Abstract
The interstitial lung diseases (ILD) include a large number of chronic, progressive, irreversible respiratory disorders involving pulmonary fibrosis, the most common of which are idiopathic pulmonary fibrosis and scleroderma lung disease (SSc ILD). Because bleomycin causes lung fibrosis when used in cancer chemotherapy, it is used to model human ILD in rodents. In most studies, bleomycin has been delivered directly into the lung by intratracheal or intraoral administration. Here we have compared the effects in mice of bleomycin delivered directly into the lungs (direct model) or systemically using osmotic minipumps (pump model) to determine which more closely resembles human ILD. The pump model is more similar to human SSc ILD in that: 1) lung injury/fibrosis is limited to the subpleural portion of the lung in the pump model and in SSc ILD, whereas the entire lung is affected in the direct model; 2) conversely, there is massive inflammation throughout the lung in the direct model, whereas inflammation is limited in the pump model and in SSc ILD; 3) hypertrophic type II alveolar epithelial cells are present at high levels in SSc ILD and in the pump model but not in the direct model; and 4) lung fibrosis is accompanied by dermal fibrosis. The pump model is also move convenient and humane than the direct model because there is less weight loss and mortality.
Keywords: fibrosis, inflammation, type II pneumocytes
interstitial lung disease (ILD) encompasses a large group of chronic, progressive, irreversible respiratory conditions marked by alveolar epithelial cell injury and hyperplasia, inflammatory cell infiltration throughout the lung, fibroblast/myofibroblast proliferation, excessive deposition of extracellular matrix (ECM) proteins, and formation of fibroblastic foci and honeycombing (29). While ILD accounts for only about 15% of respiratory diseases, it includes the most devastating of these diseases such as idiopathic pulmonary fibrosis (IPF) and systemic sclerosis (scleroderma, SSc)-related ILD (SSc ILD) (2, 24). There are no treatments approved by the Food and Drug Administration for these diseases. The pathological changes associated with ILD result in impairment of lung gas exchange and loss of pulmonary function, leading to dyspnea, respiratory failure, and death (7, 8, 15, 30).
The availability of an animal model that recapitulates the typical features of a human disease is a prerequisite for the translational study of that disease. Many rodent models have been established that mimic aspects of human lung injury/fibrosis, including exposure to bleomycin, fluorescein isothiocyanate, silica, or cigarette smoke; irradiation; and targeted overexpression of transforming growth factor-β in the lungs (3, 5, 17). Among these models, bleomycin treatment is the most widely used (16, 18, 22). Bleomycin belongs to a family of glycopeptide antibiotics originally isolated from Streptomyces verticillatus and found to be effective against squamous cell carcinomas (11, 27, 28). However, its usefulness in treating human cancers (19) is greatly limited by its pulmonary toxicity, leading to fibrosis. In most rodent studies, bleomycin has been delivered directly into the lung by either intratracheal or intraoral administrations; however, bleomycin has also been delivered systemically via intravenous, intraperitoneal, or subcutaneous routes (1, 9, 17).
Because of our interest in performing translational studies to validate novel treatments for ILD, it was critical for us to determine whether the direct delivery of bleomycin into mouse lungs or systemic delivery resulted in disease symptoms most similar to those observed in human ILD. Therefore, we have compared the effects in mice of bleomycin delivered directly into the experimental animal lung by intraoral administration (the “direct” model) and of bleomycin delivered systemically by continuous diffusion from subcutaneously implanted osmotic minipumps (the “pump” model). Our results indicate that, in addition to being a better model for human ILD by several criteria, the pump model is also more humane than the direct model.
MATERIALS AND METHODS
Bleomycin treatment of mice and harvesting of tissue.
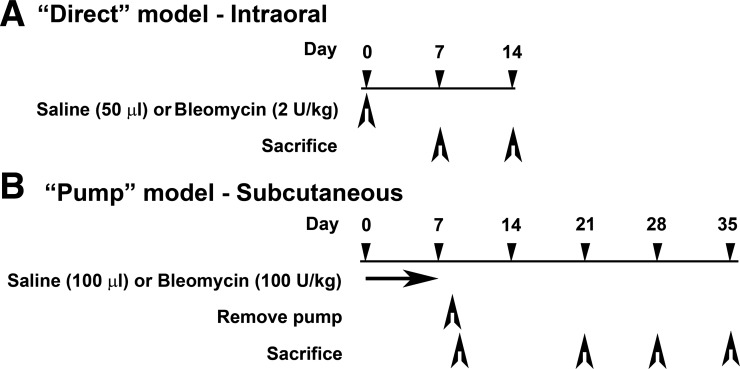
The following procedures were approved by the Medical University of South Carolina (MUSC) Institutional Animal Care and Use Committee. Ten-week-old, male CD1 mice (Charles River Laboratories, Boston, MA) maintained under pathogen-free conditions were treated with bleomycin (Teva Parenteral Medicines, Irvine, CA) by the “direct model” as previously described (26) or the “pump model.” In the pump model, osmotic minipumps (ALZET 1007D; DURECT, Cupertino, CA) containing either 100 μl saline vehicle or bleomycin (100 U/kg) designed to deliver their contents at 0.5 μl/h for 7 days were implanted under isofluorane anesthesia under the loose skin on the back of the mice slightly posterior to the scapulae. Pumps were removed on day 10 as recommended by the manufacturer. The details of these models are summarized in Fig. 1. Each experiment was performed using at least six mice per group.
Fig. 1.
Summary of experimental models. Ten-week-old, male CD1 mice were treated as indicated. A: “direct” model, mice were treated with single dose of 50 μl saline vehicle or bleomycin (2 U/kg) intraorally and killed on day 7 or on day 14. B: “pump” model, Alzet osmotic minipumps containing 100 μl saline vehicle or bleomycin (100 U/kg) were implanted subcutaneously under the loose skin on the back of mice. Pumps continuously deliver their contents at 0.5 μl/h for 7 days and are removed on day 10. Mice are killed on days 10, 21, 28, or 35.
Mice were killed at the indicated times. Under deep anesthesia, the rib cage was opened to expose the lungs. Mice were systemically perfused via the left ventricle with PBS. The left lobe was then tied off and cut off. The remaining right lobes were further perfused with buffered zinc formalin fixative (Z-Fix; Anatech, Battle Creek, MI). Fixed lung tissue was then removed and embedded in paraffin. Sections (4 μm) were stained with hematoxylin and eosin (H&E) or Masson's trichrome or immunohistochemically as described below.
Left lobes were minced and homogenized using a Tissue Tearor in 2 ml of 25 mM Tris (pH 8.0)-5 mM EDTA-5 mM EGTA plus protease inhibitors [N-ethylmaleimide (10 mM), benzamidine (5 mM), and phenylmethylsulfonyl fluoride (2 mM)] and phosphatase inhibitors [sodium fluoride (50 mM), sodium pyrophosphate (5 mM), and sodium orthovanadate (1 mM)]. The homogenate was centrifuged for 3 min at 16,000 g. Finally, the supernatant was either directly analyzed for collagen content using the Sircol Assay or was boiled for 3 min in SDS-PAGE sample buffer for use in Western blotting experiments using the indicated primary antibodies and appropriate secondary antibodies.
Fibrosis was quantitatively evaluated in light microscope images (×20 magnification) of tissue sections using a numeric fibrosis scale (Ashcroft score) (10). In brief, 20 fields/slide were evaluated for severity of lung fibrosis (independent of inflammation). Each field was assigned a score between 0 (normal lung) and 8 (total fibrosis). The average score for the 20 fields is the fibrosis score for the section.
Inflammation was scored in light microscopic images of tissue sections. In brief, 20 fields/slide were examined after H&E staining and assigned a score between 0 and 4 (0, no inflammation; 1, mild inflammation; 2, moderate inflammation; 3, severe inflammation; 4, extreme inflammation). The average score for the 20 fields is the inflammation score for the section.
Sircol assay for soluble collagen.
Soluble collagen levels in mouse lung tissue were quantified using the Sircol Collagen Assay (Biocolor) as described by the manufacturer with minor modifications. The Dye Reagent and the Acid-Salt Wash Reagent were supplemented with a final 0.2% Triton X-100. For each mouse, duplicate analyses were performed using 100 μl of Dye Reagent incubated with 10 μl of soluble extract prepared as described above. Collagen-dye pellets were washed using 100 μl of Acid-Salt Wash Reagent.
Human lung tissue.
Normal human lung tissue was obtained from the Brain and Tissue Bank for Developmental Disorders (Baltimore, MD) or from the National Disease Research Interchange (Philadelphia, PA). Under a protocol approved by the MUSC Institutional Review Board for Human Research, SSc ILD lung tissue from patients fulfilling the criteria of the American College of Rheumatology for the diagnosis of SSc with lung involvement was obtained from autopsy specimens collected by the MUSC Division of Pathology and Laboratory Medicine. All four SSc lung tissue samples used in these studies were identified as fibrotic nonspecific interstitial pneumonia by a trained pathologist.
Immunohistochemistry.
Immunofluorescent staining of mouse and human lung tissue sections was performed (25) using routine methods. Fluorescent images were acquired using a Zeiss Axio Imager M2 fluorescence microscope (Carl Zeiss). Antibodies were from eBioscience (San Diego, CA), anti-CD11b (14-0112-85) and anti-Gr-1 (14-5931); Enzo Life Sciences (Ann Arbor, MI), anti-47-kDa heat shock protein (HSP47) (ADI-SPA-470); R & D Systems (Minneapolis, MN), anti-α smooth muscle actin (ASMA) (IC1420A); Santa Cruz Biotechnology (Santa Cruz, CA), anti-caveolin-1 (sc-894); and EMD Millipore (Billerica, MA), anti-prosurfactant C (AB3786). Affinity-purified rabbit polyclonal antibodies against the collagen I α1 COOH-terminal telopeptide and against human tenascin-C were prepared in our laboratory. In some cases primary antibodies were directly labeled using an Alexa Fluor 647 Protein Labeling Kit (A-20173; Invitrogen, Carlsbad, CA) as described by the manufacturer. In other cases, appropriate fluorescent secondary antibodies were used. Nuclei were routinely stained using 4′,6-diamidino-2-phenylindole (Invitrogen). All immunohistochemical experiments were performed at least three times with similar results.
Statistical analyses.
All numerical data are expressed as averages ± SE and were analyzed using Student's t-test to evaluate statistical significance. In Figs. 1–13, statistical significance is expressed as P < 0.05, 0.01, and 0.001.
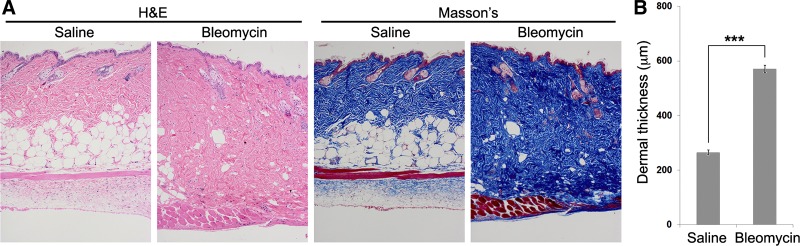
Fig. 13.
Dermal fibrosis and lipoatrophy in the pump model. Mice were treated with pumps containing saline vehicle or bleomycin and then killed on day 35. Skin above the pumps was harvested, and paraffin sections were cut and stained with H&E or Masson's trichrome. A: histological staining shows increased dermal thickness induced by bleomycin and an almost complete loss of the adipose cell layer. B: quantification of A for dermal thickness based on measurements of 5 sites/field imaged at ×100 magnification, 6 fields/section from sections from 6 mice in each category. Numbers presented are the averages ± SE. ***P < 0.001.
RESULTS
Improved weight loss and survival in the pump model.
Severe weight loss and high mortality are common problems when, as in most previous studies using bleomycin, lung fibrosis is induced by direct intratracheal or oropharyngeal administration of this drug into rodent lungs. Lung fibrosis can also be induced by systemic bleomycin treatment, but this approach has been used relatively infrequently. In this study, we have compared direct administration of bleomycin to systemic administration using osmotic minipumps to determine how the outcomes of these distinct approaches may differ and, most importantly, to determine which is a better model for human ILD.
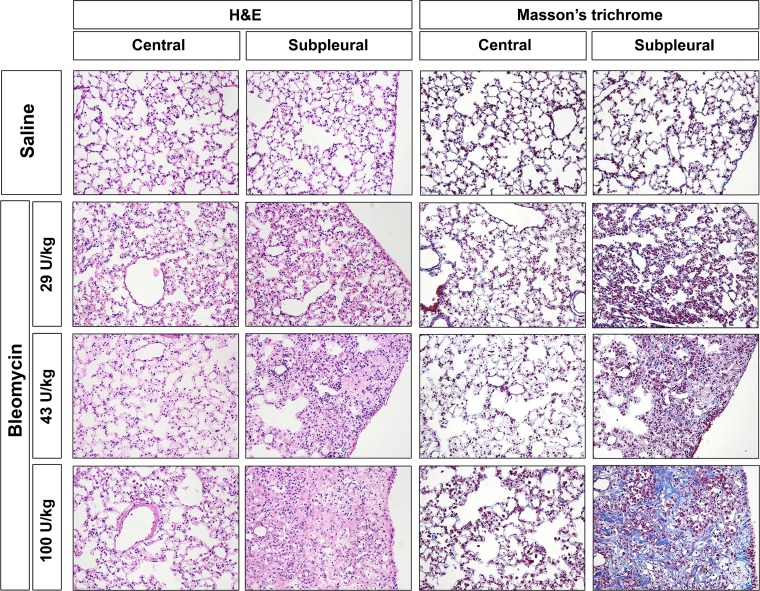
We first compared the effect of different concentrations of bleomycin in the pump model (Fig. 2). Mice treated with 29 or 43 U/kg of bleomycin showed subpleural thickening of alveolar walls and inflammation, but relatively little collagen deposition. In contrast, mice treated with 100 U/kg of bleomycin developed severe subpleural lung fibrosis while the central portion of the lung remained relatively unaffected.
Fig. 2.
Fibrosis in the pump model vs. bleomycin dose. Mice were implanted with osmotic minipumps containing saline vehicle or the indicated concentrations of bleomycin and then killed on day 35. Tissue sections were stained with hematoxylin and eosin (H&E) or with Masson's trichrome, and images were collected from the central and the subpleural lung regions. Intense blue Masson's staining indicates regions where collagen expression is robust. Similar results were obtained in three independent animals in each category.
Having determined that 100 U/kg is an appropriate dose for use in the pump model, we next compared weight loss (Fig. 3A) and survival (Fig. 3B) in the direct model and the pump model. Weight loss in bleomycin-treated mice was more severe in the direct model than in the pump model. Although recovery of weight loss did not occur in the direct model before the mice were killed on day 14, weight loss peaked in the pump model at day 14 followed by a slow recovery up until day 35 when the mice were killed. Like weight loss, survival was much more affected in the direct model than in the pump model. Whereas almost half of the mice died in the direct model, fewer than 15% died in the pump model.
Fig. 3.
Effects of bleomycin on mouse body weight and survival. A: weight change (%), average ± SE in the indicated groups on the indicated dates. Indications of statistical significance are vs. saline-treated mice in the indicated groups on indicated dates. B: survival, %survival in the indicated groups on the indicated dates. **P < 0.01, and ***P < 0.001.
Fibrosis is predominantly subpleural in the pump model and in SSc patients, but not in the direct model.
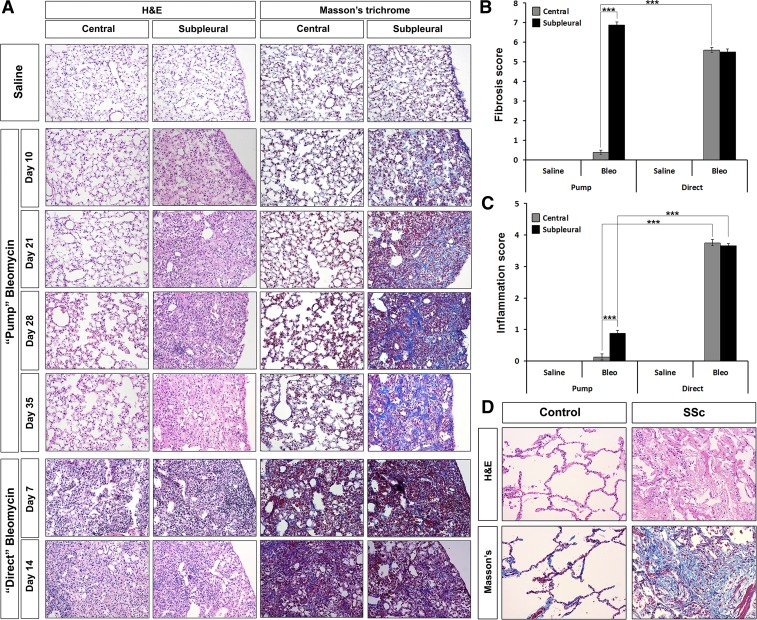
We next compared the time course of changes in lung morphology in the pump model and the direct model (Fig. 4A). In the pump model, by day 10 following bleomycin treatment, thickened alveolar septa and slightly narrowed alveolar spaces are seen in the subpleural area while little change is observed in the central portion of the lung. By day 21 large lesions with almost no alveolar spaces and with extensive inflammation and collagen deposition (visualized by Masson's Trichrome staining) are observed in the subpleural area, but little change has yet occurred in the central lung. At day 28, subpleural collagen deposition has increased somewhat while minor alveolar thickening and inflammation is observed in the central lung. At day 35, subpleural fibrosis and inflammation have resolved somewhat and alveolar spaces are slightly more apparent. Damage to the central lung is still very minor although a small amount of collagen deposition is observed as well as thickened alveolar septa. These studies were performed using CD1 mice. When we examined C57 mice to determine whether the pump model could be used in other strains of mice, we observed similar morphological changes (data not shown).
Fig. 4.
Histochemical staining of control and fibrotic mouse (pump model and direct model) and human lung tissue. Similar results were obtained in the following number of animals and human subjects: mice: saline, 11; pump day 10, 7; pump day 21, 6; pump day 28, 6; pump day 35, 7; direct day 7, 8; direct day 14, 14; humans: control, 4; SSc, 4. A: time course of fibrosis in mice. Representative images are shown for the indicated histochemical stains, lung regions, method of delivery of bleomycin, and date of death. B: fibrosis score. Average Ashcroft scores ± SE are shown for the indicated lung regions and method of delivery of bleomycin at the standard experiment termination point (35 days for pump model, 14 days for direct model). C: inflammation score. Average inflammation scores ± SE are shown for the indicated lung regions and method of delivery of bleomycin at the standard experiment termination point (35 days for pump model, 14 days for direct model). D: human lung fibrosis. Representative images are shown for the indicated histochemical stains and for control, healthy lung tissue and fibrotic scleroderma lung disease (SSc ILD) lung tissue. ***P < 0.001.
In contrast, in the direct model tissue damage is similar in the subpleural and central regions of the lung (Fig. 4A). At both 7 and 14 days, massive inflammation and obliteration of alveolar spaces has occurred in both regions. While collagen deposition is known to occur by day 14, it is difficult to observe because, in Masson's-stained sections, the massive purple staining of inflammatory cell nuclei is superimposed on the blue collagen stain and obscures it, especially at low magnification.
The reproducibility of the observations in Fig. 4A were evaluated using Ashcroft scores to rate fibrosis (Fig. 4B) and inflammation scores to rate inflammation (Fig. 4C) in several mice. While severe fibrosis was observed in both the central and subpleural regions in the direct model and in the subpleural region in the pump model, almost no fibrosis was observed in the central region in the pump model. In addition, as described above, while in the direct model extreme inflammation was observed in both the subpleural and central regions, in the pump model only modest inflammation was observed in the subpleural region and almost no inflammation was observed in the central region.
Lung sections from human SSc ILD patients and control subjects were evaluated (Fig. 4D) to determine whether samples from the direct model or the pump model more closely resembled the human disease. The fact that extensive collagen deposition is observed in SSc ILD lung while only modest inflammation is observed strongly supports the idea that the pump model is a better model for SSc ILD than is the direct model.
Immunohistochemical comparison of inflammation in the pump model, direct model, and SSc ILD.
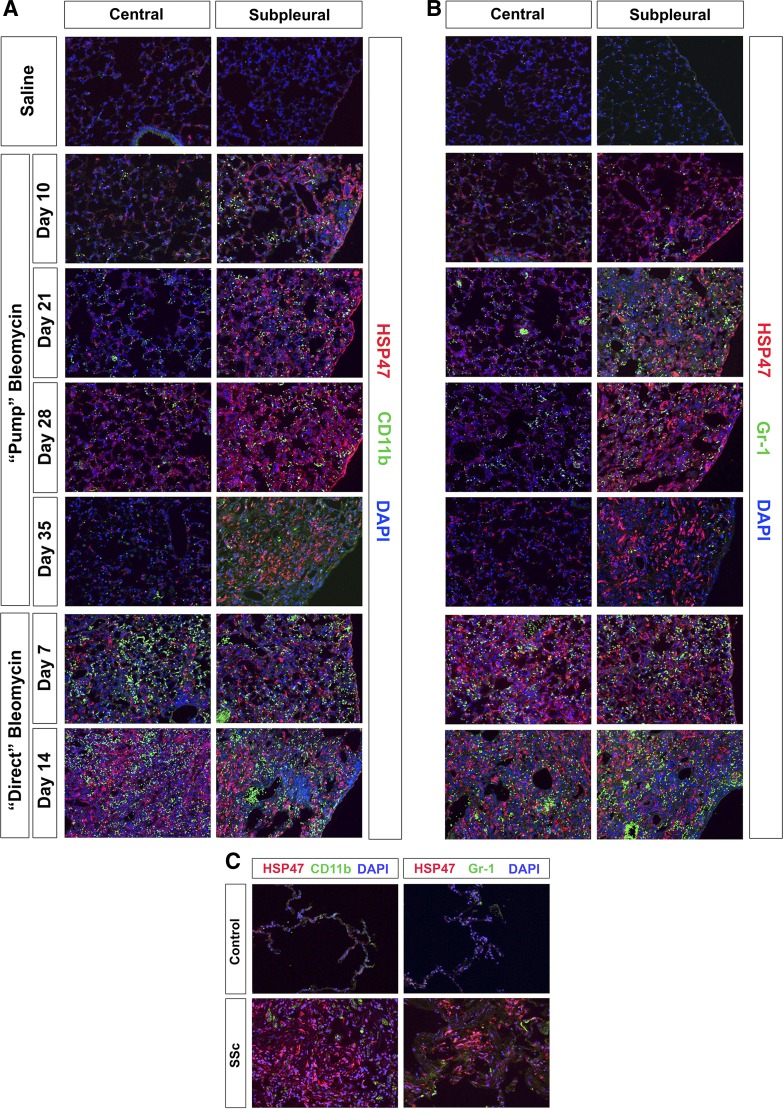
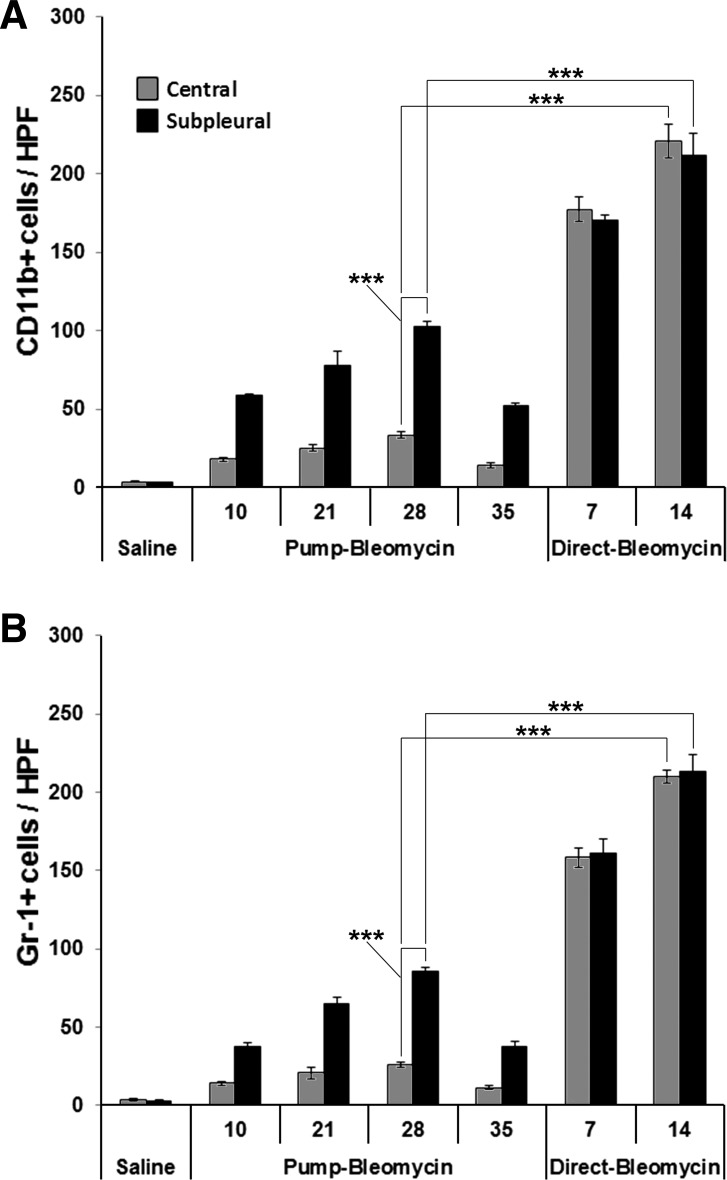
To validate the inflammation score data shown in Fig. 4C, sections were stained for the neutrophil marker Gr-1 or the monocyte marker CD11b and double-labeled with antibody against HSP47, a collagen chaperone that we find serves as a cell-associated marker for cells overexpressing collagen in fibrotic tissue (Fig. 5). Quantification of neutrophil and monocyte levels is presented in Fig. 6. Both neutrophils and monocytes were present at extremely high levels in the direct model in both the peripheral and central lung on days 7 and 14 after bleomycin treatment. In contrast, in the pump model there were relatively few neutrophils and monocytes in the central lung, although clearly far more than in control lung tissue from mice receiving saline vehicle rather than bleomycin. There was a higher level of neutrophils and monocytes in the peripheral lung that varied in number during the course of the disease but never reached the level observed in the direct model. Enhanced neutrophil and monocyte levels were apparent in the peripheral lung 10 days after bleomycin treatment and increased to their highest levels at days 21 and 28. Partial resolution of inflammation occurred at day 35 although fibrosis as detected by anti-HSP47 did not resolve.
Fig. 5.
Inflammatory cell markers in control and fibrotic mouse (pump model and direct model) and human lung tissue. Similar results were obtained in three independent animals or human subjects in each category. A and B: time course of inflammation in mice. A: representative images are shown for the indicated lung regions, method of delivery of bleomycin, and date of death double-labeled with antibodies against the collagen chaperone 47-kDa heat shock protein (HSP47) and the monocyte marker CD11b. B: representative images are shown for the indicated lung regions, method of delivery of bleomycin, and date of death, double-labeled with antibodies against the collagen chaperone HSP47 and the neutrophil marker Gr-1. C: inflammation in human lung fibrosis. Representative images are shown for healthy control lung tissue and fibrotic SSc ILD lung tissue stained with the same antibodies used in A and B.
Fig. 6.
Time course of inflammatory cell levels. Cells positive for CD11b (A) and Gr-1 (B) were counted in six high-power (×400) fields per section from both the central and subpleural regions of three mice in each category. Numbers presented are the averages ± SE per high-power field. ***P < 0.001.
When human lung sections were similarly analyzed, although massive HSP47 staining was observed in SSc ILD, very little Gr-1 or CD11b staining was observed (Fig. 5C). Thus, the level of inflammation in SSc ILD is much more similar to the level observed in the pump model than in the direct model.
Excessive ECM protein deposition.
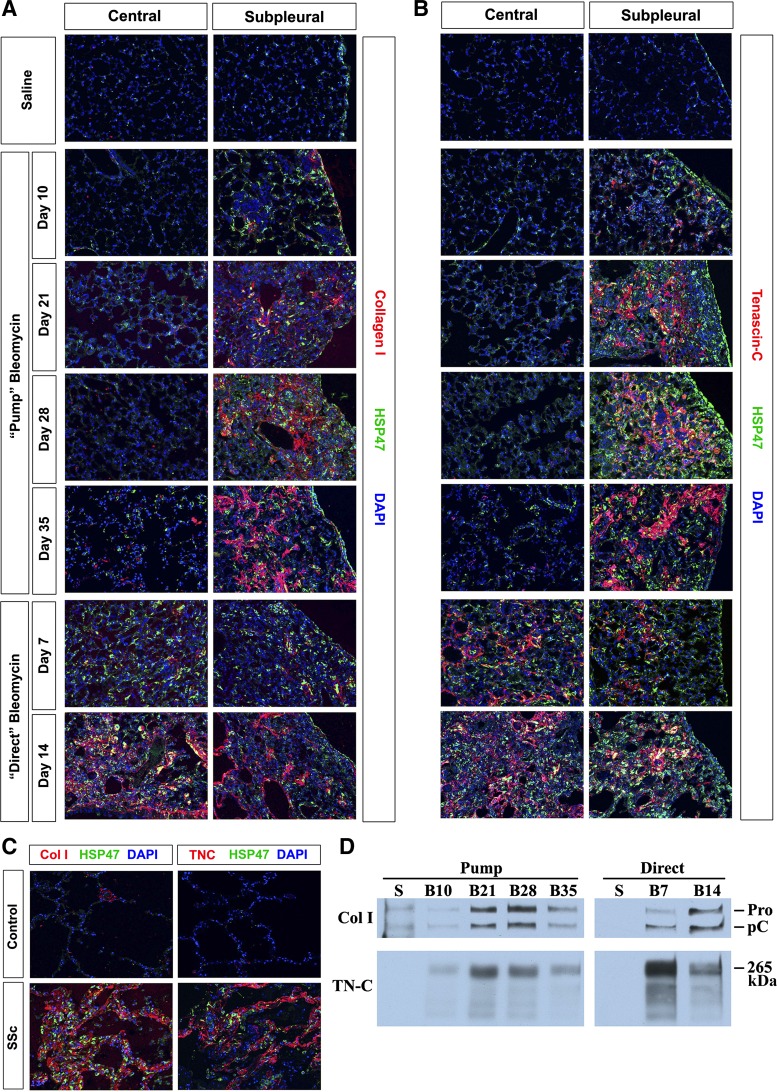
To evaluate the time course of ECM protein deposition in bleomycin-induced pulmonary fibrosis, lung sections were stained for collagen I or tenascin-C and additionally HSP47 (Fig. 7). Little staining was ever observed in control lung tissue from saline-treated mice or in the central region of the lung in the pump model. Extensive collagen I and tenascin-C staining developed gradually in the subpleural region in the pump model from day 10 through day 35, reaching maximal levels by day 28. Tenascin-C appears to precede collagen I as evidenced by stronger tenascin-C staining than collagen I staining at days 10 and 21. In the direct model, collagen I and tenascin-C staining are of similar intensity in the central and peripheral lung. The earlier appearance of tenascin-C than collagen I is much more pronounced in the direct model than in the pump model. Tenascin-C staining is strong at 7 days in both the central and peripheral lung while collagen I staining is not strong until 14 days.
Fig. 7.
Collagen I and tenascin-C in control and fibrotic mouse (pump model and direct model) and human lung tissue. Similar results were obtained in three independent animals or human subjects in each category. A and B: time course of collagen I and tenascin-C expression in mice. A: representative images are shown for the indicated lung regions, method of delivery of bleomycin, and date of death double-labeled with antibodies against HSP47 and collagen I. B: representative images are shown for the indicated lung regions, method of delivery of bleomycin, and date of death double-labeled with antibodies against HSP47 and tenascin-C. C: collagen I and tenascin-C in human lung fibrosis. Representative images are shown for control, healthy lung tissue and fibrotic SSc ILD lung tissue stained with the same antibodies used in A and B. D: collagen I and tenascin-C in lung extracts. Lung tissue extracts from the indicated method of delivery of bleomycin and date of death were prepared as described in materials and methods and Western blotted using the indicated antibodies. Samples are from pools of 6 animals in each category and represent the same percentage of a lung. S, saline; B10, bleomycin 10 days; B21, bleomycin 21 days; B28, bleomycin 28 days; B35, bleomycin 35 days; B7, bleomycin 7 days; B14, bleomycin 14 days; Col I, collagen I; TN-C, tenascin-C; Pro, procollagen I α1 bearing both NH2- and COOH-terminal propeptides; pC, collagen I α1 bearing only the COOH-terminal propeptide; 265 kDa, apparent molecular mass of the major tenascin-C form present in mouse lung tissue.
When human lung tissue was stained for collagen I, tenascin-C, and HSP47, the results obtained were equally similar to both mouse models. Very high levels of all three molecules were present in fibrotic tissue from SSc ILD patients (Fig. 7C), while almost no staining was observed in control human lung tissue.
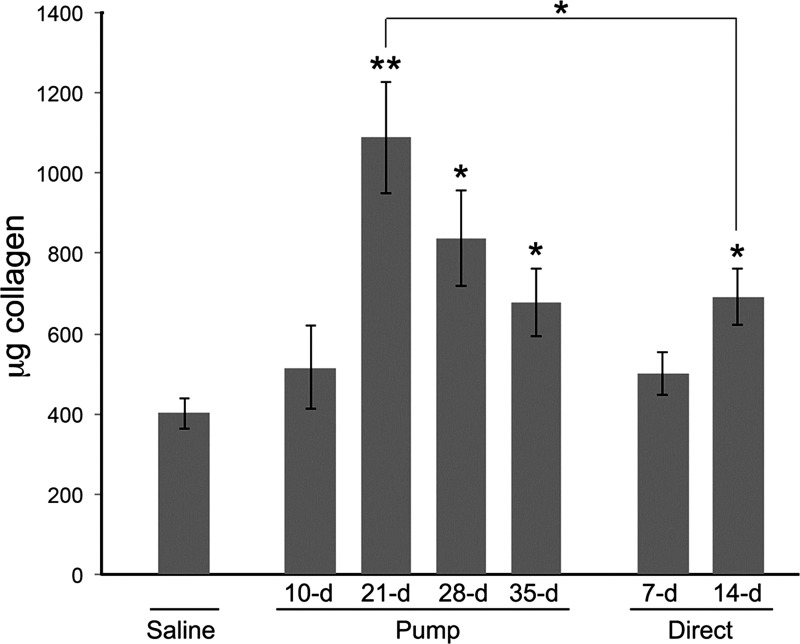
The observations on collagen I and tenascin-C expression in mouse lung tissue were validated by Western blotting (Fig. 7D) and using the Sircol Assay (Fig. 8). Newly synthesized collagen I that has not been incorporated into the ECM can be recognized on Western blots by its presence in the homogenate supernatant and its migration on SDS-PAGE as a form containing one or both propeptides. There was little newly synthesized collagen I in control lung tissue. In the pump model, there was still relatively little newly synthesized collagen I 10 days after bleomycin treatment; however, high levels were observed at days 21 and 28, which receded somewhat at day 35. In the direct model, there was a moderate level of newly synthesized collagen I at day 7 and a high level at day 14. The time course of soluble collagen levels in the pump model and in the direct model detected using the Sircol Assay (Fig. 8) was very similar to the levels detected by Western blotting (Fig. 7D). In accord with the results of Aono et al. (1), soluble collagen levels detected using the Sircol Assay were somewhat higher in the pump model than in the direct model. The difference between the peak level of soluble collagen in the pump model (day 21) and in the direct model (day 14) was statistically significant (P < 0.05).
Fig. 8.
Soluble collagen levels determined using the Sircol Assay. The Sircol Assay and sample preparation were performed as described in materials and methods. The data presented are the averages ± SE of the soluble collagen level in the entire left lobe of 4 or 5 mice in each indicated category. Statistical significance measurements are vs. the saline control except in the case where the 21-day pump model and 14-day direct model are compared. *P < 0.05 and **P < 0.01.
Tenascin-C levels were below detection by Western blotting in control mice (Fig. 7D). Bleomycin-induced increases in tenascin-C expression preceded increases in collagen I expression, particularly in the direct model. Indeed, tenascin-C expression was higher at 7 days than at 14 days in the direct model, although collagen levels were markedly higher at 14 days. In contrast, in the pump model, tenascin-C levels (like collagen levels) peaked at days 21 and 28 and decreased from the peak level at day 35.
Hypertrophic type II alveolar epithelial cells are present at high levels in SSc and the pump model but not in the direct model.
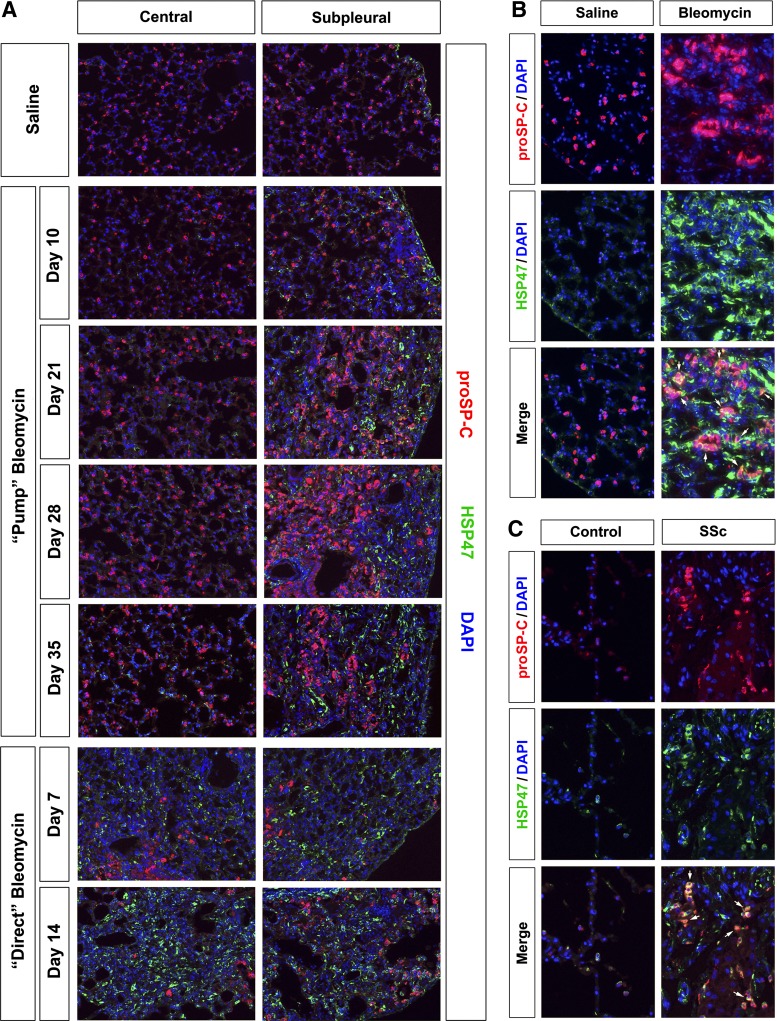
Because the accumulation of hypertrophic type II alveolar epithelial cells is a well-known feature of SSc ILD, we stained samples for the marker prosurfactant C (Fig. 9). Prosurfactant C-positive cells were uniformly distributed throughout control mouse lung tissue and in the central region in the pump model (Fig. 9A). However, prosurfactant C-positive cells proliferate and become hypertrophic in the subpleural region in the pump model 21, 28, and 35 days after bleomycin treatment. High-magnification images (Fig. 9B) make clear that many of these prosurfactant C-positive cells are also HSP47-positive. In contrast to results obtained in the pump model, the number of prosurfactant C-positive cells decreases in the direct model compared with control mice in both the central and subpleural region of the lung. In addition, while these cells are hypertrophic, they are less hypertrophic than the prosurfactant C-positive cells in the subpleural region in the pump model.
Fig. 9.
Prosurfactant-C in control and fibrotic mouse (pump model and direct model) and human lung tissue. Similar results were obtained in three independent animals or human subjects in each category. A: time course of prosurfactant-C expression in mice. Representative images are shown for the indicated lung regions, method of delivery of bleomycin, and date of death double-labeled with antibodies against HSP47 and prosurfactant-C (proSP-C). B: mouse cells double-positive for HSP47 and prosurfactant-C. Representative images are shown individually labeled for HSP47 or prosurfactant-C and merged to highlight double-labeled cells (arrows). These images are from the subpleural area in mice killed 35 days after treatment with saline vehicle or bleomycin by the pump model. C: human cells double-positive for HSP47 and prosurfactant-C. Representative images are shown for healthy control lung tissue and fibrotic SSc ILD lung tissue stained individually with HSP47 or prosurfactant-C and merged to highlight double-labeled cells (arrows).
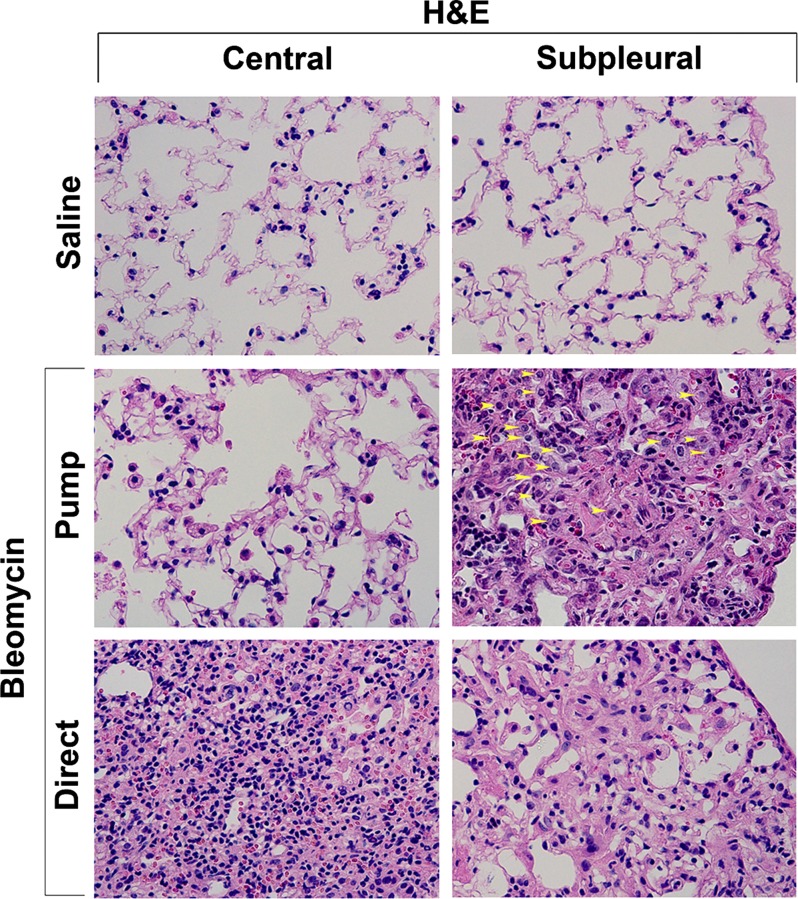
To further validate these observations, H&E-stained lung sections (Fig. 10) were carefully examined for type II alveolar epithelial cells. In accord with the prosurfactant C staining in Fig. 9, no hypertrophic type II alveolar cells were observed in control lung tissue, large numbers of hypertrophic cells were observed in the fibrotic subpleural region in the pump model, and few hypertrophic cells were observed in the direct model.
Fig. 10.
H&E staining of hypertrophic type II alveolar epithelial cells. Representative images are shown highlighting the observation that type II alveolar epithelial cells accumulate to high levels and are hypertrophic in the subpleural region in the pump model (yellow arrowheads), are not hypertrophic in the central region in the pump model, and are rarely found in the central or subpleural regions in the direct model. Pump model mice used in this experiment were killed at 35 days, and direct model mice were killed at 14 days.
When human lung tissue was examined, we found that, as in the pump model, SSc ILD lung tissue contained a high level of hypertrophic prosurfactant C-positive cells and that many of these cells were double positive for prosurfactant C and HSP47 (Fig. 9C). This raises the possibility that at least some of the cells that overexpress collagen I in fibrotic lung tissue are derived from type II alveolar epithelial cells.
Myofibroblast marker ASMA expression.
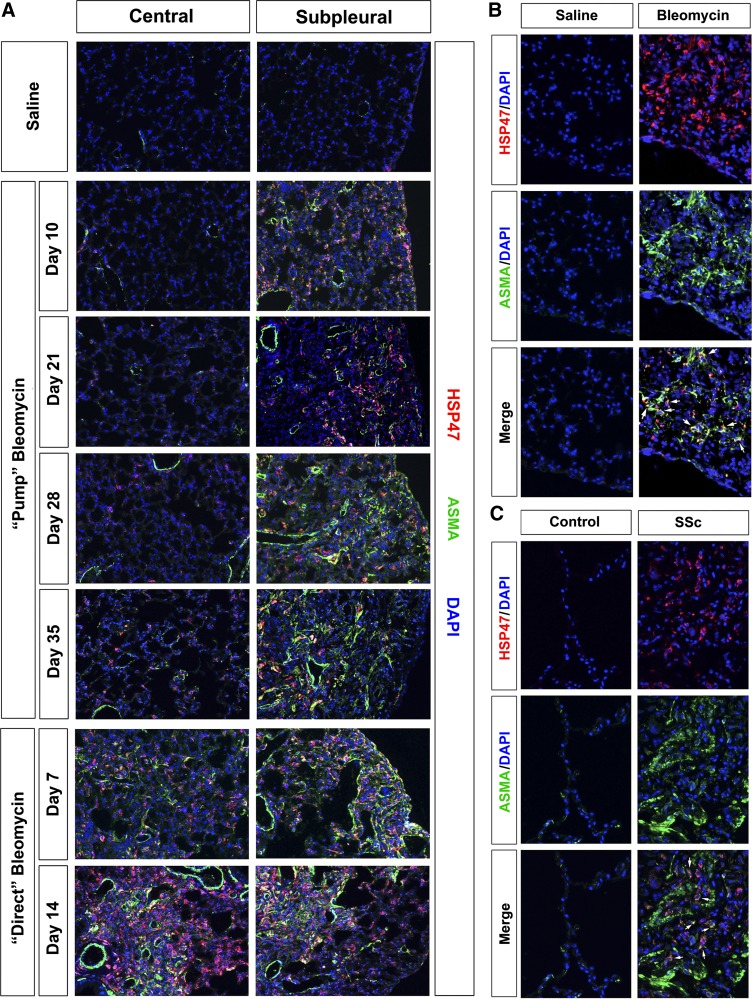
Because myofibroblasts are considered to be the primary cells that overexpress collagen I in fibrotic lung tissue, we examined the distribution of ASMA. ASMA is a marker for myofibroblasts in the sense that it is only expressed by these activated fibroblasts and not by unactivated fibroblasts. It is not at all a perfect marker for myofibroblasts because it is also expressed by smooth muscle cells associated with the lung vasculature and airways.
Consistent with the idea that ASMA expression parallels the differentiation of myofibroblasts that mediate fibrosis, ASMA expression (Fig. 11) was similar in pattern to the overexpression of collagen I (Fig. 7). Little ASMA staining was observed in control lung tissue or in the central region of the lung in the pump model. ASMA staining developed gradually in the subpleural region in the pump model and was particularly extensive at days 28 and 35. In the direct model, ASMA staining is of similar intensity in the central and peripheral lung and is extensive at both 7 and 14 days. It is noteworthy that, in addition to the increase in ASMA staining in individual myofibroblasts in fibrotic lung tissue, ASMA staining in fibrotic lung tissue is also increased in both the pump model and the direct model in the smooth muscle cell layers that encircle the vasculature and the airways.
Fig. 11.
Anti-α smooth muscle actin (ASMA) in control and fibrotic mouse (pump model and direct model) and human lung tissue. Similar results were obtained in three independent animals or human subjects in each category. A: time course of ASMA expression in mice. Representative images are shown for the indicated lung regions, method of delivery of bleomycin, and date of death double-labeled with antibodies against HSP47 and ASMA. B: mouse cells double-positive for HSP47 and ASMA. Representative images are shown individually labeled for HSP47 or ASMA and merged to highlight double-labeled cells (arrows). These images are from the subpleural area in mice killed 35 days after treatment with saline vehicle or bleomycin by the pump model. C: human cells double-positive for HSP47 and ASMA. Representative images are shown for healthy control lung tissue and fibrotic SSc ILD lung tissue stained individually for HSP47 or ASMA and merged to highlight double-labeled cells (arrows).
When human lung tissue was stained for ASMA, the results obtained were equally similar to both mouse models. Very high levels of ASMA were present in fibrotic tissue from SSc ILD patients (Fig. 11C) while almost no staining was observed in control human lung tissue. In both human and mouse fibrotic lung tissue (Fig. 11, B and C), ASMA and HSP47 double-positive cells were observed, indicating that many of the cells in which HSP47 is expressed at high levels are myofibroblasts.
HSP47-positive cells lack caveolin-1 in fibrotic lung tissue.
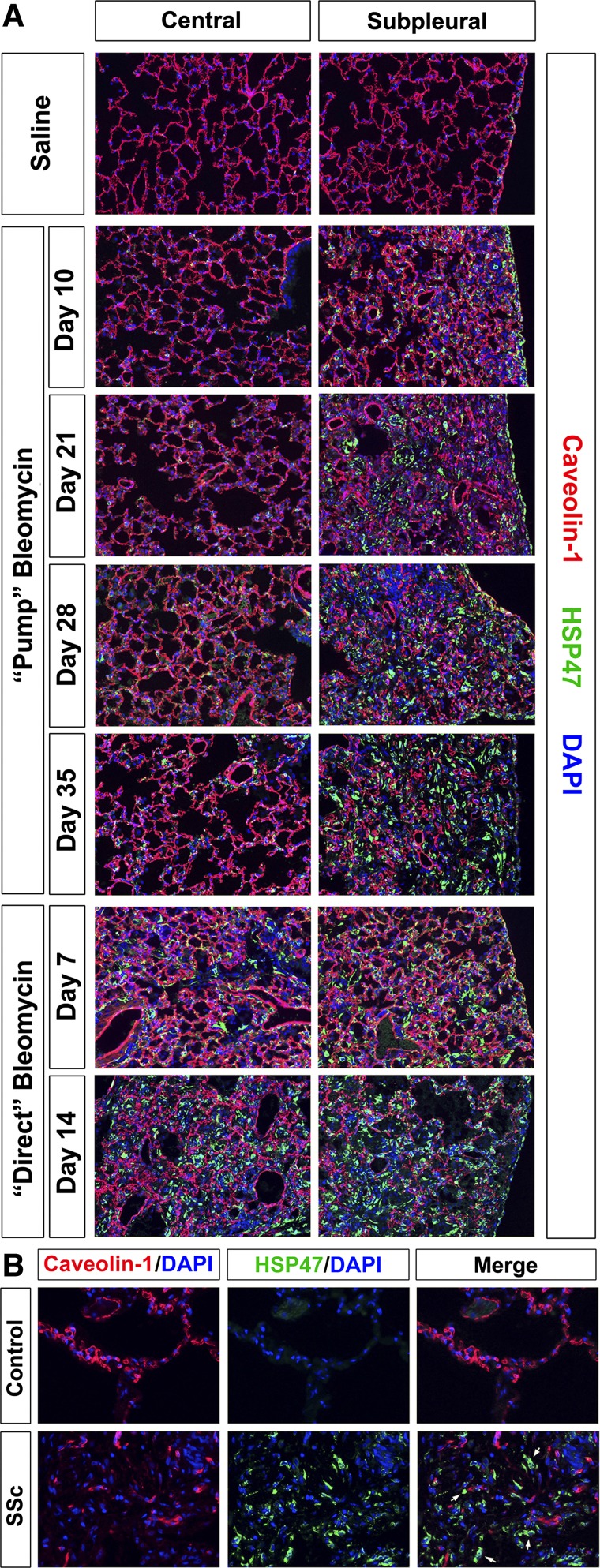
We and others have previously shown an inverse relationship between caveolin-1 levels in cells and their participation in processes associated with fibrosis (19, 21, 22). This relationship is further supported by current experiments in which sections were stained for caveolin-1 and HSP47 (Fig. 12). Intense caveolin-1 staining is observed in the alveolar epithelium of nonfibrotic lung tissue from control, saline-treated mice and from the central region of mice treated with bleomycin by the pump model. In contrast, in highly fibrotic tissue such as the peripheral region from pump model mice 21, 28, and 35 days after bleomycin treatment and in both the central and peripheral regions of direct model mice 14 days after bleomycin treatment, a patchwork of areas with HSP47 staining and areas with caveolin-1 staining of residual alveoli is observed with very little overlap (Fig. 12A). Similar results were obtained in human lung tissue (Fig. 12B), with intense caveolin-1 staining in intact alveoli in control tissue and a patchwork of staining in fibrotic SSc ILD lung tissue.
Fig. 12.
Caveolin-1 in control and fibrotic mouse (pump model and direct model) and human lung tissue. Similar results were obtained in three independent animals or human subjects in each category. A: time course of caveolin-1 expression in mice. Representative images are shown for the indicated lung regions, method of delivery of bleomycin, and date of death double-labeled with antibodies against HSP47 and caveolin-1. B: caveolin-1 in human lung fibrosis. Representative images are shown for healthy control lung tissue and fibrotic SSc ILD lung tissue stained individually for HSP47 or caveolin-1 and merged to highlight the lack of caveolin-1 in HSP47-positive cells in fibrotic lung tissue.
Dermal fibrosis and lipoatrophy in the pump model.
We also compared the pump model and direct model in terms of fibrosis of the skin and internal organs. While no fibrosis of these tissues was observed in the direct model, massive dermal fibrosis was observed in the pump model (Fig. 13), resulting in a more than twofold increase in thickness. Also noteworthy was the almost complete loss of the subdermal adipose cell layer (lipoatrophy), a phenomenon that has recently been observed in SSc patients (14). Fibrosis was also observed in a variety of internal organs (unpublished observation).
DISCUSSION
In this paper we have determined by several criteria that the delivery of bleomycin to mice systemically via osmotic minipumps produces a disease model that is much more similar to human SSC ILD than the model produced by delivering bleomycin directly into the lungs. The criteria by which the pump model is more similar than the direct model to SSc ILD are: 1) lung injury/fibrosis is limited to the subpleural portion of the lung in the pump model and in SSc ILD, whereas the entire lung is affected in the direct model; 2) conversely, there is massive inflammation throughout the lung in the direct model, whereas inflammation is limited in the pump model and in SSc ILD; 3) hyperplastic type II alveolar epithelial cells are present at high levels in SSc and in the pump model but not in the direct model; and 4) lung fibrosis is accompanied by dermal and internal organ fibrosis. The pump model and the direct model were similar to each other and to SSc ILD in terms of: 1) overexpression of ECM proteins collagen I and tenascin-C and the collagen chaperone HSP47; 2) overexpression of the myofibroblast marker ASMA; and 3) lack of expression of caveolin-1 in areas of fibrosis. In addition to the criteria above that the pump model is more similar than the direct model to SSc ILD, the pump model also has the benefit that it is more humane than the direct model because it involves less mortality and weight loss.
The relative lack of inflammation, high levels of hyperplastic type II alveolar cells, and subpleural localization of fibrosis in ILD (4, 8, 13) and the pump model (but not in the direct model) are striking and possibly interrelated observations. In another study (6) in which a single dose of intratracheal bleomycin was compared with multiple doses, it was concluded that the multiple-dose model was more similar to IPF because the multiple-dose model and IPF (but not the single-dose model) showed relatively low levels of inflammation and high levels of hyperplastic type II alveolar cells. Thus, while the mechanism remains to be determined, both Ref. 6 and the current study show a link between low levels of inflammation and high levels of hyperplastic type II alveolar cells. These observations are also noteworthy because they suggest that the simple concept that damage to the alveolar epithelium leads to inflammation, which in turn leads to fibrosis, is either not valid or is very incomplete because the high levels of inflammation we observe in the direct model do not lead to correspondingly high levels of fibrosis.
These observations also suggest that the fibrosis that occurs in the pump model and the direct model represent different diseases and may occur through distinct cellular mechanisms. As previously suggested (4), it is not surprising that the direct administration of bleomycin into the lung would injure the entire lung, resulting in massive inflammation that in turn leads to massive fibrosis such as is observed in acute lung injury/acute respiratory distress syndrome. In contrast, systemic bleomycin somehow leads to the activation of type II pneumocytes, which secrete chemokines, and cytokines that enhance the migration of fibrocytes into the lung, epithelial-mesenchymal transformation (EMT), and the activation of resident fibroblasts (13), thereby promoting fibrosis without promoting inflammation. Our observation that hyperplastic type II pneumocytes express HSP47 raises the interesting possibilities that the precursors of these cells may have undergone the equivalent of EMT or may include bone marrow-derived fibrocytes. Thus fibrosis in the direct model may be driven by inflammation while fibrosis in the pump model may be driven by the activation of type II pneumocytes.
The pump model, the direct model, and SSc ILD were all similar in the temporal and spatial expression patterns of collagen I, the collagen chaperone HSP47, the myofibroblast marker ASMA, and the master regulatory signaling molecule caveolin-1. The fact that the expression of these molecules was similar in the high-inflammation direct model and the low-inflammation pump model strongly suggests that these molecules are involved in fibrosis rather than in inflammation. HSP47 is mainly localized in the endoplasmic reticulum of collagen-producing cells and preferentially interacts with triple-helical procollagen molecules to promote its proper folding, assembly, maturation, and secretion (12, 20, 21, 23). These studies further validate HSP47 as a surrogate marker useful in identifying cells that are overexpressing collagen I in fibrotic tissues. This is particularly important in studying mouse model systems because there are currently few available antibodies against the Pro domains in mouse collagen I that could be used to directly identify mouse cells overexpressing collagen I. In contrast to collagen I expression, both immunohistochemical and Western blotting experiments suggest that the temporal and spatial expression pattern of the ECM protein tenascin-C in the direct model is correlated with inflammation. This suggests that tenascin-C may be more involved in the regulation of inflammation than in fibrosis.
Besides being a better model for SSc ILD, the pump model provides additional major benefits compared with the direct model. Much less weight loss and mortality is observed in the pump model than in the direct model. Therefore, besides the fact that the pump model is more humane, it also benefits the researcher because it allows the number of surviving animals in treatment groups to be accurately predicted, thereby making analyses of biochemical and cell biological parameters at the end of an experiment more meaningful. Finally, in the pump model we observe fibrosis in the skin and in a variety of internal organs. Therefore, this further emphasizes the point that the pump model is an accurate model for SSc plus it provides the opportunity to observe the effects of potential therapeutic treatments on fibrosis in each of the organs affected by SSc.
GRANTS
This work was supported by a postdoctoral fellowship to R. Lee [Training Grant in Inflammatory and Fibrosing Diseases National Institutes of Health (NIH) Grant T32-AR-050958 (Gary S. Gilkeson, PI)]; grants USARMY/USAMRAA W81XWH-11-1-0508, NIH Grant R21-AT-004450, and an SCTR Pilot Project (to S. Hoffman); and NIH Grants R01-AR-062078, R03-AR-056767, and K01-AR-054143 (to E. Tourkina). E. Tourkina also received a grant and the Marta Max Award from the Scleroderma Foundation. S. Hoffman also received support as a coinvestigator on NIH Grants P20-RR-016434, P20-RR-016434-09S2, and P20-RR-021949 and a grant from the Leducq Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.L., E.T., R.P.V., and S.H. conception and design of research; R.L., C.R., and M.B. performed experiments; R.L., E.T., Z.H., R.P.V., and S.H. analyzed data; R.L., E.T., Z.H., E.C.R., R.P.V., and S.H. interpreted results of experiments; R.L. and M.B. prepared figures; R.L. and S.H. drafted manuscript; R.L., M.B., R.M.S., and S.H. edited and revised manuscript; R.L., C.R., M.B., E.T., Z.H., R.M.S., R.P.V., and S.H. approved final version of manuscript.
REFERENCES
- 1.Aono Y, Ledford JG, Mukherjee S, Ogawa H, Nishioka Y, Sone S, Beers MF, Noble PW, Wright JR. Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am J Respir Crit Care Med 185: 525–536, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borchers AT, Chang C, Keen CL, Gershwin ME. Idiopathic pulmonary fibrosis-an epidemiological and pathological review. Clin Rev Allergy Immunol 40: 117–134, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol 33: 9–13, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Decologne N, Wettstein G, Kolb M, Margetts P, Garrido C, Camus P, Bonniaud P. Bleomycin induces pleural and subpleural fibrosis in the presence of carbon particles. Eur Respir J 35: 176–185, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Degryse AL, Lawson WE. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am J Med Sci 341: 444–449, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 299: L442–L452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet 380: 689–698, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Gulati M. Diagnostic assessment of patients with interstitial lung disease. Primary Care Respir J 20: 120–127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison JH, Jr, Lazo JS. High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther 243: 1185–1194, 1987 [PubMed] [Google Scholar]
- 10.Hubner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. BioTechniques 44: 507–517, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa T, Matsuda A, Miyamoto K, Tsubosaki M, Umezawa H. Biological studies on bleomycin A. J Antibiot (Tokyo) 20: 149–155, 1967 [PubMed] [Google Scholar]
- 12.Ishida Y, Nagata K. Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol 499: 167–182, 2011 [DOI] [PubMed] [Google Scholar]
- 13.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Marangoni R, Wei J, Hinchcliff M, Fang F, Tourtellotte W, Varga J. Aberrant adipogenesis in the pathogenesis of scleroderma. Arthritis Rheum 64: S341–S342, 2012 [Google Scholar]
- 15.Markart P, Wygrecka M, Guenther A. Update in diffuse parenchymal lung disease 2010. Am J Respir Crit Care Med 183: 1316–1321, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? In J Biochem Cell Biol 40: 362–382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L152–L160, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med 17: 355–361, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Muggia FM, Louie AC, Sikic BI. Pulmonary toxicity of antitumor agents. Cancer Treat Rev 10: 221–243, 1983 [DOI] [PubMed] [Google Scholar]
- 20.Nagata K. HSP47 as a collagen-specific molecular chaperone: function and expression in normal mouse development. Semin Cell Dev Biol 14: 275–282, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Nagata K. Hsp47: a collagen-specific molecular chaperone. Trends Biochem Sci 21: 22–26, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Scotton CJ, Chambers RC. Bleomycin revisited: towards a more representative model of IPF? Am J Physiol Lung Cell Mol Physiol 299: L439–L441, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Taguchi T, Razzaque MS. The collagen-specific molecular chaperone HSP47: is there a role in fibrosis? Trends Mol Med 13: 45–53, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Tan A, Denton CP, Mikhailidis DP, Seifalian AM. Recent advances in the diagnosis and treatment of interstitial lung disease in systemic sclerosis (scleroderma): a review. Clin Exp Rheumatol 29: S66–S74, 2011 [PubMed] [Google Scholar]
- 25.Tourkina E, Richard M, Gooz P, Bonner M, Pannu J, Harley R, Bernatchez PN, Sessa WC, Silver RM, Hoffman S. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 294: L843–L861, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Tourkina E, Richard M, Oates J, Hofbauer A, Bonner M, Gooz P, Visconti R, Zhang J, Znoyko S, Hatfield CM, Silver RM, Hoffman S. Caveolin-1 regulates leucocyte behaviour in fibrotic lung disease. Ann Rheum Dis 69: 1220–1226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umezawa H. Chemistry and mechanism of action of bleomycin. Fed Proc 33: 2296–2302, 1974 [PubMed] [Google Scholar]
- 28.Umezawa H, Ishizuka M, Maeda K, Takeuchi T. Studies on bleomycin. Cancer 20: 891–895, 1967 [DOI] [PubMed] [Google Scholar]
- 29.Wang ZL. Advances in understanding of idiopathic pulmonary fibrosis. Chin Med J (Engl) 122: 844–857, 2009 [PubMed] [Google Scholar]
- 30.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2: 103–121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]