Abstract
Hypothalamic proopiomelanocortin (POMC) neurons constitute a critical anorexigenic node in the central nervous system (CNS) for maintaining energy balance. These neurons directly affect energy expenditure and feeding behavior by releasing bioactive neuropeptides but are also subject to signals directly related to nutritional state such as the adipokine leptin. To further investigate the interaction of diet and leptin on hypothalamic POMC peptide levels, we exposed 8- to 10-wk-old male POMC-Discosoma red fluorescent protein (DsRed) transgenic reporter mice to either 24–48 h (acute) or 2 wk (chronic) food restriction, high-fat diet (HFD), or leptin treatment. Using semiquantitative immunofluorescence and radioimmunoassays, we discovered that acute fasting and chronic food restriction decreased the levels of adrenocorticotropic hormone (ACTH), α-melanocyte-stimulating hormone (α-MSH), and β-endorphin in the hypothalamus, together with decreased DsRed fluorescence, compared with control ad libitum-fed mice. Furthermore, acute but not chronic HFD or leptin administration selectively increased α-MSH levels in POMC fibers and increased DsRed fluorescence in POMC cell bodies. HFD and leptin treatments comparably increased circulating leptin levels at both time points, suggesting that transcription of Pomc and synthesis of POMC peptide products are not modified in direct relation to the concentration of plasma leptin. Our findings indicate that negative energy balance persistently downregulated POMC peptide levels, and this phenomenon may be partially explained by decreased leptin levels, since these changes were blocked in fasted mice treated with leptin. In contrast, sustained elevation of plasma leptin by HFD or hormone supplementation did not significantly alter POMC peptide levels, indicating that enhanced leptin signaling does not chronically increase Pomc transcription and peptide synthesis.
Keywords: arcuate nucleus, energy homeostasis, hypothalamus, metabolism, neural networks, obesity, proopiomelanocortin neurons, leptin, proopiomelanocortin
neurons in the arcuate nucleus of the hypothalamus (ARC) containing the proopiomelanocortin (POMC) gene are central to the maintenance of energy homeostasis. Pomc mRNA in these neurons encodes a 241-amino acid prohormone that is enzymatically processed into multiple bioactive peptides. Proconvertase 1/3 (PC1/3) cleaves POMC into adrenocorticotropic hormone (ACTH) and β-lipotrophin, which are further processed by PC2 and carboxypeptidase E (CPE) into α-melanocyte-stimulating hormone (α-MSH) and β-endorphin (46, 66). In the central nervous system (CNS), ACTH is relatively inactive, but α-MSH acts on melanocortin 4 receptor (MC4R) to potently downregulate appetitive behavior and increase energy expenditure, whereas β-endorphin, signaling primarily through μ-opioid receptors, has an acute stimulatory effect on feeding (13, 44).
An animal's feeding state can also tune the strength of Pomc expression and its downstream peptide products. Fasting or chronic food restriction decreases Pomc mRNA (31, 37), which translates into diminished expression of CNS ACTH, β-endorphin, and α-MSH (23, 50, 62). By contrast, a surplus of calories from fat or an exogenous injection of the fat-derived adipokine leptin have been reported to increase transcription of Pomc and the synthesis of POMC peptides (25, 34, 45, 50, 62, 70). However, extended bouts of high-fat diet (HFD) are also reported to diminish the expression of POMC peptides and the conversion of ACTH to α-MSH (8, 17, 39, 63). Likewise, diet-dependent changes in POMC peptide levels are also conferred, at least in part, by tandem regulation of peptide cleavage and peptide-modifying enzymes (8, 25, 46).
Problematically, there is a lack of consensus regarding POMC peptide levels in POMC neurons under different dietary conditions due to the use of different animal models (species, sex, and age), time points, stimuli, and experimental assays (63). To address these issues, we established acute (24–48 h) and chronic (2 wk) diet manipulations utilizing food restriction, HFD, or leptin treatment with 8- to 10-wk-old male C57BL/6 POMC-Discosoma red fluorescent protein (DsRed) transgenic reporter mice and assessed the changes in nutritional state on POMC peptide levels in the hypothalamus. At both time points, food restriction decreased circulating levels of leptin, whereas HFD or leptin treatment enhanced serum leptin levels. Our radioimmunoassay (RIA) measurements of hypothalamic extracts indicate that acute fasting and chronic food restriction generally diminished the levels of all POMC peptides, whereas only an acute exposure to HFD led to increased expression of the POMC-DsRed reporter in ARC cell bodies and increased α-MSH immunofluorescence in POMC projections throughout the hypothalamus. The latter results were replicated by acute leptin treatment of either ad libitum-fed or 24-h-fasted mice. Taken together, these findings in a mouse model indicate that hypothalamic POMC peptide expression is consistently downregulated by both acute and chronic caloric deficit in association with decreased leptin levels. However, chronically increased circulating leptin, produced either endogenously by HFD or exogenously by minipump administration, does not induce or maintain the opposite effect of upregulating the synthesis of POMC peptides.
MATERIALS AND METHODS
Animals.
All procedures were approved by the University Committee on Use and Care of Animals at the University of Michigan and followed the Public Health Service guidelines for the humane care and use of experimental animals. Eight- to 10-wk-old homozygous POMC-DsRed male mice on a C57BL/6 background were used in all experiments. We selected this strain because C57BL/6 mice are commonly used to study mouse metabolism and are prone to diet-induced obesity (DIO) with HFD (9, 61), and the POMC-DsRed transgene permits the ready identification of POMC neurons in the hypothalamus (27, 32). Mice were housed in ventilated cages under controlled temperature and photoperiod (12:12-h light-dark cycle, lights on from 0600 to 1800), with water, corncob bedding, and laboratory chow (12.1% kcal fat, 28.0% kcal protein, and 59.8% kcal carbohydrate) available ad libitum. For each component of this study (histology, RIA, and leptin ELISA), we used 8–10 animals/diet for each treatment unless stated otherwise.
Diets and treatments.
Mice were singly housed at 7.5 wk of age on aspen bedding with a nestlett and provided control chow (3.08 kcal/g; 10.2% kcal fat, 18.3% kcal protein, and 71.5% kcal carbohydrate; 58Y2; TestDiet, St. Louis, MO) for 5 days before dietary manipulation. During the acclimatization period, mice receiving injections were given a total of six mock injections (2/day for 3 days) to minimize the stress of PBS or leptin injections. After this acclimatization period, mice were divided into one of eight experimental groups. In the acute diet manipulation paradigm, control mice received 58Y2 chow plus three intraperitoneal injections of phosphate-buffered saline, pH 7.4 (PBS) 12 h apart; fasted mice were deprived of all food for 24 h; HFD mice were fed 58Y1 chow (5.1 kcal/g; 61.6% kcal fat, 18.1% kcal protein, and 20.3% kcal carbohydrate; TestDiet) for 48 h; and leptin-injected mice on 58Y2 chow received three injections of leptin 12 h apart [5 mg/kg ip in PBS (lot no. AFP1751 from Dr. A. Parlow, The National Hormone and Peptide Program, Torrance, CA)] over 24 h. The third dose was injected 30 min before tissue collection. Mice and chow were weighed daily in the acute diet paradigm. In the chronic diet modification studies, mice were subjected to food restriction, HFD, or an infusion of leptin for 2 wk. Control mice were fed 58Y2 chow and surgically implanted with a sterile PBS-secreting minipump (model 1002; Alzet, Cupertino, CA), mice receiving HFD were fed 58Y1 chow, and leptin-treated mice were fed 58Y2 chow and received 10 μg leptin/day via osmotic minipump (model 1002; Alzet). Food-restricted mice in this cohort were weighed daily and fed 50–60% of their standard daily kilocalories of 58Y2 chow to lower their body weight to ∼80% of control mice. The animals were fully fasted during the final 24 h of the experiment to maximize weight reduction/caloric restriction. Body weight and chow measurements for this cohort were taken after 24 h, 48 h, 1 wk, and 2 wk. All animal husbandry and injections were performed uniformly at 1100 and 2300, respectively.
Immunohistochemistry and antibodies.
Mice were perfused on a pressurized rig (Perfusion One; Leica, Buffalo Grove, IL) with 4% paraformaldehyde in PBS (PFA). Tissues was postfixed overnight in 4% PFA followed by successive gradients of sucrose up to 30% wt/vol in potassium phosphate-buffered saline (KPBS). Brains were sectioned into four sets on a freezing microtome stage (SM 2010R; Leica) at 30 μm/section and stored in cryoprotectant solution (25 mM PBS with 30% ethylene glycol and 20% glycerol, pH 7.3) at −20°C until use. Sections were then incubated in KBPS with 0.1% Triton X-100 (KPBS-T), 2% normal goat or donkey serum and primary antibodies overnight at 4°C. Following three washes in KPBS, the sections were incubated for 2 h at room temperature in KPBS-T with secondary antibodies, washed, and mounted on gelatin-coated glass slides. Sections were cover slipped using VectaShield hard mount (Vector Labs, Burlingame, CA) or polyvinyl alcohol mounting medium with DABCO (Sigma Aldrich). For our histological analyses, we used the following primary antibodies: rabbit-anti-ACTH (1:5,000; The National Hormone and Peptide Program), rabbit anti-β-endorphin (1:1,000; a gift from Dr. Sharon Wardlaw, Columbia University, New York, NY), and sheep anti-α-MSH [1:25,0000; a gift from Dr. Jeffrey Tatro, Tufts New England Medical Center, Boston, MA (16)]. Primary antibodies were detected using goat anti-rabbit FITC (1:500; JacksonImmuno, West Grove, PA) and donkey anti-sheep FITC (1:500; JacksonImmuno) secondary antibodies.
Imaging and analysis.
Brain sections were imaged using a Nikon 90i upright microscope (Nikon, Tokyo, Japan) equipped with an X-Cite 120Q fluorescent light source (Lumen Dyanmics, Mississauga, Ontario, Canada), a CoolSNAP HQ2 CD camera (Photometrics, Tucson, AZ), and a ×10 objective. FITC-labeled sections were imaged using a 488 excitation (ex)/525 emission (em) filter cube and an exposure time of 2 s, whereas POMC-DsRed neurons were imaged using a 525 ex/568 em filter cube with exposure times of 4 s. For image presentation, DsRed- or FITC-immunoreactive signals were adjusted to the same contrast and intensity levels post hoc in Adobe Photoshop CS6 (San Jose, CA). Each CNS nucleus was imaged along its rostral-caudal coordinates based on the mouse brain atlas of Franklin and Paxinos (21): ARC, −1.22 to −2.18; paraventricular hypothalamus (PVH), −0.58 to −1.06; and paraventricular thalamus (PVT), −0.94 to −2.06. Postimage analysis was performed in NIS-Elements AR (Nikon). Each CNS nucleus was manually outlined as a region of interest (ROI), and images were set to the same empirically derived pixel intensity established as a detectable signal to each antiserum in control animals. To selectively quantify POMC-DsRed cell bodies or POMC peptide projections, we set size thresholds of 700–2,700 μm2 (soma diameter of 15–30 μm) or <300 μm2 (fiber diameter <10 μm), respectively. The mean pixel intensity from an ROI was multiplied by the surface area of that ROI to determine the amount of fluorescent signal (in arbitrary units, AU) per image. Data were normalized to control animals to compare fold changes in fluorescent AUs.
RIA.
Hypothalamic tissue blocks were placed in 2 N acetic acid with 5 μl/ml protease inhibitor cocktail (P8340; Sigma Aldrich, St. Louis, MO) and were homogenized using an Omni TH Tissue Homogenizer on ice and then boiled for 10 min. An aliquot was used for determination of total protein content by a Bradford assay. Acid-extracted peptides were freeze-dried and then resuspended in RIA buffer. Samples were subjected to specific RIA analyses for ACTH, β-endorphin, and α-MSH as developed in our laboratory previously (8, 50) using both commercially available peptides and primary antibodies developed in the Nillni laboratory. ACTH, β-endorphin, and desacetyl-α-MSH were iodinated with 125I using the chloramine T oxidation-reduction method followed by HPLC separation, and the purified peptides were used as tracers. The ACTH and β-endorphin RIA assays were performed in 0.5 ml of RIA buffer (phosphate buffer, pH 7.4 with 500 mg/l sodium azide and 2.5 g/l BSA) containing anti-ACTH antiserum (1:30,000) or anti-β-endorphin (1:40,000) and 5,000 counts/min of 125I-labeled ACTH or 125I-labeled β-endorphin tracer. The sensitivity of the assays was ∼10 pg/tube, and the intra- and interassay variabilities were ∼5–7% and ∼10–11%, respectively. The α-MSH RIA assay was performed in 0.5 ml RIA buffer, with primary anti-α-MSH antiserum (1:20,000), and 5,000 counts/min of 125I-labeled desacetyl-α-MSH tracer. The sensitivity of the assay was ∼11.5 pg/tube, and the intra- and interassay variability was ∼10–11%. The ACTH assay detects 100% of CLIP and ACTH forms; however, this assay does not cross-react with any form of α-MSH or β-endorphin. The ACTH assay cross-reacts with the POMC precursor, although the amount of cross-reactivity is unknown. The β-endorphin antiserum does not cross-react with either ACTH or α-MSH.
Leptin ELISA.
We collected 200 μl of blood in 10 μl EDTA (75 mg/ml) from each mouse used for the hypothalamic RIA analyses and separated the plasma by centrifugation at 2,000 revolutions/min for 20 min at 4°C. Plasma leptin levels were quantified using the Mouse Leptin Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) following the manufacturer's instructions.
Statistics.
Results are presented as means ± SE. Data were analyzed using Prism 6 (GraphPad), and statistical significance between experimental conditions was determined using a repeated-measures two-way ANOVA with Dunnett's multiple-comparisons test (animal chow consumption and Δbody weight) or Student's unpaired t-test (immunohistochemistry, RIA, leptin ELISA). P values <0.05 were considered significant.
RESULTS
Effects of diet manipulation and leptin treatment on body weight and chow intake.
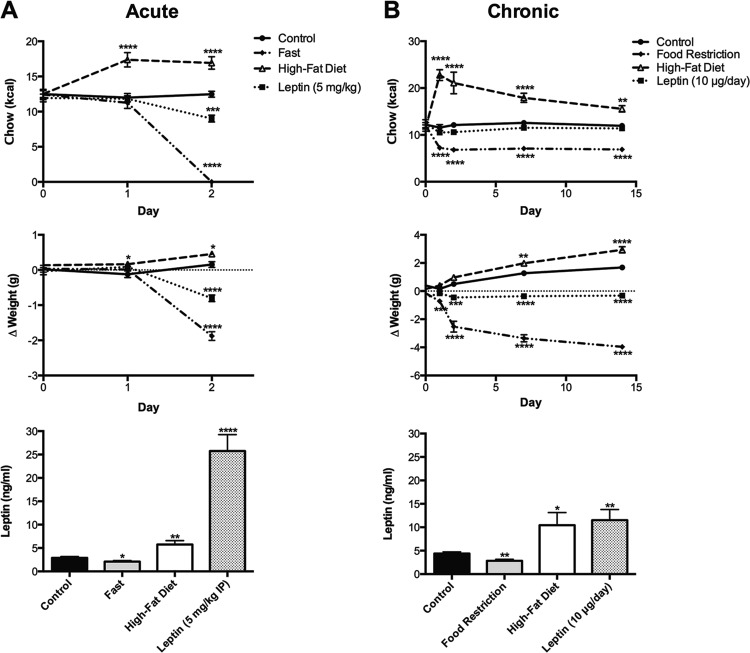
Neurons in the ARC expressing POMC-derived neuropeptides are direct modulators of energy expenditure and metabolism, but these cells are also subject to dynamic changes by an organism's feeding state (69). To determine the effects of nutritional state on the expression of POMC peptides in the ARC, we first established six experimental paradigms to modify the caloric intake or body weight of young adult male mice. Data from two separate cohorts of mice, subsequently used for either immunohistochemistry or RIA analyses, were collapsed together because an initial analysis showed no interaction of cohort with any of the measured variables (data not shown). In the acute manipulations (Fig. 1A), mice were fasted for 24 h, provided HFD for 48 h, or treated with three doses of leptin (5 mg/kg ip) over 24 h. Two-way ANOVA analyses revealed a significant interaction of time course and diet manipulation for both chow intake [F(6,210) = 35.3, P < 0.0001] and body weight [F(6,210) = 52.8, P < 0.0001]. The 24-h fast reduced body weight by 1.9 ± 0.1 g (P < 0.0001). In contrast, mice that were fed HFD for 48 h became hyperphagic and ate an additional average of 4.7 ± 0.4 kcal/day (P < 0.0001, 24 h; P < 0.0001, 48 h), resulting in a small but statistically significant increase in body mass (0.5 ± 0.1 g, P < .05). Leptin acutely reduced both body mass (−0.8 ± 0.1 g, P < 0.0001) and chow intake (−3.5 ± 0.5 kcal, P < 0.001).
Fig. 1.
Effects of food restriction, high-fat diet (HFD), and leptin treatment on body weight and chow consumption in 8- to 10-wk-old male mice. A: acute treatments, mice fasted for 24 h (diamonds) lost 7.8% body mass compared with ad libitum-fed controls (circles), whereas mice provided a HFD (triangles) for 48 h exhibited pronounced hyperphagia (+39.9% Δkcal consumed) and a small but significant weight gain (+2.4%). Mice treated with ip leptin (squares) for 24 h lost 3.5% body mass and ate 24% fewer kcal in this time period. A 24-h fast reduced plasma leptin levels by 28%, whereas 48 h of HFD and 24 h of ip leptin significantly enhanced circulating leptin to 2- and 8-fold that of control animals, respectively. B: chronic treatments, mice restricted to ∼54% kcal/day (diamonds) for 2 wk lost 17% body mass, whereas control mice (circles) gained an additional 8.5% body mass. Mice on HFD (triangles) gained 12% body mass due to heightened chow consumption. Mice outfitted with leptin minipumps (10 μg/day; squares), on the other hand, did not deviate in their overall kcal consumption but lost 2.8% body mass compared with their starting weight. Calorie restriction of mice for 2 wk decreased leptin by 35%, whereas HFD and low-level leptin infusion doubled circulating leptin levels. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 vs. control.
In the chronic manipulations (Fig. 1B), mice were food restricted (50–60% of control) for 2 wk, provided HFD for 2 wk, or treated with 10 μg/day leptin via osmotic minipump for 2 wk. Two-way ANOVA of these data revealed a significant interaction between time course and diet manipulation for both chow intake [F(12, 286) = 17.3, P < 0.0001] and body weight [F(12,300) = 52.0, P < 0.0001]. Similar to results observed after an acute fast, chronic food restriction reduced body weight by −4.0 ± 0.2 g (P < 0.0001). Mice on HFD were hyperphagic over the 2-wk time course (eating 5–12 additional kcal/time point), and while control mice gained 8.3% body mass at this age over the 2-wk period, mice eating HFD gained 11.8%. Finally, although mice receiving chronic leptin at 10 μg/day via osmotic minipump did not exhibit any measureable changes in food intake at later time points, the hormone treatment mitigated the weight gain exhibited by control mice over 2 wk and actually caused a weight loss of −2.0 ± 0.1 g compared with baseline (P < 0.0001).
Plasma leptin levels correlate with energy balance in the different treatment groups.
We focused our attention on circulating leptin levels in our diet manipulations (Fig. 1), since this hormone is both regulated by nutritional state and is commonly accepted as a primary effector of POMC neurons (54, 67). In the acute diet model, fasting reduced leptin levels to 2.1 ± 0.2 ng/ml (P < 0.05) from 2.9 ± 0.3 ng/ml in control ad libitum-fed mice, whereas HFD increased levels to 5.8 ± 0.9 ng/ml (P < 0.01). Intraperitoneal leptin induced an eightfold increase in circulating leptin levels to 25.8 ± 3.5 ng/ml (P < 0.0001). Similar results were observed in the chronic treatment paradigms. Food restriction reduced leptin levels by one-third from 4.4 ± 0.4 in PBS minipump-implanted animals to 2.8 ± 0.3 ng/ml (P < 0.01), whereas 2 wk of HFD or a 10 μg/day subcutaneous infusion of leptin doubled circulating leptin levels to 10.4 ± 2.7 (P < 0.05) and 11.5 ± 2.3 (P < 0.01) ng/ml, respectively.
Colocalization of POMC-DsRed neurons and POMC-derived peptides in the ARC.
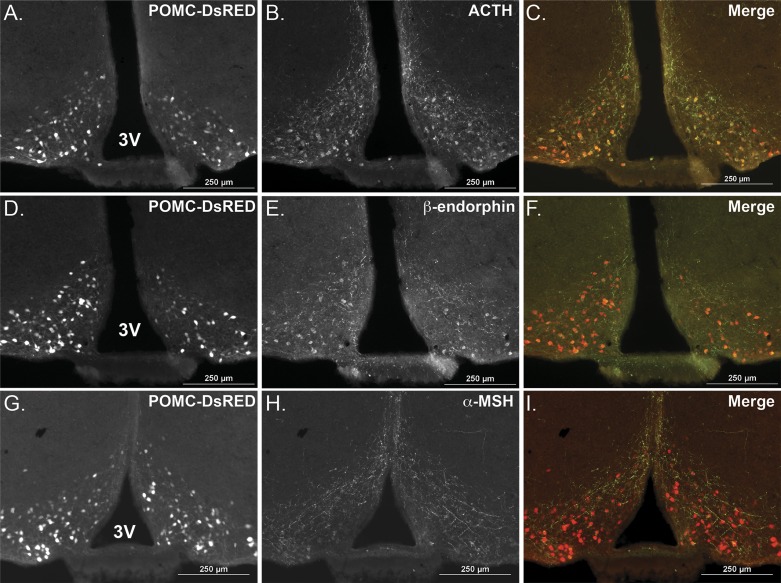
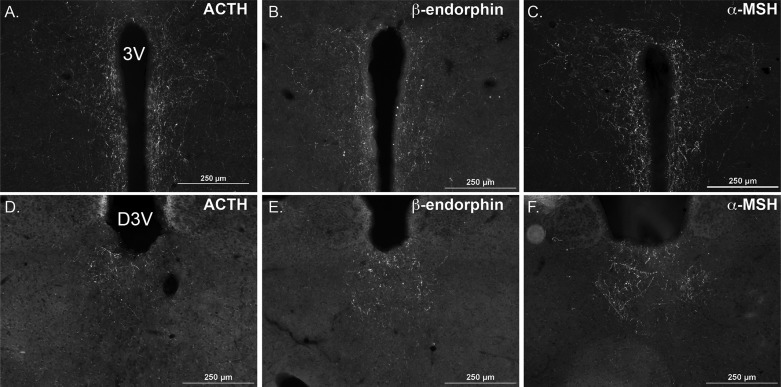
To interrogate POMC gene and peptide expression in discrete CNS nuclei using immunohistochemical methods, we quantified the POMC-DsRed signal in cell soma and ACTH, β-endorphin and α-MSH immunofluorescence in POMC fibers. The cell bodies of ∼3,000 POMC neurons span the entire rostrocaudal extent of the ARC (10). We identified these cell bodies by a DsRed fluorophore expressed under control of Pomc regulatory elements in the transgenic mice (27) (Fig. 2, A, D, and G). Brain sections were stained for ACTH (Fig. 2B), β-endorphin (Fig. 2E), or α-MSH (Fig. 2H) using antisera that all robustly label POMC peptide-containing fibers and to a variable extent the neuronal soma. This strategy allowed us to semiquantitatively measure the impact of diet on immunoreactive POMC peptide levels within the ARC and also at distinct distal target regions within the PVH (Fig. 3, A–C) and the PVT (Fig. 3, D–F) (41).
Fig. 2.
Comparison of adrenocorticotropic hormone (ACTH), β-endorphin, and α-melanocyte-stimulating hormone (α-MSH) immunoreactivity with native red fluorescent protein cloned from Discosoma (DsRed) fluorescence in the hypothalamus of proopiomelanocortin (POMC)-DsRed transgenic mice. Representative serial brain sections from an ad libitum-fed mouse showing POMC-DsRed neuron soma in the arcuate nucleus (ARC, −1.85 mm from bregma, A, D, and G). POMC-DsRed fluorescence colocalized with both ACTH (B and C) and β-endorphin (E and F) immunoreactivity in the soma, with fibers extending in the dorsal and medial directions. The α-MSH antibody (H and I) predominantly labeled immunoreactive fibers with the same dorsomedial distribution along the third ventricle as the ACTH and β-endorphin antisera. 3V, third ventricle. Scale bar = 250 μm.
Fig. 3.
POMC peptidergic projections in the paraventricular hypothalamus (PVH) and paraventricular thalamus (PVT). Robust immunopositive fibers for all three POMC peptides were detected in the PVH (−0.80 mm from bregma, A–C) and the PVT (−1.5 mm from bregma, D–F) of an ad libitum-fed mouse. 3V, third ventricle; D3V, dorsal third ventricle. Scale bar = 250 μm.
Dietary effects on POMC-DsRed fluorescence and peptide levels in discrete CNS nuclei.
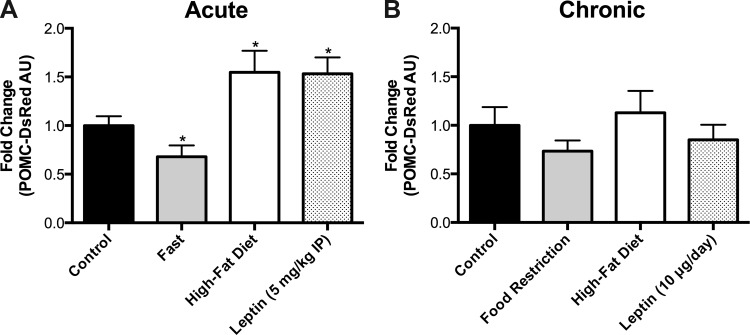
We examined the DsRed fluorescent signal for POMC cell bodies at every 120 μm along the rostrocaudal extent of the ARC, but there were no apparent differences in the signal intensity of DsRed in individual neuronal soma at different anatomical levels for each mouse, or differences in topographical distribution of the neurons between mice (data not shown). Thus, we averaged the fluorescent signals from each section to produce a single value per mouse. Acute (24 h) fasting (P < 0.05) and chronic (2 wk) caloric restriction reduced the POMC-DsRed signal compared with control mice (Fig. 4). In contrast, HFD increased POMC-DsRed signal acutely (P < 0.05, Fig. 4A) but had no effect at the 2-wk time point (Fig. 4B). Leptin induced a significant acute increase in POMC-DsRed fluorescence at 24 h (P < 0.05) but not after 2 wk of chronic infusion. Together, our data indicate that each of the experimental manipulations significantly altered Pomc-DsRed reporter gene expression acutely, but these effects were dampened or nonexistent after chronic manipulations of diet or leptin administration.
Fig. 4.
Hypothalamic POMC-DsRed fluorescence levels in response to diet and leptin treatment. A: acute treatments, a 24-h fast decreased the DsRed signal, whereas 48 h of HFD or 24 h of leptin treatment increased the DsRed signal in ARC POMC neurons. B: chronic treatments, neither chronic food restriction, HFD, nor leptin infusion by minipump for 2 wk significantly changed the levels of POMC-DsRed fluorescence compared with ad libitum-fed controls. *P < 0.05 vs. control.
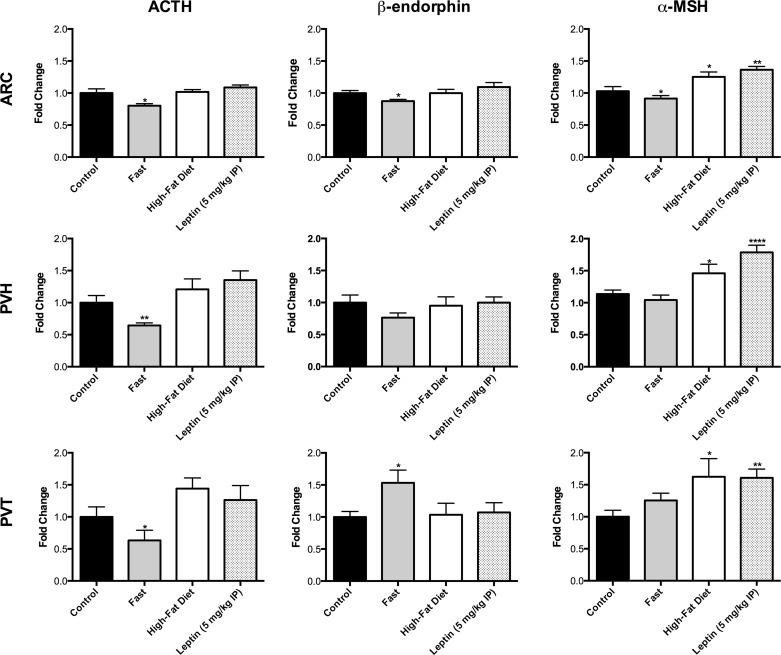
Similar to the analysis of DsRed fluorescence, we initially examined the fluorescent signal for immunoreactive POMC cell fibers at every 120 μm along the rostrocaudal extent of the hypothalamus, but there were no differences in topographical distribution of the fibers between mice from the different treatment groups for each of the brain nuclei examined (ARC, PVH, and PVT; data not shown). Therefore, we again averaged the fluorescent signals from each section to produce a single value per mouse for comparisons of diet and leptin treatment effects (Fig. 5). A 24-h fast reduced the expression of all immunoreactive POMC peptides (P < 0.05 vs. control) in the ARC, whereas 48 h HFD and 24 h leptin selectively increased levels of α-MSH in the ARC (HFD, P < 0.05; leptin, P < 0.01), PVH (HFD, P < 0.05; leptin, P < 0.0001) and PVT (HFD, P < 0.05; leptin, P < 0.01). Representative α-MSH immunofluorescent images in the ARC for the different treatments are shown in Fig. 6. Because ACTH and β-endorphin levels remained relatively static after HFD and leptin treatment, these data suggest that HFD and leptin may promote PC1/3, PC2, and CPE to actively process steady-state levels of POMC precursor peptides into more CNS bioactive α-MSH. Interestingly, fasting selectively increased, rather than decreased, β-endorphin immunoreactive fibers in the PVT (P < 0.05).
Fig. 5.
Immunoreactive POMC peptide expression in the ARC, PVH, and PVT in response to acute fasting, HFD, or leptin treatment. A 24-h fast diminished levels of ACTH in the ARC, PVH, and PVT and also decreased β-endorphin and α-MSH in the ARC. This manipulation, however, enhanced β-endorphin immunofluorescence in the PVT. HFD for 48 h and leptin for 24 h, on the other hand, did not significantly alter the expression of ACTH and β-endorphin but did enhance α-MSH fiber immunofluorescence in the ARC, PVH, and PVT. *P < 0.05, **P < 0.01, and ****P < 0.0001 vs. control.
Fig. 6.
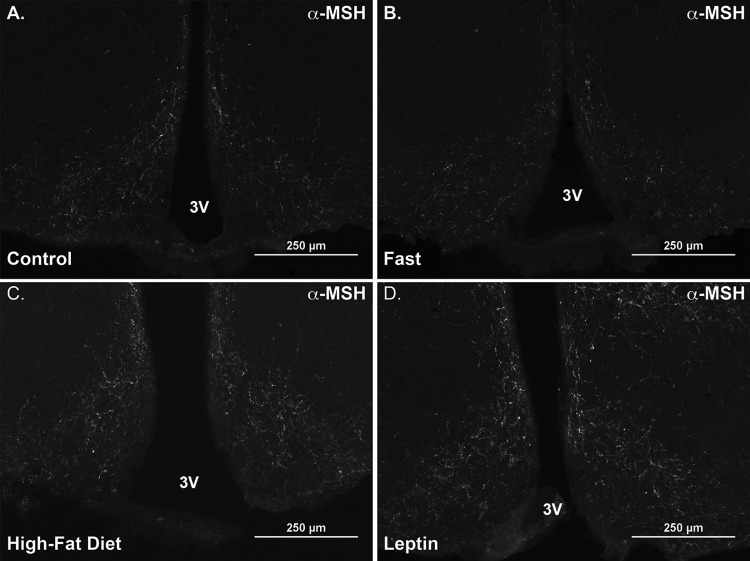
Immunoreactive α-MSH fibers in the ARC in response to acute fasting, HFD, or leptin treatment. Representative epifluorescent images of α-MSH fibers in the ARC of POMC-DsRed mice were obtained at −1.7 mm from bregma using the Nikon 90i settings described in materials and methods. All sections were imaged at ×10 magnification using an FITC filter cube and an exposure of 2 s. Images were modified simultaneously post hoc in Adobe Photoshop CS6 to optimize signal and contrast. A: a control ad libitum-fed mouse. An animal fasted for 24 h exhibits a decrease in α-MSH expression (B), whereas animals eating HFD for 48 h (C) or treated with leptin for 24 h (D) show enhanced α-MSH expression in the ARC. Scale bar = 250 μm.
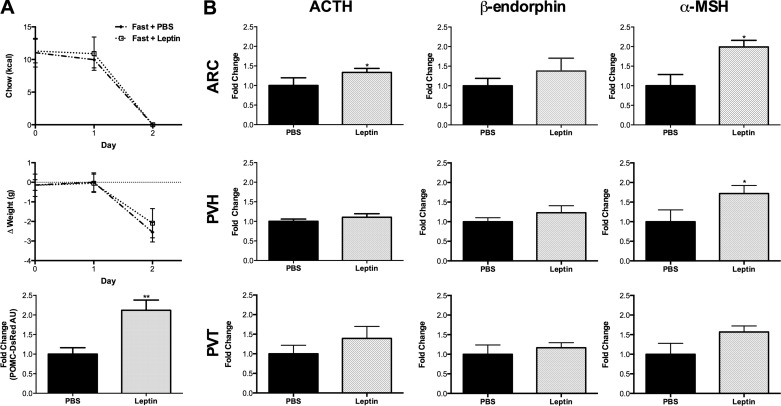
In a separate set of experiments, we further examined the interaction of acute fasting and leptin replacement (Fig. 7). Exogenous leptin treatment did not produce a more pronounced loss of body weight compared with fasted animals injected with PBS; however, it doubled the POMC-DsRed fluorescent signal in ARC POMC neuronal soma (Fig. 7A). Similarly, leptin treatment of fasted mice significantly increased ACTH immunofluorescence in the ARC and α-MSH in the ARC and PVH, with trends for increased expression of all POMC peptides in the three target nuclei examined (Fig. 7B). Thus, our data suggest that the downregulation of Pomc gene and peptide expression induced by fasting is prevented by simultaneous leptin administration. These findings are consistent with previous work from our group studying rats dosed every 6 h with leptin (0.5 mg/kg ip) during a 65-h fast (50). In that study, leptin treatment partially reversed the fasting-induced decreases in Pomc mRNA and ACTH and α-MSH peptides as measured by RT-qPCR and RIA analysis, respectively.
Fig. 7.
Simultaneous leptin treatment ameliorates fasting-induced decreases in POMC-DsRed fluorescence and POMC peptide immunoreactivity. A: fasted animals injected with PBS (diamonds; n = 5) and fasted animals injected with three doses of 5 mg/kg leptin over 24 h (open squares; n = 4) lost similar amounts of body weight (2.6 and 2.1 g, respectively) compared with their prefast baseline weights (P < 0.0001). Despite similar weight loss, animals receiving leptin exhibited significantly more robust POMC-DsRed fluorescence (checkered gray bar). B: leptin-treated mice also had significantly greater levels of ACTH immunofluorescent fibers in the ARC and α-MSH immunofluorescent fibers in the ARC and PVH compared with PBS-treated mice. *P < 0.05 and **P < 0.01 vs. fast and PBS.
For the most part, mice in the chronic treatment paradigms did not exhibit any significant alterations in immunoreactive POMC peptide expression in fibers within the ARC, PVH, or PVT compared with control mice (data not shown). The only statistically significant change in peptide expression was a decrease in ARC β-endorphin levels associated with chronic food restriction (P < 0.05). However, the biological significance of this isolated finding is not known.
Quantitative analysis of diet-induced changes in POMC peptides by RIA.
While our immunohistochemical data offer insight into relative peptide levels at an anatomical level within POMC neuron fibers, we also measured diet-induced modifications in hypothalamic POMC peptide levels using an alternative quantitative approach. Hypothalamic blocks from a second cohort of mice that underwent each of the dietary or leptin interventions were acid extracted by HPLC and analyzed using RIAs to examine the levels of ACTH, β-endorphin, and α-MSH in the combination of neuronal soma and all projecting fibers within the hypothalamus (see materials and methods for specific peptide cross-reactivity). In comparison, the ACTH antiserum used in our histological analysis detects full-length synthetic ACTH (27) with minor cross-reactivity to other processed POMC peptides (A. F. Parlow, personal communication). The β-endorphin antisera for immunohistochemistry exhibits 9% cross-reactivity to POMC (S. Wardlaw, unpublished observation), and the α-MSH antisera has <1% cross-reactivity with other POMC peptides (16). Thus, the RIA analysis provides an additional level of quality control and detection of POMC peptides to complement our semiquantitative histological analysis.
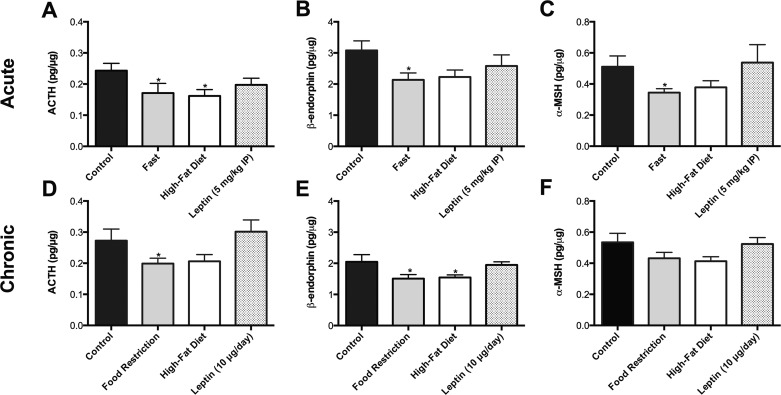
The RIA measurements showed that 24 h of food restriction decreased the levels of all three POMC peptides relative to control mice (P < 0.05), whereas chronic food restriction significantly reduced ACTH and β-endorphin levels (P < 0.05), with a trend to decreased α-MSH (Fig. 8). Both the 48-h and 2-wk HFD-treated groups also exhibited trends for decreased levels of all POMC peptides, with the decrease in ACTH after the 48-h HFD (P < 0.05) and β-endorphin levels after chronic HFD (P < 0.05) reaching significance. Neither acute nor 2 wk leptin treatments significantly modified the individual POMC peptide levels, but there were slight changes in the molar ratio of ACTH/α-MSH consistent with alterations in POMC peptide processing (8, 50).
Fig. 8.
Radioimmunoassay quantitation of POMC peptides from hypothalamic extracts. In both acute (A, B, and C) and chronic (D, E, and F) states of calorie restriction, all POMC peptides [ACTH (A and D), β-endorphin (B and E), and α-MSH (C and F)] decreased compared with control values, whereas there was a trend for mice on either acute or chronic HFD to also exhibit decreased levels of POMC peptides. Data are presented as means ±1 SE pg peptide content/μg total protein/extracted hypothalamus. *P < 0.05 vs. control.
DISCUSSION
Our purpose in designing these experiments was 1) to examine different diet manipulations at short- and long-term time points in an established rodent model, 2) to quantify the anatomical and biochemical changes in hypothalamic POMC peptides, and 3) to correlate the peptide levels with circulating levels of leptin. The chosen manipulations in a transgenic mouse model produced robust and consistent changes in body weight and food intake. Both an acute 24-h fast or 2-wk period of chronic food restriction decreased the quantities of hypothalamic POMC-DsRed, ACTH, β-endorphin, and α-MSH, with the exception of increased β-endorphin immunoreactivity in the PVT of acutely fasted mice. We postulate that enhanced opioid activity in the PVT may integrate feeding information to cortical reward centers after a fasted animal is refed (5, 6, 30, 38). HFD, on the other hand, transiently increased POMC-DsRed and α-MSH content in POMC projections, particularly in the PVH and PVT, but the total hypothalamic POMC peptide content measured by RIAs was decreased. Finally, acute but not chronic injections of leptin increased both POMC-DsRed and α-MSH fiber immunofluorescence during ad libitum feeding and reversed the fasting-induced decreases of POMC-DsRed and α-MSH. These observations are consistent with a previous study showing that another mammalian prohormone, prothyrotropin-releasing hormone, is initially cleaved in the trans Golgi network by PC1, and its peptide products are delivered to different vesicles of the secretory pathway (49). Our findings suggest that, even though all POMC neurons express the Pomc gene and process the prohormone to ACTH, β-endorphin, and α-MSH, the POMC peptides may be differentially processed and trafficked to specific target sites throughout the CNS in a diet-dependent manner (53).
The observed changes in the levels of DsRed fluorescence in POMC neuronal soma closely match the magnitude and valence of changes in Pomc mRNA reported previously from similar experimental manipulations (Low laboratory, unpublished data) (31, 37, 50). These similarities support our use of the relatively stable DsRed transcriptional reporter protein as a surrogate for direct measurements of Pomc mRNA. Taken together, the present results show that nutritional state can modify levels of hypothalamic Pomc expression and POMC peptides, but these effects are not consistently related to the corresponding plasma leptin levels.
The conundrum of HFD chow and POMC peptide levels.
We originally hypothesized that a surplus of calories from HFD would homeostatically drive Pomc transcription (70) and the synthesis of α-MSH to downregulate the hyperphagic behavior associated with this regimen. However, only anatomical data from the acute 48-h HFD exposure, but not the 2-wk HFD, are consistent with this hypothesis. Similar to previous studies in C57BL/6 mice, HFD acutely increased hypothalamic Pomc mRNA but chronically had no effect in transgenic C57BL/6 POMC-DsRed mice (19, 70). However, the total POMC peptide contents in the hypothalamus as measured by RIA was either decreased or unchanged in both the acute and chronic HFD groups. Perhaps the simplest explanation may be that acute HFD prompts POMC neurons to synthesize and release α-MSH at distal target sites to mitigate the abrupt increase in caloric intake. As discussed above, the RIAs quantify all POMC peptides in the soma and their hypothalamic projections, and therefore decreased peptide content in the soma of HFD animals may mask more subtle changes in α-MSH immunoreactivity localized to the projecting fibers.
Rats or mice chronically fed a HFD have been reported to exhibit decreased POMC peptide levels and either no change or a decrease in Pomc expression (8, 17, 24, 26, 29, 36). Decreased levels of POMC peptides in these rodent models have been attributed to hypothalamic inflammation, endoplasmic reticulum (ER) stress, and enhanced autophagy (8, 11, 42, 47, 60). The consensus is that animals persistently exposed to a HFD, regardless of the absolute fat content of the chow (63), develop cellular stress and impaired processing of POMC peptides. Our “chronic HFD” rodent model, on the other hand, is weeks to months shorter than those used in the aforementioned studies, and POMC neurons in the mice may not be subject to the same level of cellular stress. On a much shorter time scale, HFD is reported to rapidly mitigate the beneficial metabolic effects of intracerebroventricular leptin treatment in Lepob/ob mice through a proposed inflammatory pathway (33), but it is not known if this physiology applies to nonobese animals as well. Although we cannot rule out the possibility of inflammation or ER stress contributing to decreased POMC peptide expression in the 2 wk chronic HFD mice, a secondary explanation may be that, while our HFD animals were initially hyperphagic, they slowly decreased the total grams of chow consumed per day in response to the higher kilocalorie content of the HFD chow and may simply be maintaining their new body weight set point (12, 59). Consequently, the necessity to drive Pomc and α-MSH synthesis becomes less crucial to compensate for the state of positive energy balance as the mice begin to normalize their chow intake, despite the small but statistically significant increase in body weight. Whereas HFD has been studied as a mechanism for modifying POMC peptide content, other reports suggest that HFD induces alterations in synaptic scaffold proteins, as well as changes in the synaptic inputs to POMC neurons (3, 45, 60). However, the interplay between these mechanisms and POMC peptide synthesis is not clear.
Increased circulating leptin levels do not drive POMC peptide synthesis in ad libitum-fed animals.
It is well established that genetic ablation of the genes encoding leptin, POMC, or MC4R lead to a similar phenotype of pronounced hyperphagia, weight gain, and decreased energy expenditure (2, 20) and that leptin can directly affect POMC neurons through both tyrosine kinase signaling and fast synaptic mechanisms downstream of the long from of the leptin receptor (10, 15, 28, 52, 65). A central dogma in the field of CNS control of metabolism is that leptin stimulates POMC neurons to release α-MSH, which acts on downstream melanocortin receptors to increase energy expenditure and decrease food intake. However, enhanced levels of circulating leptin may not necessarily further increase the activity of ARC POMC neurons. In our experiments, acute fasting, acute HFD feeding, and acute leptin administration, to either fasted or ad libitum-fed animals, predictably altered plasma leptin levels, Pomc expression assessed by DsRed levels, and α-MSH immunoreactivity localized to POMC fibers. On the other hand, chronic leptin administration, which mimicked the endogenous plasma levels associated with chronic HFD, dissociated the changes in body weight and POMC peptide content from each other. This suggests that, while a minimum threshold of leptin signaling in POMC neurons is required to maintain energy balance, glucose homeostasis, and insulin sensitivity (1, 4, 68), increased circulating leptin levels induced by diet or hormone treatment do not necessarily increase melanocortin peptide synthesis in ARC POMC neurons. In a rat model of HFD-based DIO, animals exhibited decreased α-MSH expression in the hypothalamus despite a hyperleptinemic phenotype (48). In this model, it was later discovered that ER stress dampened the conversion of ACTH to α-MSH, despite enhanced transcription of Pomc (8). In contrast, chronic treatment of ad libitum-fed C57BL/6 mice with a long-lasting leptin antagonist resulted in profound metabolic dysfunction, including significant weight gain after 12 wk of treatment and profound hyperphagia in the first two weeks of the experiment (55). The metabolic phenotype was similar to that of HFD-induced DIO C57BL/6 mice, but the combined study using the leptin antagonist treatment in the presence of HFD-DIO animals has not been performed to date. Regardless, Solomon et al. (55) reported that Pomc expression was decreased after 4 and 12 wk of treatment with the leptin antagonist, but whether this is due directly to a lack of bioavailable leptin or is secondary to the obesity phenotype remains unclear.
Genetic ablation of leptin receptors specifically from POMC neurons results in hyperphagia, weight gain, and impaired glucose handling (1, 4). Alternatively, overexpression of leptin receptors or the leptin effector signal transducer and activator of transcription 3 (STAT3) in POMC neurons upregulates suppressor of cytokine signaling 3 (SOCS3), which binds to phosphotyrosine residues on the leptin receptor and subsequently downregulates its signaling by second messengers (18, 22, 43, 64). The conflicting STAT3/SOCS3 signals in POMC neurons result in mice that are much more prone to HFD-induced obesity. Paradoxically, these different rodent models exhibit metabolic dysfunction despite either an absence or overabundance of leptin receptor signaling in POMC neurons. While we did not assay our animals for phospho-STAT3 and -SOCS3 expression in the ARC, enhanced levels of leptin, particularly in the chronic HFD and leptin minipump models, may be upregulating SOCS3 expression, and thus contributing to a state of cellular leptin resistance. Similarly, it has been reported that chronic leptin supplementation fails to ameliorate DIO in C57BL/6 mice and aged rats (7, 56, 58). Although these studies did not examine hypothalamic POMC peptide content, the authors did note a failure of leptin to increase energy expenditure or drive anorexigenic behavior in their animal model. On the contrary, centrally administered or transgenically overexpressed α-MSH can induce anorexia, catabolism, and energy expenditure across a range of ages and diets in different rodent models (14, 35, 40, 51, 57), presumably because these interventions bypass leptin resistance.
Based on the current and previously published studies, our primary conclusion is that rodents maintain appropriate leptin levels to preserve physiological leptin signaling, Pomc transcription, and α-MSH peptide synthesis and release. Although POMC-derived α-MSH can potently regulate energy balance, the feedback pathway connecting food intake, circulating leptin levels, and peptide release from ARC POMC neurons is subject to change by nutritional state. Consequently, circulating leptin levels do not directly correlate with Pomc expression and POMC peptide synthesis under all conditions.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants F32-DK-098051 (to A. J. Mercer), R01-DK-085916 (to E. A. Nillni), and R01-DK-066604 (to M. J. Low). The Molecular Imaging and Analysis Core of the Michigan Diabetes Research Center is funded by NIDDK Grant P30-DK-020572.
DISCLOSURES
The authors have no financial or professional conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: A.J.M., V.O.-C., and M.J.L. conception and design of research; A.J.M., R.S., C.A.A., and V.O.-C. performed experiments; A.J.M., R.S., C.A.A., E.A.N., and M.J.L. analyzed data; A.J.M., E.A.N., and M.J.L. interpreted results of experiments; A.J.M. prepared figures; A.J.M. drafted manuscript; A.J.M., C.A.A., E.A.N., and M.J.L. edited and revised manuscript; A.J.M., R.S., C.A.A., E.A.N., and M.J.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sharon Wardlaw (Columbia University) for providing β-endorphin antisera and Dr. Jeffrey Tatro (Tufts New England Medical Center) for providing α-MSH antisera for our immunofluorescence studies. We also thank Dr. Steve Lentz at the Molecular Imaging and Analysis Core (University of Michigan) for advice on our semiquantitative immunofluorescence analysis, Dr. Jessica Mercer (University of Michigan) for critical reading of the manuscript, and Eva Yokosawa (University of Michigan) for assistance with animal husbandry, perfusions, and immunohistochemistry.
REFERENCES
- 1.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42: 983–991, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet 3: 589–600, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Benani A, Hryhorczuk C, Gouaze A, Fioramonti X, Brenachot X, Guissard C, Krezymon A, Duparc T, Colom A, Nedelec E, Rigault C, Lemoine A, Gascuel J, Gerardy-Schahn R, Valet P, Knauf C, Lorsignol A, Penicaud L. Food intake adaptation to dietary fat involves PSA-dependent rewiring of the arcuate melanocortin system in mice. J Neurosci 32: 11970–11979, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berglund ED, Vianna CR, Donato J, Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest 122: 1000–1009, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155: 1337–1350, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatnagar S, Dallman MF. The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res 851: 66–75, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Bowen H, Mitchell TD, Harris RB. Method of leptin dosing, strain, and group housing influence leptin sensitivity in high-fat-fed weanling mice. Am J Physiol Regul Integr Comp Physiol 284: R87–R100, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cakir I, Cyr NE, Perello M, Litvinov BP, Romero A, Stuart RC, Nillni EA. Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing. J Biol Chem 288: 17675–17688, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champy MF, Selloum M, Zeitler V, Caradec C, Jung B, Rousseau S, Pouilly L, Sorg T, Auwerx J. Genetic background determines metabolic phenotypes in the mouse. Mamm Genome 19: 318–331, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001 [DOI] [PubMed] [Google Scholar]
- 11.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146: 4192–4199, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy-volunteers. Am J Clin Nutr 56: 641–655, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Dutia R, Meece K, Dighe S, Kim AJ, Wardlaw SL. beta-Endorphin antagonizes the effects of alpha-MSH on food intake and body weight. Endocrinology 153: 4246–4255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eerola K, Nordlund W, Virtanen S, Dickens AM, Mattila M, Ruohonen ST, Chua SC, Jr, Wardlaw SL, Savontaus M, Savontaus E. Lentivirus mediated alpha-melanocyte stimulating hormone overexpression in the hypothalamus decreases diet induced obesity in mice. J Neuroendocrinol 10.1111/jne.12109 [DOI] [PubMed] [Google Scholar]
- 15.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23: 775–786, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 402: 442–459, 1998 [PubMed] [Google Scholar]
- 17.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Munzberg H, Hampel B, Alber J, Kloppenburg P, Bruning JC, Wunderlich FT. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci 29: 11582–11593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan CN, Liu XL, Shen WW, Deckelbaum RJ, Qi KM. The regulation of leptin, leptin receptor and pro-opiomelanocortin expression by N-3 PUFAs in diet-induced obese mice is not related to the methylation of their promoters. Nutr Metab 8: 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farooqi IS, O'Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab 4: 569–577, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Franklin KB, Paxinos G. The Mouse Brain in Sterotaxic Coordinates, Compact (3rd Ed.). Philadelphia, PA: Elsevier, 2008, p. 256 [Google Scholar]
- 22.Gamber KM, Huo L, Ha S, Hairston JE, Greeley S, Bjorbaek C. Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity. PLoS One 7: e30485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gambert SR, Garthwaite TL, Pontzer CH, Hagen TC. Fasting associated with decrease in hypothalamic beta-endorphin. Science 210: 1271–1272, 1980 [DOI] [PubMed] [Google Scholar]
- 24.Gout J, Sarafian D, Tirard J, Blondet A, Vigier M, Rajas F, Mithieux G, Begeot M, Naville D. Leptin infusion and obesity in mouse cause alterations in the hypothalamic melanocortin system. Obesity (Silver Spring) 16: 1763–1769, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Guo L, Munzberg H, Stuart RC, Nillni EA, Bjorbaek C. N-acetylation of hypothalamic alpha-melanocyte-stimulating hormone and regulation by leptin. Proc Natl Acad Sci USA 101: 11797–11802, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen MJ, Schioth HB, Morris MJ. Feeding responses to a melanocortin agonist and antagonist in obesity induced by a palatable high-fat diet. Brain Res 1039: 137–145, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci 29: 13684–13690, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Bronneke HS, Levin BE, Diano S, Cowley MA, Tschop MH. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA 107: 14875–14880, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang XF, Han M, South T, Storlien L. Altered levels of POMC, AgRP and MC4-R mRNA expression in the hypothalamus and other parts of the limbic system of mice prone or resistant to chronic high-energy diet-induced obesity. Brain Res 992: 9–19, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493: 72–85, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Kim EM, Welch CC, Grace MK, Billington CJ, Levine AS. Chronic food restriction and acute food deprivation decrease mRNA levels of opioid peptides in arcuate nucleus. Am J Physiol Regul Integr Comp Physiol 270: R1019–R1024, 1996 [DOI] [PubMed] [Google Scholar]
- 32.King CM, Hentges ST. Relative number and distribution of murine hypothalamic proopiomelanocortin neurons innervating distinct target sites. PLoS One 6: e25864, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch CE, Lowe C, Pretz D, Steger J, Williams LM, Tups A. High fat diet induces leptin resistance. J Neuroendocrinol 26: 58–67, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Korner J, Chua SC, Jr, Williams JA, Leibel RL, Wardlaw SL. Regulation of hypothalamic proopiomelanocortin by leptin in lean and obese rats. Neuroendocrinology 70: 377–383, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Lee M, Kim A, Chua SC, Jr, Obici S, Wardlaw SL. Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab 293: E121–E131, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res 875: 89–95, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 73: 511–522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci 30: 14102–14115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marco A, Kisliouk T, Weller A, Meiri N. High fat diet induces hypermethylation of the hypothalamic Pomc promoter and obesity in post-weaning rats. Psychoneuroendocrinology 38: 2844–2853, 2013 [DOI] [PubMed] [Google Scholar]
- 40.McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular α-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol 279: R695–R703, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Mercer AJ, Hentges ST, Meshul CK, Low MJ. Unraveling the central proopiomelanocortin neural circuits. Front Neurosci 7: 19, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, Velloso LA. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One 4: e5045, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Mountjoy KG. Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes. Biochem J 428: 305–324, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Newton AJ, Hess S, Paeger L, Vogt MC, Fleming Lascano J, Nillni EA, Bruning JC, Kloppenburg P, Xu AW. AgRP innervation onto POMC neurons increases with age and is accelerated with chronic high-fat feeding in male mice. Endocrinology 154: 172–183, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nillni EA. Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin. Endocrinology 148: 4191–4200, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9: 35–51, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Perello M, Cakir I, Cyr NE, Romero A, Stuart RC, Chiappini F, Hollenberg AN, Nillni EA. Maintenance of the thyroid axis during diet-induced obesity in rodents is controlled at the central level. Am J Physiol Endocrinol Metab 299: E976–E989, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perello M, Stuart R, Nillni EA. Prothyrotropin-releasing hormone targets its processing products to different vesicles of the secretory pathway. J Biol Chem 283: 19936–19947, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perello M, Stuart RC, Nillni EA. Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. Am J Physiol Endocrinol Metab 292: E1348–E1357, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Petervari E, Szabad AO, Soos S, Garami A, Szekely M, Balasko M. Central alpha-MSH infusion in rats: disparate anorexic vs. metabolic changes with aging. Regul Pept 166: 105–111, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304: 110–115, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Pritchard LE, White A. Neuropeptide processing and its impact on melanocortin pathways. Endocrinology 148: 4201–4207, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Shi H, Sorrell JE, Clegg DJ, Woods SC, Seeley RJ. The roles of leptin receptors on POMC neurons in the regulation of sex-specific energy homeostasis. Physiol Behav 100: 165–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solomon G, Atkins A, Shahar R, Gertler A, Monsonego-Ornan E. Effect of peripherally administered leptin antagonist on whole-body metabolism, and bone microarchitecture and biomechanical properties in mouse. Am J Physiol Endocrinol Metab 306: E14–E27, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Soos S, Balasko M, Jech-Mihalffy A, Szekely M, Petervari Anorexic vs E. metabolic effects of central leptin infusion in rats of various ages and nutritional states. J Mol Neurosci 41: 97–104, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Soos S, Petervari E, Szekely M, Jech-Mihalffy A, Balasko M. Complex catabolic effects of central alpha-MSH infusion in rats of altered nutritional states: differences from leptin. J Mol Neurosci 43: 209–216, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Surwit RS, Edwards CL, Murthy S, Petro AE. Transient effects of long-term leptin supplementation in the prevention of diet-induced obesity in mice. Diabetes 49: 1203–1208, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Tam J, Fukumura D, Jain RK. A mathematical model of murine metabolic regulation by leptin: energy ealance and defense of a stable body weight. Cell Metab 9: 52–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Muller TD, Munzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsujii S, Nakai Y, Fukata J, Nakaishi S, Takahashi H, Usui T, Imura H. Effects of food deprivation and high fat diet on immunoreactive beta-endorphin levels in brain regions of Zucker rats. Endocrinol Jpn 34: 903–909, 1987 [DOI] [PubMed] [Google Scholar]
- 63.van den Heuvel JK, van Rozen AJ, Adan RA, la Fleur SE. An overview on how components of the melanocortin system respond to different high energy diets. Eur J Pharmacol 660: 207–212, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Villanueva EC, Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes (Lond) 32, Suppl 7: S8–S12, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71: 142–154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol 660: 213–219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warne JP, Xu AW. Metabolic transceivers: in tune with the central melanocortin system. Trends Endocrinol Metab 24: 68–75, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 15: 1350–1355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeltser LM, Seeley RJ, Tschop MH. Synaptic plasticity in neuronal circuits regulating energy balance. Nat Neurosci 15: 1336–1342, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS. Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 279: E838–E845, 2000 [DOI] [PubMed] [Google Scholar]