Abstract
Hepatocyte growth factor (HGF) is expressed as an angiogenic factor in adipose tissue. However, the molecular mechanism of Hgf expression remains largely unknown in the tissue. We addressed the issue by studying Hgf expression in adipocytes and macrophages. Hgf was expressed more in the stromal-vascular fraction than the adipocyte fraction. The expression was fivefold more in macrophages than the stromal-vascular faction and was reduced by 50% after macrophage deletion in adipose tissue. The expression was reduced by differentiation in adipocytes and by tumor necrosis factor-α or lipopolysaccharide treatment in macrophages. The expression was suppressed by nuclear factor (NF)-κB in C57BL/6 mice with NF-κB p65 overexpression under the aP2 gene promoter (aP2-p65 mice) but enhanced by inactivation of NF-κB p65 in mouse embryonic fibroblasts. The Hgf gene promoter was suppressed by p65 overexpression, which blocked peroxisome proliferator-activated receptor-γ (PPARγ) interaction with RNA polymerase II. The p65 activity was abolished by knockdown of histone deacetylase 3. Hgf expression was upregulated by hypoxia in vitro and in vivo. Compared with vascular endothelial growth factor (Vegf), which was predominately expressed in mature adipocytes, Hgf was mainly expressed in nonadipocytes, suggesting that Hgf and Vegf may have different cell sources in adipose tissue. In mechanism, Hgf expression is inhibited by NF-κB through suppression of PPARγ function in the Hgf gene promoter. Both Hgf and Vegf are induced by hypoxia. The study provides a molecular mechanism for the difference of inflammation and hypoxia in the regulation of angiogenic factors.
Keywords: vascular endothelial growth factor, macrophage infiltration, nuclear factor-κB, mice with nuclear factor-κB p65 overexpression under the Ap2 gene promoter, inflammation, adipose tissue hypoxia, peroxisome proliferator-activated receptor-γ
macrophage infiltration into adipose tissue contributes to the chronic inflammatory response in obesity (39, 41). Our study suggests that macrophages may play an important role in the regulation of angiogenesis in adipose tissue by secretion of angiogenic factors (26, 40). In obesity, adipose tissue growth leads to a hypoxia response (45) that is responsible for chronic inflammation, adipocyte death, and reduced adipogenesis (46, 47). A reduction in adipose tissue blood supply is a major mechanism of the hypoxia response (33, 35, 43). Insufficient angiogenic activity is likely one of the risk factors for the reduced blood supply (20, 26, 32). Hepatocyte growth factor (HGF) is expressed by adipose tissue, and the expression is increased in obese conditions (4, 44). HGF elevation is associated with metabolic disorder and insulin resistance in humans and mice (1, 14, 36). Although adipocytes secrete HGF (3, 29), the cell source of HGF remains unclear in the adipose tissue.
HGF was originally identified as a mitogenic stimulator of hepatocytes but was later found as an angiogenic factor (25). HGF is required for embryonic morphogenesis, tumor progression, and tissue regeneration with the angiogenic activity. Studies suggest that HGF has anti-inflammatory effects in various injury and disease models, including obesity (12, 30). The mechanism is inhibition of nuclear factor (NF)-κB by HGF (9, 11). The angiogenic activity of HGF may contribute to the pathogenesis of certain forms of renal disease, cardiovascular disease, and cancers, etc. (25).
Regulation of Hgf expression remains largely unknown in adipose tissue. At the molecular level, some transcription factors have been reported in the regulation of Hgf expression. Estrogen receptor, specificity protein 1, specificity protein 3, CCAAT/enhancer-binding protein, upstream stimulatory factor, peroxisome proliferator-activated receptor-γ (PPARγ), and hypoxia-inducible factor 1 (HIF-1) were reported to enhance Hgf expression. Nuclear factor 1 or activator protein 2 and the chicken ovalbumin upstream promoter transcription factor were found to inhibit the expression (6, 16, 21). However, all of the studies were performed in fibroblasts like NIH/3T3 cells, mesenchymal stem cells, or glioma cells in cancer or vascular research (5, 17, 27). It is not known if these transcription factors regulate Hgf in macrophages or adipocytes in relevance to adipose tissue. We addressed this issue with a focus on NF-κB and PPARγ.
In the current study, we determined Hgf expression in adipocytes and macrophages. Our data suggest that Hgf mRNA was suppressed by NF-κB in response to inflammation. NF-κB inhibited PPARγ activity in the transcriptional regulation of Hgf gene promoter. The study provides a molecular mechanism for the difference of inflammation and hypoxia in the regulation of angiogenesis.
MATERIALS AND METHODS
Obese mice.
Male ob/ob mice (B6.V-Lepob/Lepob, stock no.: 000632) and C57BL/6 (B6) mice were purchased from the Jackson Laboratory at the age of 4–5 wk and used at 8 wk in this study for genetic obesity. The ob/ob mice were fed on normal chow diet (11% kcal in fat), and the gender-matched wild-type (WT) littermates were used as the lean control. In diet-induced obesity (DIO), male B6 mice at 5 wk of age were fed a high-fat diet (HFD, D12331; Research Diets, New Brunswick, NJ) in which 58% of the calorie is from fat. In the lean control, mice (age- and gender-matched B6) were fed on normal chow diet. After 16 wk on HFD, the mice were used in experiments. All of the mice were housed in the animal facility at the Pennington Biomedical Research Center with a 12:12-h light-dark cycle and constant temperature (22–24°C). The mice had free access to food and water. All procedures were performed in accordance with National Institutes of Health guidelines for the care and use of animals and approved by the Institute Animal Care and Use Committee at the Pennington Biomedical Research Center.
Macrophage deletion.
Macrophages were deleted in the adipose tissue by a single injection of clodronate-liposome. Clodronate-liposome was prepared and administrated at 150 mg/kg intraperitoneally in B6 mice as described elsewhere (37). The deletion efficacy was confirmed in adipose tissue at day 4 after the injection.
aP2-p65 mice.
B6 mice with NF-κB p65 overexpression under the aP2 gene promoter were generated as described elsewhere (34). In the mice, p65 is overexpressed in adipocytes and macrophages. Male transgenic mice and their WT littermates were used in this study.
Mouse embryonic fibroblasts and cell lines.
The p65−/− mouse embryonic fibroblasts (MEFs) were used previously (7). HEK-293 epithelial cells, 3T3-L1 preadipocytes, and NIH/3T3 fibroblasts were purchased from the American Type Culture Collection (Manassas, VA). The MEFs and cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 4 mM glutamine, and 50 mg/l gentamicin in an atmosphere of 5% CO2 at 37°C. For adipogenesis, 3T3-L1 preadipocytes were grown to confluence in a 100-mm plate and then treated with the adipogenic cocktail (5 μg/ml insulin, 0.5 mmol/l isobutyl methylxanthine, and 10 μmol/l dexamethasone) for 4 days. This was followed by incubation in insulin-supplemented medium for an additional 3 days. The normal medium was used on day 7 to maintain the adipocytes.
Primary adipocytes.
Primary adipocytes were prepared from epididymal fat pads of lean B6 mice. The cell isolation was conducted using a protocol described previously (45). Briefly, the fat pads were collected immediately from the mice after cervical dislocation, minced, and digested with collagenase (1 mg/ml; Sigma C6885) at 37°C for 60 min. The cell suspension was centrifuged at 400 g for 2 min, and the cells in the top fraction of medium were collected as primary adipocytes. The precipitation fraction was collected as primary stromal-vascular cell. After being washed two times in DMEM, the cells were incubated in 35-mm dishes in DMEM with 10% FBS. The cells were used in gene expression assays 48 h later.
Peritoneal macrophages.
Primary peritoneal macrophages were isolated from B6 mice using the starch protocol (45). The macrophages were harvested 3 days later with 20 ml of cold PBS in lavage and then cultured in RPMI-1640 (supplemented with 10% FBS and 50 μg/ml gentamicin). The cells were used in gene expression assays or treatment 48 h later. The cells were exposed to hypoxia in serum-free RPMI-1640 medium.
Hypoxia treatment.
In vitro, ambient hypoxia was generated by filling in a sealed metal chamber (self-designed) with a low oxygen air, which contains 1% oxygen, 5% CO2, and 94% nitrogen. In the treatments, the cells were maintained in serum-free DMEM supplemented with 0.25% bovine serum albumin and 25 μM HEPES as described elsewhere (45). The hypoxia degree was monitored with an oxygen meter, and the chamber was maintained in a 37°C water bath. Mice were exposed to hypoxia as previously described (19). Briefly, mice were simultaneously fasted and exposed to 6 h of stable hypoxia from 8:00 A.M. until 2:00 P.M. A gas regulatory system controlled the flow of nitrogen into customized Plexiglas cages, resulting in a stable oxygen concentration of 17%.
Real-time quantitative RT-PCR.
Total RNA was extracted from homogenized fat pads or cells using the TRI reagent (T9424; Sigma) according to the manufacturer's instruction. Real-time quantitative RT-PCR was conducted using an ABI 7900HT fast real-time PCR system (Applied Biosystems, Foster City, CA). The following primer and probe were ordered from Applied Biosystems: Hgf (Mm01135177_m1), vascular endothelial growth factor (Vegf; Mm00437304_m1), tumor necrosis factor (Tnf)-α (Mm00443258_m1), p65 (Mm00501346_m1), Cd11b (Mm00434455_m1), and F4/80 (Mm00802530_m1). The mRNA signal was normalized over 18S rRNA signal. A mean value of triplicates was used for relative mRNA level or calculation of mRNA fold induction.
Western blot.
The epididymal fat pads of WT and aP2-p65 mice were collected, homogenized, and sonicated in a lysis buffer, and Western blot was conducted as described elsewhere (8). Lipids were removed from the lysate before the protein assay was conducted. Antibodies to NF-κB p65 and actin were from Abcam (Cambridge, MA). Horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG were purchased from GE Healthcare (HP7 9NA; Buckinghamshire, UK).
Serum TNF-α.
Serum TNF-α was measured using a multiplex kit (catalog no. MADPK71k-03; Linco Research, St. Charles, MO).
Transfection and luciferase assay.
Transient transfection was conducted in triplicate in 12-well plates. HEK-293 or NIH/3T3 cells (1.5 × 105/well) were transfected with plasmid DNA utilizing Lipofectamine 2000. In the transfection, 0.2 μg/well of reporter DNA were used. Hgf gene promoter activity was determined with a luciferase reporter plasmid containing a Hgf promoter fragment that was made from a Hgf-Cat reporter plasmid (a gift from Dr. Reza Zarnegar at the University of Pittsburgh) (2). In all of the transient transfection experiments, the internal control was 0.1 μg/well of SV40 Renilla luciferase reporter plasmid, and the total DNA concentration was corrected in each well with a control vector plasmid. The luciferase assay was conducted using the dual luciferase substrate system (Promega) with a 96-well luminometer. The Hgf luciferase activity was normalized with the internal control Renilla luciferase, and a mean value together with a SE of the triplicate samples were used to determine the reporter activity. Each experiment was repeated at least three times.
Chromatin immunoprecipitation.
The chromatin immunoprecipitation (ChIP) assay was performed using a protocol as described elsewhere (7). Cells were maintained in a 100-mm cell culture plate, treated with TNF-α (20 ng/ml) after overnight serum starvation. The chromatin DNA was extracted, broken into fragments of 400–200 bp in length by sonication, and then immunoprecipitated with antibodies to polymerase II. IgG was used in immunoprecipitation as a control for nonspecific signal. DNA in the immunoprecipitation product was amplified in RT-PCR with the ChIP assay primers that cover the PPARγ response element site in the HGF gene promoter (18): forward, 5′-AGCTCCAGCTTCCAAATTGC-3′; reverse, 5′-CACACTCCCCCTCCCAGAA-3′. iTaq Universal SYBR Green Supermix from Bio-Rad (Hercules, CA) was used to quantify the PCR product.
Statistical analysis.
In Figs. 1–6, a mean value and SE of multiple data points were used to represent the final result. Student's t-test or one-way ANOVA was used in statistical analysis of the data with significance P < 0.05.
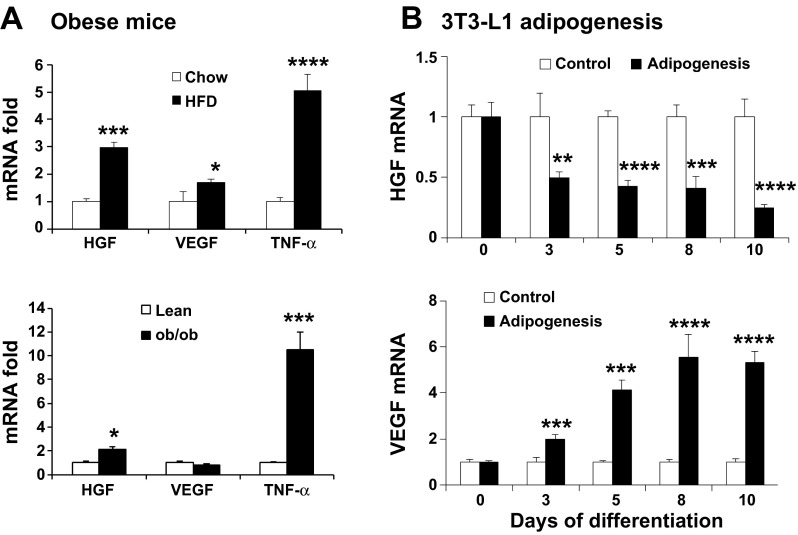
Fig. 1.
Hepatocyte growth factor (HGF) has a low expression in mature adipocytes although the expression is increased in adipose tissue in obesity. A: mRNA of Hgf, vascular endothelial growth factor (Vegf), and tumor necrosis factor (Tnf)-α in the epididymal fat of diet-induced obesity (DIO) and ob/ob mice. The test was conducted in DIO mice at 16 wk on HFD and ob/ob mice at 8 wk of age. B: regulation of Hgf expression during adipogenesis. Confluent 3T3-L1 preadipocytes were treated with the adipogenic cocktail (5 μg/ml insulin, 0.5 mmol/l isobutyl methylxanthine, and 10 μmol/l dexamethasone) for 4 days followed by insulin-supplemented medium for an additional 3 days. The normal medium was used at day 7 to maintain the adipocytes. Hgf and Vegf mRNA levels were detected in the undifferentiated and differentiated cells. Data are expressed as means ± SE (n = 4). Compared with chow or lean mice in A or control in B: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
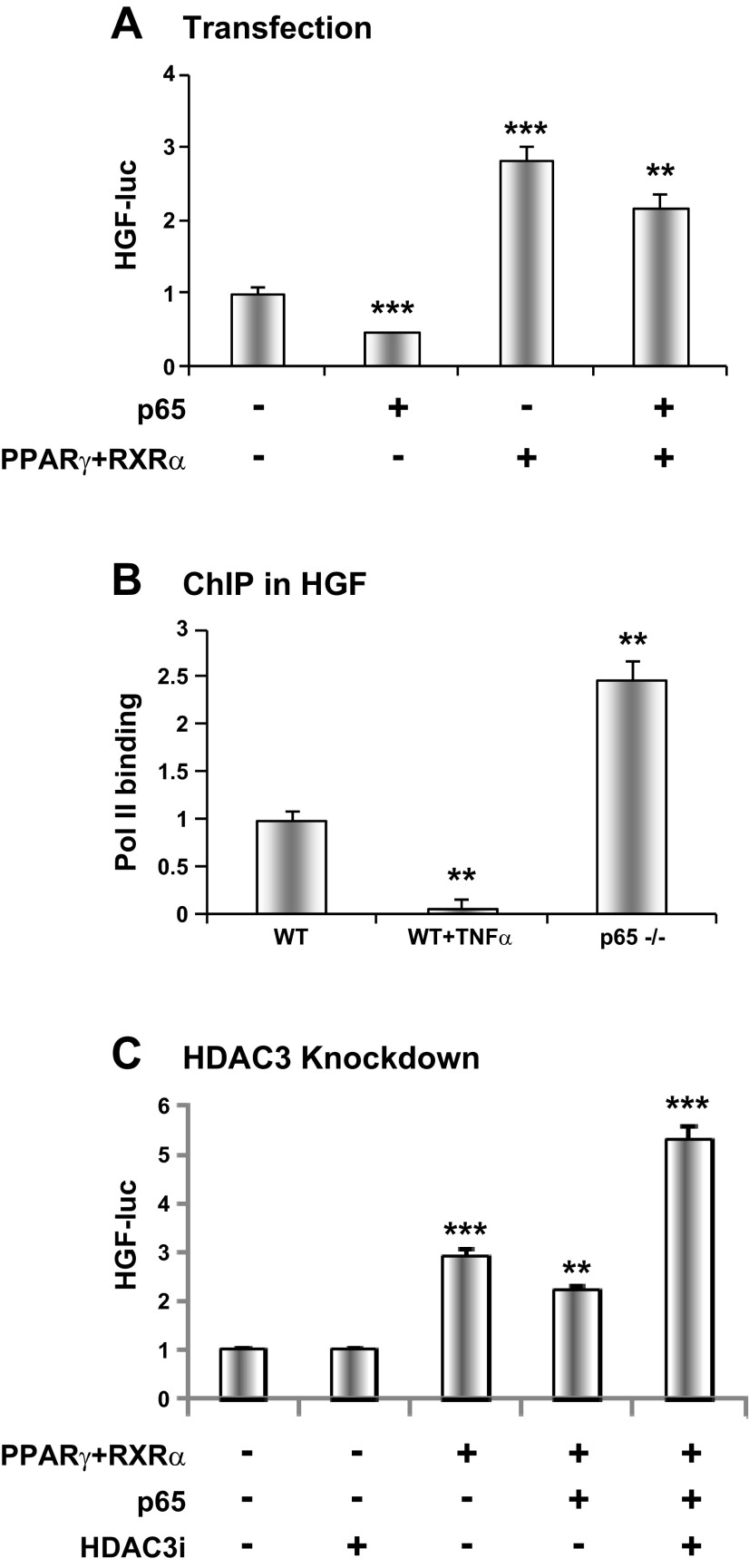
Fig. 6.
Transcriptional regulation of HGF by NF-κB and peroxisome proliferator-activated receptor-γ (PPARγ). A: NF-κB p65 inhibition of the HGF gene promoter. The test was conducted using the Hgf-luc reporter in HEK 293 cells. The promoter was induced by PPARγ plus retinoid X receptor (RXR) α. p65 was used to enhance NF-κB activity in cotransfection. B: RNA polymerase II (Pol II) signal in the Hgf promoter. Chromatin immunoprecipitation (ChIP) assay was conducted at the PPARγ response element. MEFs were treated with TNF-α (20 ng/ml) for 30 min. C: HDAC3 in NF-κB activity. Inhibition of PPARγ activity by p65 was examined in cotransfection. The Hgf-luc reporter was used to determine the inhibition in cotransfection of HEK 293 cells. Vector based-Hdac3 RNAi (HDAC3i) was used in cotransfection. The results are expressed as means ± SE (n = 3). Compared with control or WT: **P < 0.01 and ***P < 0.001.
RESULTS
HGF mRNA in adipose tissue of obese mice.
HGF mRNA was determined in white adipose tissue of mice after 4 mo on HFD. A threefold elevation was observed in epididymal fat pads of DIO mice relative to the lean control (Fig. 1). A similar increase was observed in ob/ob, a genetic obese mouse model (Fig. 1). As a control, TNF-α mRNA was increased by 5- and 10-fold in DIO and ob/ob mice, respectively (P < 0.001 and P < 0.0001). VEGF was increased in DIO mice, but not in ob/ob mice. These results suggest that HGF expression was increased significantly in the visceral fat tissue of obese mice and the increase was associated with chronic inflammation.
Hgf reduction by adipogenesis.
To study Hgf expression in adipocytes, we examined Hgf mRNA in 3T3-L1 cells during adipocyte differentiation. 3T3-L1 preadipocytes were differentiated in standard adipogenic cocktail, and mRNA expression was examined at 3, 5, 8, and 10 days. Hgf mRNA was decreased gradually in a time-dependent manner with adipocyte differentiation. In mature adipocytes, Hgf mRNA was only 25% of that of preadipocytes (Fig. 1B). In contrast, Vegf expression was induced dramatically by the differentiation. The data suggest that mature adipocytes may be the major source of VEGF, but not HGF, in the fat tissue.
HGF expression in macrophages.
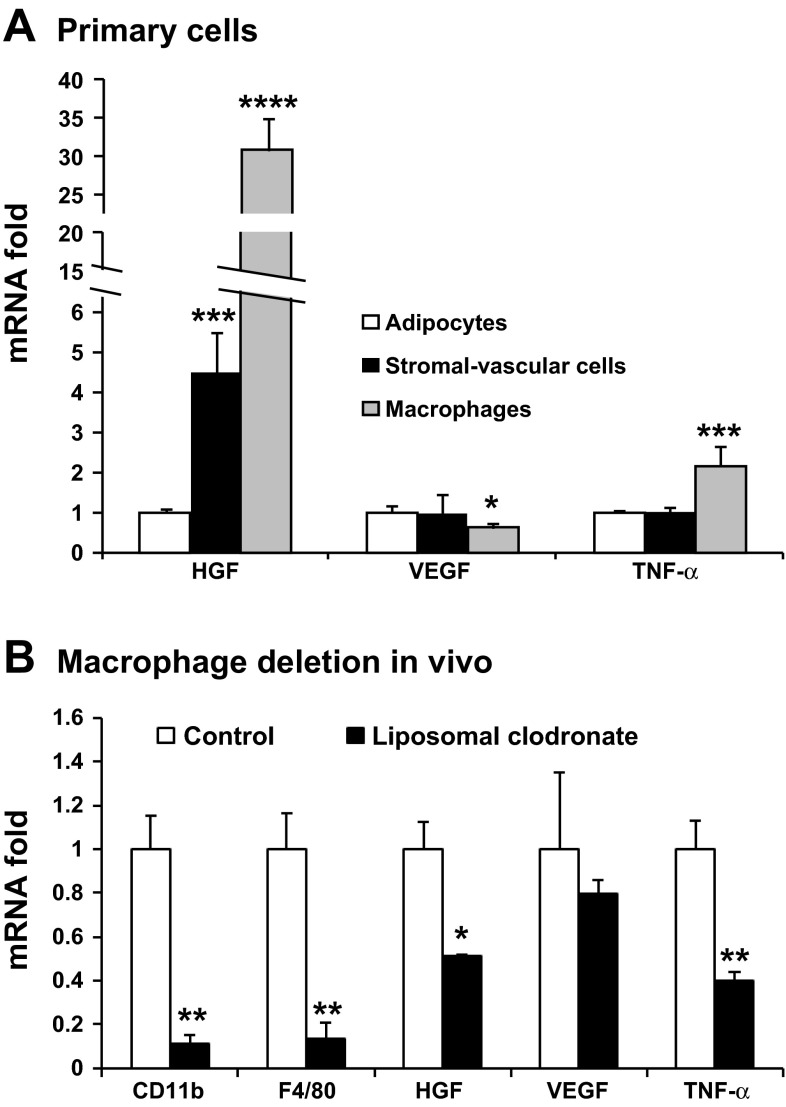
Residential macrophage is a major source of angiogenic factor platelet-derived growth factor (PDGF) in fat tissue (26). It is not known if Hgf is expressed by macrophages. To address this issue, epididymal fat was used to generate the adipocyte and stromal-vascular fractions after collagenase digestion. The two portions were compared in Hgf expression. The adipocyte fraction has a very low Hgf expression (Fig. 2A). The stromal-vascular fraction exhibited 4.5-fold of Hgf mRNA over the adipocyte (Fig. 2A). In contrast, the two fractions did not show a difference in mRNA expression for Vegf or Tnf-α. Macrophages are a component of the stromal-vascular fraction; therefore, we determined Hgf expression in macrophages. Peritoneal macrophages exhibited an expression at 30-fold higher than that of the adipocyte fraction (Fig. 2A). Macrophages expressed a lower level of Vegf, but higher Tnf-α, relative to the adipocyte fraction.
Fig. 2.
HGF expression in macrophages. A: gene expression in the adipocytes, stromal-vascular fraction, and peritoneal macrophages. Adipocytes and stromal-vascular fractions were made from epididymal fat of lean C57BL/6 (B6) mice. The gene expression was determined after 48 h culture in serum-containing medium. Primary macrophages were isolated from B6 mice after ip injection of starch solution. B: gene expression in epididymal fat after macrophage deletion in mice by liposomal clodronate injection. The results are expressed as means ± SE (n = 4). Compared with adipocytes (A) or control (B): *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
To test the macrophage activity in vivo, Hgf was examined in adipose tissue after macrophage deletion in B6 mice. Clodronate-liposome was used to deplete macrophages. Four days after the injection, 90% of macrophages were removed from the adipose tissue as indicated by the macrophage markers, such as Cd11b and F4/80 (Fig. 2B). In the tissue, mRNA of Hgf and Tnf-α was reduced by 49 and 60%, respectively (Fig. 2B). As a control, Vegf was not altered significantly by macrophage deletion. These data suggest that macrophages are an important source of HGF in adipose tissue.
HGF inhibition by NF-κB.
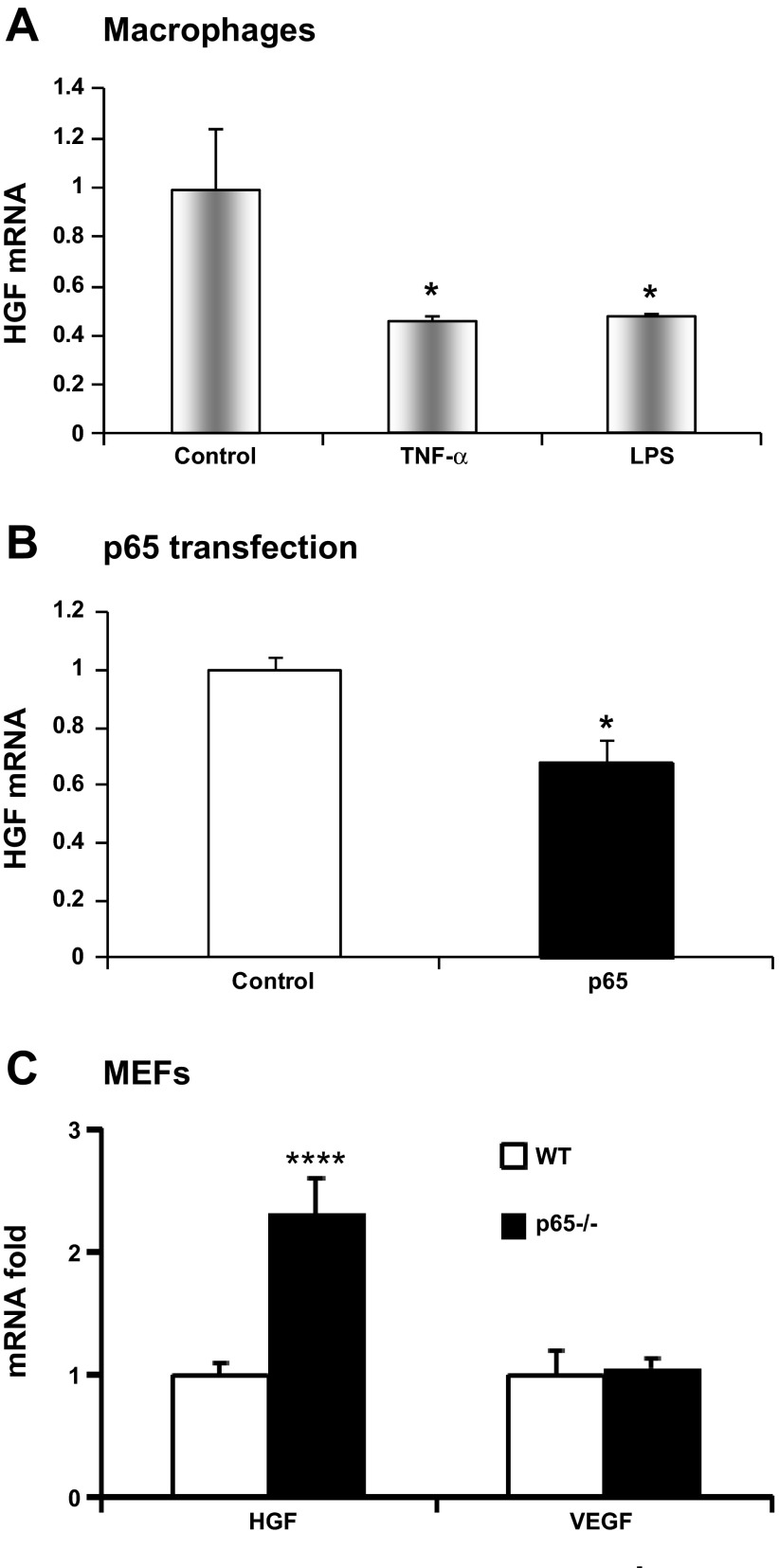
We conducted a series of tests to understand signals that regulate Hgf in macrophages. In the first set of assay, Hgf was examined in macrophages after treatment with inflammatory signals such as TNF-α (20 ng/ml) or lipopolysaccharide (LPS, 1 μg/ml) for 4 h. In response, Hgf mRNA was reduced by >50% in macrophages in either condition, suggesting that Hgf expression is inhibited by proinflammatory signals. NF-κB was investigated in the inhibition, since it is activated by both TNF-α and LPS. Induction of NF-κB activity by overexpression of p65 subunit reduced Hgf by 32% in a transient transfection in NIH/3T3 cells (Fig. 3B). The effect of NF-κB inhibition was tested in p65 null MEFs. Hgf expression was increased by 2.3-fold in the p65 null cells (Fig. 3C). Vegf expression was not altered in the null cells. These results suggest that Hgf expression is inhibited by NF-κB in response to TNF-α or LPS.
Fig. 3.
Inhibitory effect of nuclear factor NF-κB on HGF expression. A: HGF mRNA levels in the primary peritoneal macrophages treated with TNF-α (20 ng/ml) or lipopolysaccharide (LPS, 1 μg/ml) for 4 h. B: inhibition of Hgf by NF-κB in p65 transfection of NIH/3T3 cells. C: Hgf and Vegf mRNA levels in p65 null mouse embryonic fibroblasts (MEFs). The results are expressed as means ± SE (n = 3). Compared with control or WT: *P < 0.05 and ****P < 0.001.
HGF reduction in adipose tissue of aP2-p65 transgenic mice.
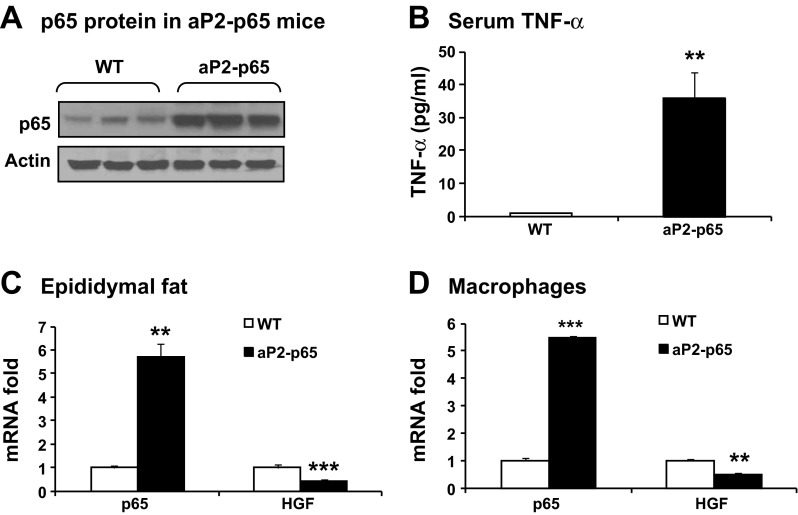
We examined NF-κB effect on HGF expression in vivo using aP2-p65 transgenic mice in which NF-κB subunit p65 is overexpressed in adipose tissue under the aP2 (Fabp4) gene promoter. The P65 protein was increased by about twofold in the epididymal fat of aP2-p65 mice (Fig. 4A). In the plasma, TNF-α was increased for 35.8-fold (Fig. 4B). As expected, p65 mRNA was increased five- to sixfold in fat tissue and macrophages of the mice (Fig. 4, C and D). In fat tissue, Hgf mRNA was decreased by 57.6% (Fig. 4C). In macrophages, Hgf was reduced by 53.4% (Fig. 4D). These data suggest that NF-κB inhibits Hgf expression in vivo.
Fig. 4.
HGF reduction in fat tissue of mice with NF-κB p65 overexpression under the aP2 gene promoter (aP2-p65). A: p65 protein in the epididymal fat of aP2-p65 mice. The wide-type (WT) littermates were used in the control. B: serum TNF-α in aP2-p65 mice. C: p65 and Hgf mRNA in the epididymal fat of aP2-p65 mice. D: p65 and Hgf mRNA in primary peritoneal macrophages of aP2-p65 mice. The results are expressed as means ± SE (n = 3). Compared with WT: **P < 0.01 and ***P < 0.001.
HGF induction by hypoxia.
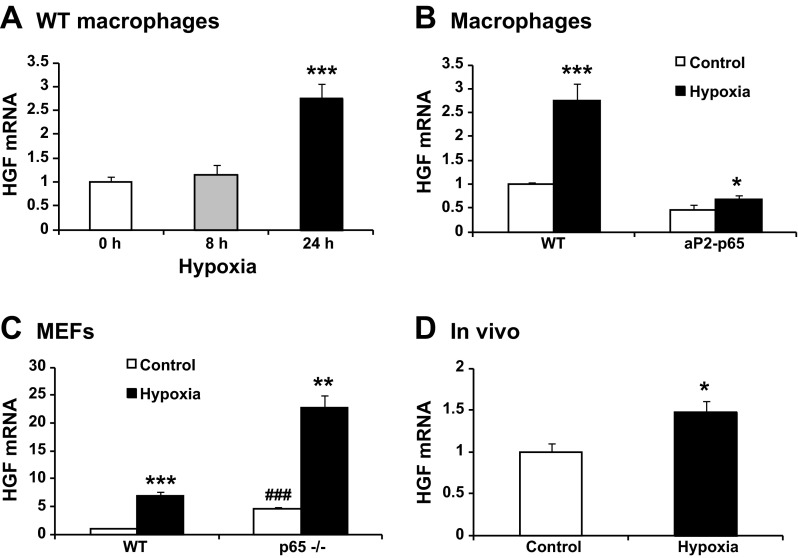
The above results suggest that chronic inflammation is not responsible for the enhanced Hgf expression in adipose tissue in obesity. Adipose hypoxia is another stress signal in obesity, and it is not clear if the Hgf expression is induced by hypoxia. To address this issue, Hgf expression was determined in the peritoneal macrophages after hypoxia treatment for 8 or 24 h. The expression was not significantly increased at 8 h, but a 2.8-fold increase was observed at 24 h (Fig. 5A). In macrophages of aP2-p65 mice, the hypoxia treatment overcame the inhibitory effect of NF-κB (Fig. 5B). The NF-κB effect was further tested in p65 null MEFs. In normoxia, the p65 null MEFs expressed 4.9 times more Hgf over WT MEFs (Fig. 5C). In response to hypoxia, the null MEFs maintained the response in Hgf expression similar to WT MEFs (Fig. 5C). To test the hypoxia effect in vivo, B6 mice were exposed to mild hypoxia (17% O2) for 6 h. The treatment induced a 46.5% increase in Hgf mRNA in epididymal fat of the mice (Fig. 5D). These results suggest that hypoxia is able to stimulate Hgf expression, and the response is stronger in the absence of NF-κB activity.
Fig. 5.
Hypoxia induction of HGF expression. A: Hgf mRNA induction by hypoxia. The peritoneal macrophages were exposed to hypoxia (1% oxygen) (n = 3). B: Hgf mRNA in aP2-p65 macrophages. HGF was tested after 24 h hypoxia treatment (n = 3). C: Hgf mRNA in the p65−/− MEFs. The MEFs were exposed to hypoxia for 24 h (n = 3). D: Hgf mRNA in adipose tissue. The Hgf mRNA was determined in epididymal fat of the mice after modest hypoxia (17% O2) treatment for 6 h (n = 5). The results are expressed as mean values ± SE. Compared with 0 h (A) or control (B, C, and D): *P < 0.05, **P < 0.01, and ***P < 0.001; compared with WT control (C): ###P < 0.001.
Mechanism of hgf inhibition by NF-κB.
PPARγ was reported to induce Hgf expression through transcriptional regulation (18). We know that NF-κB inhibits PPARγ activity through activation of corepressor HDAC3 (7). It is not known if the interaction occurs in the Hgf gene promoter. We addressed this issue using a Hgf luciferase reporter in a transient transfection of HEK-293 cells. The Hgf promoter activity was induced by PPARγ and reduced by NF-κB p65 in cotransfection (Fig. 6A). The inhibitory activity of p65 was blocked by PPARγ in cotransfection. In ChIP assay, polymerase II activity in the Hgf promoter was inhibited by TNF-α in WT MEFs but enhanced in p65−/− MEFs (Fig. 6B). The activity was decreased by 95% at 30 min of TNF treatment in WT cells, but increased by 2.5-fold in the null cells. To test whether HDAC3 plays a role in the HGF regulation by NF-κB, the promoter activity was examined after Hdac3 knockdown with vector-based RNAi (HDAC3i). The knockdown blocked the inhibitory activity of p65 in the Hgf promoter and increased the reporter activity by about twofold (Fig. 6C). These data suggest that NF-κB inhibited PPARγ activity in the Hgf gene promoter, and HDAC3 is required for the inhibition.
DISCUSSION
We provide evidence that macrophages may be an important source of HGF in the adipose tissue of obese mice. Plasma HGF level is positively associated with adiposity in obese subjects (1, 30, 36). It is generally believed that adipose tissue is responsible for the plasma HGF elevation in obesity. However, it was not clear which cell type is responsible for the HGF expression in the adipose tissue. In this study, we observed that Hgf expression was increased significantly in the visceral fat of obese mice, and the stromal vascular fraction accounts for the major part of Hgf expression. In the stromal vascular section, macrophages are an important source of HGF, since the expression was decreased by one-half after macrophage deletion in the fat tissue (Fig. 2B). The data support that macrophages may be a cell source of HGF in the adipose tissue.
This study supports that macrophages may contribute to angiogenesis in the adipose tissue in obesity by expressing HGF. In obesity, macrophage infiltration is increased in adipose tissue (39, 41). Regarding function of the adipose tissue macrophages, many studies suggest that the macrophages promote the chronic inflammation in adipose tissue. Our studies suggest that the macrophages also stimulate angiogenesis by secretion of angiogenic factors. In one study, we reported that the macrophages secret PDGF to induce tube formation of mouse endothelial cells (26). In another study, we observed that macrophage deficiency in expression of angiogenic factors was associated with angiogenic defects in sirtuin 1 knockout mice (40). In the current study, our data suggest that the macrophages may be an important source of Hgf in the adipose tissue. This possibility remains to be verified by studying adipose tissue macrophages.
Our study suggests that NF-κB p65 mediates the proinflammatory signal to inhibit Hgf expression in macrophages. The conclusion is supported by reduced HGF expression in macrophages in vitro, macrophages and fat tissues of aP2-p65 mice. Consistently, inactivation of NF-κB dramatically enhanced Hgf mRNA in p65 null MEFs. The data consistently suggest that inflammation inhibits Hgf expression through NF-κB. The conclusion is different from that of two other studies in which TNF-α induced Hgf expression in mesenchymal stem cells (42, 43), in which the NF-κB activity was not tested in vivo.
We tested the anti-inflammatory activities of HGF in this study and failed to observe the activity in our models (data not shown). HGF was reported to inhibit the inflammatory response in hepatitis, acute respiratory distress syndrome, and acute renal failure in vivo (25). NF-κB activity was suppressed by HGF in vitro through blocking nuclear translocation of p65 in some studies (23, 24). However, the activity was not observed in this study, since HGF was not able to block NF-κB-mediated cytokine expression or luciferase reporter activity. We suggest that HGF may inhibit inflammation by stimulating angiogenesis in vivo, which attenuates the hypoxia response. This possibility remains to be tested in vivo.
Our results suggest that NF-κB inhibits Hgf expression by targeting PPARγ. To explore the mechanism of Hgf regulation, we examined the Hgf promoter activity using a luciferase reporter. There is no NF-κB binding site in the promoter DNA according to sequence analysis in this study. There is a PPARγ-responsive element that was induced by overexpression of PPARγ in cotransfection, which supports the activity of a PPARγ ligand in the regulation of Hgf (18). The PPARγ activity was inhibited by NF-κB through recruitment of corepressor HDAC3, which is a component of PPARγ corepressor (7, 42).
Our data suggest that Hgf expression is induced by hypoxia. Several groups including ours have demonstrated that hypoxia exists in adipose tissue of obese mice (10, 15, 28, 31). The hypoxia concept provides a common mechanism for the adipose response in obesity, such as chronic inflammation, macrophage infiltration, adiponectin reduction, and adipocyte death in obesity (10, 15, 28, 38, 45). However, it is unclear if the hypoxia regulates Hgf expression in adipose tissue. In the current study, Hgf mRNA was induced by hypoxia in vitro and in vivo, suggesting a role of HIF-1 in the induction of HGF. This conclusion is consistent with the observation in pancreatic tumor cells (21).
This study reveals difference of inflammation and hypoxia in the regulation of angiogenesis in adipose tissue. Inflammation and hypoxia both enhance angiogenesis, but their activities are different. The difference was demonstrated in the regulation of the angiogenic factor HGF. Inflammation inhibits Hgf expression but promotes Vegf expression through NF-κB (13). Hypoxia induces expression of both Hgf and Vegf through HIF-1. In obese conditions, Hgf elevation in adipose tissue is likely a result of integration of multiple signals, including inflammation (NF-κB), hypoxia (HIF-1), insulin (HIF-1) (13), and fatty acids (PPARγ). We observed that Hgf expression was higher in DIO mice than ob/ob mice (3- vs. 2-fold in Fig. 1A). The higher expression was associated with lower TNF-α expression in DIO mice (5- vs. 10-fold in Fig. 1A). In another study, we reported that NF-κB induced 11β-hydroxysteroid dehydrogenase type 1 expression (22). The reduction in Hgf and an increase in 11β-HDS1 may contribute to suppression of angiogenic activity in adipose tissue under strong inflammation.
Expression of Hgf and Vegf was compared side by side in adipocytes and macrophages in the current study. The two angiogenic factors exhibited distinct patterns in expression. Hgf mRNA is largely expressed in macrophages and nondifferentiated adipocytes. The expression was dramatically reduced in fat tissue by macrophage deletion or in adipocyte after differentiation. In contracts, Vegf mRNA is predominately expressed by mature adipocytes. The expression was elevated dramatically during adipocyte differentiation and was not decreased in adipose tissue after macrophage deletion. The data suggest that VEGF is mainly produced by adipocytes in the adipose tissue.
In summary, adipocytes express Hgf, but the expression is reduced by adipocyte differentiation. This pattern is opposite to that of Vegf whose expression is induced by adipocyte differentiation (13). Peritoneal macrophage expresses a high level of Hgf. The expression was suppressed by inflammatory signal through activation of NF-κB, which inhibits Hgf transcription by targeting PPARγ. NF-κB inhibits the PPARγ function through activation of HDAC3. Hgf expression in adipose tissue in obesity is likely a result of HIF-1 and PPARγ activation by hypoxia, fatty acids, and insulin. Adipose tissue macrophage may be a potential source of Hgf, and this possibility remains to be tested in the adipose tissue macrophages. In addition, the study provides a mechanism for the difference of inflammation and hypoxia in the regulation of angiogenesis.
GRANTS
This work was supported by grants from the National Institutes of Health (NIH) (DK-68036 and DK-085495) to J. Ye, the Pujiang Project (11PJ1407700) and National Science Foundation of China fund (31171128) to J. Yin, and the Center of Biomedical Research Excellence (COBRE) (NIH 2P20-RR-021945) to Z. Gao. The quantitative RT-PCR was in the Genetic Core that is supported in part by COBRE (NIH 2P20-RR-021945) and Nutrition Obesity Research Center (NIH 2P30-DK-072476) center grants from the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J. Yin, J.H.L., J.Z., and V.Y.P. performed experiments; J. Yin, J.H.L., J.Z., Z.G., V.Y.P., and J. Ye analyzed data; J. Yin prepared figures; J. Yin and J. Ye drafted manuscript; J.Z., Z.G., V.Y.P., and J. Ye interpreted results of experiments; Z.G. and J. Ye conception and design of research; J. Ye edited and revised manuscript; J. Ye approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Reza Zarnegar for the kind gift of HGF-CAT reporter plasmid and technical support from Tianyi Tang.
REFERENCES
- 1.Araujo TG, Oliveira AG, Carvalho BM, Guadagnini D, Protzek AO, Carvalheira JB, Boschero AC, Saad MJ. Hepatocyte growth factor plays a key role in insulin resistance-associated compensatory mechanisms. Endocrinology 153: 5760–5769, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Bell AW, Jiang JG, Chen Q, Liu Y, Zarnegar R. The upstream regulatory regions of the hepatocyte growth factor gene promoter are essential for its expression in transgenic mice. J Biol Chem 273: 6900–6908, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bell LN, Cai L, Johnstone BH, Traktuev DO, March KL, Considine RV. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metab 294: E336–E344, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 117: 2362–2368, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu SH, Feng DF, Ma YB, Zhu ZA, Zhang H, Qiu JH. Stabilization of hepatocyte growth factor mRNA by hypoxia-inducible factor 1. Mol Biol Rep 36: 1967–1975, 2009 [DOI] [PubMed] [Google Scholar]
- 6.de Mol P, Fokkert MJ, de Vries ST, de Koning EJ, Dikkeschei BD, Gans RO, Tack CJ, Bilo HJ. Metabolic effects of high altitude trekking in patients with type 2 diabetes. Diabetes Care 35: 2018–2020, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of nuclear translocation of hdac3 by ikba is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor γ function. J Biol Chem 281: 4540–4547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3–L1 adipocytes. Mol Endocrinol 18: 2024–2034, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Giannopoulou M, Dai C, Tan X, Wen X, Michalopoulos GK, Liu Y. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-kappaB signaling. Am J Pathol 173: 30–41, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE, Targeting Inflammation Using Salsalate in Type 2 Diabetes Study. T. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med 159: 1–12, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong R, Rifai A, Ge Y, Chen S, Dworkin LD. Hepatocyte growth factor suppresses proinflammatory NFkappaB activation through GSK3beta inactivation in renal tubular epithelial cells. J Biol Chem 283: 7401–7410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong R, Rifai A, Tolbert EM, Biswas P, Centracchio JN, Dworkin LD. Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. J Am Soc Nephrol 15: 2868–2881, 2004 [DOI] [PubMed] [Google Scholar]
- 13.He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1a activity in adipose tissue by obesity-associated factors: adipogenesis, insulin and hypoxia. Am J Physiol Endocrinol Metab 300: E877–E885, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiratsuka A, Adachi H, Fujiura Y, Yamagishi SI, Hirai Y, Enomoto M, Satoh A, Hino A, Furuki K, Imaizumi T. Strong association between serum hepatocyte growth factor and metabolic syndrome. J Clin Endocrinol Metab 90: 2927–2931, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and Inhibitory Domains of Hypoxia-inducible Factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem 272: 19253–19260, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Jiang JG, Chen Q, Bell A, Zarnegar R. Transcriptional regulation of the hepatocyte growth factor (HGF) gene by the Sp family of transcription factors. Oncogene 14: 3039–3049, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Jiang JG, Johnson C, Zarnegar R. Peroxisome proliferator-activated receptor gamma-mediated transcriptional up-regulation of the hepatocyte growth factor gene promoter via a novel composite cis-acting element. J Biol Chem 276: 25049–25056, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Jun JC, Shin MK, Yao Q, Bevans-Fonti S, Poole J, Drager LF, Polotsky VY. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab 303: E377–E388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol Cell Biol 29: 1575–1591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitajima Y, Ide T, Ohtsuka T, Miyazaki K. Induction of hepatocyte growth factor activator gene expression under hypoxia activates the hepatocyte growth factor/c-Met system via hypoxia inducible factor-1 in pancreatic cancer. Cancer Sci 99: 1341–1347, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Gao Z, Ye J. Regulation of 11β-HSD1 expression during adipose tissue expansion by hypoxia through different activities of NF-kB and HIF-1α. Am J Physiol Endocrinol Metab 304: E1035–E1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher JJ. Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest 91: 2244–2252, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min JK, Lee YM, Kim JH, Kim YM, Kim SW, Lee SY, Gho YS, Oh GT, Kwon YG. Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial ICAM-1 and VCAM-1 by inhibiting the nuclear factor-kappaB pathway. Circ Res 96: 300–307, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: much more than a growth factor. J Gastroenterol Hepatol 26, Suppl 1: 188–202, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab 295: E313–E322, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plaschke-Schlutter A, Behrens J, Gherardi E, Birchmeier W. Characterization of the scatter factor/hepatocyte growth factor gene promoter. Positive and negative regulatory elements direct gene expression to mesenchymal cells. J Biol Chem 270: 830–836, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32: 451–463, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Saiki A, Watanabe F, Murano T, Miyashita Y, Shirai K. Hepatocyte growth factor secreted by cultured adipocytes promotes tube formation of vascular endothelial cells in vitro. Int J Obes (Lond) 30: 1676–1684, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Shintani Y, Aoki H, Nishihara M, Ohno S, Furusho A, Hiromatsu S, Akashi H, Imaizumi T, Aoyagi S. Hepatocyte growth factor promotes an anti-inflammatory cytokine profile in human abdominal aortic aneurysm tissue. Atherosclerosis 216: 307–312, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Simler N, Grosfeld A, Peinnequin A, Guerre-Millo M, Bigard AX. Leptin receptor-deficient obese Zucker rats reduce their food intake in response to hypobaric hypoxia. Am J Physiol Endocrinol Metab 290: E591–E597, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Sun K, Asterholm IW, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA 109: 5874–5879, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 121: 2094–2101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Mynatt R, Martin RJ, Keenan M, Gao Z, Ye J. Uncoupling of inflammation and insulin resistance by NF-kB in transgenic mice through induction of energy expenditure. J Biol Chem 285: 4637–4644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 93: 1–21, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Tsukagawa E, Adachi H, Hirai Y, Enomoto M, Fukami A, Ogata K, Kasahara A, Yokoi K, Imaizumi T. Independent association of elevated serum hepatocyte growth factor levels with development of insulin resistance in a 10-year prospective study. Clin Endocrinol (Oxf) 79: 43–48, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83–93, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455: 479–492, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu F, Burk D, Gao Z, Yin J, Zhang X, Weng J, Ye J. Angiogenic deficiency and adipose tissue dysfunction are associated with macrophage malfunction in SIRT1−/− mice. Endocrinology 153: 1706–1716, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan J, Gao Z, Yu G, He Q, Weng J, Ye J. Nuclear corepressor is required for inhibition of phosphoenolpyruvate carboxykinase expression by tumor necrosis factor-α. Mol Endocrinol 21: 1630–1641, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Ye J. Adipose tissue vascularization: its role in chronic inflammation. Curr Diab Rep 11: 203–210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes 33: 54–66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye J, Gao Z, Yin J, He H. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Yin J, Gao Z, He Q, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab 296: E333–E342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell 2: 331–341, 2002 [DOI] [PubMed] [Google Scholar]