The goal of this study was, therefore, to determine the effect of diabetes on endothelial angiogenic and biomechanical responses and the role of the extracellular microenvironment in mediating these effects. The hypothesis is that a proangiogenic nanofiber microenvironment can augment diabetes-induced deficiencies in angiogenic potential and response to mechanical strain by cardiac endothelial cells. Endothelial responses were quantified for two models of diabetic conditions: 1) an in vitro acute and chronic hyperglycemic model where normal [wild-type (WT)] cardiac endothelial cells were cultured in high-glucose conditions, and 2) an in vivo chronic diabetes model where the cells were harvested from the hearts of rats with type I [streptozotocin (STZ)-induced] diabetes. Capillary morphogenesis, VEGF expression, cell morphology, orientation, proliferation, apoptosis, and nitric oxide (NO) expression were determined for cells cultured on Matrigel or proangiogenic nanofiber hydrogel. The effects of biomechanical stimulation were assessed following cell exposure to uniaxial strain (Flexcell System).
MATERIALS AND METHODS
STZ type I diabetes rat model.
All animal procedures were performed using protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee. Type I diabetes was induced in 8-wk-old female Sprague-Dawley rats (SAS SD Strain 400; Charles River, Wilmington, MA) using a single intraperitoneal injection of STZ (70 mg/kg; Sigma-Aldrich, St. Louis, MO). The STZ rat model was chosen because it closely mimics the time-dependent disease progression of diabetic cardiomyopathy (7, 40, 61). Immediate onset of diabetes was confirmed with serum glucose levels >300 mg/dl.
Assessment of cardiac function.
Echocardiography and electrocardiography (ECG) were performed on diabetic and WT animals to assess cardiac function and electric activity of the heart, respectively. Rats were anesthetized by the combination of ketamine and xylazine injection, with normothermia being maintained by heating pad. Echocardiographies were performed using iE33 Ultrasound System (Phillips) at 0, 35, and 60 days post-STZ injection. A 15-MHz pediatric probe (optimized and dedicated to rodent studies) placed in the parasternal, short axis orientation recorded left ventricle systolic and diastolic internal dimensions. Three loops of M-mode transthoracic echocardiography data were captured for each animal, and data were averaged from at least five-beat cycles/loop. Parameters were determined using the American Society for Echocardiography leading-edge technique in blinded fashion. These parameters allowed the determination of left ventricular fractional shortening (FS) by the equation: FS = [(LVIDd − LVIDs)/LVIDd ] × 100%; where LVID refers to the LV internal dimension at diastole (d) and systole (s). Transmitral flow waveforms were recorded using continuous wave Doppler oriented in the parasternal, apical view. The following measurements were performed: mitral peak flow velocity of the early filling wave (E), peak flow velocity of atrial filling wave (A), and the E/A-wave ratio. All transmitral peak flow measurements represent the mean of at least three consecutive cardiac cycles. Heart rate and electrical activity were measured by placing ECG leads on both forepaws and on the tail to monitor heart rate.
Cardiac endothelial cell isolation.
Diabetic and WT animals were killed 8 wk after STZ injection. The hearts were perfused with PBS and then harvested and stored in cold PBS. For endothelial cell isolation, the aortic arch was removed from the heart to avoid smooth muscle cell contamination. The myocardium tissue was finely chopped and digested using trypsin (1 mg/ml; Sigma-Aldrich) and collagenase I (172 U/ml; Worthington Biochemical, Lakewood, NJ) (45). Cell-containing supernatant was separated from remaining tissue using a 70-μm disposable cell strainer (Becton Dickinson Labware, Bedford, MA) and centrifuged at 800 rpm for 5 min at 4°C. The cell pellet was resuspended and added to 1% gelatin-coated cell culture dishes and incubated overnight in Medium 199 (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA), 1% antibiotic-Antimycotic (Atlanta Biologicals, Lawrenceville, GA), 10 μg/ml heparin (Sigma-Aldrich), and 0.2 ng/ml endothelial cell growth supplement (Sigma-Aldrich). Cardiac endothelial cells were isolated from cardiac cell mixture using sequential sorting with PECAM-1- and ICAM-2-conjugated magnetic beads (Invitrogen) (35). Endothelial phenotype was confirmed using lectin and von Willebrand Factor staining (Sigma-Aldrich), with >95% cells positively stained. Cell cultures were maintained at 37°C in >95% humidified air containing 5% CO2. Cells from passage 4–13 were used in all experiments.
Experimental design.
At the beginning of experiments, the following four experimental groups were created. The first group consisted of WT cardiac endothelial cells cultured in standard low-glucose media (WT; cell culture media supplemented with 5.5 mM d-glucose; Sigma-Aldrich). The other three groups represented diabetic conditions: the WT endothelial cells cultured in high-glucose media [for 24 h (HG) or 17 days (HG chronic), with cell culture media supplemented with 22.2 mM d-glucose], and endothelial cells were harvested from diabetic animals and cultured in standard low-glucose media (db; cell culture media supplemented with 5.5 mM d-glucose). An additional group of WT cells was cultured in osmotic control media (l-control; cell culture media supplemented with 5.5 mM d-glucose and 15 mM l-glucose; Sigma-Aldrich).The d- and l- glucose are stereoisomers of glucose, with only the d-glucose isomer occurring naturally, and the l-glucose isomer commonly used as a control group.
Application of cyclic strain.
Cardiac endothelial cells were trypsinized and seeded onto six-well collagen I-precoated UniFlex Culture plates (Flexcell International, Hillsborough, NC) at a seeding density of 200,000 cells/well in the appropriate experimental group culture media. For static controls, cells were cultured in sixwell tissue culture plates also coated with collagen I (PureCol; Advanced BioMatrix, San Diego, CA). Collagen I was used as a culture substrate because it constitutes 80% of the total collagen in the heart (37). All culture plates were incubated overnight before stimulation to allow for cell adhesion. UniFlex Culture Plates were then loaded onto the Flexcell FX-5000 Tension System outfitted with ArcTangleLoading Stations (24 mm; Flexcell International) for application of uniaxial strain and housed at 37°C in >95% humidified air containing 5% CO2. Strain parameters were set to 5% elongation at 1 Hz for 24 h. These strain parameters were selected based on physiological in vivo cardiac parameters (23) and matched those used in the previous studies (3, 39, 51). Static controls were maintained in the same incubator for the duration of the experiments.
Endothelial cell morphology and orientation.
After 24 h, the cultured media from the strained and static cardiac endothelial cell samples were collected and stored at −20°C. The cell samples were fixed with 2% paraformaldehyde and stained with phalloidin-tetramethylrhodamine-B isothiocyanate (phalloidin-TRITC; Sigma-Aldrich) to assess actin reorganization, followed by DAPI nuclear staining (Invitrogen, Carlsbad, CA). Images were obtained (n = 5 per sample) using an inverted fluorescent microscope equipped with appropriate filter cubes and CCD camera (Olympus IX81; Olympus America, Center Valley, PA).
Capillary morphogenesis.
Immediately following exposure to strain, cardiac endothelial cells (WT, HG, and db) were trypsinized, labeled with CellTracker Dye (Invitrogen, Carlsbad, CA) or with phalloidin-TRITC (Sigma Aldrich), and seeded on Matrigel or RAD16-II peptide nanofiber hydrogel (10 mg/ml) in cell culture plate inserts (13-mm diameter, 0.4-μm pore size; Millipore, Billerica, MA) at a seeding density of 30,000–50,000 cells/insert as described previously (41). Samples were cultured for 24 h to allow spontaneous formation of capillary-like structures. After fixation with 2% paraformaldehyde, images were taken (n = 5 per sample) using an inverted fluorescent microscope (Olympus IX81; Olympus America, Center Valley, PA). Correlation analysis with MATLAB custom-written code (The MathWorks, Natick, MA) was used to characterize capillary-like endothelial network size as described previously (26, 41).
VEGF expression by cardiac endothelial cells.
VEGF protein expression by cardiac endothelial cells was quantified for media samples collected from different experiments using VEGF ELISA kit per manufacturer's protocol (R&D Systems, Minneapolis, MN). Additional controls of the cell culture medium alone (containing 10% serum) were included to confirm that growth factors in the serum would not affect protein expression and detection, with no differences observed between medium samples and the 0 pg/ml standard.
Cardiac endothelial cell proliferation and apoptosis.
After application of strain for 24 h, cells from both strained and static controls were trypsinized and seeded on either nanofibers or gelatin-coated 24-well tissue culture plates at a seeding density of 20,000 cells/well (n = 6 per experimental group). Cells were fixed with 2% paraformaldehyde and immunostained with Ki67 and active caspase-3 to assess cell proliferation and apoptosis, respectively; the total number of cells in each sample was determined by DAPI staining (Invitrogen). The average absolute number and percentages (number of positive cells to the total number of cells) of proliferating or apoptotic cells per well were quantified in three nonoverlapping, randomly selected fields. For analyses of cell apoptosis following chronic cell exposure to high-glucose (d- or l-glucose) conditions, caspase-Glo 3/7 activity assay (Promega) was used.
NO experiments.
To elucidate the role of NO in cell responses to diabetic environment and mechanical strain, the NO concentration in the cell culture media was measured using Abcam's nitric oxide assay kit (Colorimetric). To examine the role of endothelial nitric oxide synthase (eNOS) signaling, cells were treated with 200 μM Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME; eNOS inhibitor; Abcam), and capillary morphogenesis was quantified. The inhibitor was added to the culture supernatant and preincubated for 1 h to equilibrate respective target blocking. All analyses were done in duplicates/triplicates.
Statistical analyses.
Single factor ANOVA was used to compare ventricle dimensions, FS, EF, and transmitral flow parameters of diabetic rats with WT controls. Each in vitro experiment was performed in triplicate and repeated at least three times (N = 3, minimum n = 9). ANOVA and post hoc tests with Bonferroni corrections were used to determine the effects of the microenvironment (Matrigel or nanofiber), mechanical strain (static or cyclic strain), and diabetic condition (WT, HG, HG chronic, or db) on angiogenic responses (capillary morphogenesis, proliferation, apoptosis, and VEGF expression). All tests were run at a significance level of α = 0.05. All results are reported as average ± SD.
RESULTS
Effect of diabetes on cardiac function.
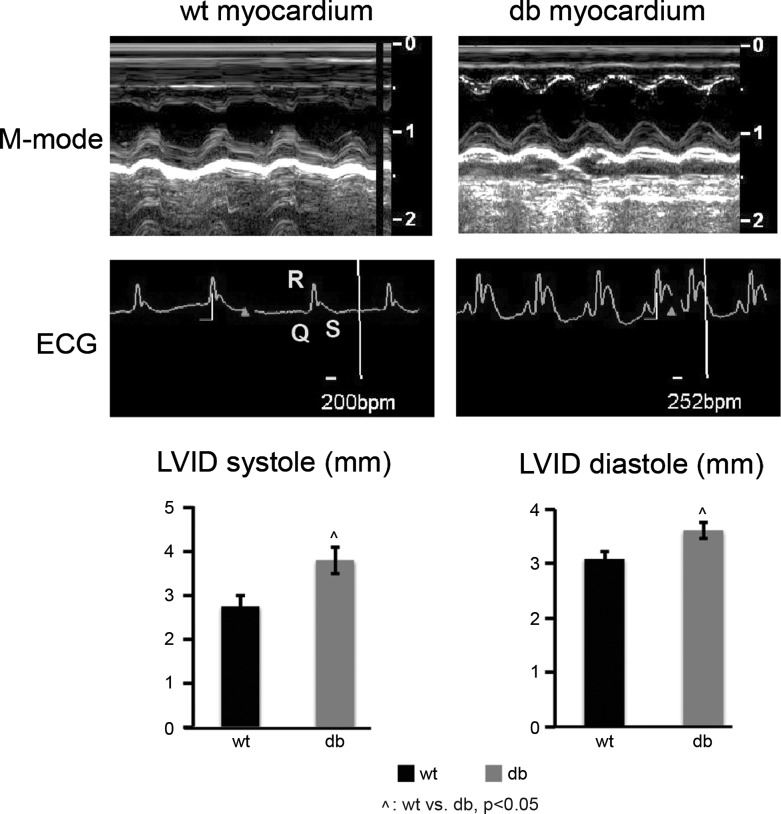
To examine ventricular hypertrophy, echocardiography was performed on diabetic as well as WT control animals. Cardiac performance parameters derived from M-mode images (Fig. 1) clearly indicate that in the diabetic group both septal and posterior wall motion are altered, which results in increased LVID at systole and diastole after 60 days of diabetes onset (P < 0.05). Analyses of ECGs show that diabetes results in a significantly increased heart rate (db: 252 vs. WT: 200 beats/min) and a significantly wider QRS complex than the WT group by day 60 of diabetes onset (P < 0.05). These results demonstrate the development of moderate left ventricular dilation and impaired ventricle contractile function in diabetic rats.
Fig. 1.
Two-dimensional echocardiography and ECG analysis. Impaired contractility and left ventricular dilation are observed in the diabetic hearts. Top: M-mode echocardiography conducted at day 60 post-streptozotocin (post-STZ) injection. Left ventricular dilation is detected with significant increases in left ventricular inner dimensions at diastole (LVIDd) and systole (LVIDs) at day 60 post-STZ. No significant age-related effects on ventricle dimensions are observed in wild-type group. ECGs shows faster heart rate and wider QRS complex in diabetic (db) rats compared with wild type (WT; n = 3 per group; ^P < 0.05, WT vs. db).
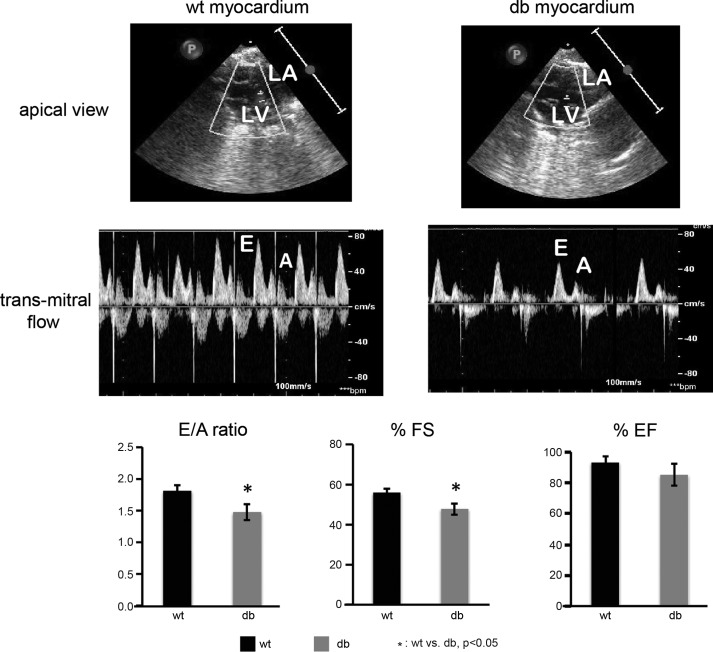
Transmitral blood flow was measured to determine E/A ratio (E: early ventricular filling; A: atrial ventricular filling), which indicates abnormalities in cardiac diastolic function. Reduction in E/A ratio suggests pseudonormal filling dynamics of the left ventricle in the diabetic hearts and the development of moderate diastolic dysfunction (Fig. 2). Reduction in FS indicates mild systolic dysfunction by day 60 of diabetes onset. The pattern of moderate diastolic dysfunction preceding mild systolic dysfunction is similar to the pattern observed in diabetic patients.
Fig. 2.
Transmitral flow was assessed during passive ventricular filling as a measure of diastolic function at day 60 post-STZ. The early ventricular filling (E) and transmitral filling (A) are severely impaired in the diabetic group, resulting in a significantly reduced E/A ratio, which indicates pseudonormal diastolic dysfunction in the diabetic heart. Fractional shortening is significantly decreased in diabetic animals at 60 days following STZ injection compared with controls, indicative of mild systolic dysfunction. There is a trend (not significant) of a reduced ejection fraction of the diabetic heart. No significant age-related or heart rate effects are observed in E/A, %fractional shortening (%FS), and %ejection fraction (%EF) parameters in control group. LA, left atrium; LV, left ventricle (n = 3 per group; *P < 0.05, WT vs. db).
Consistent with the results of altered E/A ratio and hypertrophy in diabetic hearts (Figs. 1 and 2), there is evidence of a reduced ejection fraction (%EF) in the diabetic heart, although this trend is not statistically significant.
Effect of diabetes on endothelial angiogenic and biomechanical responses.
Immediately following 24-h exposure to strain, wells from both static and strained groups were stained with phalloidin to visualize cytoskeletal organization within endothelial cells (Fig. 3). No qualitative differences were apparent in the cytoskeletal organization and cell morphology between WT and db cells in both static and strained groups. Interestingly, in both acute and chronic HG groups, the morphology was significantly compromised, with decreased cell spreading evident in both static condition (Fig. 3, top) and after strain application (Fig. 3, bottom). In the chronic HG group, there were significant problems with cell detachment and survival following trypsinization and subsequent seeding for the strain application.
Fig. 3.
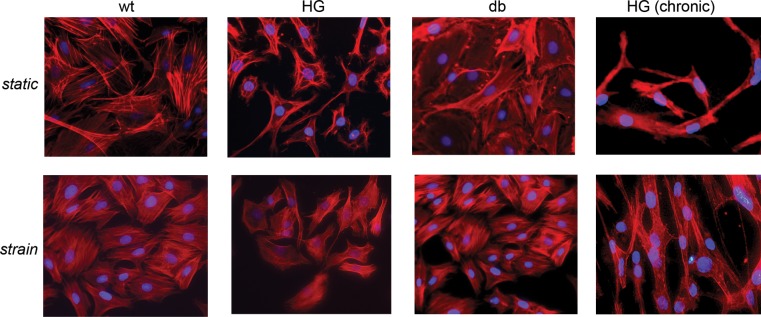
Phalloidin staining of static (top) and strained (bottom) samples at acute [high glucose (HG), 24-h culture] and chronic [HG (chronic), 17-day culture] stages. No differences are apparent in cell morphology and cytoskeletal organization between WT and db groups in either static conditions or strain conditions. However, WT cells exposed to high-glucose medium (HG and HG chronic) show altered cell morphology (2nd and 4th columns).
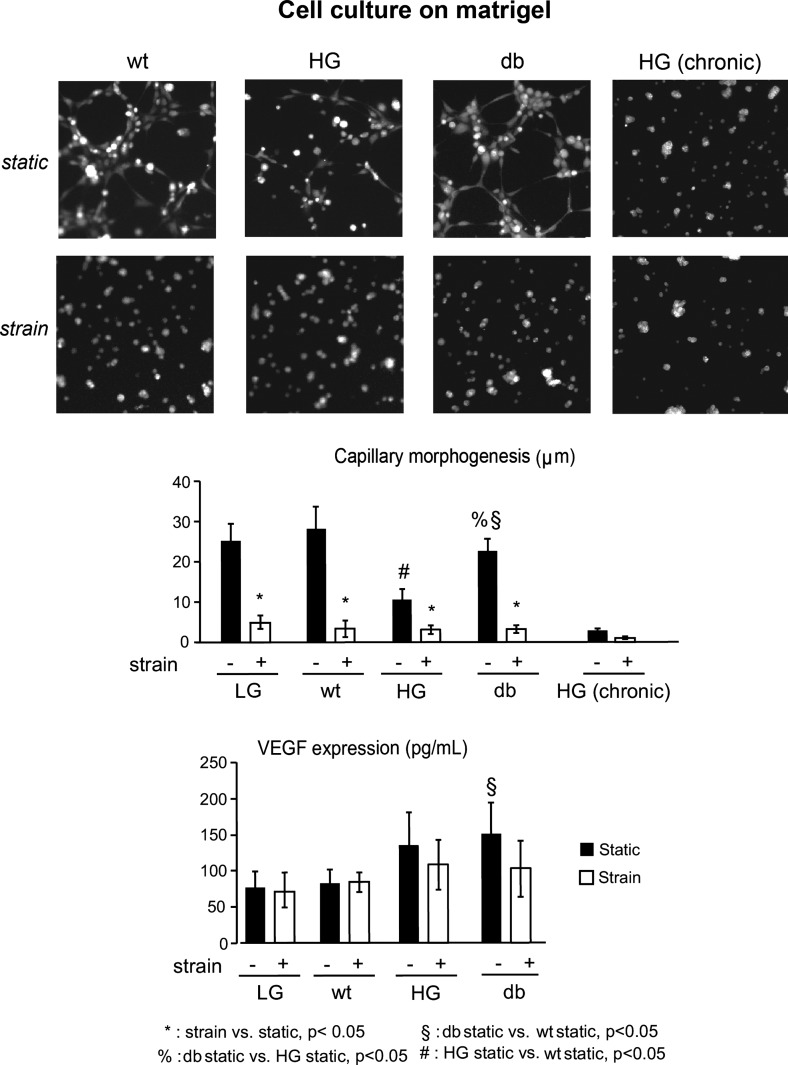
The effect of diabetes on capillary morphogenesis was determined using Matrigel assay, and the quantitative analysis of the characteristic network sizes was performed as described previously (10, 41). Under static conditions, capillary morphogenesis was significantly reduced in both db and acute HG groups, with a larger decrease in HG group compared with the db group (Fig. 4, top; P < 0.05). In chronic HG group, capillary morphogenesis was completely abolished (Fig. 4). Interestingly, cell exposure to cyclic strain before seeding on the Matrigel resulted in complete absence of capillary morphogenesis in all experimental groups (wt, acute and chronic HG, and db; Fig. 4, middle, P < 0.05).
Fig. 4.
Effect of strain on angiogenic response of WT and diabetic cardiac endothelial cells. Quantitative analysis shows significant reduction in capillary morphogenesis in db and HG group compared with WT controls under static conditions. Capillary morphogenesis was also significantly reduced in samples with chronic (17 day) HG exposure. Mechanical strain completely abolishes capillary morphogenesis in all experimental groups. VEGF expression by db cells is significantly higher compared with WT cells under static conditions. This difference, however, is not observed after cells are exposed to strain, with the VEGF protein levels for all groups similar to that of static WT controls.
Vascular endothelial growth factor (VEGF) protein expression in the sample media was measured using ELISA (Fig. 4). Under static conditions, cells in the diabetic groups tend to have an increased VEGF expression (the trend is significant for the db group only). This difference, however, is not observed after cells are exposed to strain, with the VEGF protein levels for all groups similar to that of static WT controls.
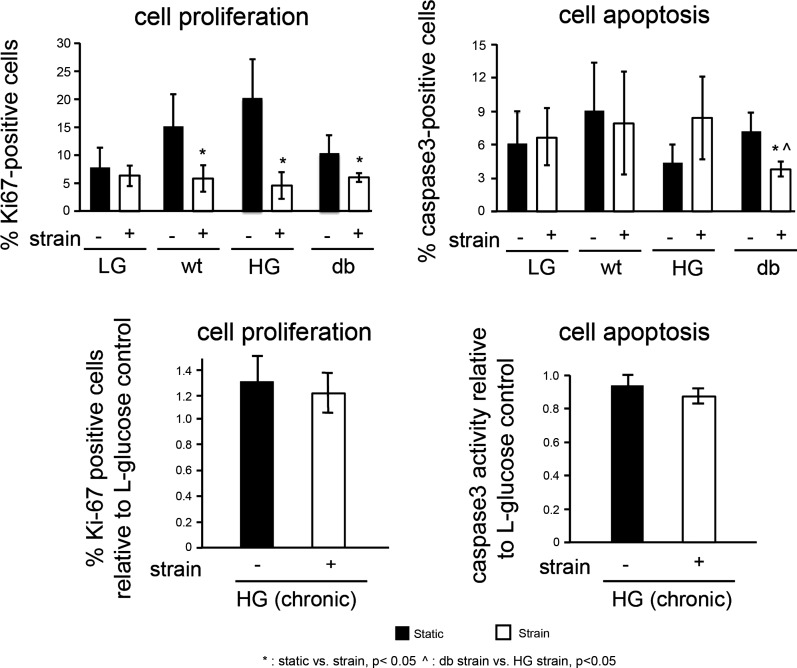
Ki67 staining was performed to examine endothelial proliferation in samples from static and strained cultures. Under static conditions, there was no significant difference in percentage of proliferating cells among WT, acute HG, and db groups. However, under strained conditions, the percentage of proliferating cells was significantly reduced compared with static conditions in all experimental groups (P < 0.05; Fig. 5, top). Interestingly, there was a differential response of acute HG and db groups following cell exposure to strain, with proliferation in the HG group being more sensitive to strain than in the db group. Similar trends were observed following chronic cell exposure to HG conditions (Fig. 5, bottom); however, these trends were not significant.
Fig. 5.
Effect of strain on cell proliferation and apoptosis in normal and diabetic cardiac endothelial cells. Ki67 and caspase-3 staining is performed on static controls and cells subjected to 24 h of strain to determine the percentage of proliferating and apoptotic cells. In WT, HG and db experimental groups, cell proliferation was significantly higher in static samples than it was in strained samples (top left). The percentage of apoptotic cells in db group was significantly less under strained conditions compared with static controls. Chronic exposure (17 day) to high-glucose (HG) did not effect endothelial proliferation or apoptosis under either static or strain conditions (bottom).
Endothelial cell apoptosis was measured in static and strained cell samples under normal and diabetic conditions by staining for the cell apoptosis marker active caspase-3 (WT, acute HG, and db groups) or measuring cell caspase-3 activity (Promega; chronic HG group). Under static conditions (Fig. 5, bottom), no difference in cell apoptosis was observed among WT, HG, and db groups. Similar to the findings for capillary morphogenesis and proliferation, there was a difference between the HG and db group responses to strain. While cell apoptosis in the acute HG group remained unchanged, the percentage of apoptotic cells was significantly reduced in db groups (Fig. 5; P < 0.05), with a similar trend in chronic HG group. This may indicate that strain provides a protective effect against apoptosis in cells with chronic exposure to diabetic hyperglycemia.
Overall, these results suggest that under the experimental conditions of this study, the general effect of strain on cardiac endothelial cells tends to be a reduction in cell angiogenic response that is present in both WT and diabetic groups.
Nanofiber microenvironment restores diabetes-induced endothelial deficiencies and biomechanical responses of cardiac endothelial cells.
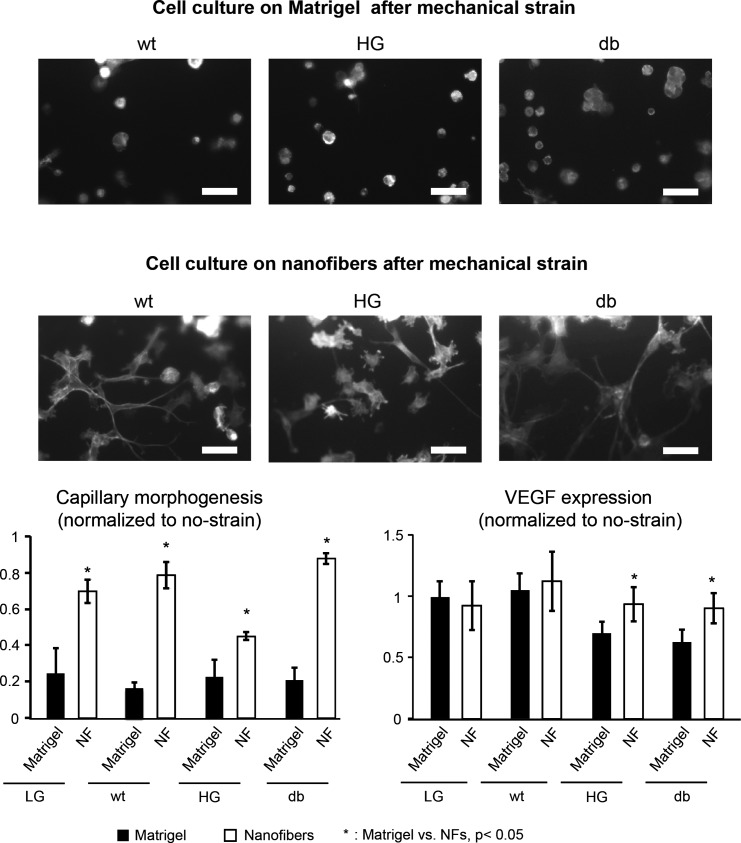
To examine the effect of the substrate microenvironment on cytoskeletal reorganization of cells after the application of cyclic strain, endothelial cells from all experimental groups were trypsinized and surface seeded on nanofibers or on Matrigel (control). Interestingly, nanofibers are able to restore the cytoskeletal organization that is impaired by cell exposure to strain (Fig. 6), resulting in enhanced cell spreading and improved cell morphology in WT, acute HG, and db groups. In WT and db groups, a complete restoration of the capillary morphogenesis to the static levels by the nanofibers is observed, while in the acute HG group, it is restored to ∼50% of the control levels. In contrast, impaired capillary morphogenesis in the chronic HG group cannot be restored by the nanofibers (Fig. 6). VEGF expression by HG and db cells cultured on nanofibers is significantly greater than the cells cultured on Matrigel controls (Fig. 6, bottom right; P < 0.05), while no significant effect of the substrate on the VEGF expression is observed in WT cells.
Fig. 6.
Effect of nanofibers (NFs) on cardiac endothelial cell cytoskeletal organization, capillary morphogenesis and VEGF expression following exposure to mechanical strain. On Matrigel surface, the cytoskeletal organization by endothelial cells in all experimental groups was completely impaired after strain and the cells remained in clusters with no cell spreading (top). On the other hand, NFs enhanced cytoskeletal organization with significant improvement in cell spreading and morphology (scale bar = 10 μm). In addition, the NFs restored capillary morphogenesis in all experimental groups (bottom left). VEGF expression levels by the cells cultured on NFs were significantly higher in HG and db groups (P < 0.05), while no significant differences were observed in WT group (bottom right).
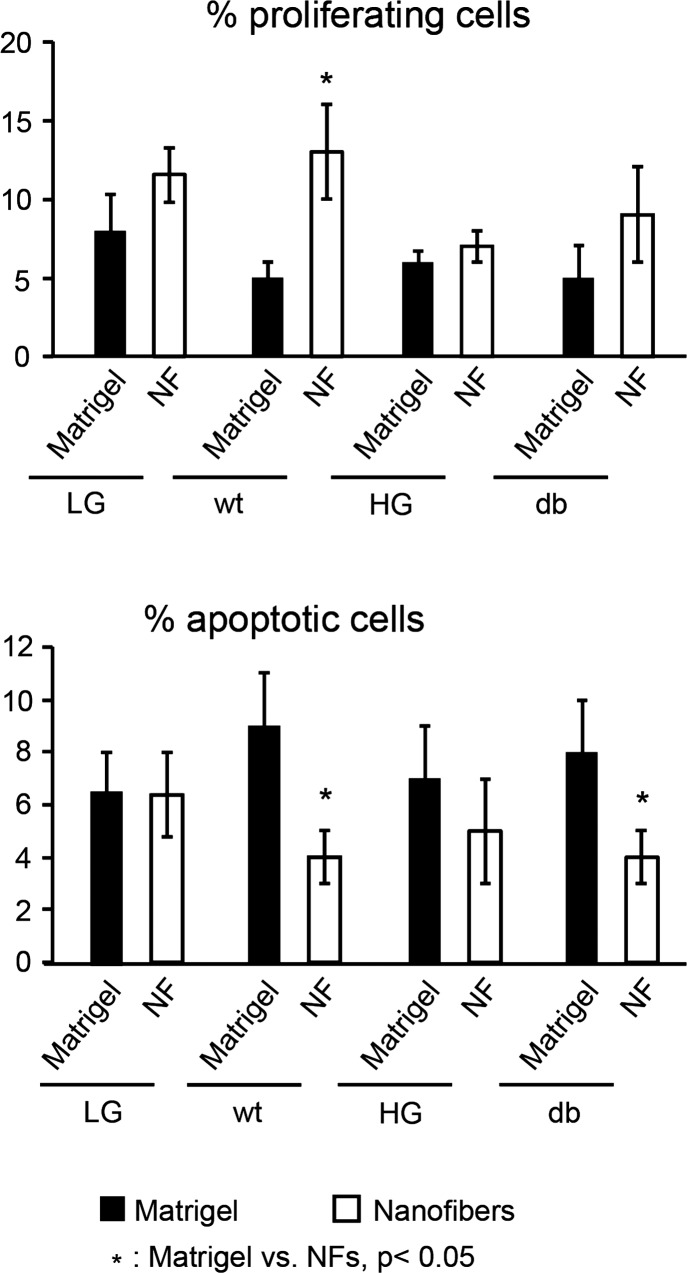
When cells are cultured on the nanofibers after cyclic strain application, the percentage of proliferating cells is significantly increased in the WT group compared with Matrigel controls (Fig. 7, top; P < 0.05). No significant differences in proliferation are observed between nanofibers and Matrigel controls in HG and db groups. Caspase-3 staining showed that nanofibers significantly decreased cell apoptosis in all experimental groups (wt, HG and db) compared with Matrigel controls (Fig. 7, bottom; P < 0.05).
Fig. 7.
Effect of NFs on cell proliferation and apoptosis. Cell proliferation and apoptosis are measured using Ki-67 and caspase-3 staining. Cell proliferation is significantly increased in WT cells seeded on NF compared with Matrigel after strain application (P < 0.05). No differences are observed in HG and db groups (top). The percentages of apoptotic cells are significantly reduced in WT and db cells when cultured on NFs compared with Matrigel controls (bottom).
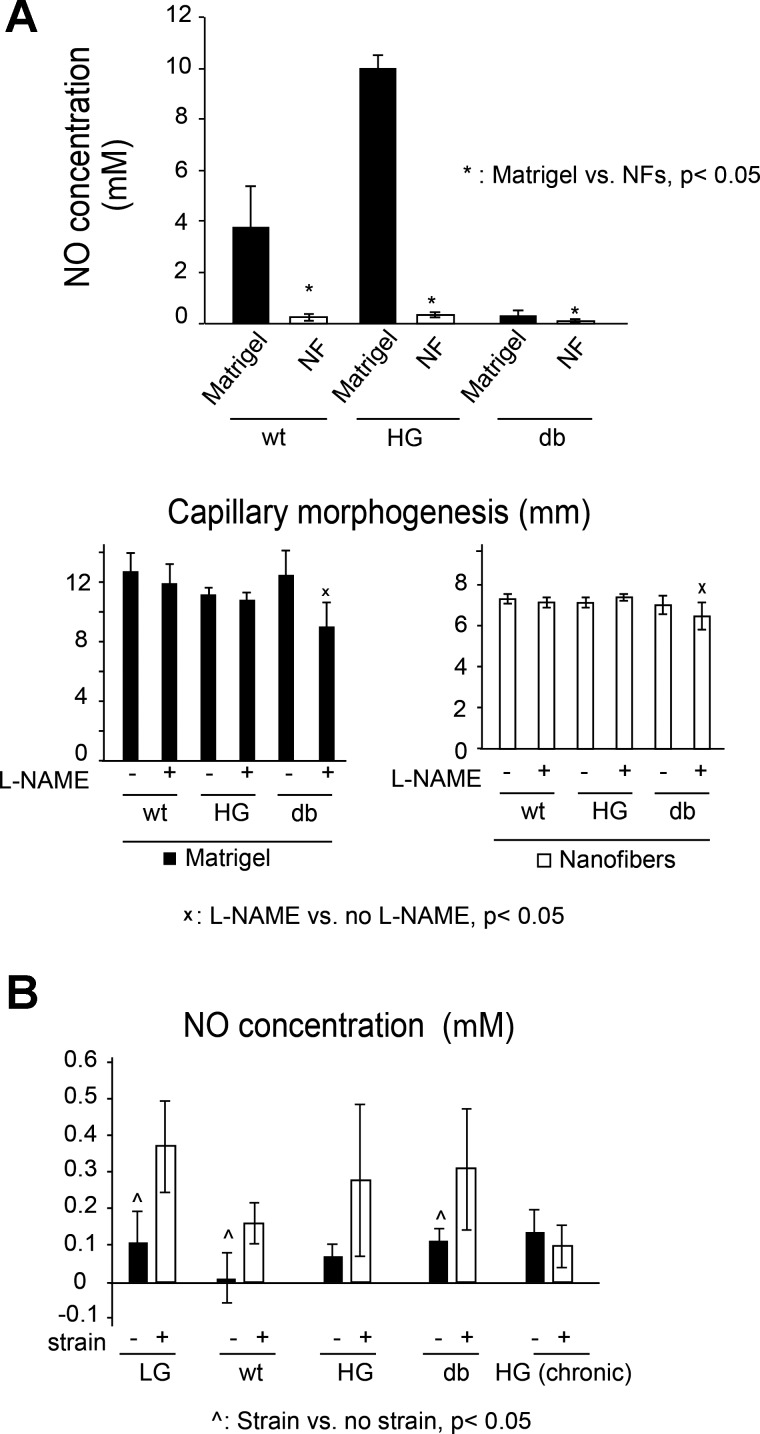
To investigate if the nitric oxide-reactive oxygen species signaling may play a role in the observed endothelial responses to strain and diabetic microenvironment, experiments were performed to 1) quantify the effects of the diabetic conditions, substrate and strain on the levels of NO production by endothelial cells, and 2) determine the effect of eNOS inhibition by l-NAME on capillary morphogenesis. Our results demonstrate (Fig. 8A) that Matrigel stimulates significantly higher levels of NO production in all groups, compared with the nanofibers cultures. This increase in NO levels is the strongest in WT and HG groups, with a much smaller NO expression in the db cells. Interestingly, eNOS inhibition using l-NAME suggests that NO does not likely play any major role in capillary morphogenesis in neither the nanofiber nor the Matrigel environment, with the small l-NAME induced inhibition observed in the db groups only (Fig. 8A, middle). Consistent with previous studies by other research groups (for example, see Ref. 4 and 55), cell exposure to strain results in increase in NO expression in all groups (difference is significant for low glucose, WT, and db; Fig. 8B) except chronic HG.
Fig. 8.
Effects of different substrate (A) and strain application (B) on nitric oxide (NO) expression. A: NO expression is significantly higher on Matrigel than on NFs in WT and db groups (P < 0.05). Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), a potent inhibitor of NO synthesis does not play a significant role in capillary morphogenesis (bottom). B: application of strain significantly increases NO expression in LG and db groups, with a similar trend for the HG cells; in contrast, strain appears to have no effect on NO expression in the chronic HG group.
DISCUSSION
The focus of this study is to determine the effects of diabetes on endothelial cell angiogenic and biomechanical responses and the role of the substrate microenvironment as a mediator of these responses. A rat model of DCM is used to represent type I chronic diabetes (db), where endothelial cells are exposed to diabetic conditions in vivo for 6 wk before subsequent cell isolation from the myocardium of the diabetic animal and culture in standard media. This model demonstrates significant diabetes-induced alterations in contractile properties and left ventricle dimensions, as well as the pseudonormal filling dynamics of the left ventricle, consistent with clinical studies of patients with DCM (6, 25) and with previous in vivo studies (11, 40, 61). In addition to the chronic in vivo model, both acute and chronic hyperglycemic in vitro models are also utilized, where normal (WT) cardiac endothelial cells undergo a short-term (acute or HG) or long-term (chronic HG) exposure to high-glucose media in culture. The results demonstrate that there are differential angiogenic and biomechanical responses of cardiac endothelial cells between these two models and that a proangiogenic environment can augment diabetes-induced deficiencies in the angiogenic potential of these cells, supporting the study hypothesis. It is shown that the HG condition causes alterations in cytoskeleton reorganization and significant reduction in capillary morphogenesis compared with the WT control group. In contrast, cardiac endothelial cells chronically exposed to diabetic condition (db group) have a normal cytoskeleton organization, and the reduction in capillary morphogenesis of these cells is not as dramatic as in HG condition. In addition, there is a significant increase in VEGF expression in the db group in static conditions compared with WT controls, consistent with previous studies in retinal endothelial cells, renal mesangial cells and vascular smooth muscle cells (31, 32, 43). However, this trend is not significant in the acute HG group. Further, the results of this study demonstrate that Matrigel environment induces high levels of NO expression in WT cells or “recent WT” cells that have been subjected to HG conditions in vitro; at the same time, Matrigel is unable to stimulate high NO production by the db cells. The results also show that strain has a significant effect on NO concentration; namely that the NO concentration is higher under strained conditions compared with static conditions for WT, acute HG, and db groups. These findings are consistent with previous studies that have shown that NO levels and NOS activity increase with strain application in both bovine aortic endothelial cells and human microvascular endothelial cells (4, 55). However, in our study, this effect is not seen in the chronic HG group. While db cells maintain their ability to respond to strain in a normal manner, the chronic HG group does not retain this ability. Importantly, these findings of the differences between in vitro and in vivo models are consistent with the concept that the endothelial cells have a long-term “hyperglycemic memory” of the physiological environment in the body (14, 20), as well as with the previously described endothelial cell “memory” of inflammatory stimulation (59). This indicates that acute cell exposure to high-glucose conditions may not be an ideal model for studying generalized effects of a diabetic environment on vascular cell responses.
The results of the biomechanical studies show that the application of mechanical strain before seeding on the substrate results in almost complete inhibition of capillary morphogenesis and endothelial cell proliferation in all experimental groups (WT, HG, and db), as well as a decrease in VEGF expression levels, compared with static controls. These results of decreased cell function are consistent with previous reports of reduced extracellular matrix deposition and matrix metalloproteinase (MMP) expression under diabetic and mechanical strain conditions in cardiac fibroblasts (27). They are also consistent with reports of decreased VEGF production and cell proliferation and migration in other cell types under diabetic conditions (13, 16, 65). Overall, these results suggest that a combined effect of diabetes and altered mechanical environment (e.g., increased stretching) in the fibrotic tissue may further exacerbate vascular deficiency in the remodeling diabetic heart.
Interestingly, previous studies by several groups (Table 1) have shown that the cell substrate can play a role in endothelial cell response to strain. In these studies, both pro- and antiangiogenic responses were observed, with the results depending on the substrate type (27, 28, 38, 56–58, 64). Substrates such as collagen, gelatin, fibronectin, pronectin, and silicone elastomers appeared to stimulate angiogenic responses, resulting in increased levels of VEGF, MMP-2, and enhanced proliferation and vascular network formation (28, 56, 62, 64). On the other hand, substrates such as fibrin and Matrigel resulted in the inhibition of angiogenic responses with strain application, including decreased network lengths and diminished network branching (19, 38, 58). The responses varied between two- and three-dimensional cultures (19, 58, 64). Overall, these studies suggest that, at least for nondiabetic endothelial cells, substrate may mediate strain-induced alterations in angiogenic cell responses. Therefore, in our studies, diabetic and normal cell responses were quantified for two substrates, Matrigel and the proangiogenic nanofiber hydrogel. We have previously shown that these nanofibers support cellular function in multiple cardiac cell types including cardiomyocytes, endothelial cells, and fibroblasts (26, 42), and that their proangiogenic mechanism of action involves sustained angiogenic activation of endothelial cells through αvβ3-integrins (10). The results of the present study demonstrate that the stimulating effect of the nanofiber microenvironment on endothelial angiogenic and biomechanical responses is present under both normal and diabetic conditions. In the latter case, the application of the nanofiber microenvironment results in the augmentation of the diabetes-associated deficiencies in cardiac endothelial cell responses. Our experiments with eNOS inhibitor l-NAME further suggest that the positive effect of the nanofiber microenvironment on capillary morphogenesis is likely to be independent from NO signaling. Overall, an important novel aspect of these findings is that diabetes-associated deficiencies in endothelial cell responses can be addressed, at least to a certain extent, by the appropriate choice of an extracellular microenvironment, suggesting hyperglycemic memory can be reversed.
In summary, the results of this study show that 1) diabetes alters cardiac endothelium angiogenic response, with differential effects of acute, chronic in vitro, and chronic in vivo exposure to high-glucose conditions, 2) endothelial cells subjected to mechanical strain may have a greatly reduced angiogenic potential, and 3) both of these deficiencies can be at least partially augmented by the proangiogenic nanofiber microenvironment. These findings may contribute to the development of novel approaches to reverse hyperglycemic memory of endothelial cells and enhance vascularization of the diabetic heart, where improved angiogenic and biomechanical endothelial responses can be the key factor to successful therapy.
GRANTS
This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R21-DK-078814-01A1 (to D. A. Narmoneva), Sigma Xi Research-In-Aid Grant (to A. Q. Sheikh), Interdisciplinary Faculty Research Grant (University of Cincinnati Research Council; to D. A. Narmoneva), and Nanomedicine Fellowship (University of Cincinnati Nanomedicine Group; to A. Q. Sheikh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.Q.S. and D.A.N. conception and design of research; A.Q.S., C.K., T.T., J.R.H., and W.H. performed experiments; A.Q.S., C.K., and D.A.N. analyzed data; A.Q.S., C.K., R.B.H., and D.A.N. interpreted results of experiments; A.Q.S. prepared figures; A.Q.S. and D.A.N. drafted manuscript; A.Q.S., C.K., and D.A.N. edited and revised manuscript; A.Q.S., Y.W., and D.A.N. approved final version of manuscript.
REFERENCES
- 1. Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 121: 748–757, 2008 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. ADA Fact Sheet 2011. Alexandria, VA: American Diabetes Association, 2011 [Google Scholar]
- 3. Awolesi MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest 96: 1449–1454, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Awolesi MA, Widmann MD, Sessa WC, Sumpio BE. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery 116: 439–444; discussion 444–435, 1994 [PubMed] [Google Scholar]
- 5. Balaji S, Vaikunth SS, Lang SA, Sheikh AQ, Lim FY, Crombleholme TM, Narmoneva DA. Tissue-engineered provisional matrix as a novel approach to enhance diabetic wound healing. Wound Repair Regen 20: 15–27, 2012 [DOI] [PubMed] [Google Scholar]
- 5a. Barron V, Brougham C, Coghlan K, McLucas E, O'Mahoney D, Stenson-Cox C, McHugh PE. The effect of physiological cyclic stretch on the cell morphology, cell orientation and protein expression of endothelial cells. J Mater Sci Mater Med 18: 1973–1981, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 115: 3213, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Models Mech 2: 454–466, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol 28: 1606–1613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension 31: 162–169, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Cho H, Balaji S, Sheikh AQ, Hurley JR, Tian YF, Collier JH, Crombleholme TM, Narmoneva DA. Regulation of endothelial cell activation and angiogenesis by injectable peptide nanofibers. Acta Biomater 8: 154–164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Jonathan Lederer W, Matlib MA. Defective intracellular Ca2+ signaling contributes to cardiomyopathy in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 283: H1398–H1408, 2002 [DOI] [PubMed] [Google Scholar]
- 11a. Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, Meade G, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol 26: 62–68, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 111: 442, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Ftesi S, Chang EI, Longaker MT, Gurtner GC. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg 123: 475–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205: 2409–2417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen 14: 558–565, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Garcia MJ, McNamara PM, Gordon T, Kannell WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow up study. Diabetes 23: 105, 1974 [DOI] [PubMed] [Google Scholar]
- 18. Garin G, Berk BC. Flow-mediated signaling modulates endothelial cell phenotype. J Endo Cell Res 13: 375–384, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Gassman AA, Kuprys T, Ucuzian AA, Brey E, Matsumura A, Pang Y, Larson J, Greisler HP. Three-dimensional 10% cyclic strain reduces bovine aortic endothelial cell angiogenic sprout length and augments tubulogenesis in tubular fibrin hydrogels. J Tissue Eng Regen Med 5: 375–383, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goettsch C, Goettsch W, Arsov A, Hofbauer LC, Bornstein SR, Morawietz H. Long-term cyclic strain downregulates endothelial Nox4. Antioxid Redox Signal 11: 2385–2397, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Gordon EE, Kira Y, Demers LM, Morgan HE. Aortic pressure as a determinant of cardiac protein degradation. Am J Physiol Cell Physiol 250: C932–C938, 1986 [DOI] [PubMed] [Google Scholar]
- 23. Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res 72: 375–383, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech 40: 947–960, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 107: 539–557, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Hurley JR, Balaji S, Narmoneva DA. Complex temporal regulation of capillary morphogenesis by fibroblasts. Am J Physiol Cell Physiol 299: C444–C453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hurley JR, Sheikh AQ, Huang W, Wang Y, Narmoneva DA. Effects of diabetes on matrix protein expression and response to cyclic strain by cardiac fibroblasts. Cell Mol Bioeng 5: 173–183, 2012 [Google Scholar]
- 28. Hurley NE, Schildmeyer LA, Bosworth KA, Sakurai Y, Eskin SG, Hurley LH, McIntire LV. Modulating the functional contributions of c-Myc to the human endothelial cell cyclic strain response. J Vasc Res 47: 80–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kämpfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and -2 and the Tie-1 and -2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest 81: 361–373, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Kemeny SF, Figueroa DS, Clyne AM. Hypo- and hyperglycemia impair endothelial cell actin alignment and nitric oxide synthase activation in response to shear stress. PLoS One 8: e66176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim NH, Jung HH, Cha DR, Choi DS. Expression of vascular endothelial growth factor in response to high glucose in rat mesangial cells. J Endocrinol 165: 617–624, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Kim NH, Oh JH, Seo JA, Lee KW, Kim SG, Choi KM, Baik SH, Choi DS, Kang YS, Han SY, Han KH, Ji YH, Cha DR. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int 67: 167–177, 2005. [DOI] [PubMed] [Google Scholar]
- 32a. Korff T, Ernst E, Nobiling R, Feldner A, Reiss Y, Plate KH, Fiedler U, Augustin HG, Hecker M. Angiopoietin-1 mediates inhibition of hypertension-induced release of angiopoietin-2 from endothelial cells. Cardiovasc Res 94: 510–518, 2012. [DOI] [PubMed] [Google Scholar]
- 33. Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 162: 303–312, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewko B, Bryl E, Witkowski JM, Latawiec E, Angielski S, Stepinski J. Mechanical stress and glucose concentration modulate glucose transport in cultured rat podocytes. Nephrol Dial Transplant 20: 306–311, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr, Luscinskas FW. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol 162: 1591, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu XM, Ensenat D, Wang H, Schafer AI, Durante W. Physiologic cyclic stretch inhibits apoptosis in vascular endothelium. FEBS Lett 541: 52–56, 2003 [DOI] [PubMed] [Google Scholar]
- 37. MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res 46: 257–263, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Matsumoto T, Yung YC, Fischbach C, Kong HJ, Nakaoka R, Mooney DJ. Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng 13: 207–217, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Metzler SA, Pregonero CA, Butcher JT, Burgess SC, Warnock JN. Cyclic strain regulates proinflammatory protein expression in porcine aortic valve endothelial cells. J Heart Valve Dis 17: 571–577; discussion 578, 2008 [PubMed] [Google Scholar]
- 40. Mihm MJ, Seifert JL, Coyle CM, Bauer JA. Diabetes related cardiomyopathy time dependent echocardiographic evaluation in an experimental rat model. Life Sci 69: 527–542, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Narmoneva DA, Oni O, Sieminski AL, Zhang S, Gertler JP, Kamm RD, Lee RT. Self-assembling short oligopeptides and the promotion of angiogenesis. Biomaterials 26: 4837, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: Implications for cardiac regeneration. Circulation 110: 962, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Natarajan R, Bai W, Lanting L, Gonzales N, Nadler J. Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 273: H2224–H2231, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Nerem RM. Role of mechanics in vascular tissue engineering. Biorheology 40: 281–287, 2002 [PubMed] [Google Scholar]
- 45. Nishida M, Carley WW, Gerritsen ME, Ellingsen O, Kelly RA, Smith TW. Isolation and characterization of human and rat cardiac microvascular endothelial cells. Am J Physiol Heart Circ Physiol 264: H639–H652, 1993 [DOI] [PubMed] [Google Scholar]
- 46. Paneni F, Mocharla P, Akhmedov A, Costantino S, Osto E, Volpe M, Luscher TF, Cosentino F. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ Res 111: 278–289, 2012 [DOI] [PubMed] [Google Scholar]
- 47. Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Exp Rev Mol Med 11: e2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest 60: 884–899, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riser BL, Ladson-Wofford S, Sharba A, Cortes P, Drake K, Guerin CJ, Yee J, Choi ME, Segarini PR, Narins RG. TGF-beta receptor expression and binding in rat mesangial cells: modulation by glucose and cyclic mechanical strain. Kidney Int 56: 428–439, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Sadoshima JI, Jahn L, Takahashi T, Kulik TJ, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem 267: 10551–10560, 1992 [PubMed] [Google Scholar]
- 51. Sato M, Suzuki K, Ueki Y, Ohashi T. Microelastic mapping of living endothelial cells exposed to shear stress in relation to three-dimensional distribution of actin filaments. Acta Biomater 3: 311–319, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Shatti SJ, Ginsberg MH. Integrin signaling in vascular biology. J Clin Invest 100: S91–S95, 1997 [PubMed] [Google Scholar]
- 53. Sheikh AQ, Taghian T, Hemingway B, Cho H, Kogan AB, Narmoneva DA. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low-amplitude electric field. J R Soc Interface 10: 20120548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Toffoli B, Bernardi S, Candido R, Zacchigna S, Fabris B, Secchiero P. TRAIL shows potential cardioprotective activity. Invest New Drugs 30: 1257–1260, 2012 [DOI] [PubMed] [Google Scholar]
- 55. Upchurch GR, Jr, Leopold JA, Welch GN, Loscalzo J. Nitric oxide alters human microvascular endothelial cell response to cyclic strain. J Cardiovasc Pharmacol Therap 3: 135–142, 1998 [DOI] [PubMed] [Google Scholar]
- 56. von Offenberg Sweeney N, Cummins P.M, Birney YA, Cullen JP, Redmond EM, Cahill PA. Cyclic strain-mediated regulation of endothelial matrix metalloproteinase-2 expression and activity. Cardiovasc Res 63: 625–634, 2004 [DOI] [PubMed] [Google Scholar]
- 57. von Offenberg Sweeney N, Cummins P.M, Cotter EJ, Fitzpatrick PA, Birney YA, Redmond EM, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochem Biophys Res Commun 329: 573–582, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Wilson CJ, Kasper G, Schutz MA, Duda GN. Cyclic strain disrupts endothelial network formation on Matrigel. Microvasc Res 78: 358–363, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Wolff B, Burns AR, Middleton J, Rot A. Endothelial cell “memory” of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med 188: 1757–1762, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yano M, Yamamoto T, Kobayashi S, Ikeda Y, Matsuzaki M. Defective Ca2+ cycling as a key pathogenic mechanism of heart failure. Circ J 72: A22, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 111: 2073–2085, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Yung YC, Chae J, Buehler MJ, Hunter CP, Mooney DJ. Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc Natl Acad Sci USA 106: 15279–15284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol 20: 674–682, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Zheng W, Christensen LP, Tomanek RJ. Differential effects of cyclic and static stretch on coronary microvascular endothelial cell receptors and vasculogenic/angiogenic responses. Am J Physiol Heart Circ Physiol 295: H794–H800, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zykova SN, Jenssen TG, Berdal M, Olsen R, Myklebust R, Seljelid R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 49: 1451–1458, 2000 [DOI] [PubMed] [Google Scholar]