Abstract
Acute respiratory distress syndrome (ARDS) is a devastating disease with distinct pathological stages. Fundamental to ARDS is the acute onset of lung inflammation as a part of the body's immune response to a variety of local and systemic stimuli. In patients surviving the inflammatory and subsequent fibroproliferative stages, transition from injury to resolution and recovery is an active process dependent on a series of highly coordinated events regulated by the immune system. Experimental animal models of acute lung injury (ALI) reproduce key components of the injury and resolution phases of human ARDS and provide a methodology to explore mechanisms and potential new therapies. Macrophages are essential to innate immunity and host defense, playing a featured role in the lung and alveolar space. Key aspects of their biological response, including differentiation, phenotype, function, and cellular interactions, are determined in large part by the presence, severity, and chronicity of local inflammation. Studies support the importance of macrophages to initiate and maintain the inflammatory response, as well as a determinant of resolution of lung inflammation and repair. We will discuss distinct roles for lung macrophages during early inflammatory and late resolution phases of ARDS using experimental animal models. In addition, each section will highlight human studies that relate to the diverse role of macrophages in initiation and resolution of ALI and ARDS.
Keywords: activation, ARDS, inflammation, macrophage
alveolar macrophages form the first line of defense against airborne particles and microbes and use a variety of pattern recognition and scavenger receptors to sense and phagocytose pathogens. Macrophages remain in constant exposure to the outside world with close contact to both air- and blood-borne materials (98). Since the introduction of the macrophage system by Metchnikoff in the late 19th century when it was first described as a phagocytic cell, dynamic factors have been uncovered to define a heterogeneous cell population identified by differences in maturation, tissue migration, phenotypes, and cellular interaction (35, 160, 179). The presence and severity of tissue inflammation is paramount in defining macrophage subpopulations.
In the lung and alveolar space, inflammation is intimately tied to macrophage phenotype and function, a process well described during the acute inflammatory stage of acute respiratory distress syndrome (ARDS) and now increasingly being recognized during resolution and recovery from ARDS (63). Studies performed over 10 years ago in humans with ARDS using serial bronchoalveolar lavages (BAL) determined that increased alveolar macrophage number and a mature cellular phenotype were associated with a favorable outcome (156, 172); however, a causative role for macrophages in enhancing resolution and repair from human ARDS remains unknown. Using diverse experimental acute lung injury (ALI) and resolution models, investigators have identified important cellular mechanisms and molecular pathways of macrophage response to help define their roles in lung homeostasis, acute inflammation, resolution, and repair (Fig. 1). We will focus on key aspects of lung macrophage biology (phenotype, differentiation, function, and fate) within the context of acute inflammation and resolution responses. We will identify areas of controversy and those with therapeutic potential and will summarize our understanding in humans.
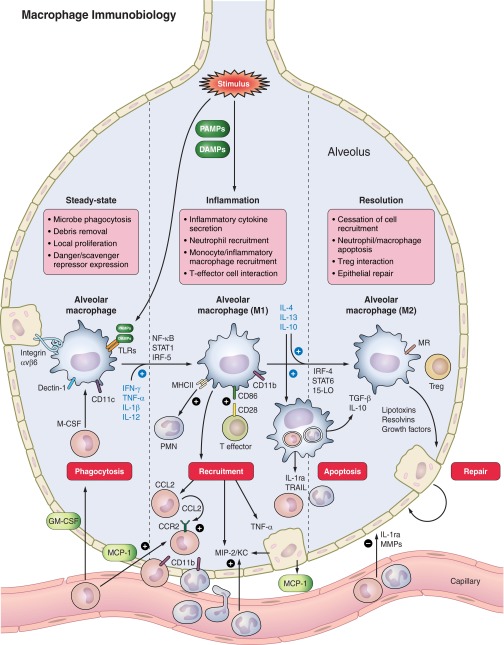
Fig. 1.
A schematic displaying major alveolar macrophage phenotypes and functions during steady state, inflammation, and resolution. During quiescence, macrophages are tethered to epithelial cells, with persistent expression of pattern recognition (TLRs) and scavenger (Dectin-1) receptors. Turnover is slow and driven primarily by M-CSF/GM-CSF induced local proliferation, with minimal recruitment from circulating monocytes. With increased foreign antigen stimulation (PAMPs, DAMPs), macrophage activation occurs, skewing resident macrophages toward an M1 activation state (transcription factors NF-κB, STAT1, IRF-5; cytokines IFN-γ, TNF-α, IL-1β, IL-12) hallmarked by secretion of proinflammatory cytokines capable of neutrophil (MIP-2/KC) and inflammatory monocyte/macrophage recruitment (MCP-1/CCL2), as well as stimulation of alveolar epithelial cells (TNF-α) and effector T cells (CD86-CD28) to help promote and sustain the inflammatory response. With sufficient control of foreign antigen, macrophages reprogram toward an M2 activation state (transcription factors STAT6, STAT3, IRF-4, 15-LO; cytokines IL-4, IL-13, IL-10) and coordinate a series of regulated responses to abrogate inflammation, and enhance resolution/repair. Key processes include cessation of inflammatory cell recruitment (IL-1ra, MMPs); apoptosis of inflammatory cells (IL-1ra, TRAIL, IL-10, TGF-β); interaction with Tregs; and secretion of lipoxins, resolvins, and growth factors to promote epithelial repair and type II to type I AEC transition. AEC, alveolar epithelial cell; CCL2, chemokine (C-C motif) ligand 2; CCR2, chemokine (C-C motif) receptor 2; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN-γ, interferon-γ; IL, interleukin; IL-1ra, interleukin 1 receptor antagonist; iNOS, inducible nitric oxide synthase; IRF, interferon regulator factor; LO, lipoxygenase; M-CSF, macrophage colony-stimulating factor; MCP-1, monocyte chemoattractant protein-1; MIP-2, macrophage inflammatory protein-2; MMPs, matrix metalloproteinases; MR, mannose receptor (MRC1); NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; PMN, polymorphonuclear leukocyte (neutrophil); STAT, signal transducer and activator of transcription; TGF-β, transforming growth factor β; TLRs, Toll-like receptors; TNF-α, tumor necrosis factor-α; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; Treg, regulatory T cell.
Macrophage Phenotype at Steady State
Murine.
At a portal of entry to the body, alveolar and lung macrophages encounter constant exposure to foreign particles and infectious agents (182). During steady state, lung macrophages express relatively high levels of pathogen-associated molecular pattern (PAMP) and danger-associated molecular pattern (DAMP) receptors arming the cell with the means to initiate a prompt and robust immune response (58, 182). To aid in removal of inhaled pathogens and particles, steady-state alveolar macrophages express mannose receptor (CD206) and β-glucan specific receptor (Dectin-1), potent scavenger receptors that also help define a M2, alternative-activated, anti-inflammatory cell activation state (35, 182). Quiescent alveolar macrophages express high levels of CD11c, a marker not typically expressed by other tissue macrophages (60, 195), and express low levels of CD11b, a marker with higher expression on most other tissue macrophages (60). Kasahara et al. (84) demonstrated that newborn mice exposed to microbial extracts increased CD11c+ cells in the airway, introducing a possible mechanism for induction of alveolar macrophage CD11c expression due to persistent airway pathogen exposure into adulthood. Using adoptive transfer experiments, Guth et al. (60) demonstrated the influential effects of the lung and airway microenvironment on macrophage phenotype, including local granulocyte-macrophage colony-stimulating factor (GM-CSF) secretion.
Morphologically, alveolar macrophages resemble other tissue macrophages with their large size and increased cytoplasm/nucleus ratio. Lung interstitial macrophages are smaller, with reduced granularity and autofluorescence (98, 128), and may represent an intermediate cell population between blood monocytes and terminally differentiated alveolar macrophages (17, 68, 98). Even though interstitial and alveolar macrophages can both express CD11c and F4–80 with high intensity (76, 128, 203), CD11b expression is generally higher on interstitial macrophages (128, 203). Macrophage form and function are also related, as McWhorter et al. (120) recently demonstrated that M1-polarizing stimuli (endotoxin, IFN-γ) induced a rounded cell shape whereas M2-polarizing stimuli (IL-4, IL-13) induced an elongated cell shape. Furthermore, regulating cell shape by a micropatterning technique demonstrated that cell elongation, without other stimuli, enhanced a M2 phenotype and anti-inflammatory cytokine production in an actin-dependent manner.
DEFINING SUBPOPULATIONS.
Concise definitions of different lung macrophage subpopulations need optimization and should include a standardized list of cell surface flow markers that accurately distinguish dendritic cells from macrophages as well. With the advancement of immunological tools including multiparameter flow cytometry, knowledge and quantification of membrane glycoproteins to immunophenotype macrophages is attainable, especially when verified by gene assessment of traditional macrophage markers (Table 1). Flow cytometric analysis is limited by macrophage autofluorescence, necessitating techniques to minimize its interference with other exogenously applied fluorophores. Approaches to circumvent this problem include avoidance of the fluorescein or FITC fluorophores that exhibit similar wavelength spectra (43), or, as demonstrated by Soroosh et al. (168), using relative cell autofluorescence to help identify macrophages and dendritic cells. Most studies suggest a combination of cell surface markers F4–80+, CD11b+/−, CD11c+, and major histocompatibility complex (MHC) IIlow to identify mature or interstitial macrophages in the lung and alveolar space (58, 81, 103, 182, 189). Although macrophages and dendritic cells both express CD11c, dendritic cells express higher levels of MHC II, and macrophages express higher levels of F4–80. Recently, Zaynagetdinov et al. (203) demonstrated that CD68, an intracellular marker that requires cell permeabilization, was more specific for alveolar macrophages (CD68hi, F4–80+, CD11b−) and helped to distinguish them from dendritic cells (CD68hi, CD11c+, F4–80−), interstitial macrophages (CD68low, F4–80+, CD11b+, CD11c+, CD14low), or monocytes (CD68low, F4–80+, CD11b+, CD11c−, CD14−) during steady state.
Table 1.
Macrophage/monocyte activation markers
| Marker | Classification | Primary Function(s) | Stimulus/Receptor | Human Expression | Murine Expression |
|---|---|---|---|---|---|
| Identification | Cell expression | ||||
| CD11b (Mac-1a, Itgam) | ++ IM/MO, ± AM, + DC | cell adhesion/migration, complement-opsonized phagocytosis | ICAM-1 (CD54) | nonresolving ARDS (156) | lower in alveolar space; increased in migrated cells |
| CD11c (Itgax) | ± IM/MO, ++AM, +/++ DC | cell adhesion, associates with CD18 | ICAM-1 fibrinogen and iC3b | ++ iMO, + rMO | BAL expression higher than most tissues |
| CD68 (macrosialin) | +IM/MO, ++ AM | IC glycoprotein, specific mac expression (203), cell-cell interaction | + MO, IM, AM | good marker, but intracellular staining by flow; ++ DC | |
| F4-80 (EMR1) | +IM/MO, +/++ AM | glycoprotein coupling EC to IC signaling, signaling during tolerance/priming | downregulation by IFN-γ | 70% murine homolog, gene level only, ++ Eos | not as alveolar macrophage-specific |
| CD14 | ++ MO, + IM, ± AM | LPS-binding protein, TLR signaling, apoptotic cell receptor (182) | apoptotic cells | ++ (-CD16) = murine ++ Ly6c MO, + acute LPS | does not readily define mac subtypes in mice |
| CD16 | ++ AM, ++ iMO, + PMN | Fc receptor (FcγIII). NK cell activation, ADCC | IgG, IgG-antigen complex | ++ (and + CD14) = murine ± Ly6c MO | B cells, MO, AM, NK cells, PMN |
| Siglec F | +/++ AM, ++ Eos | inhibits Eos proliferation (30), virus/bacteria cell entry | Sialic acid | convergent paralog to Siglec-8 (human) (30) | |
| CD33 (Siglec-3) | + MO (human), + PMN (murine) | regulates fcn via glycan recognition, pathogen uptake | Sialic acid | MO and myeloid precursors | primarily on PMNs |
| Siglec 1 (Sialoadhesin, CD169) | ++ AM | recognition/phagocytosis of bacteria (182) | Sialic acid | macrophages, ?AM | |
| FcyR1 (CD64) | ++ IM/AM, + MO | phagocytosis, Ag capture, ADCC, signals through ITAM motif | IgG immune complex | 3 distinct genes (CD64A, B, C) | |
| CD115 (M-CSFR, CSF1R) | ++ MO (human iMO, rMO), +AM,IM | cell production, maturation, and fcn via M-CSF | M-CSF | ++ on iMO and rMO (58) | ++ MO, useful for microbead isolation |
| Gr-1 (Ly6g, Ly6c) | ++ MO, ± IM (Ly6c), ++ PMN (Ly6g) | precursor for IM (Ly6c++ MO) and AM, AAM? (Ly6+/−) | Ly6c− monocyte; Ly6g− neutrophil; Gr-1 both | ||
| 7/4 | ++MO (+ CCR2, ± CX3CR1), +PMN | ? inflammatory response | |||
| CD62L (L-selectin) | ++ iMO (also ++ Ly6c), ++ PMN | monocyte recruitment, tethering and rolling | glycoproteins (CD34, GYLCAM1, MADCAM1) | present on iMO | |
| CX3CR1 | ++MO, +AM/IM | migration, survival signal (96), patrolling (8) | CX3CL1 | Ly6C++ = rMO human; Ly6c− = iMO human | |
| M1 activation | M1 (CAM) | Microbicidal, tissue damage, cellular immunity | TNF-α, IL-12, IFN-γ | ||
| MHC II (I-A/I-E, HLA-DR) | ++ rMO, + iMO, also ++ DC | Antigen presentation | IFN-γ, but also IL-4/13 | HLA-DR: + iMO, ++ rMO, ++ resolve ARDS (156) | + IM, stimulated AM expression |
| CD80 | T-cell / PMN signaling | IFN-γ | |||
| CD86 | also + DC | T-cell / PMN signaling | IFN-γ | ++ rMO, + iMO (distinct from murine) | may be distinct than human effect |
| iNOS (Nos2) | produce NO, kill bacteria | minimal in AM | |||
| Cox2 (Ptgs2) | inflammation and pain | ||||
| IL-12 | Induces Th1 T-cell, M1 stimulus | IFN-γ | |||
| CCL15 | chemoattractant for MO, lymphs, and Eos | ||||
| CCL20 | chemoattractant for T cells | ||||
| CXCL9 | T-cell trafficking | ||||
| CXCL10 (IP-10) | induces Th1 T cells, NK cells | ||||
| IL23a (IL23p19) | enhances IFN-γ by memory T cells and induces IL-17 by Th17 cells. | ||||
| CXCL11 | inductes Th1 T cells, NK cells | ||||
| CCR2 | Ex. macrophages and monocytes | lung migration, deficiency impairs ++Ly6c MO | MCP-1 (CCL2), CCL7 | + iMO | |
| CCR5 | Ex. macrophages and monocytes | rercuitment into or within inflamed tissues (56) | CCL3, CCL5, inhibited by IL-10 (56) | + rMO | |
| 27E10 (MRP8/MRP14) | + human IM/MO | migration and adhesion, chemotactic for PMNs | nonresolving ARDS (156) | ||
| MARCO | ++AM, nonspecific for M1 | scavenger receptor, particulate ingestion (titanium dioxide) (182) | TLR signaling, microbial stimuli | expressed by steady-state AM | |
| CD36 | ++ AM, nonspecific for M1 | apoptotic cell clearance, primarily LDL uptake | IFN-γ | ||
| M2 activation | M2 (AAM) | Tissue repair, humoral immunity, allergic response | IL-4, IL-13, IL-10 | ||
| Mannose receptor (Mrc1,CD206) | also + DC (myeloid), −MO/PMN | phagocytosis of ligands (MPO, HIV Ag, bacteria (56), present Ag | downregulated by IFN-γ | ||
| Scavenger receptor A (CD204) | −MO and + DC subsets | apoptotic cell clearance | M-CSF | ||
| Dectin-1 | β-glucan receptor, TLR2 interaction (182), phagocytosis, proliferate T cells | ||||
| IL-4ra | IL-4/IL-13 signaling | IL-10 (56), + feedback with IL-4/13 | |||
| Arg1 | counteracts Nos2 (iNO) (56) | induced by IFN-γ/STAT3 too (56) | Not upregulated | ||
| 12,15-lipoxygenase (LOX) | mediates PPARγ upregulation by IL-4 | ||||
| TfR (CD71) (79) | carrier protein for transferrin, iron uptake, cell proliferation | ||||
| OX2R (CD200R) | bind epithelial CD200 to dampen AM inflammation | mainly IL-10, TGF-β | |||
| IL-10 | not specific, ?Mreg subpopulation | deactivate resp. burst and TNF-α, ? fibrogenesis | TLR activation | ||
| TGF-b | key role in fibrosis | IL-13 (56) | |||
| IL-1ra (IL-1 decoy receptor) | Anti-IL-1 effects | Expressed by Gr-1+CCR2+ ex. Macs (64) | |||
| Cxcl13 | B cell chemoattractant | ||||
| CCL12 | monocyte chemoattactant, recruitment of fibrocytes | ||||
| CCL17 (TARC) | binds CCR4, ++ MO, ++ DC | drives fibrogenesis with IL-10, acts on CCR4+ subset of CD4 T cells (56) | IL-4/IFN-γ antagonistic; STAT6/1-binding (56) | ||
| CCL18 (AMAC1) | also + DC | attracts lymphocytes, monocytes | IL-10 | ||
| CCL22 (MDC) | attracts CCR4+ Th cells to polarize Th2 response | downregulated by IFN-γ and IL-10 | |||
| CCL24 | chemoattractant for eosinophils, mast cells and basophils | ||||
| YM1 (Chi3l3) | IC staining by flow cytometry | chitinase-like protein, binds to ECM, heparan sulfate | IL-4/IFN-γ antagonistic; STAT6/1-binding (56) | ||
| RELMa (Fizz1) | IC staining by flow cytometry | resistin-like secreted protein (56), promote ECM deposition | IL-4/IFN-γ antagonistic; STAT6/1-binding (56) | Not expressed | + Eos, epithelial cells |
| Mac-2 (galectin-3) | ++MO, AM | CD98 binding activates PI3K, + feedback loop with IL-4 | Inhibited by LPS | ||
| 25F9 | resident macs | late inflammatory macrophage | ++ resolving ARDS (156) | ||
| RM3/1 | ++ MO, AM, CD163 family | membrane glycoprotein, late inflammatory macrophage | ++ resolving ARDS (156) |
Identification of macrophage/monocyte markers and activation states, including primary function, key stimuli/receptor, and known details about human or murine expression. Top: identification of macrophage and monocyte subpopulations in mice and humans are based on single or multiple combinations of surface and intracellular markers. We present correlative data on human leukocyte expression for each marker. Middle: surface and intracellular markers of M1 macrophage activation, along with primary function in addition to general characteristics including microbiocidal activity, tissue damage, and cellular immunity. Transcription factors that promote an M1 activated phenotype include NF-κB, IRF5, STAT1. Stimuli common to the group include IFN-γ, TNF-α, and IL-12. Bottom: surface and intracellular markers of M2 macrophage activation, along with primary function in addition to general characteristics including wound healing, tissue repair, humoral immunity, and allergic response. Transcription factors that promote an M2-activated phenotype include IRF4, KLF4, PPAR, and STAT6. Stimuli common to the group include IL-4, IL-13, and IL-10, although some consider IL-10 programmed macrophages to behave as a distinct, regulatory phenotype (MReg).
AAM, alternatively activated macrophage (M2); ADCC, antibody-dependent cell-mediated cytotoxicity; Ag, antigen; AM, mature alveolar macrophages; ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage; CAM, classically activated macrophage (M1); Cox2, cyclooxygenase-II enzyme; CSF, colony stimulating factor; CX3CL1, fractaline; CX3CR1, CX3C chemokine receptor 1; DC, dendritic cell; EC, extracellular; ECM, extracellular matrix; Eos, eosinophil; Ex., exudative; fcn, function; FcRγ1, high-affinity Fc γ receptor; HLA, human leukocyte antigen; IC, intracellular; ICAM-1, intercellular adhesion molecule 1; IL-4ra, IL-4 receptor α; IM, immature alveolar macrophage/interstitial macrophages; iMO, human inflammatory (classical) monocyte; iNOS, inducible nitric oxide synthase; IP-10, interferon γ-induced protein 10; ITAM, immune-receptor tyrosine activation motif; Itgam, integrin αM; Itgax, integrin αX; LPS, lipopolysaccharide (endotoxin); LDL, low-density lipoprotein; mac, macrophage; MARCO, macrophage receptor collagenous domain; M-CSF, macrophage colony-stimulating factor; MHC, major histocompatibility complex; MO, monocyte; MReg, regulatory macrophage, possible subset of AAM; NK, natural killer; PMN, neutrophil; PPAR, peroxisome proliferator-activated receptor; rMO, human resident (nonclassical; intermediate) monocyte; siglec, sialic acid-binding immunoglobulin-type lectins; TfR, transferrin receptor; TLR, Toll-like receptor; 27E10, calgranulin A/B.
Cell immunophenotyping in other tissues may be more refined. Meredith and coworkers (126, 127) utilized expression of zinc finger transcription factor to distinguish bone marrow-derived classical dendritic cells from plasmacytoid dendritic cells and monocyte/macrophages, and Rivollier et al. (155) distinguished dendritic cells (CD103+) from macrophages (CX3CR1hi) in colonic lamina propria using surface markers. For the majority of macrophage surface markers (Table 1, top), the functional significance is not well known. Exceptions include CD11b, which is required for monocyte migration to sites of inflammation (152), and CX3CR1, which is required for patrolling and monocyte survival (8, 96). Additionally, CD115 is important for a number of functions as the macrophage colony-stimulating factor (M-CSF) receptor, including macrophage proliferation and differentiation.
Human.
AUTOFLUORESCENCE.
Phenotypic characterization of human lung macrophages at steady state is hindered by a lack of accessibility to samples. In addition, the intensity of autofluorescence in lung macrophages is comparable to exogenously applied fluorophores and is only partially overcome with advances in flow cytometry. Edelson et al. (43) described the autofluorescence spectrum: excitation at wavelengths 370 and 490 nm, and maximum emission at 541 nm with a shoulder at 580 nm; heterogeneity in intensity and autofluorescent wavelengths between subjects further complicates the assessment (199). Intracellular storage of fluorescent flavoprotein, lipofuscein, cigarette smoke hydrocarbons, and other factors prevalent because of the unique position of lung macrophages at the interface of the external environment can all explain the increased autofluorescence. Techniques to minimize its effects include use of dyes exciting at and/or emitting light of higher wavelengths (allophycocyanin, cyanine5/cyanine7, or Alexa Fluor 647), quenching of autofluorescence using eta-octyl-β-d-galactopyranoside or crystal violet (requires cell permeabilization), and either enzymatic or immunological signal amplification.
SUBPOPULATIONS.
Use of flow cytometry to rigorously define human lung macrophage subpopulations is underutilized. Macrophage markers CD68, CD163, and Mac2 (Galectin 3) are expressed in most tissues based on immunohistochemistry but have not been well defined with use of flow cytometry. Monocyte populations are better characterized and are typically divided into “inflammatory” and “resident.” Inflammatory (classical) monocytes are CD14hiCD16−CCR2+ and resident monocytes are CD14+CD16+CCR5+. A comparison of gene expression profiles between human and murine monocyte populations revealed similarity between human inflammatory monocytes (CD14hiCD16−) with murine CD115+Ly6chi monocytes, and between human resident monocytes (CD14+CD16+) with murine CD115+Ly6clow monocytes (73). Among what is known about human lung macrophages, important differences in phenotypic markers defining subpopulations exist compared with murine lung. Compared with murine macrophages, human macrophages produce relatively little arginase (Arg1) following IL-4 stimulation and little nitric oxide synthase [inducible nitric oxide synthase (iNOS), Nos2] following IFN-γ stimulation.
Macrophage Phenotype with Inflammation
Murine.
The presence of lung inflammation complicates the characterization of macrophage subpopulations. Using bleomycin as a murine lung injury model, Misharin et al. (128) found that F4–80 was less specific for alveolar macrophages since it was also expressed on interstitial macrophages, monocytes, eosinophils, or CD11b+ DCs. Instead, Siglec F (a member of the family of sialic-acid-binding immunoglobulin-like lectins) (30), in combination with CD11c or CD64, accurately identified alveolar macrophages when verified using morphological analysis. During lung inflammation, two main states of differentiation exist, characterized by classically activated macrophages (CAM) and alternatively activated macrophages (AAM). CAMs display the M1 macrophage phenotype and produce high levels of proinflammatory cytokines IL-1β, TNF-α, IL-12, and iNOS in response to paracrine signaling from the T helper 1 (Th1) cytokine IFN-γ and also in response to autocrine signaling by IFN-β (99, 191). AAMs display the M2 macrophage phenotype and produce anti-inflammatory cytokines IL-10 and IL-1ra in response to signaling from Th2 cytokines IL-4 and IL-13. Macrophages skewed toward an M1 activation state express surface markers including but not limited to MHC II (IA/IE), CD80, CD86, and CCR2. M2-skewed macrophages express markers including but not limited to mannose receptor (MR), Dectin-1, TfR (transferrin receptor) (81), and CD200R. Expression of different STAT transcription factors or suppressors of cytokine signaling (SOCS) proteins drives M1 (STAT1, SOCS2) vs. M2 (STAT6/STAT3, SOCS3) expression (169).
LIMITATIONS OF PHENOTYPIC MARKERS.
Relying on one or even several markers to define the presence of M1 or M2 macrophages creates problems, often magnified during inflammation. Macrophages demonstrate significant plasticity, allowing them to transition between distinct phenotypes or even to a hybrid phenotype based on environmental signals. For example, M1-to-M2 macrophage transition occurs after phagocytosis of apoptotic neutrophils or in the presence of IL-4 (50, 81, 181). However, when macrophages were stimulated with immune complexes in the presence of IL-4, they produced high levels of IL-10, a prominent feature of regulatory macrophages (Mregs). Mregs are a distinct macrophage subtype, yet in this case the cells also retained features of M2 macrophages (44). Another example in which phenotypic markers don't necessarily reflect true cell function is the high expression of the M2 markers Dectin-1 and mannose receptor on macrophages in the alveolar space at baseline, in the setting of persistent, albeit low-level, antigen exposure. The functions of an unchallenged macrophage are likely quite different from an alveolar macrophage expressing the same M2 markers during the resolution phase of experimental lung injury. Therefore, it is probably more effective to define macrophage subpopulations during disease states on the basis of function including cytokine profiles, phagocytosis, and TH lymphocyte skewing and proliferation.
Human.
Extensive characterization of human alveolar macrophage differentiation and phenotypes has not been performed, but there are a few noteworthy studies. Rosseau performed serial BALs in ventilated ARDS patients compared with one BAL in ventilated cardiogenic pulmonary edema (CPE) patients or in nonventilated healthy volunteers (156). In ARDS patients, total alveolar macrophage number and expression of CD14 and CD11b increased, and expression of CD71, human leukocyte antigen (HLA)-DR, and 25F9 (mature tissue macrophage markers) decreased compared with healthy volunteers or CPE patients. Furthermore, alveolar macrophages from ARDS patients resembled blood monocytes with smaller size and less autofluorescence compared with mature alveolar macrophages. With increased 27E10, a marker that recognizes acute-phase monocyte/macrophages (15), and decreased RM3/1, a marker denoting dampened inflammation (205), alveolar macrophages from ARDS patients skewed toward a more proinflammatory, M1 activation phenotype compared with other groups. Interestingly, persistent expression of M1 proinflammatory macrophage surface markers as well as increased BAL monocyte chemoattractant protein-1 (MCP-1), a proinflammatory cytokine that is also a monocyte chemoattractant, portended a worse prognosis (156).
To model the early phase of ARDS, Brittan et al. (19) exposed healthy volunteers to inhaled lipopolysaccharide (LPS, 60 μg, vehicle saline), followed by blood and BAL collection up to 8 h later. In contrast to similar monocyte subpopulations in the blood, BAL from LPS-challenged patients revealed a significant increase in total alveolar macrophages (CD14+), consisting primarily of pulmonary monocyte-like cells (PMLC) defined by smaller size and less granularity compared with mature alveolar macrophages. PMLCs were predominantly recruited (CD16−), not resident (CD16+) monocytes (204). Brittan et al. defined recruited monocytes by differences in forward/side scatter and CD14+ expression, and determined that these cells expressed HLA-DR at higher levels compared with resident monocytes. In contrast, Rosseau et al.'s study (157) suggested an increase in HLA-DR expression on more mature, proresolution macrophages. Expression and abundance of a broader array of human lung macrophage markers needs is necessary during homeostasis and inflammatory responses.
Macrophage Recruitment and Proliferation
Murine.
BASELINE CONDITIONS.
Since the identification of macrophage systems, scientists have debated the relative importance of local proliferation vs. recruitment from blood monocytes to help maintain tissue homeostasis. Evidence in humans and mice supports dual and perhaps complementary mechanisms to repopulate the alveolar macrophage pool, skewed by the presence and severity of inflammation (35, 36, 201). A recent fate-mapping study using mice with Cre recombinase in their CX3CR1 gene loci confirmed alveolar macrophages to be of prenatal origin, devoid of blood monocyte input for tissue renewal at baseline (201). During unchallenged states, alveolar macrophage turnover is slow. Local proliferation regulates cell replacement and is primarily mediated by GM-CSF and M-CSF (CSF-1) signaling through macrophage CSF-1R (58, 71, 77). Recently, however, Jenkins et al. (77) showed that IL-4 can induce pleural macrophage proliferation in a CSF-1-independent manner.
Studying macrophage turnover under true baseline conditions is difficult, since even mild inflammation can skew lung and alveolar macrophage cell replacement toward repopulation from the bone marrow (1, 58, 97, 114, 146). Chimera studies offer some insight into the influence of inflammation on macrophage turnover. Whole body irradiated mice undergo donor alveolar macrophage replacement within a few weeks (53, 80, 97, 110, 114, 177). In contrast, mice irradiated with a lung shield had stable host alveolar macrophage presence even at 1 year (176, 177), demonstrating that tissue damage and inflammation from irradiation accelerate cell turnover. To try and study cell turnover without irradiation, Landsman and Jung (97) used systemic diphtheria toxin (DT) to deplete CD11c+ alveolar/lung macrophages and CD11c+ dendritic cells in CD11c-DTR mice. In doing so, they observed an intermediate lung macrophage population derived from blood monocytes that proliferated in the lung and migrated to the alveolar space to replace alveolar macrophages. Repeated injections of DT have been shown to induce injury (159), however, and likely also increase lung inflammation compared with baseline. Furthermore, rapid depletion of resident macrophages may enhance bone marrow recruitment signals.
INFLAMMATION.
More significant inflammation due to stimuli such as intratracheal LPS increases alveolar macrophage numbers, and bone marrow recipient mice demonstrate accelerated replacement of a significant percentage of lung and alveolar macrophages by donor cells (1, 114, 146). With inflammation, alveolar macrophage recruitment and differentiation occur due to local secretion of growth factors (GM-CSF and M-CSF) and other cytokines (CCL2-CCR2 axis), and through communication with activated resident lung macrophages and epithelial cells (65, 170). During the inflammatory response, local cytokine profiles influence transition toward either an M1 or M2 macrophage phenotype as follows: secreted IFN-γ or GM-CSF (M1, CAM), IL-4 or IL-13 (M2, AAM), or cell-based IL-25 or IL-33 (M2) (56, 65).
Human.
INFLAMMATION ALTERS RECRUITMENT.
As is the case in murine models, regulation of human alveolar macrophage replacement may be based on the severity of inflammation. In patients with ARDS, Rosseau et al. (156) observed an increase in total BAL macrophages without a concomitant increase in macrophage proliferation assessed by staining of a nuclear antigen, Ki67, and suggested that MCP-1 may mediate macrophage recruitment. Studies in human recipients of allogeneic bone-marrow transplants (allo-BMT) demonstrate recruitment of blood monocyte precursors, followed by local proliferation to repopulate lung leukocytes (137, 186). Alveolar macrophage size and numbers were similar to nonsmoking controls until day 50 after allo-BMT; after day 50, a threefold increase in alveolar macrophage numbers occurred in the transplant recipients, along with a significant increase in expression of proliferating cell nuclear antigen (PNCA) (137). The importance of local proliferation in vivo is supported by in vitro studies demonstrating GM-CSF-enhanced proliferation of alveolar macrophages (136). Despite utilizing recruitment and local proliferation to replace host macrophages, patients remain susceptible to infection after transplantation. Donor macrophages express less HLA-DR and mount an inadequate response to inflammatory stimuli, similar to endotoxin tolerance (16, 97, 129, 130). However, susceptibility to infection may be related to pharmacological immunosuppression or decreased presence of alveolar neutrophils rather than inadequate numbers or function of macrophages.
IMPLICATIONS OF A GM-CSF TRIAL.
Based on a study observing higher levels of BAL GM-CSF in patients with improved outcome following ARDS (111), as well as the effects of GM-CSF on macrophage proliferation and maturation, alveolar surfactant removal, epithelial cell survival, and protection in experimental lung injury models (171), GM-CSF was proposed as therapy in ARDS patients. However, a randomized clinical trial of systemic GM-CSF in patients with ARDS did not demonstrate a benefit on the primary outcome of ventilator-free days or on mortality (21); GM-CSF did increase blood leukocytes, but alveolar leukocytes were not enumerated or phenotyped. GM-CSF, along with granulocyte colony-stimulating factor (G-CSF), can inhibit neutrophil apoptosis in the lungs of patients with ARDS, which may have blunted any positive effects on macrophage maturation or epithelial survival in the study (111). Other therapeutic options to consider include localized tissue delivery and use of a growth factor such as M-CSF (CSF-1) capable of inducing macrophage proliferation and shifting the balance toward anti-inflammatory, proresolution M2 macrophages (70). In addition, a more rigorous assessment of inflammatory cells in the alveolar space during ARDS may help identify biomarkers critical for resolution and help determine which patients would benefit most from therapy.
Macrophage Function: Murine
Homeostasis.
During homeostasis, alveolar macrophages phagocytose dust, allergens, and senescent cells and perform other trophic duties (56). Compared with other tissue macrophages, alveolar macrophages possess a reduced capacity to present antigen (190). Expression of M2, anti-inflammatory proteins, and a reduced capacity to present antigen may be adaptive responses to limit deleterious inflammation in the presence of constant low-level stimuli (185). However, alveolar macrophages also express higher levels of proinflammatory genes even at baseline and are thus prepared to initiate a prompt innate immune response when necessary (56, 57).
Inflammation.
With pattern recognition of sufficient foreign substances, alveolar macrophages initiate a proinflammatory cascade to clear airway and alveolar pathogens and propagate the innate immune response. Early response cytokines including IFN-γ, TNF-α, and IL-1β potentiate chemokine secretion to recruit neutrophils, exudative macrophages, and lymphocytes (63). Along with neutrophils, recruited macrophages are essential for pathogen clearance through release of toxic mediators, reactive species, and phagocytosis of infected particles or other inflammatory debris. In the absence of intrinsic anti-inflammatory pathways (3) or with persistent inflammatory stimuli, tissue resident and recruited proinflammatory, M1 macrophages can directly induce tissue damage through excessive release of toxic species including nitric oxide (NO), superoxide, and matrix metalloproteinases (MMPs) and also indirectly augment tissue damage through excessive recruitment of neutrophils and TH17 cells. A chronic venous ulcer skin model demonstrated that excessive iron taken up by macrophages induced a persistent M1 macrophage phenotype and worse tissue injury (166). Recruited macrophages express proinflammatory genes (97, 170) but also express high levels of immunosuppressive genes (56, 57) and thus exhibit significant reprogramming potential as an adaptive response to mitigate ongoing inflammation.
Resolution and repair.
Resolution of lung inflammation and injury is an active, coordinated process dependent on pathogen clearance, reduced proinflammatory signaling, and removal of proinflammatory neutrophils and macrophages as well as transition to an anti-inflammatory and proreparative milieu. Resident and recruited macrophages are central to the resolution of lung inflammation with their ability to transition from proinflammatory to anti-inflammatory, tissue-reparative phenotypes (63, 76). Specific recruited macrophage populations have been shown to be critical for resolution. Following direct LPS or bacterial stimulus, Gr-1highCCR2high exudative macrophages migrate to the lung and express high levels of IL-1ra (IL-1 receptor antagonist) (14), a M2 marker that antagonizes the M1 cytokine IL-1β and may also induce neutrophil removal (64).
EFFEROCYTOSIS AND MACROPHAGE PHENOTYPE.
Engulfment of apoptotic inflammatory cells, or efferocytosis, shifts macrophages toward a M2, anti-inflammatory phenotype by abrogating release of proinflammatory mediators (88, 150, 194) and augmenting TGF-β (47, 72) and IL-10 production (23) to promote resolution and wound repair. Efferocytosis reduces NO by increasing the arginase-to-iNOS ratio (50, 54) via PPAR-δ and PPAR-γ signaling (50, 79, 134). Additionally, M2-derived cytokines including IL-4, IL-13, and IL-10 increase mannose receptor expression to further augment efferocytosis (7, 90). Inflammatory macrophage clearance is also important for lung injury resolution. In direct lung injury models using streptococcus (108), LPS (76), or influenza (76), inflammatory (exudative) macrophage-dependent caspase or Fas-mediated apoptosis and clearance was critical for resolution of inflammation. This is particularly important work, and a potential effect of inflammatory macrophage apoptosis on clearance of other inflammatory cell populations warrants further study.
LIPID MEDIATORS.
Macrophages secrete many classes of lipid mediators, i.e., lipoxins, resolvins, and protectins, generally via 15-lipoxygenase (15-LO)-mediated signaling to actively promote resolution of inflammation (7). Efferocytosis upregulates 15-LO expression and activity (50, 161), as do cytokines IL-4 and IL-13. By signaling through the ALX/FPR2 receptor expressed on alveolar macrophages and epithelial cells, exogenous aspirin-triggered resolvin D1 reduced murine lung injury induced by hydrochloric acid or LPS; the mechanism appears to involve dampening NF-κB activation (45, 104).
PROLONGED M1 OR M2 MACROPHAGE ACTIVATION.
Persistent activation of either M1 or M2 macrophages appears to be detrimental to wound healing and tissue repair (165, 166). Production of lipid mediators is antifibrotic, however, and can balance the profibrotic effects of a sustained M2 macrophage phenotype that can occur with prolonged IL-10 or IL-13 exposure (125, 135, 173, 174). We found that macrophage-derived iNOS (a marker for CAM or M1 activation state) was critical for resolution of experimental lung injury (33). iNOS−/− mice had unremitting lung inflammation and injury following intratracheal LPS, and alveolar macrophages demonstrated a sustained M1 phenotype. In addition to iNOS, exploration of transcription factors or pathways responsible for this transition could reveal important targets to accelerate resolution of lung inflammation and promote lung repair after injury (81). Sufficient inflammation may be necessary to trigger pathways responsible for the transition from M1 to an M2 phenotype, and further investigation is warranted to determine whether macrophage transition from M1 to M2 phenotype is critical for resolution of lung inflammation and injury.
Macrophage Function During Human ARDS
Acute, inflammatory phase.
Human studies characterizing macrophage function in ARDS are limited and primarily conjectural. Existing knowledge centers on the contribution of macrophage-derived cytokines to the injury and resolution phases of ARDS. With onset of inflammation, resident alveolar macrophages are thought to be the primary source of IL-1β and TNF-α, which then stimulate neighboring cells to propagate the inflammatory response characteristic of acute-phase ARDS. Jacobs et al. (74) found that total IL-1 and subtype IL-1β were secreted in increased amounts by alveolar macrophages from ARDS patients than from healthy patients or patients with pneumonia without ARDS; a similar pattern was observed when those alveolar macrophages were isolated and rechallenged with LPS and IFN-γ. Donnelly et al. (41) noted that increased BAL IL-8 secretion and increased alveolar macrophage IL-8 expression was associated with progression to ARDS among at-risk patients with significant systemic inflammation. Additionally, increased BAL IL-8 was associated with increased mortality in patients with established ARDS (31, 116, 121).
Resolution phase.
Any role for alveolar macrophages in resolution of ARDS remains quite speculative. Steinberg et al. (172) demonstrated that among patients with sepsis-induced ARDS, a persistent increase in alveolar neutrophils and a persistent decrease in alveolar macrophages (both by percentage of total BAL cells and by absolute number) at days 7 and 14 were associated with worse outcome. Interestingly, Matute-Bello et al. (112) found that rates of neutrophil apoptosis were low throughout phases of ARDS and did not predict survival in established ARDS patients. To our knowledge, macrophage efferocytosis or rates of macrophage apoptosis have not been assessed during any stage of human ARDS, investigation of which is limited by the fleeting presence of apoptotic inflammatory cells in animals and humans. In addition to efferocytosis, another potential mechanism for macrophage-derived resolution is through secretion of epithelial growth factors including KGF, HGF, and GM-CSF to promote epithelial repair. The cellular source(s) of these growth factors remain unknown in ARDS. Human fibroblasts have been shown to be a source of KGF in response to inflammatory stimuli (18, 149). Human macrophages may secrete HGF to help regenerate lung epithelium as was shown in a mouse influenza model (138). To enhance repair, human lung epithelial cells may be a source of GM-CSF when stimulated by macrophage-derived TNF-α as shown in murine cells by Carakova et al. (24).
Macrophage Fate
Murine.
As is the case with differentiation, phenotype, and function of alveolar macrophages, inflammation regulates macrophage cell fate. Alveolar macrophages either become apoptotic or they migrate to draining lymph nodes. Although both may occur in mice (89, 180), only macrophage apoptosis appears to be critical for resolution of acute inflammation (76).
Both pathogen-specific and host defense mechanisms can alter rates of macrophage apoptosis during the acute infectious or inflammatory period. In direct bacteria models, alveolar macrophage apoptosis may be a pathogen-derived mechanism utilized primarily by intracellular organisms (Shigella, Salmonella) to evade the immune system (66, 206). Alternatively, the host can promote alveolar macrophage apoptosis to limit intracellular bacterial replication by Mycobacterium or Chlamydia species (85, 95, 145), or to enhance clearance of extracellular bacteria such as Streptococcus (39). Recruited macrophages initially behave similarly to neutrophils in their ability to kill pathogens and in their susceptibility to apoptosis. Apoptosis of recruited macrophages can also enhance killing of engulfed bacterial pathogens and help terminate proinflammatory responses (47, 95, 107, 198). Additionally, as inflammation resolves, recruited macrophages can further differentiate into a mature phenotype to increase their half-life far beyond resolution of lung inflammation (40).
Migration of alveolar macrophages to draining lung lymph nodes has been suggested in the context of pathogen removal and antigen transport, but not during resolution of significant inflammation; this contrasts the fate of peritoneal macrophages following resolution of inflammatory peritonitis (13). Kirby et al. (89) delivered low-dose fluorescently labeled Streptococcus (104 CFUs, intratracheal) to mice, which caused mild lung inflammation without neutrophil alveolitis, and demonstrated increased migration of alveolar macrophages to draining lymph nodes within 6–12 h. In contrast, a model using an intratracheal LPS dose sufficient to induce prominent neutrophil alveolitis did not demonstrate significant alveolar macrophage migration to draining lymph nodes when assessed 6 days later (76); notably, markers to identify macrophages were different between the two studies. Wissinger et al. (200) suggested that limiting alveolar macrophage migration to lymph nodes may shield the immune system from the constant barrage of inhaled antigens. Some controversy exists as to whether alveolar macrophages migrate to lymph nodes in any circumstance, or rather do cells with a similar phenotype arise in the lymph nodes by alternate means (35, 75).
Human.
To our knowledge, there are no human studies assessing alveolar macrophage fate during resolution phases of ARDS. The study by Steinberg et al. (172) suggested that an insufficient number of alveolar macrophages impaired ARDS survival, implying either protracted recruitment or accelerated apoptosis and removal; neither mechanism has been investigated in human studies.
Macrophage Cellular Interactions During Inflammation and Resolution
Alveolar macrophages interact with other macrophage subtypes and several other cell types during inflammation, resolution, and tissue repair. We will highlight four main interactions: macrophage-inflammatory cell (neutrophil and recruited macrophages), macrophage-epithelial, macrophage-lymphocyte, and macrophage-mesenchymal stem cell. Macrophages do interact with additional cell types during inflammation and resolution, such as macrophage-derived reactive oxygen species causing damage to endothelial cells (48); however, we will focus on those four interactions.
Macrophage-Neutrophil/Recruited Macrophage
Murine.
To initiate the immune response, binding of pattern recognition receptors activates alveolar macrophage NF-κB and IRF-5 transcription factors leading to secretion of early response cytokines type 1 interferon, TNF-α, and IL-1β to stimulate chemokine secretion from neighboring macrophages and other cells (63). To potentiate the innate immune response, macrophages secrete chemokines macrophage inflammatory protein-2 (MIP-2) and keratinocyte-derived chemokine (KC) to recruit neutrophils to the alveolar space (42, 158, 178, 196, 202), and secrete MCP-1 (CCL2) to recruit monocytes (113, 115). Multiple pathways within macrophages downstream of Toll-like receptor signaling appear to be involved in this response. Hasan et al. (62) and Cornell et al. (29) recently demonstrated that the macrophage proteins geranylgeranyl transferase and mitogen-activated protein kinase phosphatase 2, respectively, mediate neutrophil recruitment in experimental ARDS models. After rapid decline of MIP-2 and KC, persistent, or second-phase, neutrophil alveolitis can be maintained by inflammatory macrophages (38, 113) and by SDF-1 (CXCL12) secreted primarily by alveolar epithelial cells (AECs) (151).
Transition to the resolution phase involves a series of coordinated events primarily regulated by resident and recruited macrophage populations (6, 63, 64). Macrophage-derived MMPs 1, 3, 8, and 12 cleave CXC chemokines (MIP-2) and CC chemokines (CCL2, 3, 7, 8, 9, and 12) to mitigate neutrophil and exudate macrophage recruitment in various lung injury models (37, 118, 119, 139, 153).
A cornerstone stage in resolution of inflammation is apoptosis of neutrophils and clearance by macrophages (20, 160, 179). Studies have demonstrated the importance of neutrophil apoptosis for resolution in experimental lung injury models (34, 117). During acute inflammation, survival signals G-CSF and IL-1β enhance the half-life of neutrophils (83); however, IL-1ra, a prominent source of which is recruited macrophages, helps modulate neutrophil influx and augment neutrophil apoptosis. High levels of macrophage-derived TNF-α can also induce neutrophil apoptosis (192), as can alveolar macrophage death ligand TRAIL expression (117). Rapid recognition of apoptotic neutrophils by macrophages hastens removal to limit tissue damage from contents released by necrotic neutrophils. Even though impaired clearance of apoptotic neutrophils can exacerbate inflammation and worsens mortality (183), studies have yet to definitively demonstrate that enhancing clearance of apoptotic neutrophils can improve outcome in ALI.
Human.
ARDS studies investigating important macrophage communication with other inflammatory cells have focused primarily on the acute phase, where alveolar neutrophil recruitment is predicated largely on IL-8 secretion via alveolar macrophages (41). To attempt to assess the contribution of circulating monocytes toward alveolar neutrophil recruitment, Barr et al. (10) randomized patients to receive leukapheresis of mononuclear cells at intervals after inhaled LPS, followed by a BAL at 9 h. Leukapheresis was successful in significantly reducing, but not eliminating, monocytes (and lymphocytes, platelets) in the blood and BAL but did not significantly reduce circulating or alveolar neutrophil numbers. BAL and serum levels of IL-8, the predominant neutrophil chemokine, and MCP-1, the predominant monocyte chemokine, were similar in the sham and leukapheresis groups (10). The study was limited by a short exposure interval, relatively mild inflammation, incomplete elimination of monocytes, and inability to assess the contribution of resident alveolar macrophages.
Differences in the alveolar milieu ARDS during acute vs. resolution phases may alter clearance of inflammatory cells. Compared with BAL fluid from patients in late-phase ARDS when inflammation was resolving, only BAL fluid from patients in early-phase ARDS inhibited neutrophil apoptosis in vitro (111), thought to be mediated by IL-8 or G-CSF (2). Interestingly, rates of alveolar neutrophil apoptosis were not predictive of outcome in human ARDS subjects; however, a direct assessment of macrophage efferocytosis, or actual clearance of apoptotic neutrophils, was not performed (111).
Macrophage-Epithelial Cell
Murine.
ONSET OF INFLAMMATION.
At baseline, cell contact between macrophages and the alveolar epithelium appears to be regulated by epithelial integrin αvβ6 (94, 164, 200) which activates macrophage TGF-β to inhibit secretion of TNF-α and IL-6. During the innate immune response, alveolar macrophages detach from alveolar epithelial cells and secrete more TNF-α to stimulate AECs (5, 26, 163). In response to TNF-α or direct Toll-like receptor binding, AECs secrete KC and LPS-induced chemokine (LIX) to recruit PMNs (9, 78), secrete MCP-1 to recruit monocytes (143, 163, 187), and express ICAM-1 and MCP-1 to promote macrophage mobility and phagocytic potential (11, 82, 100, 124, 147, 162). Viruses with direct epithelial infectivity, such as influenza A, enhance monocyte recruitment by inducing AECs to secrete CCL2 and CCL5 and by facilitating monocyte β1 and 2 integrin contact with integrin-associated protein (CD47), ICAM-1, VCAM-1, and junctional adhesion molecule-on the AEC surface (12, 63, 157). After monocytes and macrophages migrate into the alveolar space during acute inflammation, cellular interactions are not well understood. Mice deficient in epithelial integrin αvβ6 demonstrated persistent alveolar macrophage activation due to decreased lung TGF-β (132), but specific interactions occurring between inflammatory macrophages and the apical AEC surface are not known.
Resolution and repair.
To dampen inflammation and transition toward resolution, exudative macrophages secrete IL-1ra, which competitively inhibits IL-1β binding to IL-1r1, its receptor on AECs, reducing AEC damage and decreasing ICAM-1 secretion. With reduced stimulus from ICAM-1, macrophages secrete less MIP-2; reduced ICAM-1 and MIP-2 attenuate alveolar neutrophil recruitment (22).
To repair and regenerate the airway and alveolar epithelial barriers, type II AECs and Clara cells are progenitor cells that regulate proliferation and differentiation into type I AECs. Traditionally, primary lung epithelial cells have been easier to isolate from rats, so we have included some of this important work. In murine or rat lung injury models, lung growth factors, matrix components, and other soluble mediators can contribute to epithelial repair by mitogenic and nonmitogenic pathways, but the cellular source(s) are frequently not macrophages or in some cases remain unknown. According to more definitive work in the muscle (105), gut (175), kidney (102), and heart (61), M2-predominant lung macrophages may also contribute to lung epithelial repair, but a direct effect has not been determined by use of macrophage depletion studies. Morimoto et al. (131) postulated an indirect effect of hepatocyte growth factor (HGF) based on macrophage production after efferocytosis of apoptotic neutrophils in a bacterial pneumonia model; efferocytosis can promote a M2 macrophage phenotype, but this was not specifically determined. HGF is a potent mitogen for rat alveolar epithelial cells (109, 149), but specific mechanisms of epithelial recovery in ALI models have not been thoroughly examined. Even macrophage-derived TNF-α, a predominantly proinflammatory cytokine, can stimulate AEC-production of GM-CSF, leading to reparative downstream signaling (24), strengthening support for GM-CSF as an integral cytokine in epithelial repair (69, 148). Proresolution, M2 macrophages have also been shown to secrete EGF and IL-1ra to enhance alveolar fluid clearance by increasing active sodium transport through apical epithelial Na+ (ENaC) and basolateral Na-K-ATPase channels (63, 133, 184). In a ventilator-induced lung injury model, antibody-mediated blockade of MMP-2 impaired epithelial wound closure (MLE-12 cell line); MMP-2 was expressed by epithelial cells and intra-alveolar inflammatory cells (55).
Human
Macrophage contribution to epithelial repair has been described in animal models, but direct evidence in humans is incomplete and hard to interpret. KGF and HGF, which can be secreted by multiple cell types including macrophages, were present in pulmonary edema fluid in the acute phase of ALI (193). HGF was significantly higher in ALI patients compared with those with hydrostatic pulmonary edema. Somewhat surprisingly, increased HGF was associated with increased mortality; the authors hypothesized that higher HGF levels were present as a failed attempt to combat the severity of ALI, as the mortality in this group was 73%. Geiser et al. (52) used BAL fluid from ARDS patients to test epithelial repair following scratch assay in an epithelial cell line. Interestingly, he found that IL-1β, a macrophage-derived cytokine critical for the acute phase of ALI, accelerated epithelial repair especially when the fluid source originated from BAL of early (<12 h) compared with late (12–48 h) ARDS patient samples. TGF-β and KGF have also been shown to enhance epithelial repair in vitro (86). Using BAL samples from ARDS patients, Gonzalez-Lopez et al. (55) showed that inhibition of MMP-2 abrogated epithelial wound healing in A549 cells, a human alveolar epithelial cell line.
Outside of the ARDS realm, stimulated human alveolar macrophages have been shown to enhance epithelial growth. Conditioned media from silica-exposed alveolar macrophages identified platelet-derived growth factor (PDGF), insulin-like growth factor (IGF-1), and fibroblast-derived growth factor (FGF) as potential mitogens toward rat type II pneumocytes on the basis of depletion studies (123).
Macrophage-Lymphocyte
Murine.
As antigen-presenting cells (APC), macrophages coordinate the adaptive immune response using cognate antigenic peptide presentation by MHC molecules to T cell receptors (TCR) at the immunological synapse (27, 28). APCs synergize with the TCR to form a crucial ligand-receptor cosignaling molecule interaction that regulates T cell activation, differentiation, effector function, and cell survival using an expanding list of ligand-receptor combinations (25, 28, 93). In murine models of direct lung injury including intratracheal LPS (33, 34, 67, 92), low-dose intratracheal LPS plus moderate hyperoxia (4), gram-negative or gram-positive bacterial infection (106, 165), and a sepsis model of indirect lung injury (141, 142), macrophage expression of costimulatory molecules including MHC II, CD40, CD80, and CD86 helped augment lung inflammation through innate and adaptive immune responses. The low oxygen tension of the lung interstitium may blunt T cell proinflammatory cytokine (IFN-γ) release to mitigate collateral tissue damage as an adaptive mechanism (46, 144, 167).
Signaling and recruitment of lymphocytes is an emerging, critical component of lung injury resolution. Although a number of studies have demonstrated lung and alveolar influx of lymphocytes during various stages of inflammation and resolution (49, 81, 93, 154), only a few have demonstrated important roles for lymphocytes in lung injury resolution. We have shown that a subset of lymphocytes, regulatory T cells (Tregs, defined as CD4+CD25+ and transcription factor Foxp3+), can enhance neutrophil apoptosis and decrease the fibroproliferative response in LPS-induced murine lung injury models (34, 51, 197). Proresolution Treg effects may occur through direct action and through communication with other immune cells, such as macrophages, as demonstrated in culture by a Treg-contact dependent decrease in macrophage TNF-α and increase in reparative TGF-β secretion (34). Others have suggested that lymphocyte-derived IFN-γ can stimulate alveolar macrophage MMP-9 production, which in turn activates latent TGF-β to help achieve macrophage homeostasis and adherence to the alveolar epithelium (94).
Human.
Possible interactions between macrophages and T cells in human ARDS remain purely theoretical at present. Human Foxp3+ Tregs can induce an M2, alternatively activated macrophage phenotype and blunt their subsequent response to LPS in culture (188). In addition, alveolar Tregs are present in patients with ARDS (34), but specific function(s) remain unknown in ARDS. Somewhat limited by the practicality of isolating sufficient Tregs for therapeutic use during acute disease processes, gaining a better understanding of the reparative role for Tregs in experimental ARDS models may provide a more feasible therapeutic option in humans.
Macrophage-Mesenchymal Stem Cell
Murine.
Mesenchymal stem cells (MSCs), also known as multipotent mesenchymal stromal cells or bone marrow stromal cells (BMSCs), make up one class of adult stem cells with immunomodulatory properties. Immune suppression occurs primarily through modulation of other innate and adaptive immune cells (100), including macrophages and monocytes. MSCs have been shown to enhance macrophage phagocytic activity in mice (122). Using cecal ligation and puncture as a sepsis model, Nemeth et al. (140) showed that PGE2 secretion derived from MSCs reprogrammed alveolar macrophages to secrete IL-10, which enhanced murine survival and organ function (100, 140). In a direct lung injury model using endotoxin or live bacteria, MSCs improved survival and lung injury parameters. Alveolar MIP-2 and TNF-α levels were decreased, and alveolar IL-10 was increased, leaving the authors to postulate a macrophage effect mediated by paracrine MSC signaling (59).
Human.
MSCs present potentially attractive therapy for ARDS patients according to animal work and preliminary studies in humans. Support is also generated by studies demonstrating that human MSCs can enhance human macrophage (87) and murine monocyte (91) phagocytic activity and can reprogram macrophages toward a M2 phenotype in a coculture system (87). Human MSCs have been tested ex vivo by use of an isolated human lung perfusion system. Following LPS instillation, Lee et al. (101) demonstrated that MSCs or conditioned media derived from MSCs reduced endothelial permeability and lung wet-to-dry ratios and increased alveolar fluid clearance; specific effects on human macrophages were not assessed. A phase 1 clinical trial testing the therapeutic effect of systemic MSCs in patients with severe ARDS is in progress, with plans for phase 2 starting in 2014. KGF, a key product in MSC-conditioned media given its protective effect in experimental large-animal ALI studies (101), is also being tested in a phase 2, multicenter trial in sepsis-induced ALI in humans (32).
Conclusion
Abundant in the lung and alveolar space during steady state, macrophages are critical to the initiation and resolution of lung inflammation, during which biological signatures are defined by their differentiation, phenotype, function, and cell-cell interactions. Extensive work in animals has helped dissect numerous processes critical for the development of ARDS using experimental models. More recent work has also focused on the determinants of resolution and repair. With a myriad of potential cellular reprogramming targets, macrophages represent an attractive target for intervention at various stages of ARDS. However, our understanding of macrophage biology during human ARDS, especially during resolution, remains quite limited. More elaborate lung immunophenotyping during different phases of ARDS with a concurrent functional assessment of macrophage subsets may be critical for identifying patients in whom therapeutic intervention could be most beneficial.
GRANTS
This work was supported by NHLBI HL089346 and the Johns Hopkins Bayview Scholars Program (L. S. King), R00HL103793 (F. R. D'Alessio), FTF7280014 AHA (N. R. Aggarwal), and FAMRI YCSA (N. R. Aggarwal).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.R.A., L.S.K., and F.R.D. conception and design of research; N.R.A. interpreted results of experiments; N.R.A. and F.R.D. prepared figures; N.R.A. drafted manuscript; N.R.A., L.S.K., and F.R.D. edited and revised manuscript; N.R.A., L.S.K., and F.R.D. approved final version of manuscript.
REFERENCES
- 1.Adamson IY, Bowden DH. Role of monocytes and interstitial cells in the generation of alveolar macrophages II. Kinetic studies after carbon loading. Lab Invest 42: 518–524, 1980 [PubMed] [Google Scholar]
- 2.Aggarwal A, Baker CS, Evans TW, Haslam PL. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J 15: 895–901, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal NR, D'Alessio FR, Eto Y, Chau E, Avalos C, Waickman AT, Garibaldi BT, Mock JR, Files DC, Sidhaye V, Polotsky VY, Powell J, Horton M, King LS. Macrophage A2A adenosinergic receptor modulates oxygen-induced augmentation of murine lung injury. Am J Respir Cell Mol Biol 48: 635–646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal NR, D'Alessio FR, Tsushima K, Files DC, Damarla M, Sidhaye VK, Fraig MM, Polotsky VY, King LS. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 298: L371–L381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen GL, Menendez IY, Ryan MA, Mazor RL, Wispe JR, Fiedler MA, Wong HR. Hyperoxia synergistically increases TNF-α-induced interleukin-8 gene expression in A549 cells. Am J Physiol Lung Cell Mol Physiol 278: L253–L260, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Ariel A, Maridonneau-Parini I, Rovere-Querini P, Levine JS, Muhl H. Macrophages in inflammation and its resolution. Front Immunol 3: 324, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariel A, Serhan CN. New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front Immunol 3: 4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Balamayooran G, Batra S, Cai S, Mei J, Worthen GS, Penn AL, Jeyaseelan S. Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. Am J Respir Cell Mol Biol 47: 104–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barr LC, Brittan M, Morris AC, McAuley DF, McCormack C, Fletcher AM, Richardson H, Connell M, Patel D, Wallace WA, Rossi AG, Davidson DJ, Manson L, Turner M, Hirani N, Walsh TS, Anderson NH, Dhaliwal K, Simpson AJ. A randomized controlled trial of peripheral blood mononuclear cell depletion in experimental human lung inflammation. Am J Respir Crit Care Med 188: 449–455, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Beck-Schimmer B, Madjdpour C, Kneller S, Ziegler U, Pasch T, Wuthrich RP, Ward PA, Schimmer RC. Role of alveolar epithelial ICAM-1 in lipopolysaccharide-induced lung inflammation. Eur Respir J 19: 1142–1150, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Beck-Schimmer B, Schimmer RC, Madjdpour C, Bonvini JM, Pasch T, Ward PA. Hypoxia mediates increased neutrophil and macrophage adhesiveness to alveolar epithelial cells. Am J Respir Cell Mol Biol 25: 780–787, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol 157: 2577–2585, 1996 [PubMed] [Google Scholar]
- 14.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol 181: 3733–3739, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Bhardwaj RS, Zotz C, Zwadlo-Klarwasser G, Roth J, Goebeler M, Mahnke K, Falk M, Meinardus-Hager G, Sorg C. The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur J Immunol 22: 1891–1897, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 30: 475–487, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Blusse van Oud Alblas A, van Furth R. The origin of pulmonary macrophages. Immunobiology 161: 186–192, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Brauchle M, Angermeyer K, Hubner G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene 9: 3199–3204, 1994 [PubMed] [Google Scholar]
- 19.Brittan M, Barr L, Conway Morris A, Duffin R, Rossi F, Johnston S, Monro G, Anderson N, Rossi AG, McAuley DF, Haslett C, Hirani N, Dhaliwal K, Simpson AJ. A novel subpopulation of monocyte-like cells in the human lung after lipopolysaccharide inhalation. Eur Respir J 40: 206–214, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nat Rev Immunol 13: 59–66, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Burnham EL, Hyzy RC, Paine R, 3rd, Coley C, 2nd, Kelly AM, Quint LE, Lynch D, Janssen WJ, Moss M, Standiford TJ. Chest CT features are associated with poorer quality of life in acute lung injury survivors. Crit Care Med 41: 445–456, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler MW, Robertson I, Greene CM, O'Neill SJ, Taggart CC, McElvaney NG. Elafin prevents lipopolysaccharide-induced AP-1 and NF-κB activation via an effect on the ubiquitin-proteasome pathway. J Biol Chem 281: 34730–34735, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol 168: 1968–1977, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Cakarova L, Marsh LM, Wilhelm J, Mayer K, Grimminger F, Seeger W, Lohmeyer J, Herold S. Macrophage tumor necrosis factor-α induces epithelial expression of granulocyte-macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am J Respir Crit Care Med 180: 521–532, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Cao Q, Wang Y, Zheng D, Sun Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC. IL-10/TGF-β-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol 21: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-κB activation and TNF-α gene transcription induced by hypoxia and endotoxin. J Immunol 165: 1013–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr, Wynn TA, Gause WC. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 18: 260–266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13: 227–242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornell TT, Fleszar A, McHugh W, Blatt NB, Le Vine AM, Shanley TP. Mitogen-activated protein kinase phosphatase 2, MKP-2, regulates early inflammation in acute lung injury. Am J Physiol Lung Cell Mol Physiol 303: L251–L258, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol 7: 255–266, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Cross LJ, Matthay MA. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin 27: 355–377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cross LJ, O'Kane CM, McDowell C, Elborn JJ, Matthay MA, McAuley DF. Keratinocyte growth factor in acute lung injury to reduce pulmonary dysfunction—a randomised placebo-controlled trial (KARE): study protocol. Trials 14: 51, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Alessio FR, Tsushima K, Aggarwal NR, Mock JR, Eto Y, Garibaldi BT, Files DC, Avalos CR, Rodriguez JV, Waickman AT, Reddy SP, Pearse DB, Sidhaye VK, Hassoun PM, Crow MT, King LS. Resolution of experimental lung injury by monocyte-derived inducible nitric oxide synthase. J Immunol 189: 2234–2245, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 119: 2898–2913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 14: 986–995, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun 4: 1886, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, −7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood 112: 3455–3464, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Dhaliwal K, Scholefield E, Ferenbach D, Gibbons M, Duffin R, Dorward DA, Morris AC, Humphries D, MacKinnon A, Wilkinson TS, Wallace WA, van Rooijen N, Mack M, Rossi AG, Davidson DJ, Hirani N, Hughes J, Haslett C, Simpson AJ. Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am J Respir Crit Care Med 186: 514–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, Whyte MK. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol 171: 5380–5388, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Dockrell DH, Whyte MK. Regulation of phagocyte lifespan in the lung during bacterial infection. J Leukoc Biol 79: 904–908, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Grant IS, Pollok AJ, Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 341: 643–647, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Duffy AJ, Nolan B, Sheth K, Collette H, De M, Bankey PE. Inhibition of alveolar neutrophil immigration in endotoxemia is macrophage inflammatory protein 2 independent. J Surg Res 90: 51–57, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Edelson JD, MacFadden DK, Klein M, Rebuck AS. Autofluorescence of alveolar macrophages: problems and potential solutions. Med Hypotheses 17: 403–407, 1985 [DOI] [PubMed] [Google Scholar]
- 44.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80: 1298–1307, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer MA, Levy BD. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol 6: 256–266, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med 364: 656–665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest 101: 890–898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farley KS, Wang L, Mehta S. Septic pulmonary microvascular endothelial cell injury: role of alveolar macrophage NADPH oxidase. Am J Physiol Lung Cell Mol Physiol 296: L480–L488, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med 152: 1666–1672, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-β, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem 281: 38376–38384, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Garibaldi BT, D'Alessio FR, Mock JR, Files DC, Chau E, Eto Y, Drummond MB, Aggarwal NR, Sidhaye V, King LS. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol 48: 35–43, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geiser T, Atabai K, Jarreau PH, Ware LB, Pugin J, Matthay MA. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1β-dependent mechanism. Am J Respir Crit Care Med 163: 1384–1388, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Godleski JJ, Brain JD. The origin of alveolar macrophages in mouse radiation chimeras. J Exp Med 136: 630–643, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 18: 1716–1718, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Lopez A, Astudillo A, Garcia-Prieto E, Fernandez-Garcia MS, Lopez-Vazquez A, Batalla-Solis E, Taboada F, Fueyo A, Albaiceta GM. Inflammation and matrix remodeling during repair of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 301: L500–L509, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179: 1855–1863, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 296: L936–L946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci USA 108: 1827–1832, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasan Z, Rahman M, Palani K, Syk I, Jeppsson B, Thorlacius H. Geranylgeranyl transferase regulates CXC chemokine formation in alveolar macrophages and neutrophil recruitment in septic lung injury. Am J Physiol Lung Cell Mol Physiol 304: L221–L229, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2: 65, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, Steinmueller M, Lohmeyer J. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med 183: 1380–1390, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel WA, Mack M, Srivastava M, Seeger W, Maus UA, Lohmeyer J. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol 177: 1817–1824, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA 96: 2396–2401, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol 4: 1223–1229, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Holt PG, Warner LA, Papadimitriou JM. Alveolar macrophages: functional heterogeneity within macrophage populations from rat lung. Aust J Exp Biol Med Sci 60: 607–618, 1982 [DOI] [PubMed] [Google Scholar]
- 69.Huffman Reed JA, Rice WR, Zsengeller ZK, Wert SE, Dranoff G, Whitsett JA. GM-CSF enhances lung growth and causes alveolar type II epithelial cell hyperplasia in transgenic mice. Am J Physiol Lung Cell Mol Physiol 273: L715–L725, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119: 1810–1820, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Hume DA, Pavli P, Donahue RE, Fidler IJ. The effect of human recombinant macrophage colony-stimulating factor (CSF-1) on the murine mononuclear phagocyte system in vivo. J Immunol 141: 3405–3409, 1988 [PubMed] [Google Scholar]
- 72.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Invest 109: 41–50, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115: e10–e19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobs RF, Tabor DR, Burks AW, Campbell GD. Elevated interleukin-1 release by human alveolar macrophages during the adult respiratory distress syndrome. Am Rev Respir Dis 140: 1686–1692, 1989 [DOI] [PubMed] [Google Scholar]
- 75.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol 176: 3578–3584, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 184: 547–560, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, Maizels RM, Hume DA, Allen JE. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med 210: 2477–2491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 32: 531–539, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johann AM, von Knethen A, Lindemann D, Brune B. Recognition of apoptotic cells by macrophages activates the peroxisome proliferator-activated receptor-γ and attenuates the oxidative burst. Cell Death Differ 13: 1533–1540, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Johnson KJ, Ward PA, Striker G, Kunkel R. A study of the origin of pulmonary macrophages using the Chediak-Higashi marker. Am J Pathol 101: 365–374, 1980 [PMC free article] [PubMed] [Google Scholar]
- 81.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol 47: 417–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kannan S, Huang H, Seeger D, Audet A, Chen Y, Huang C, Gao H, Li S, Wu M. Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. aeruginosa infection. PLoS One 4: e4891, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kantari C, Pederzoli-Ribeil M, Witko-Sarsat V. The role of neutrophils and monocytes in innate immunity. Contrib Microbiol 15: 118–146, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Kasahara K, Matsumura Y, Ui K, Komatsu Y, Mikasa K, Kita E. Intranasal priming of newborn mice with microbial extracts increases opsonic factors and mature CD11c+ cells in the airway. Am J Physiol Lung Cell Mol Physiol 303: L834–L843, 2012 [DOI] [PubMed] [Google Scholar]
- 85.Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun 65: 298–304, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kheradmand F, Folkesson HG, Shum L, Derynk R, Pytela R, Matthay MA. Transforming growth factor-α enhances alveolar epithelial cell repair in a new in vitro model. Am J Physiol Lung Cell Mol Physiol 267: L728–L738, 1994 [DOI] [PubMed] [Google Scholar]
- 87.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37: 1445–1453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 21: 643–653, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Kirby AC, Coles MC, Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol 183: 1983–1989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Front Immunol 2: 57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 302: L1003–L1013, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol 12: 231–238, 2011 [DOI] [PubMed] [Google Scholar]
- 93.Kugathasan K, Roediger EK, Small CL, McCormick S, Yang P, Xing Z. CD11c+ antigen presenting cells from the alveolar space, lung parenchyma and spleen differ in their phenotype and capabilities to activate naive and antigen-primed T cells. BMC Immunol 9: 48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambrecht BN. Alveolar macrophage in the driver's seat. Immunity 24: 366–368, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7: 433–444, 1997 [DOI] [PubMed] [Google Scholar]
- 96.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113: 963–972, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol 179: 3488–3494, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol 70: 163–170, 2001 [PubMed] [Google Scholar]
- 99.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11: 750–761, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Lee JH, Del Sorbo L, Uhlig S, Porro GA, Whitehead T, Voglis S, Liu M, Slutsky AS, Zhang H. Intercellular adhesion molecule-1 mediates cellular cross-talk between parenchymal and immune cells after lipopolysaccharide neutralization. J Immunol 172: 608–616, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106: 16357–16362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang J, Jung Y, Tighe RM, Xie T, Liu N, Leonard M, Gunn MD, Jiang D, Noble PW. A macrophage subpopulation recruited by CC chemokine ligand-2 clears apoptotic cells in noninfectious lung injury. Am J Physiol Lung Cell Mol Physiol 302: L933–L940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir Res 13: 110, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu H, Huang D, Ransohoff RM, Zhou L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J 25: 3344–3355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004 [DOI] [PubMed] [Google Scholar]
- 107.Marriott HM, Bingle CD, Read RC, Braley KE, Kroemer G, Hellewell PG, Craig RW, Whyte MK, Dockrell DH. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Invest 115: 359–368, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marriott HM, Hellewell PG, Cross SS, Ince PG, Whyte MK, Dockrell DH. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J Immunol 177: 6480–6488, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mason RJ, Leslie CC, McCormick-Shannon K, Deterding RR, Nakamura T, Rubin JS, Shannon JM. Hepatocyte growth factor is a growth factor for rat alveolar type II cells. Am J Respir Cell Mol Biol 11: 561–567, 1994 [DOI] [PubMed] [Google Scholar]
- 110.Matute-Bello G, Lee JS, Frevert CW, Liles WC, Sutlief S, Ballman K, Wong V, Selk A, Martin TR. Optimal timing to repopulation of resident alveolar macrophages with donor cells following total body irradiation and bone marrow transplantation in mice. J Immunol Methods 292: 25–34, 2004 [DOI] [PubMed] [Google Scholar]
- 111.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Hudson LD, Martin TR. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med 28: 1–7, 2000 [DOI] [PubMed] [Google Scholar]
- 112.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 156: 1969–1977, 1997 [DOI] [PubMed] [Google Scholar]
- 113.Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlondorff D, Seeger W, Lohmeyer J. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med 166: 268–273, 2002 [DOI] [PubMed] [Google Scholar]
- 114.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol 35: 227–235, 2006 [DOI] [PubMed] [Google Scholar]
- 115.Maus UA, Koay MA, Delbeck T, Mack M, Ermert M, Ermert L, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. Am J Physiol Lung Cell Mol Physiol 282: L1245–L1252, 2002 [DOI] [PubMed] [Google Scholar]
- 116.McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care 12: R41, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGrath EE, Marriott HM, Lawrie A, Francis SE, Sabroe I, Renshaw SA, Dockrell DH, Whyte MK. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol 90: 855–865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 289: 1202–1206, 2000 [DOI] [PubMed] [Google Scholar]
- 119.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 100: 1160–1167, 2002 [PubMed] [Google Scholar]
- 120.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci USA 110: 17253–17258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 β and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 107: 1062–1073, 1995 [DOI] [PubMed] [Google Scholar]
- 122.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 182: 1047–1057, 2010 [DOI] [PubMed] [Google Scholar]
- 123.Melloni B, Lesur O, Bouhadiba T, Cantin A, Martel M, Begin R. Effect of exposure to silica on human alveolar macrophages in supporting growth activity in type II epithelial cells. Thorax 51: 781–786, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mendez MP, Morris SB, Wilcoxen S, Greeson E, Moore B, Paine R., 3rd. Shedding of soluble ICAM-1 into the alveolar space in murine models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 290: L962–L970, 2006 [DOI] [PubMed] [Google Scholar]
- 125.Meneghin A, Hogaboam CM. Infectious disease, the innate immune response, and fibrosis. J Clin Invest 117: 530–538, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med 209: 1153–1165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meredith MM, Liu K, Kamphorst AO, Idoyaga J, Yamane A, Guermonprez P, Rihn S, Yao KH, Silva IT, Oliveira TY, Skokos D, Casellas R, Nussenzweig MC. Zinc finger transcription factor zDC is a negative regulator required to prevent activation of classical dendritic cells in the steady state. J Exp Med 209: 1583–1593, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of the macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49: 503–510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mokart D, Guery BP, Bouabdallah R, Martin C, Blache JL, Arnoulet C, Mege JL. Deactivation of alveolar macrophages in septic neutropenic ARDS. Chest 124: 644–652, 2003 [DOI] [PubMed] [Google Scholar]
- 130.Mokart D, Kipnis E, Guerre-Berthelot P, Vey N, Capo C, Sannini A, Brun JP, Blache JL, Mege JL, Blaise D, Guery BP. Monocyte deactivation in neutropenic acute respiratory distress syndrome patients treated with granulocyte colony-stimulating factor. Crit Care 12: R17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, Nagatake T. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 24: 608–615, 2001 [DOI] [PubMed] [Google Scholar]
- 132.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin αvβ6-mediated TGF-β activation causes Mmp12-dependent emphysema. Nature 422: 169–173, 2003 [DOI] [PubMed] [Google Scholar]
- 133.Morty RE, Eickelberg O, Seeger W. Alveolar fluid clearance in acute lung injury: what have we learned from animal models and clinical studies? Intensive Care Med 33: 1229–1240, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, Nguyen KD, Steinman L, Michie SA, Chawla A. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med 15: 1266–1272, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nakata K, Akagawa KS, Fukayama M, Hayashi Y, Kadokura M, Tokunaga T. Granulocyte-macrophage colony-stimulating factor promotes the proliferation of human alveolar macrophages in vitro. J Immunol 147: 1266–1272, 1991 [PubMed] [Google Scholar]
- 137.Nakata K, Gotoh H, Watanabe J, Uetake T, Komuro I, Yuasa K, Watanabe S, Ieki R, Sakamaki H, Akiyama H, Kudoh S, Naitoh M, Satoh H, Shimada K. Augmented proliferation of human alveolar macrophages after allogeneic bone marrow transplantation. Blood 93: 667–673, 1999 [PubMed] [Google Scholar]
- 138.Narasaraju T, Ng HH, Phoon MC, Chow VT. MCP-1 antibody treatment enhances damage and impedes repair of the alveolar epithelium in influenza pneumonitis. Am J Respir Cell Mol Biol 42: 732–743, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nelson MP, Christmann BS, Dunaway CW, Morris A, Steele C. Experimental Pneumocystis lung infection promotes M2a alveolar macrophage-derived MMP12 production. Am J Physiol Lung Cell Mol Physiol 303: L469–L475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, Karulf MR, Rom WN, Weiden MD, Gold JA. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PLoS One 4: e6600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, Gold JA. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am J Respir Crit Care Med 177: 301–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O'Brien AD, Standiford TJ, Christensen PJ, Wilcoxen SE, Paine R., 3rd. Chemotaxis of alveolar macrophages in response to signals derived from alveolar epithelial cells. J Lab Clin Med 131: 417–424, 1998 [DOI] [PubMed] [Google Scholar]
- 144.Ohta A, Diwanji R, Kini R, Subramanian M, Sitkovsky M. In vivo T cell activation in lymphoid tissues is inhibited in the oxygen-poor microenvironment. Front Immunol 2: 27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ojcius DM, Souque P, Perfettini JL, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol 161: 4220–4226, 1998 [PubMed] [Google Scholar]
- 146.Ojielo CI, Cooke K, Mancuso P, Standiford TJ, Olkiewicz KM, Clouthier S, Corrion L, Ballinger MN, Toews GB, Paine R, 3rd, Moore BB. Defective phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol 171: 4416–4424, 2003 [DOI] [PubMed] [Google Scholar]
- 147.Paine R, 3rd, Morris SB, Jin H, Baleeiro CE, Wilcoxen SE. ICAM-1 facilitates alveolar macrophage phagocytic activity through effects on migration over the AEC surface. Am J Physiol Lung Cell Mol Physiol 283: L180–L187, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Paine R, 3rd, Wilcoxen SE, Morris SB, Sartori C, Baleeiro CE, Matthay MA, Christensen PJ. Transgenic overexpression of granulocyte macrophage-colony stimulating factor in the lung prevents hyperoxic lung injury. Am J Pathol 163: 2397–2406, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Panos RJ, Rubin JS, Csaky KG, Aaronson SA, Mason RJ. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest 92: 969–977, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Patel VA, Longacre A, Hsiao K, Fan H, Meng F, Mitchell JE, Rauch J, Ucker DS, Levine JS. Apoptotic cells, at all stages of the death process, trigger characteristic signaling events that are divergent from and dominant over those triggered by necrotic cells: implications for the delayed clearance model of autoimmunity. J Biol Chem 281: 4663–4670, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, Rai PR, Brown KK, Weiss DJ, Poynter ME, Suratt BT. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol 178: 8148–8157, 2007 [DOI] [PubMed] [Google Scholar]
- 152.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol 156: 191–211, 1994 [DOI] [PubMed] [Google Scholar]
- 153.Quintero PA, Knolle MD, Cala LF, Zhuang Y, Owen CA. Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1 α to reduce acute lung inflammation and injury in mice. J Immunol 184: 1575–1588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol 182: 7264–7271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med 209: 139–155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rosseau S, Hammerl P, Maus U, Walmrath HD, Schutte H, Grimminger F, Seeger W, Lohmeyer J. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 279: L25–L35, 2000 [DOI] [PubMed] [Google Scholar]
- 157.Rosseau S, Selhorst J, Wiechmann K, Leissner K, Maus U, Mayer K, Grimminger F, Seeger W, Lohmeyer J. Monocyte migration through the alveolar epithelial barrier: adhesion molecule mechanisms and impact of chemokines. J Immunol 164: 427–435, 2000 [DOI] [PubMed] [Google Scholar]
- 158.Rovai LE, Herschman HR, Smith JB. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol 64: 494–502, 1998 [DOI] [PubMed] [Google Scholar]
- 159.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol 19: 746–750, 2001 [DOI] [PubMed] [Google Scholar]
- 160.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 83: 865–875, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur J Immunol 41: 366–379, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schmal H, Czermak BJ, Lentsch AB, Bless NM, Beck-Schimmer B, Friedl HP, Ward PA. Soluble ICAM-1 activates lung macrophages and enhances lung injury. J Immunol 161: 3685–3693, 1998 [PubMed] [Google Scholar]
- 163.Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-α during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 293: L105–L113, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Sheppard D. Roles of αv integrins in vascular biology and pulmonary pathology. Curr Opin Cell Biol 16: 552–557, 2004 [DOI] [PubMed] [Google Scholar]
- 165.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121: 985–997, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1α and adenosine receptors. Nat Rev Immunol 5: 712–721, 2005 [DOI] [PubMed] [Google Scholar]
- 168.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med 210: 775–788, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Spence S, Fitzsimons A, Boyd CR, Kessler J, Fitzgerald D, Elliott J, Gabhann JN, Smith S, Sica A, Hams E, Saunders SP, Jefferies CA, Fallon PG, McAuley DF, Kissenpfennig A, Johnston JA. Suppressors of cytokine signaling 2 and 3 diametrically control macrophage polarization. Immunity 38: 66–78, 2012 [DOI] [PubMed] [Google Scholar]
- 170.Srivastava M, Jung S, Wilhelm J, Fink L, Buhling F, Welte T, Bohle RM, Seeger W, Lohmeyer J, Maus UA. The inflammatory vs. constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J Immunol 175: 1884–1893, 2005 [DOI] [PubMed] [Google Scholar]
- 171.Standiford LR, Standiford TJ, Newstead MJ, Zeng X, Ballinger MN, Kovach MA, Reka AK, Bhan U. TLR4-dependent GM-CSF protects against lung injury in gram-negative bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol 302: L447–L454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med 150: 113–122, 1994 [DOI] [PubMed] [Google Scholar]
- 173.Strieter RM. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc Am Thorac Soc 5: 305–310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, Shanley TP. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol 300: L341–L353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takaba J, Mishima Y, Hatake K, Kasahara T. Role of bone marrow-derived monocytes/macrophages in the repair of mucosal damage caused by irradiation and/or anticancer drugs in colitis model. Mediators Inflamm 2010: 634145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tarling JD, Coggle JE. The absence of effect on pulmonary alveolar macrophage numbers during prolonged periods of monocytopenia. J Reticuloendothel Soc 31: 221–224, 1982 [PubMed] [Google Scholar]
- 177.Tarling JD, Coggle JE. Evidence for the pulmonary origin of alveolar macrophages. Cell Tissue Kinet 15: 577–584, 1982 [DOI] [PubMed] [Google Scholar]
- 178.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun 69: 2017–2024, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tauber AI. The immunological self: a centenary perspective. Perspect Biol Med 35: 74–86, 1991 [DOI] [PubMed] [Google Scholar]
- 180.Taut K, Winter C, Briles DE, Paton JC, Christman JW, Maus R, Baumann R, Welte T, Maus UA. Macrophage turnover kinetics in the lungs of mice infected with Streptococcus pneumoniae. Am J Respir Cell Mol Biol 38: 105–113, 2008 [DOI] [PubMed] [Google Scholar]
- 181.Taylor EL, Rossi AG, Dransfield I, Hart SP. Analysis of neutrophil apoptosis. Methods Mol Biol 412: 177–200, 2007 [DOI] [PubMed] [Google Scholar]
- 182.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol 23: 901–944, 2005 [DOI] [PubMed] [Google Scholar]
- 183.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 296: 155–158, 2002 [DOI] [PubMed] [Google Scholar]
- 184.Temelkovski J, Kumar RK, Maronese SE. Enhanced production of an EGF-like growth factor by parenchymal macrophages following bleomycin-induced pulmonary injury. Exp Lung Res 23: 377–391, 1997 [DOI] [PubMed] [Google Scholar]
- 185.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 170: 499–509, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science 192: 1016–1018, 1976 [DOI] [PubMed] [Google Scholar]
- 187.Thorley AJ, Ford PA, Giembycz MA, Goldstraw P, Young A, Tetley TD. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type II epithelial cells and macrophages. J Immunol 178: 463–473, 2007 [DOI] [PubMed] [Google Scholar]
- 188.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA 104: 19446–19451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tighe RM, Liang J, Liu N, Jung Y, Jiang D, Gunn MD, Noble PW. Recruited exudative macrophages selectively produce CXCL10 after noninfectious lung injury. Am J Respir Cell Mol Biol 45: 781–788, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Toews GB, Vial WC, Dunn MM, Guzzetta P, Nunez G, Stastny P, Lipscomb MF. The accessory cell function of human alveolar macrophages in specific T cell proliferation. J Immunol 132: 181–186, 1984 [PubMed] [Google Scholar]
- 191.Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, Vogel SN. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat Immunol 3: 392–398, 2002 [DOI] [PubMed] [Google Scholar]
- 192.van den Berg JM, Weyer S, Weening JJ, Roos D, Kuijpers TW. Divergent effects of tumor necrosis factor α on apoptosis of human neutrophils. J Leukoc Biol 69: 467–473, 2001 [PubMed] [Google Scholar]
- 193.Verghese GM, McCormick-Shannon K, Mason RJ, Matthay MA. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. Biologic and clinical significance. Am J Respir Crit Care Med 158: 386–394, 1998 [DOI] [PubMed] [Google Scholar]
- 194.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature 390: 350–351, 1997 [DOI] [PubMed] [Google Scholar]
- 195.von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, Holt PG, Stumbles PA. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol 175: 1609–1618, 2005 [DOI] [PubMed] [Google Scholar]
- 196.Wagner JG, Driscoll KE, Roth RA. Inhibition of pulmonary neutrophil trafficking during endotoxemia is dependent on the stimulus for migration. Am J Respir Cell Mol Biol 20: 769–776, 1999 [DOI] [PubMed] [Google Scholar]
- 197.Wang L, Zhao L, Lv J, Yin Q, Liang X, Chu Y, He R. BLT1-dependent alveolar recruitment of CD4+CD25+ Foxp3+ regulatory T cells is important for resolution of acute lung injury. Am J Respir Crit Care Med 186: 989–998, 2012 [DOI] [PubMed] [Google Scholar]
- 198.Webster SJ, Daigneault M, Bewley MA, Preston JA, Marriott HM, Walmsley SR, Read RC, Whyte MK, Dockrell DH. Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease. J Immunol 185: 2968–2979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Weyde J, Wassermann K, Schell-Frederick E. Analysis of single and double-stained alveolar macrophages by flow cytometry. J Immunol Methods 207: 115–123, 1997 [DOI] [PubMed] [Google Scholar]
- 200.Wissinger EL, Saldana J, Didierlaurent A, Hussell T. Manipulation of acute inflammatory lung disease. Mucosal Immunol 1: 265–278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zamjahn JB, Quinton LJ, Mack JC, Frevert CW, Nelson S, Bagby GJ. Differential flux of macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant from the lung after intrapulmonary delivery. Am J Physiol Lung Cell Mol Physiol 301: L568–L574, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol 49: 180–189, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood 116: e74–e80, 2010 [DOI] [PubMed] [Google Scholar]
- 205.Zwadlo G, Voegeli R, Schulze Osthoff K, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol 55: 295–304, 1987 [DOI] [PubMed] [Google Scholar]
- 206.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature 358: 167–169, 1992 [DOI] [PubMed] [Google Scholar]