Abstract
Mechanical loading is an important regulator in skeletal growth, maintenance, and aging. Estrogen receptors have a regulatory role in mechanically induced bone adaptation. Estrogen receptor-α (ERα) is known to enhance load-induced bone formation, whereas ERβ negatively regulates this process. We hypothesized that ERβ regulates mechanical signaling in osteoblasts. We tested this hypothesis by subjecting primary calvarial cells isolated from wild-type and ERβ-knockout mice (BERKO) to oscillatory fluid flow in the absence or presence of estradiol (E2). We found that the known responses to fluid shear stress, i.e., phosphorylation of the mitogen-activated protein kinase ERK and upregulation of COX-2 expression, were inhibited in BERKO cells in the absence of E2. Flow-induced increase in prostaglandin E2 (PGE2) release was not altered in BERKO cells in the absence of E2, but was increased when E2 was present. Additionally, immunofluorescence analysis and estrogen response element luciferase assays revealed increased ERα expression and flow- and ligand-induced nuclear translocation as well as transcriptional activity in BERKO cells in both the presence and absence of E2. Taken together, these data suggest that ERβ plays both ligand-dependent and ligand-independent roles in mechanical signaling in osteoblasts. Furthermore, our data suggest that one mechanism by which ERβ regulates mechanotransduction in osteoblasts may result from its inhibitory effect on ERα expression and function. Targeting estrogen receptors (e.g., inhibiting ERβ) may represent an effective approach for prevention and treatment of age-related bone loss.
Keywords: estrogen receptor-β, osteoblast, mechanobiology, cyclooxygenase-2, prostaglandin E2
mechanical loading is an important regulator in skeletal growth, maintenance, and aging (25, 41, 47). Increased bone formation is observed typically with increased mechanical loading, whereas bone loss is observed with decreased mechanical loading or disuse (27, 48). Current evidence suggests that mechanical stress is sensed by osteocytes, which then signal osteoblasts and osteoclasts to either add or remove bone, respectively (5). The mechanism by which a physical stimulus is transformed into a biochemical signal, termed mechanotransduction, remains unclear (6).
Estrogen is a key regulator of bone formation. During growth, radial expansion of long bones occurs at similar rates in boys and girls. However, at the onset of puberty, when estrogen levels increase in girls, periosteal expansion is inhibited (44). Conversely, at the onset of menopause, when estrogen levels fall in women, radial expansion recommences, suggesting that estrogen inhibits periosteal bone apposition (19). Estrogen also appears to inhibit exercise-induced periosteal expansion (4, 25).
The mechanism for opposing effects of estrogen on bone formation appears to be related to signaling through distinct receptor isoforms. Estrogen signals via two receptors, estrogen receptor (ER)α and ERβ, which are members of the superfamily of ligand-regulated nuclear receptors (20). The amino acid sequence homology between the two receptors is ∼97% in the DNA-binding domain and ∼56% in the ligand-binding domain. Ligand binding induces a conformational change, receptor dimerization, and translocation to the nucleus, where the activated receptor complex binds to DNA and recruits coregulator proteins to affect gene transcription. ERs can activate transcription by binding directly to DNA at the classical estrogen response element (ERE) or indirectly through interactions with the Jun/Fos family of transcription factors, which bind at activator protein-1 (AP-1) genomic elements (26). Transcriptional activity of ERα and ERβ is mediated by two transcription activation functions (AF), AF-1 and AF-2, that can exhibit differential activity levels, depending on the ligand, receptor type, and enhancer element combination (37). ERβ has been shown to reverse or inhibit ERα activation at AP-1 sites, suggesting that ERβ has an overall suppressive action on transcription of certain genes modulated by ERα (30).
ERα and ERβ are expressed in human, rat, and mouse osteoblasts and osteocytes (8, 36). Damien et al. (12) showed that a selective estrogen receptor modulator and ICI-182,780, a pure ER antagonist, inhibit the proliferative response of osteoblasts to strain and estrogen. In addition, they showed that mechanical strain results in the phosphorylation of ERα, which is dependent on the phosphorylation of extracellular signal-regulated kinase (ERK), a member of the mitogen-activated protein kinase (MAPK) family that is important in cell proliferation, differentiation, and apoptosis (21). Both strain and estrogen result in a pronounced translocation of ERα from the cytoplasm to the nucleus in osteoblasts and osteocytes (56). In addition, both strain and estrogen result in an increase in ER transcriptional activity, as measured by an ERE luciferase activity assay (55), providing further evidence for a role for ERs in mechanical signaling in osteoblasts.
The specific role of each ER isoform in mechanically induced bone adaptation has been implicated in studies using ER-knockout mice. Lee et al. (28) showed that strain-induced periosteal bone formation was almost completely absent in ERα-knockout (ERKO) mice. Conversely, similar studies in female ERβ knockout (BERKO) mice exhibited an enhanced load-induced response suggesting that signaling via ERβ normally suppresses periosteal bone formation (42, 43). These findings were supported by in vitro studies in which primary long bone osteoblasts isolated from ERKO mice exhibited no strain-related increase in proliferation, whereas osteoblasts isolated from BERKO mice exhibited a 125% increase in cell number following mechanical strain (29). Finally, Galea et al. (18) has provided evidence that both strain and estrogen work through ERβ to downregulate Sost expression, which in turn enhances osteogenic activity (49a). Taken together, these data suggest that both ERα and ERβ can regulate mechanical signaling in bone and influence bone adaptation and that the ERβ itself or signaling via ERβ may normally oppose mechanical signaling via ERα in osteoblasts.
The aim of this work was to further elucidate mechanisms by which ERβ regulates osteoblast function in response to mechanical stimulation. We isolated primary calvarial cells from wild-type (WT) and BERKO neonates and subjected them to oscillatory fluid flow (OFF) for varying periods in the absence and presence of estradiol (E2) and evaluated ERK, cyclooxygenase-2 (COX-2), and prostaglandin (PGE2) signaling as well as expression of receptor levels and ER transcriptional activity.
MATERIALS AND METHODS
Cell culture.
All experimental procedures involving primary mouse osteoblasts were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. Primary mouse calvarial cells were isolated as described previously (54). Briefly, calvaria from 3- to 5-day-old WT and BERKO mice (14) were isolated aseptically and exposed to seven consecutive 10-min digestions in 0.2% collagenase-0.25% trypsin. Cells released in the second through seventh digestions were pooled and maintained in phenol red-free Dulbecco's minimum essential medium (DMEM) supplemented with 10% fetal calf serum (FCS), 4 mM l-glutamine, and 1% penicillin-streptomycin antibiotics (Gibco-Invitrogen, Carlsbad, CA). At 80–90% confluence, cells were passaged by brief trypsinization, with the medium changed every day. The osteoblastic phenotype was confirmed by treatment with ascorbic acid (50 μg/ml), resulting in positive staining for alkaline phosphatase expression (data not shown). For all experiments passages 4–6 were used, and cells were in a proliferative, nondifferentiated stage of growth.
OFF.
All fluid flow experiments were performed under sterile conditions. Primary calvarial osteoblasts from WT and BERKO neonates were plated at 2,500/cm2 onto glass slides coated with rat type I collagen and grown to 80% confluence. Twenty-four hours before flow, cells were cultured in serum-free medium (phenol red-free DMEM + 0% FCS + 4 mM l-glutamine + 1% penicillin-streptomycin) with ICI-182 780 (10−6 M), a pure ER antagonist (Sigma Aldrich, St. Louis, MO), when appropriate. On the day of flow, slides were maintained in static culture or loaded individually into parallel plate flow chambers (17) containing E2-free or E2-supplemented (10−7 M) flow medium. Chambers were connected in series to rigid-walled tubing and a Hamilton glass syringe and plunger. The plunger was driven by a custom-built oscillatory loading device, which produced fluid-flow shear stress (15 dyn/cm2, 1 Hz). Depending on the outcome measurement, varying flow periods were used. For p-ERK analysis, 30-min flows were performed. For PGE2 release and COX-2 expression, 4-h flows were performed, followed by a 1-h postflow incubation. For ERα nuclear translocation, 1-h flows were performed. For ERE luciferase activity, 12-h flows were performed.
Immunoblot analysis.
For immunoblot analysis, cells were collected in SDS-PAGE sample buffer, and protein concentration was determined using an amido black assay (45). Equal protein (20 μg) was separated by SDS-PAGE and transferred to nitrocellulose for immunoblotting. The following primary antibodies were used: rabbit polyclonal anti-ERK1 and -2 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-phospho-ERK (Santa Cruz Biotechnology), rabbit polyclonal anti-COX-2 (Cayman Chemical, Ann Arbor, MI), mouse monoclonal anti-actin (Sigma-Aldrich, St. Louis, MO), and mouse monoclonal anti-vinculin (Sigma-Aldrich). Appropriate anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were used. Horseradish peroxidase chemiluminescence was detected using a Fujifilm LAS-3000 image detection system (Fujifilm, Tokyo, Japan), and densitometric analysis was performed using Fujifilm MultiGauge (version 2) densitometric analysis software (Fujifilm). Actin or vinculin expression was used to normalize gene expression. A minimum of three samples per experimental group was quantified.
PGE2 release.
For assessment of prostaglandin release in response to fluid shear stress, cells were subjected to either static conditions or OFF for 4 h. After flow, slides were overlaid with 1 ml pf flow medium and placed in the incubator for 1 h. After 1 h, the medium was collected and assessed for PGE2 concentration using an enzyme immunoassay per the manufacturer's instructions (Amersham Biosciences, Piscataway, NJ). Results were normalized to total cellular protein, as determined by an amido black assay. A minimum of four samples per experimental group was quantified.
Immunofluorescence analysis.
WT and BERKO primary calvarial cells were subjected to static conditions or OFF for 1 h, and cells were fixed in cold 4% paraformaldehyde and permeabilized in 0.2% Triton in PBS. A primary antibody against ERα (Affinity Bioreagents, Golden, CO) and a FITC-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) were used to visualize ERα, and DAPI stain (Sigma Chemical, St. Louis, MO) was used to visualize nuclei. Images were captured using a SPOT RT digital camera (Diagnostic Instruments) attached to an Optiphot-2 Nikon epifluorescent microscope with a PlanApo ×40 Nikon objective (Nikon, Tokyo, Japan). At least three separate flow experiments were performed for each group, and ∼10 fields per experiment (slide) were captured. ERα expression in WT and BERKO cells was quantified by digital image analysis (Image J; National Institutes of Health) using a threshold technique that captured the number of pixels representing fluorescing protein with each cell.
ERE luciferase reporter.
An ERE luciferase reporter plasmid (0.5 μg; Panomics) together with an internal control thymidine kinase-driven Renilla luciferase vector plasmid (pRL-TK, 0.5 μg; kindly provided by Dr. B. Paul Herring) were cotransfected into WT and BERKO cells. Cells were plated onto glass slides at 50% confluence. The following day, cells were incubated with a total of 1 μg of plasmid DNA and 2 μl of FuGENE 6 transfection reagent (Roche Diagnostics) in 100 μl of phenol red-free, additive-free DMEM (Gibco-Invitrogen). At 48 h posttransfection, cells were subjected to either static conditions or OFF for 12 h. We chose the 12-h time point because 12-h E2 treatment was shown to induce a detectable increase (∼2.5-fold) in relative luciferase activity (firefly/Renilla) in WT cells overexpressing ERα (data not shown). After flow, cell lysates were collected. Reporter gene firefly luciferase activities were normalized to internal control Renilla luciferase activities using a Dual Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer's instructions. Data are presented as normalized relative light units. A minimum of three samples per experimental group were analyzed.
Data analysis.
Differences were tested for significance using a three-factor ANOVA, with genotype, OFF, and E2 treatment serving as main factors. Statistical significance was assumed for P < 0.05. Data are presented as means ± SE.
RESULTS
BERKO calvarial cells exhibit reduced OFF-induced ERK phosphorylation, which is restored by E2 treatment.
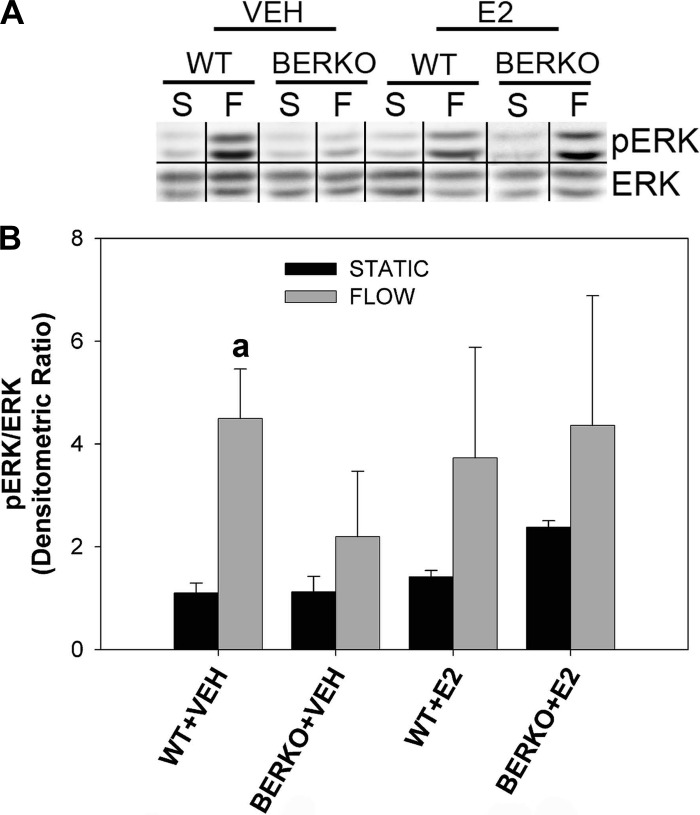
To determine the role of ERβ in mechanical signaling in osteoblasts, primary calvarial cells were subjected to 30 min of OFF in the absence and presence of E2. Phosphorylation of ERK, a well-known reaction to fluid flow, was determined. In the absence of E2, WT cells (WT + VEH) subjected to OFF exhibited an approximately fourfold significant increase in ERK phosphorylation normalized by ERK compared with static controls (Fig. 1). Under the same conditions, BERKO cells exhibited an increase of approximately twofold, only half of that observed in WT + VEH cells. The absolute value of p-ERK/ERK in BERKO + VEH exposed to OFF was approximately twofold lower than that observed in WT + VEH flowed cells. Interestingly, in the presence of E2, flow-induced ERK phosphorylation was restored to near-control levels in BERKO cells, although the difference between groups did not reach significance.
Fig. 1.
ERK phosphorylation in primary wild-type (WT) and estrogen receptor (ER)β-knockout (BERKO) calvarial cells. A: Western blot analysis of anti-ERK phosphorylation (p-ERK; Y site) and ERK in WT and BERKO cells exposed to static conditions (S) or 30 min of oscillatory fluid flow (OFF) (F) in the absence (VEH) and presence of estradiol (E2). Representative bands from experimental groups are shown. Bands taken from noncontiguous lanes are divided by black lines. B: quantification of p-ERK/ERK densitometric ratios, normalized by the mean of p-ERK/ERK for the WT S VEH group (thus shown as “1”), is presented. Experimental groups contain 2–3 samples. Bars represent normalized means ± SE. Genotype, F, and E2 were not significant factors by a 3-way ANOVA; however, WT + VEH F was significantly greater than WT + VEH S when compared using a Student t-test. aP < 0.05 vs. WT + VEH S.
Flow-induced COX-2 upregulation in BERKO cells in the absence of E2 is attenuated but restored in the presence of E2.
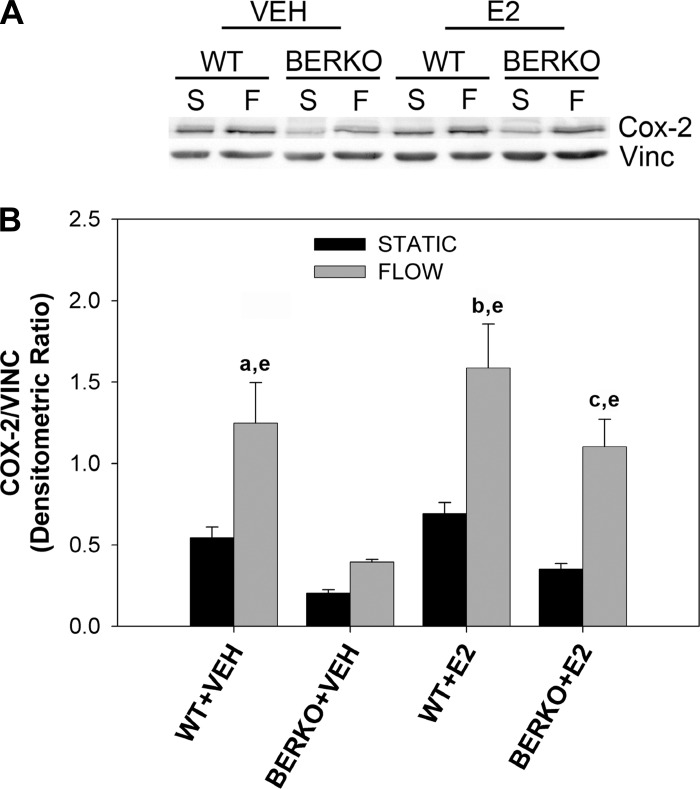
Upregulation of COX-2 protein, the rate-limiting enzyme in the production of PGE2 and an important mediator of in vivo load-induced bone formation (16), was determined by Western blot analysis. Primary calvarial cells were subjected to 4 h of OFF in the absence and presence of E2. Basal COX-2 expression levels in WT cells were ∼1.7-fold that of BERKO cells regardless of treatment with E2 (Fig. 2). COX-2 expression levels were ∼2.3-fold greater in WT + VEH cells subjected to OFF, a significant increase compared with static controls (Fig. 2B). In BERKO + VEH cells subjected to OFF, no significant increase in COX-2 expression was detected compared with BERKO + VEH cells kept in static conditions. However, in the presence of E2 (BERKO + E2), OFF resulted in a significant, 5.9-fold increase in COX-2 expression (Fig. 2B).
Fig. 2.
Expression of cyclooxygenase (COX-2) in primary WT and BERKO calvarial osteoblasts. A: Western blot analysis of anti-COX-2 and anti-vinculin (Vinc) in wild-type and BERKO cells exposed to S or 4 h of F in the absence (VEH) and presence of E2. Representative examples are shown. B: quantification of COX-2/Vinc densitometric ratios in WT and BERKO cells. Experiment was performed in triplicate. Bars represent means ± SE. Genotype (P < 0.001), F (P < 0.0001), and E2 (P < 0.01) had significant main factor effects by a 3-way ANOVA. aP < 0.01 vs. WT + VEH S; bP < 0.001 vs. WT + E2 S; cP < 0.01 vs. BERKO + E2 S; eP < 0.05 vs. BERKO + VEH F by Fisher's protected least significant difference (PLSD) post hoc analysis.
OFF-induced PGE2 release is greatest in BERKO cells in the presence of E2.
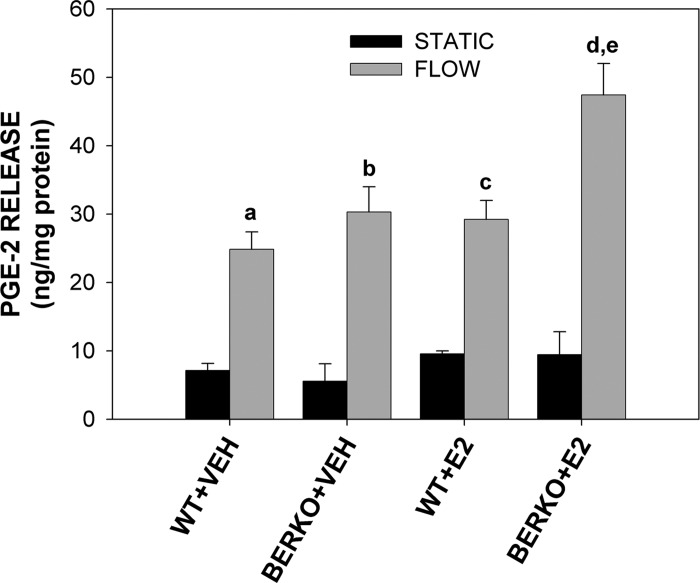
PGE2 is an important factor in load-induced bone formation. Therefore, we analyzed PGE2 release in WT and BERKO cells in response to OFF. To assess ligand-independent and ligand-dependent effects of ERβ in PGE2 release, WT and BERKO cells were subjected to 4 h of OFF in the absence and presence of E2, and the amount of soluble PGE2 released 1 h postflow was determined. PGE2 values for WT + VEH and WT + E2 cells subjected to OFF was ∼3.5-fold and ∼3.1-fold that of static controls, respectively, with absolute values of 24.9 and 29.2 ng/mg of protein (Fig. 3). BERKO + VEH and BERKO + E2 cells exhibited a ∼5.6-fold and ∼5.0-fold increase, respectively, in PGE2 in response to OFF; however, total PGE2 released was significantly greater in BERKO + E2 cells subjected to OFF (47.4 ng/mg protein) when compared with all other groups, both static and OFF. That WT and BERKO cells subjected to OFF exhibit similar levels of PGE2 in the absence of E2 suggests that ERβ has no ligand-independent effect on flow-induced PGE2 release. Conversely, the robust PGE2 release in BERKO + E2 cells subjected to OFF indicates that ligand-dependent signaling via ERβ normally suppresses flow-induced PGE2 release in osteoblasts.
Fig. 3.
Prostaglandin E2 (PGE2) release from primary WT and BERKO calvarial osteoblasts. PGE2 release normalized to total protein in WT and BERKO cells exposed to S or 4 h of F in the absence (VEH) and presence of E2. Each group was comprised of 4–7 replicates. Bars represent means ± SE. Genotype (P < 0.05), F (P < 0.0001), E2 (P < 0.01), and genotype × F (P < 0.05) had significant main factor effects by a 3-way ANOVA. aP < 0.01 vs. WT + VEH S; bP < 0.0001 vs. BERKO + VEH S; cP < 0.001 vs. WT + E2 S; dP < 0.0001 vs. BERKO + E2; eP < 0.0001 vs. all other S and F groups by Fisher's PLSD post hoc analysis.
BERKO osteoblasts exhibit greater ERα expression and enhanced cytoplasmic and nuclear localization.
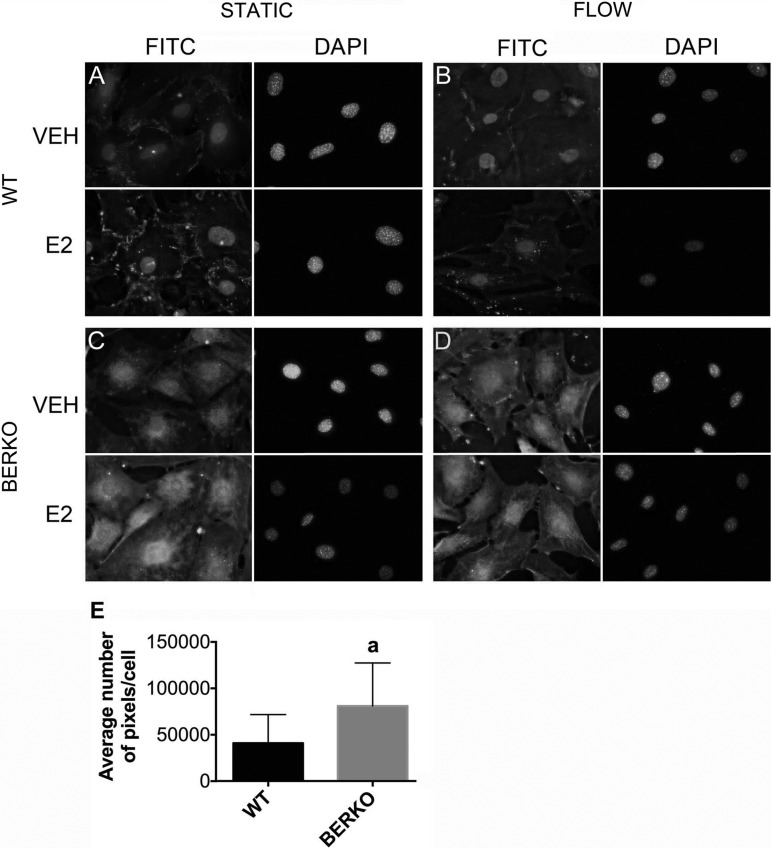
Activation of ERs typically involves receptor translocation to the nucleus, where it binds to the ERE, activating ERE-driven gene transcription. Thus, we next determined the role of ERβ in ERα cellular localization in response to OFF. WT and BERKO calvarial cells were exposed to 1 h of OFF in the absence and presence of E2, fixed, and stained for ERα and DAPI. By immunofluorescence, we observed that in WT cells exposed to static conditions in the absence of E2 (WT + VEH), ERα was localized primarily in the nucleus, with punctate cell-cell junction and cytoplasmic staining also observed (Fig. 4A). One hour of OFF appeared to slightly enhance nuclear staining in WT + VEH cells with less membrane staining, indicating a possible ERα translocation from cell-cell junctions to the nucleus (Fig. 4B). E2 treatment also appeared to increase nuclear staining, whereas the combination of OFF and E2 did not further enhance ERα nuclear localization. In BERKO + VEH cells exposed to static conditions, there was robust cytoplasmic and nuclear ERα staining compared with control cells (Fig. 4C). Both OFF and E2 appeared to similarly enhance intracellular ERα staining, with very little additional enhancement in BERKO cells exposed to the combination of OFF and E2 (Fig. 4, C and D). These data suggest that ERβ may normally help localize ERα to the cell membrane, and in the absence of ERβ there is cytoplasmic and nuclear translocation.
Fig. 4.
Expression of ERα in WT and BERKO primary calvarial cells exposed to static conditions or 1 h of OFF in the absence (VEH) and presence of E2. Following 1 h of OFF, cells were fixed and stained with anti-ERα antibody, FITC-conjugated secondary antibody, and DAPI for visualization of ERα localization. ERα (FITC) and DAPI images are presented side by side. A: WT cells treated with VEH or E2 and exposed to static conditions. B: WT cells treated with VEH or E2 and exposed to 1 h of OFF. C: BERKO cells treated with VEH or E2 and exposed to static conditions. D: BERKO cells treated with VEH or E2 and exposed to 1 h of OFF. Images illustrate representative ERα localization for each experimental group. Magnification, ×400. E: quantification of ERα expression in WT and BERKO cells. Expression of ERα is significantly greater in BERKO vs. WT cells by a Student t-test. aP < 0.05 vs. WT cells.
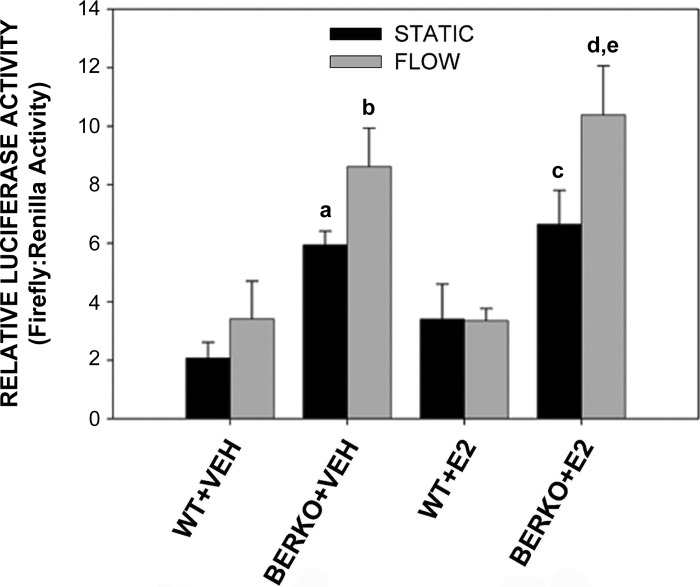
Basal and flow-induced ER transcriptional activity increased in BERKO osteoblasts in the presence or absence of ligand.
To determine whether enhanced ERα intracellular and nuclear localization observed in BERKO cells translates into changes in transcriptional activity, WT and BERKO cells were cotransfected with an ERE-driven luciferase reporter construct and exposed to static conditions or 12 h of OFF in the absence and presence of E2. A small and insignificant increase in relative luciferase activity was observed in both WT and BERKO cells in the absence of E2 (Fig. 5). Interestingly, ERE luciferase activity in BERKO cells under both static and flow conditions was significantly greater than activity observed in control cells (P < 0.05, P < 0.01). E2 treatment also resulted in a small and insignificant increase in luciferase activity in WT cells, but the combination of E2 and OFF did not further increase activity (Fig. 5). E2 treatment alone did not result in increased activity in BERKO cells; however, OFF did lead to significantly greater activity in BERKO + E2 cells compared with BERKO + E2 static controls. Again, both basal and OFF-induced luciferase activities were significantly increased in BERKO cells compared with controls in the presence of E2. Thus, the increased ERα localization to the cytoplasm and nucleus in BERKO cells corresponds with greater ERE activity observed in these cells, suggesting that ERβ normally inhibits ERα transcriptional activity.
Fig. 5.
Relative (firefly/Renilla) luciferase activity in WT and BERKO primary calvarial cells. Cells were cotransfected with an estrogen response element luciferase (firefly) reporter plasmid and a control thymidine kinase-driven Renilla luciferase vector plasmid, treated with VEH or E2, and subjected to static conditions or 12 h of OFF. Each group was comprised of 3–4 replicates. Bars represent means ± SE. Genotype (P < 0.001) and OFF (P < 0.05) had significant main factor effects by a 3-way ANOVA. E2 treatment had no significant effect. aP < 0.05 vs. WT + VEH S; bP < 0.01 vs. WT + VEH F; cP < 0.05 vs. WT + E2 S; dP < 0.001 vs. WT + E2 F; eP < 0.05 vs. BERKO + E2 S by Fisher's PLSD post hoc analysis.
DISCUSSION
ERβ plays a significant role in regulating important cellular mechanotransduction events of ERK phosphorylation, COX-2 upregulation, and PGE2 release. We show that BERKO cells exhibit higher levels of ERα expression, an increase in ER transcriptional activity, and flow- and ligand-induced PGE2 release.
Important mechanical signaling events in osteoblasts include activation of the MAPK pathway, an increase in COX-2 expression, and release of prostaglandins. Phosphorylation of ERK, a member of the MAPK family, peaks within the first 15 min after application of fluid flow shear stress in osteoblasts and endothelial cells (50, 53) and, as mentioned previously, is known to be important in cell proliferation, differentiation, and apoptosis. COX-2 is an important enzyme in the conversion of arachidonic acid into prostaglandins. In vitro, COX-2 mRNA and protein levels increase in response to fluid flow shear stress, making it an important indicator of mechanotransduction in osteoblasts (3, 39). Prostaglandins are lipid mediators that are involved in the inflammatory response (38). PGE2 is the most abundant prostaglandin in the human body, with known anabolic effects in bone (49). Significant increases in cellular production of PGE2 in response to fluid flow shear stress have been shown to affect TGFβ release, an important mediator of bone remodeling (24, 40).
Recent data suggest that ERα and ERβ can regulate mechanical signaling in bone, and ERβ itself or signaling via ERβ may normally oppose ERα in osteoblasts. Adult female BERKO mice have increased trabecular bone volume (46), significantly higher cortical bone content and periosteal circumference (23), and enhanced load-induced bone formation (43), indicating that ERβ negatively regulates radial bone apposition during growth and in response to mechanical stimuli.
Our results indicate that ERβ can regulate signaling pathways shown to be important in mechanical signaling in osteoblasts, including ERK phosphorylation, COX-2 expression, PGE2 release, and ERα localization. Of these, only PGE2 release appears to be affected by ligand-dependent ERβ signaling. BERKO cells exhibit reduced OFF-induced ERK phosphorylation compared with WT cells in the absence of E2. Because ERK phosphorylation is involved in increased cell proliferation and differentiation (52), this result suggests that ERβ may be important for osteoblast proliferation in response to mechanical stimuli in the absence of ligand. This hypothesis is in contrast to previously published data showing that primary osteoblasts isolated from female BERKO mice exhibit greater proliferation rates in response to mechanical strain compared with WT cells in vitro (125 vs. 61%) (29), suggesting that ERβ is antiproliferative. In fact, other studies have shown that ERβ can directly inhibit ERα-mediated cyclin D1 gene activation, a key regulator for entry into the proliferative stage of the cell cycle (31). Although we did not assess flow-induced proliferation in this study, we did not observe significant differences in basal growth rate in BERKO cells by both manual counts and a biochemical assay (data not shown). The means of mechanical stimulation used in the two studies (strain vs. OFF) could explain these conflicting data. This result is not in agreement with in vivo data in female BERKO mice. That is, if ERK phosphorylation played a direct role in the enhanced load-induced bone formation observed in female BERKO mice, then p-ERK levels would be greatest in BERKO + E2 cells. The fact that p-ERK levels appear to be similar in BERKO + E2 cells compared with WT + E2 suggests that mechanisms other than ERK phosphorylation are important in enhanced load-induced bone formation in BERKO mice.
COX-2 is an important enzyme in the conversion of arachidonic acid into prostaglandins (7, 11, 39). In vitro, COX-2 mRNA and protein levels increase in response to fluid flow shear stress (3). In addition, selective inhibition of COX-2 effectively blocks flow-induced PGE2 release in vitro (9) and load-induced bone formation in vivo (10). That there is an increase in flow-induced COX-2 expression in WT but not BERKO cells in the absence of E2 suggests that ERβ is normally permissive of flow-induced COX-2 expression in a ligand-independent manner. Furthermore, the concomitant increase in both COX-2 protein and PGE2 release in WT but not BERKO cells suggests that sustained COX-2 synthesis is not necessary for PGE2 release in BERKO cells (Figs. 2 and 3). We have not ruled out the possibility that COX-2 expression was upregulated at an earlier time point and then decreased over the course of the 4-h flow, which could alternatively account for the increased PGE2 release. Thus, the COX-2 and PGE2 data taken together suggest that, in the absence of ligand, the magnitude of the COX-2 and PGE2 responses is not tightly coupled in BERKO cells. Further studies are needed to determine whether ERβ regulates the relationship between COX-2 expression and PGE2 release.
BERKO + E2 cells showed significantly greater PGE2 release when compared with all other experimental groups. PGE2 is a known anabolic agent whose increased cellular production may result in greater osteogenic activity. In vivo administration of PGE2 to young rats results in increased trabecular bone volume and greater proliferation and differentiation of progenitors in the marrow (51). Treatment of rat calvarial osteoblasts with PGE2 results in a dose-dependent increase in the osteoblast differentiation markers, alkaline phosphatase activity, and mineralized bone nodule formation (22). Enhanced PGE2 release observed in BERKO cells may provide a mechanism for enhanced load-induced bone formation observed in female BERKO mice.
ERs are localized mainly in the cellular membrane and nucleus, with some cytoplasmic localization (32). Upon ligand binding, ERs form homo- or heterodimers and translocate to the nucleus, where they affect gene transcription. We observed significantly greater ERα expression in BERKO cells, an observation supported by data showing that ERβ causes a significant increase in ligand-dependent ERα degradation in T47D human breast carcinoma cells within 3 h of E2 treatment (33). We also observed greater cell-cell junction localization of ERα in WT cells, suggesting that ERβ may normally affect the subcellular localization of ERα. The mechanism by which subpopulations of estrogen receptors localize to the membrane is unclear, and further studies are needed to address the possibility that ERβ is involved in membrane localization of ERα.
The overall increase in ERE luciferase reporter activity in BERKO cells supports the observed increased nuclear ERα localization (Fig. 5). Additionally, these studies confirm significantly increased ER transcriptional activity in response to flow in BERKO cells, a phenomenon we did not observe in WT cells. These data are in agreement with studies showing that ERβ can negatively regulate estrogen-mediated transcriptional activity in bone. Microarray analysis of estrogen-mediated gene expression in the humerus of ovariectomized BERKO mice was shown to be 85% higher compared with WT controls (30). Animal data are supported by studies showing a negative regulatory role for ERβ in human bone metabolism. Japanese postmenopausal women who harbored one or two alleles of a specific ERβ gene polymorphism had significantly higher lumbar bone mineral density compared with control subjects not possessing the polymorphism (34). The mechanism of action on bone mineral density is unclear, but the Ogawa et al. (35) have suggested that the polymorphism may lead to changes in ERβ protein function, which could affect the action of E2 on bone. Taken together, these data suggest that ERβ normally acts to negatively regulate ERα transcriptional activity. It is clear that ERα plays an important role in bones' response to mechanical loading. In addition, ERα appears to also be required for Wnt/β-catenin signaling (2). Galea et al. (18) showed that flow activated nuclear accumulation of β-catenin and activated the TCF/LEF promoter in ROS 17/2.8 cells. This response was inhibited by treatment with the ER modulator ICI-182,780 and the GSK-3β inhibitor lithium chloride. In addition, this response was not observed in ERα−/− mice, suggesting that ERα is required for Wnt/β-catenin signaling in response to mechanical loading. More recently, the same group showed that both strain and estrogen work through ERβ to downregulate Sost expression (18), a gene that inhibits Wnt signaling and attenuates osteoblast bone formation. Thus, Wnt signaling can be regulated by both ERα and ERβ. If the loss of ERβ results in upregulation of ERα, then it may also enhance Wnt/β-catenin signaling, providing another mechanism by which BERKO mice exhibit enhanced mechanical adaptation.
One interesting observation that should be addressed is the fact that E2 treatment did not activate ERE activity. One possible explanation for this is that endogenous ER expression in osteoblasts is low (15), potentially resulting in relatively low ERE activity levels. In a separate experiment, we showed that ERE activity increased in a time-dependent manner only after cells were transfected with ERs (data not shown). This response increased significantly after 12 h of treatment. Keeping in mind that BERKO cells appear to have greater ERα expression, it is curious they did not exhibit increased ERE activity with E2 treatment when they clearly exhibited greater basal ERE activity relative to WT cells. One possible explanation for this observation is that the effect of E2 and OFF may require different treatment periods before significant changes in ERE activity transpire. Zaman et al. (56) showed that, in osteocytes, strain and estrogen resulted in transient changes in ERα mRNA levels; however, the effects of strain were larger and occurred earlier than those associated with estrogen treatment. They also showed that 36 h of E2 treatment, at similar doses used in the current study, resulted in a significant increase in ERE-CAT reporter gene activity in ROS.SMER no. 14 cells, with less of an effect observed at 18 h (55), suggesting that our 12-h treatment period may have been too short to allow significant accumulation of the ERE luciferase product.
The aim of this work was to determine the mechanisms by which ERβ regulates mechanical signaling in bone. We observed a suppression of OFF-induced increases in BERKO cells in the absence of ligand of both ERK and COX-2, two molecules associated with load-induced increases in bone formation, suggesting that a different mechanism may be at play. Increased expression and transcriptional activity of ERα in BERKO cells is perhaps the means by which enhancement of load-induced bone formation occurs. Additionally, we observed no difference in the ability of BERKO cells to release PGE2 in response to flow in the absence of E2, and in fact, we observed a synergistic effect of flow and E2. Considering the importance of prostaglandins in load-induced bone formation, this result could explain increased bone formation observed in BERKO mice. Both ERα and ERβ can regulate mechanical signaling in bone and influence bone adaptation, and in targeting ERs (e.g., inhibiting ERβ), it may represent an effective approach for prevention and treatment of age-related bone loss.
GRANTS
This work was supported by National Institutes of Arthritis and Musculoskeletal and Skin Disease Grant No. AR-046530. (C. H. Turner) and Veterans Affairs Career Development Award-2 No. A6842W (A. B. Castillo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B.C. and C.H.T. conception and design of research; A.B.C. performed experiments; A.B.C. analyzed data; A.B.C., J.W.T., F.M.P., and C.H.T. interpreted results of experiments; A.B.C. prepared figures; A.B.C. drafted manuscript; A.B.C. and J.W.T. edited and revised manuscript; A.B.C., J.W.T., F.M.P., and C.H.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jennifer Doyle for assistance with imaging, Dr. Robert Bigsby for helpful discussion, and Rita O'Riley for technical help with cell culture.
REFERENCES
- 1.Alam I, Warden SJ, Robling AG, Turner CH. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J Bone Miner Res 20: 438–446, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem 282: 20715–20727, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bakker AD, Klein-Nulend J, Burger EH. Mechanotransduction in bone cells proceeds via activation of COX-2, but not COX-1. Biochem Biophys Res Commun 305: 677–683, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res 17: 2274–2280, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Burger EH, Klein-Nulend J. Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J 13: S101–S112, 1999 [PubMed] [Google Scholar]
- 6.Castillo AB, Jacobs CR. Skeletal mechanobiology. In: Mechanobiology Handbook, edited by Nagatomi J. Boca Raton, FL: CRC, 2011, Vol. 9, p. 179–228 [Google Scholar]
- 7.Chambers TJ, Fox S, Jagger CJ, Lean JM, Chow J. The role of prostaglandins and nitric oxide in the response of bone to mechanical forces. Osteoarthritis Cartilage 7: 422–423, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Chen FP, Hsu T, Hu CH, Wang WD, Wang KC, Teng LF. Expression of estrogen receptors alpha and beta in human osteoblasts: identification of exon-2 deletion variant of estrogen receptor beta in postmenopausal women. Chang Gung Med J 27: 107–115, 2004 [PubMed] [Google Scholar]
- 9.Cheng B, Kato Y, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. PGE(2) is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology 142: 3464–3473, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Chow JW, Chambers TJ. Indomethacin has distinct early and late actions on bone formation induced by mechanical stimulation. Am J Physiol Endocrinol Metab 267: E287–E292, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Chow JW, Fox SW, Lean JM, Chambers TJ. Role of nitric oxide and prostaglandins in mechanically induced bone formation. J Bone Miner Res 13: 1039–1044, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Damien E, Price JS, Lanyon LE. Mechanical strain stimulates osteoblast proliferation through the estrogen receptor in males as well as females. J Bone Miner Res 15: 2169–2177, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127: 4277–4291, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, Riggs BL. Evidence of estrogen receptors in normal human osteoblast-like cells. Science 241: 84–86, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res 11: 1688–1693, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng 32: 1053–1060, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H, Stein GS, van Wijnen AJ, Lanyon LE, Price JS. Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J Biol Chem 288: 9035–9048, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaney RP, Barger-Lux MJ, Davies KM, Ryan RA, Johnson ML, Gong G. Bone dimensional change with age: interactions of genetic, hormonal, and body size variables. Osteoporos Int 7: 426–431, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87: 905–931, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Jessop HL, Rawlinson SC, Pitsillides AA, Lanyon LE. Mechanical strain and fluid movement both activate extracellular regulated kinase (ERK) in osteoblast-like cells but via different signaling pathways. Bone 31: 186–194, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kaneki H, Takasugi I, Fujieda M, Kiriu M, Mizuochi S, Ide H. Prostaglandin E2 stimulates the formation of mineralized bone nodules by a cAMP-independent mechanism in the culture of adult rat calvarial osteoblasts. J Cell Biochem 73: 36–48, 1999 [PubMed] [Google Scholar]
- 23.Ke HZ, Brown TA, Qi H, Crawford DT, Simmons HA, Petersen DN, Allen MR, McNeish JD, Thompson DD. The role of estrogen receptor-b in the early age-related bone gain and later age-related bone loss in female mice. J Musculoskel Neuron Interact 2: 479–488, 2002 [PubMed] [Google Scholar]
- 24.Klein-Nulend J, Semeins CM, Burger EH. Prostaglandin mediated modulation of transforming growth factor-beta metabolism in primary mouse osteoblastic cells in vitro. J Cell Physiol 168: 1–7, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Kontulainen S, Sievänen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res 18: 352–359, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74: 311–317, 2000 [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact 7: 33–47, 2007 [PubMed] [Google Scholar]
- 28.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature 424: 389, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Lee KC, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta. J Endocrinol 182: 193–201, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Lindberg MK. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in Mice. Mol Endocrinol 17: 203–208, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Liu MM. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem 277: 24353–24360, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Lu Q, Ebling H, Mittler J, Baur WE, Karas RH. MAP kinase mediates growth factor-induced nuclear translocation of estrogen receptor alpha. FEBS Lett 516: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Matthews J. Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol 20: 534–543, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Ogawa S, Hosoi T, Shiraki M, Orimo H, Emi M, Muramatsu M, Ouchi Y, Inoue S. Association of estrogen receptor beta gene polymorphism with bone mineral density. Biochem Biophys Res Commun 269: 537–541, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res 26: 3505–3512, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onoe Y, Miyaura C, Ohta H, Nozawa S, Suda T. Expression of estrogen receptor beta in rat bone. Endocrinology 138: 4509–4512, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277: 1508–1510, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol 119: 229–240, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Pead MJ, Lanyon LE. Indomethacin modulation of load-related stimulation of new bone formation in vivo. Calcif Tissue Int 45: 34–40, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Pfeilschifter J, Seyedin SM, Mundy GR. Transforming growth factor beta inhibits bone resorption in fetal rat long bone cultures. J Clin Invest 82: 680–685, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. Med Sci Sports Exerc 34: 196–202, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Saxon LK, Galea G, Meakin L, Price J, Lanyon LE. Estrogen receptors α and β have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology 153: 2254–2266, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Saxon LK, Robling AG, Castillo AB, Mohan S, Turner CH. The skeletal responsiveness to mechanical loading is enhanced in mice with a null mutation in estrogen receptor-β. Am J Physiol Endocrinol Metab 293: E484–E491, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Schiessl H, Frost HM, Jee WS. Estrogen and bone-muscle strength and mass relationships. Bone 22: 1–6, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Sheffield JB, Graff D, Li HP. A solid-phase method for the quantitation of protein in the presence of sodium dodecyl sulfate and other interfering substances. Anal Biochem 166: 49–54, 1987 [DOI] [PubMed] [Google Scholar]
- 46.Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 30: 18–25, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Smith EL, Gilligan C, McAdam M, Ensign CP, Smith PE. Deterring bone loss by exercise intervention in premenopausal and postmenopausal women. Calcif Tissue Int 44: 312–321, 1989 [DOI] [PubMed] [Google Scholar]
- 48.Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res 7: 761–769, 1992 [DOI] [PubMed] [Google Scholar]
- 49.Suponitzky I, Weinreb M. Differential effects of systemic prostaglandin E2 on bone mass in rat long bones and calvariae. J Endocrinol 156: 51–57, 1998 [DOI] [PubMed] [Google Scholar]
- 49a.ten Dijke P, Krause C, de Gorter DJ, Löwik CW, van Bezooijen RL. Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J Bone Joint Surg Am 90, Suppl 1: 31–35, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Tseng H, Peterson TE, Berk BC. Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ Res 77: 869–878, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Weinreb M, Suponitzky I, Keila S. Systemic administration of an anabolic dose of PGE2 in young rats increases the osteogenic capacity of bone marrow. Bone 20: 521–526, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Yang SH, Sharrocks AD, Whitmarsh AJ. MAP kinase signalling cascades and transcriptional regulation. Gene 513: 1–13, 2013 [DOI] [PubMed] [Google Scholar]
- 53.You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem 276: 13365–13371, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Young SR, Hum JM, Rodenberg E, Turner CH, Pavalko FM. Non-overlapping functions for Pyk2 and FAK in osteoblasts during fluid shear stress-induced mechanotransduction. PLoS One 6: e16026, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaman G, Cheng MZ, Jessop HL, White R, Lanyon LE. Mechanical strain activates estrogen response elements in bone cells. Bone 27: 233–239, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS, Lanyon LL. Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res 21: 1297–1306, 2006 [DOI] [PubMed] [Google Scholar]