Abstract
Interleukin-6 (IL-6) is an important myokine that is highly expressed in skeletal muscle cells upon exercise. We assessed IL-6 expression in response to electrical stimulation (ES) or extracellular ATP as a known mediator of the excitation-transcription mechanism in skeletal muscle. We examined whether the canonical signaling cascade downstream of IL-6 (IL-6/JAK2/STAT3) also responds to muscle cell excitation, concluding that IL-6 influences its own expression through a positive loop. Either ES or exogenous ATP (100 μM) increased both IL-6 expression and p-STAT3 levels in rat myotubes, a process inhibited by 100 μM suramin and 2 U/ml apyrase. ATP also evoked IL-6 expression in both isolated skeletal fibers and extracts derived from whole FDB muscles. ATP increased IL-6 release up to 10-fold. STAT3 activation evoked by ATP was abolished by the JAK2 inhibitor HBC. Blockade of secreted IL-6 with a neutralizing antibody or preincubation with the STAT3 inhibitor VIII reduced STAT3 activation evoked by extracellular ATP by 70%. Inhibitor VIII also reduced by 70% IL-6 expression evoked by ATP, suggesting a positive IL-6 loop. In addition, ATP increased up to 60% the protein levels of SOCS3, a negative regulator of the IL-6 signaling pathway. On the other hand, intracellular calcium chelation or blockade of IP3-dependent calcium signals abolished STAT3 phosphorylation evoked by either extracellular ATP or ES. These results suggest that expression of IL-6 in stimulated skeletal muscle cells is mediated by extracellular ATP and nucleotide receptors, involving IP3-dependent calcium signals as an early step that triggers a positive IL-6 autocrine loop.
Keywords: myokines, muscle plasticity, exercise, signal transducer and activator of transcription 3, purinergic signaling
interleukin-6 (IL-6) is a proinflammatory cytokine that has been related to several processes in skeletal muscle cells, including in vitro proliferation and differentiation, regeneration of damaged adult fibers, atrophy, and hypertrophy, among others (7, 35, 71, 86). Variations of IL-6 expression levels have been related to metabolic changes in skeletal muscle undergoing different exercise protocols (17, 66, 77). It is known that IL-6 plasma concentration in volunteers performing exercise can reach increases of up to 100-fold, depending on the exercise characteristics (endurance/strength) and duration (reviewed in Ref. 61). The main source of plasma IL-6 during exercise is the skeletal muscle fibers themselves, although other cell types such as cells from the immune system and subcutaneous adipose tissue cannot be ruled out as IL-6 producers (2, 8, 41, 58).
Several intracellular signaling pathways have been related to IL-6 expression in skeletal muscle. It has been suggested that intracellular free Ca2+, acting as a second messenger, regulates the expression of IL-6 in skeletal muscle, possibly through the Ca2+-dependent phosphatase calcineurin (5, 8, 87). Also, another Ca2+-dependent process, possibly through the action of p38 MAPK and Ca2+/calmodulin-dependent kinase, has been reported to play a role on IL-6 expression related to intracellular glycogen content (17, 87). The role for NF-κB, a classic activator of proinflammatory interleukin expression, in IL-6 expression mediated by muscle activity is less clear, as we and others have obtained disparate results (4, 45, 89). Whereas we reported a NF-κB activation in C2C12 cells after a 45-Hz electrical stimulation (ES) (4, 45), Whitham et al. (89) did not find any differences in IL-6 expression between control and cells pharmacologically treated for IKK inhibition after 1-Hz ES. This discrepancy might represent a fine-tuned regulation of IL-6 expression that is strongly dependent on a stimulation pattern that might have a role in the muscle plasticity process. On the other hand, AP-1 appears to have a major role in IL-6 expression. Recently, it has been shown that the JNK/AP-1 pathway plays an important role in IL-6 expression in muscle cells (89). Whitham et al. (89) demonstrated that contraction of skeletal muscle cells induced by electric pulse stimulation increases the JNK phosphorylation, the activity of an AP-1 luciferase reporter, and the expression of IL-6. All of these effects were abolished upon pharmacological inhibition of JNK. The same results were obtained when they analyzed p-JNK levels and IL-6 expression in control and knockout mice for JNK1 at rest and after 30 min of exercise (89). In agreement with the role of AP-1 on IL-6 expression, we have demonstrated previously that depolarization of primary skeletal muscle cells from rats, as well as C2C12 cells, increases both IL-6 mRNA levels and activity of an IL-6 reporter. The mutation of the AP-1 response element in IL-6 reporter fully abolished reporter activity after membrane depolarization, reinforcing the major role of AP-1 in IL-6 expression. (45). Furthermore, the increase in IL-6 expression as a consequence of membrane depolarization was abolished using inositol 1,4,5-trisphosphate (IP3) pathway inhibitors, indicating a role for Ca2+ from intracellular deposits (45).
Nerve activity over skeletal muscle, in addition to promoting contraction of muscle fibers, drives a process known as excitation-transcription coupling (ETC) that induces changes in transcription of genes of several pathways (metabolic, structural, endocrine, etc.) (6, 59). We have demonstrated that the ETC process involves the participation of dihydropyridine receptor (Cav1.1) as the membrane voltage sensor coupled to ATP release from skeletal muscle cells through pannexin-1 hemichannels (6, 11, 24). We have established that extracellular ATP is a relevant mediator between membrane depolarization and signaling pathways leading to gene expression both in rat newborn-derived myotubes and in mouse adult skeletal fibers (11, 16, 42, 43). Extracellular ATP activates metabotropic P2Y receptors; β/γ-subunits of the attached heterotrimeric G protein subsequently activate phosphatidylinositol 3-kinase (PI3K) and phospholipase C (PLC), increasing the intracellular IP3 levels and cytosolic Ca2+ concentration via IP3 receptor (IP3R) activation in the sarcoplasmic reticulum membrane (11, 23, 24). This calcium signal is mainly nuclear in distribution (12) and has been related to expression of early genes (Fos/Jun, Egr1), late genes as IL-6, and structural genes as Troponin I via activation of several signal transduction cascades (ERK1/2, CREB, NF-κB, AP-1) in skeletal muscle cells (11–13, 16, 45, 62, 85). We have also reported that direct stimulation of rat myotubes with exogenous ATP induces an increment in IL-6 mRNA levels (11). Beyond the role of the depicted transcription factors, we cannot rule out the participation of other players in IL-6 expression in muscle cells.
A number of studies in different tissues have demonstrated that expression of IL-6 can be the result of the action of extracellular IL-6 itself through an autoregulatory mechanism (30, 48, 73, 87). After receptor binding, IL-6 induces the activation of Janus-activated kinase 2 (JAK2) tyrosine kinase and the signal transducer and activator of transcription 3 (STAT3) (74). This signaling cascade has been related directly to the expression of IL-6, since pharmacological inhibition of the JAK2/STAT3 pathway in the malignant fibrous histiocytomma cell line blocks both the expression and secretion of IL-6 (73). The same result was obtained when the suppressor of cytokine signaling 3 (SOCS3), a natural inhibitor of the pathway, was overexpressed (73). The expression of IL-6 was also promoted in rat osteoblasts by 100 ng/ml of exogenous IL-6; however, this induction is dependent on the presence of a soluble IL-6 receptor. Moreover, intact response elements on IL-6 promoter for NF-κB, NF-IL-6, and CREB transcription factors are necessary for IL-6 expression, indicating that these proteins play a major role on the expression of the cytokine (30). In muscle tissue, positive feedback for IL-6 has been demonstrated in vivo as well as in vitro (48, 87). In healthy people, an IL-6 infusion right into the femoral artery was translated into a large increase of IL-6 mRNA levels in vastus lateralis muscle (>120-fold compared with control) (48). Another report showed that stimulation of C2C12 cells with IL-6 also provoked an increase in IL-6 that was partially attributed to p38 MAPK and rises in intracellular Ca2+ concentration (87). Notwithstanding that STAT3 activation on muscle cells has been widely described in response to exercise (82, 83) and also after IL-6 stimulation (3, 14, 71), a role for this pathway on autoregulation of IL-6 in response to skeletal muscle activity has not been assigned.
The aim of this work was to confirm that IL-6 expression responds to the events described for the mechanism of ETC in skeletal muscle. Additionally, we examined whether the canonical signaling cascade downstream of IL-6 (IL-6/JAK2/STAT3) also responds to ETC and whether it participates in the autocrine regulation of the cytokine expression. We demonstrated that depolarization of skeletal muscle cells induces not only expression of IL-6 mediated by ATP signaling but also secretion of the cytokine to extracellular medium. Furthermore, expression of IL-6 depends on an IP3-derived Ca2+ signal, acting as an early step to promote a positive IL-6 loop via the JAK2/STAT3 pathway. These results expand the understanding of the ETC mechanisms in skeletal muscle and the regulation of IL-6 expression due to muscle activity.
EXPERIMENTAL PROCEDURES
Reagents
ATP, ADP, UTP, UDP, apyrase grade VII from potato, suramin, cytosine arabinoside, penicillin, streptomycin, amphotericin B, LY-290042, U-73122, cycloheximide, actinomycin D, and mouse anti-β-actin antibody were obtained from Sigma-Aldrich (St. Louis, MO). Dulbecco's modified Eagle's medium-F-12, bovine serum, and fetal calf serum were from Invitrogen (Carlsbad, CA). Collagenase type II was from Worthington Biochemical (Lakewood, NJ). Recombinant rat IL-6 was from PeproTech (Rocky Hill, NJ). Complete Mini Protease Inhibitors were from Roche Applied Science (Indianapolis, IN). Antibodies against p-Tyr705/STAT3 and SOCS3 were from Cell Signaling Technology (Beverly, MA). Anti-rat IL-6-neutralizing antibody was from R & D Systems (Minneapolis, MN). Secondary horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies were from Pierce Biotechnology (Rockford, IL). Enhanced chemiluminescence (ECL) reagents were from Amersham Biosciences (Piscataway, NJ). Pharmacological inhibitors 2-aminoethoxydiphenyl borate (2-APB) and 1,2,3,4,5,6-hexabromocyclehexane (HBC) were from Tocris Bioscience (Bristol, UK). STAT3 inhibitor VIII (5,15-diohenylporphirin) was from Santa Cruz Biotechnology (Dallas, TX). Xestospongin B was kindly donated by Dr. Jordi Molgó (Laboratoire de Neurobiologie Cellulaire et Moléculaire, Institut Fédératif de Neurobiologie Alfred Fessard, CNRS, France). The cell permeant chelator BAPTA-AM was from Molecular Probes (Eugene, OR). Plasmid coding for parvalbumin protein with cytosolic localization (PV-NES-DsRed) was kindly provided by Dr. Manuel Estrada (25). p-DsRed-Monomer control plasmid was purchased from Clontech Laboratories, (Mountain View, CA).
Newborn-Derived Rat Myotube Culture
Animal care, manipulation, and procedures were in agreement with protocols approved by the Bioethical Committee of the Facultad de Medicina, Universidad de Chile. Neonatal derived rat myotubes were cultured as described previously (40). Briefly, muscle tissue from the hindlimbs of 12- to 24-h postnatal Sprague-Dawley rat pups was dispersed mechanically and then treated with 0.2% (wt/vol) collagenase for 15 min with mild agitation. The suspension was filtered through a Nytex membrane or lens tissue paper and spun down at low speed. Ten to fifteen minutes of preplating was performed for the enrichment of myoblasts; cells were plated at densities of 3.5 × 105 cells/dish (35 mm). The plating medium was Dulbecco's modified Eagle's medium-Ham's F-12, 10% bovine serum, 25% fetal calf serum, 100 mg/l penicillin, and 50-mg/l streptomycin. After 36 h in culture, fetal calf serum concentration was reduced to 1.8% to induce differentiation. Myotubes in the dish, some spontaneously contracting, with an estimated purity of 90% were visible after the 5th day of culture; these were used for experiments after 5–7 days in culture. When required, transfections were performed on myoblasts on the 4th day. Two micrograms of plasmids was used to transfect cells with Lipofectamine 2000 (Invitrogen) according to the supplier's instructions.
Whole Muscle Dissection and Skeletal Fiber Isolation
Flexor digitorum brevis (FDB) muscles were dissected from 5- to 7-wk-old BalbC mice. Either whole muscle treatment with exogenous ATP or skeletal fiber isolation was carried out. Isolated muscle fibers were obtained by enzymatic digestion with collagenase type II, as described by Casas et al. (16). Isolated fibers were seeded in matrigel-coated dishes and used 20 h after seeding.
Cell Treatments
Depolarization assays were performed as reported previously (24). In brief, cells were washed and incubated in Krebs buffer (145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 10 mM HEPES, pH 7.4) for 30 min. Then, electrical field stimulation (ES) of the whole dish was carried out with a handmade stimulation device connected to a GRASS S48 stimulator. The tetanus protocol used was 45 Hz, 400 pulses, 1 ms each (24). Alternatively, 0.1–500 μM of exogenous nucleotides (ATP, ADP, UTP, or UDP) was added to culture media by the indicated times. The blockers and inhibitors were preincubated by different time periods and maintained during the stimulus (ATP, ES, or recombinant IL6), as follows: apyrase, 5 min; suramin, 30 min; cycloheximide or actinomycin D, 2 h; HBC or inhibitor VIII, overnight.
Western Blot Analysis
Stimulated cells were lysed in 60 μl of ice-cold lysis buffer (20 mM Tris·HCl, pH 7.4, 1% Triton X-100, 2 mM EDTA, 10 mM Na3VO4, 20 mM NaF, 10 mM sodium pyrophosphate, 150 mM NaCl, 1 mM PMSF, and a protease inhibitor mixture). Cell lysates were separated in 10% SDS-polyacrylamide gels and transferred to polyvinylidenedifluoride membranes (Millipore). Membranes were blocked at room temperature for 1 h in Tris-buffered saline containing 3% fat-free milk, with or without 0.5% Tween-20, and then incubated overnight with the appropriate primary antibody. Membranes were incubated with the secondary antibody at room temperature for 1.5 h. The immunoreactive proteins were detected using ECL reagents according to the manufacturer's instructions. For loading control, membranes were stripped in buffer containing 0.2 M glycine (pH 2) and 0.05% Tween-20 at room temperature for 30 min, blocked as described above, and assessed with the corresponding control antibody.
mRNA Determinations
Total RNA from either skeletal myotubes, isolated fibers, or whole muscles was extracted with TRIzol reagent (19). The reverse transcription (RT) reaction was performed with 1 μg of total RNA using an oligo(dT) primer. Conventional PCR (semiquantitative) was carried out using forward and reverse primers specific for IL-6: IL-6 forward primer, 5′-CCAATTTCCAATGCTCTCCT-3′; IL-6 reverse primer, 5′-ACCACAGTGAGGAATGTCCA-3′. GAPDH mRNA amplification was used as the internal control: GAPDH forward primer, 5′-CAACTTTGGCATCGTGGAAG-3′; GAPDH reverse primer, 5′-CTGCTTCACCACCTTCTT-3′. After an initial 10-min denaturing at 94°C, amplifications were carried out for 25–30 cycles as follows: denaturing at 94°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 30 s. After completion of the cycles, a final 10-min extension at 72°C was carried out. PCR products were analyzed by electrophoresis in 1.5% agarose gels. Amplifications without the RT step were made to exclude possible contamination with genomic DNA. Quantitative PCR (qPCR) was performed in Mx3000P Thermocycler (Stratagene, La Jolla, CA) using the Brilliant SYBR Green QPCR Core Reagent Kit, also from Stratagene. The primers used to amplify IL-6 or GAPDH mRNA were the same used in conventional RT-PCR experiments. PCR amplification of the housekeeping gene GAPDH or β-actin was performed as a control. The β-actin forward primer was 5′-TCTACAATGAGCTGCGTGTG-3′, and the β-actin reverse primer was 5′-TACATGGCTGGGGTGTTGAA-3′. Expression values were calculated using the 2−ΔΔCT method (55).
ELISA
The concentration of IL-6 released to the culture media at different times was assessed by rat IL-6 Enzyme-Linked Immuno Sorbent Assay Quantikine Rat IL-6 (R & D Systems) according to the manufacturer's instructions. The absorbance was read at 450 nm (corrected at 540 nm) in a Synergy 2 Multi-Mode Microplate Reader (Biotek). Results were expressed as total picograms of IL-6 at the supernatant per total milligrams of protein in the cell extract (pg IL-6/mg protein).
Statistical Analysis
Data of n experiments were expressed as means ± SE. The significance of difference among treatments was evaluated using a t-test for unpaired data or analysis of variance followed by Dunnett's posttest for multiple comparisons or by one-way ANOVA test followed by Bonferroni's posttest. A P value of <0.05 was considered statistically significant.
RESULTS
IL-6 Expression Evoked By ES in Rat Myotubes: Dependence on Extracellular ATP and Nucleotide Receptor Activation
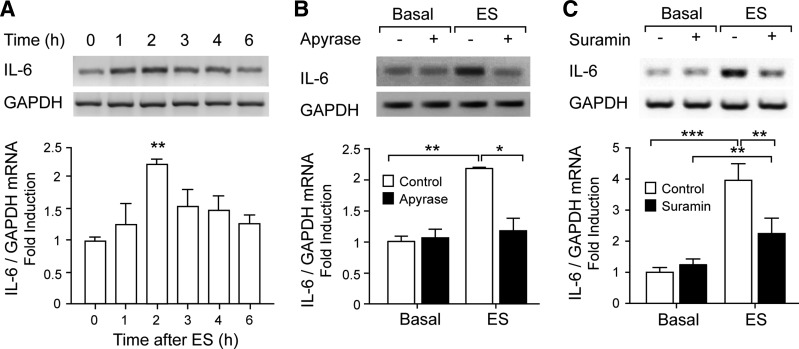
ES of skeletal myotubes evoked a significant and transient increase in IL-6 mRNA levels detected by conventional RT-PCR. Two hours after the stimulus, IL-6 mRNA doubled its basal level (Fig. 1A). Previously, we demonstrated that ATP is endogenously released during myotube or adult fiber ES, acting as a relevant mediator between membrane depolarization and cell signaling leading to gene expression (11, 42). In light of those results, we looked for a possible role of extracellular ATP-mediating IL-6 mRNA expression evoked by ES. Both extracellular ATP/ADP metabolization using apyrase (2 U/ml) and P2Y/P2X nucleotide receptor blockade using the general blocker suramin (100 μM) strongly reduced the rise in IL-6 mRNA expression evoked by ES (Fig. 1, B and C). These results suggest that extracellular ATP, activating P2Y/P2X receptors, is a mediator between membrane depolarization and IL-6 expression changes in skeletal cells. Interexperiment variability in ES-evoked IL-6 mRNA increase was observed, as can be seen comparing Fig. 1, A and B, with Fig. 1C. For that reason, we performed proper controls for each experimental set and compared treatments with their own controls.
Fig. 1.
Electrical stimulation (ES) induced ATP-dependent IL-6 expression in rat myotubes. Rat myotubes were electrically stimulated (45 Hz, 400 pulses, 1 ms each). Total RNA was isolated at the indicated times. IL-6 mRNA expression was assessed by conventional semiquantitative RT-PCR. A: IL-6 mRNA levels increase with ES. B: extracellular nucleotide metabolization abolished IL-6 expression evoked by ES. IL-6 expression increased 2 h after ES, and this increase was blocked after ATP metabolization using 2 U/ml apyrase for 30 min prior to and during the protocol. C: nucleotide receptor blockade strongly reduced IL-6 expression evoked by ES. The general P2Y/P2X antagonist suramin (100 μM), incubated for 30 min prior to and during the protocol, significantly reduced IL-6 expression increased 2 h after ES. Top: representative agarose gels for RT-PCR products from IL-6 mRNA amplifications with their corresponding GAPDH control. Bottom: correspondence to intensity quantization of each IL-6 band normalized to GAPDH expression, presented as fold increase of untreated control cells (means ± SE; n = 3–6). *P < 0.05, **P < 0.01, and ***P < 0.001, analysis of variance followed by Dunnett's multiple comparison test.
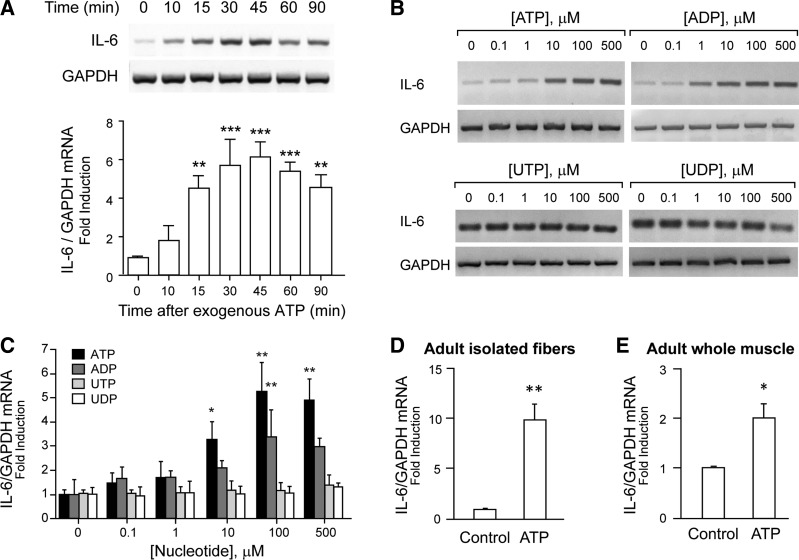
Subsequently, we studied the effect of exogenous ATP addition on IL-6 expression detected by conventional RT-PCR. ATP rose IL-6 mRNA significantly in a time- and concentration-dependent manner (Fig. 2, A–C). One-hundred micromolars of ATP increased IL-6 mRNA in just 15 min, reaching a rise of up to sixfold after 45 min of exposure (Fig. 2A). The rise was maintained ≤90-min incubation with ATP. In the concentration-response curve, 10–500 μM ATP incubated for 30 min significantly increased IL-6 mRNA levels (Fig. 2, B and C). Considering that ATP has the ability to activate all the P2Y/P2X receptor subtypes, we used a pharmacological strategy, tending to discriminate between receptor subtypes involved in IL-6 expression in rat myotubes. ADP, an agonist that activates only some P2Y receptor subtypes (P2Y1, P2Y12, P2Y13), evoked a concentration-dependent rise in IL-6 mRNA, reaching ≤3.5-fold increase at 100 μM (Fig. 2, B and C). In a previous study, we demonstrated that P2Y1 but not P2Y12 or P2Y13 mRNA was detected in our model of newborn rat myotubes (11), suggesting a role for P2Y1 in the ADP-evoked signaling. Otherwise, the pyrimidine nucleotides UTP and UDP, which activate P2Y2, P2Y4, and P2Y6 receptors, failed to increase IL-6 mRNA in a concentration range of 0.1–500 μM (Fig. 2, B and C). To validate the IL-6 expression evoked by ATP in adult muscle, we stimulated mouse adult fiber isolated from FDB muscles with 100 μM ATP for 4 h and assessed IL-6 mRNA by qPCR. A 10-fold increase in IL-6 mRNA level was observed with this treatment (Fig. 2D). Moreover, a twofold increase in IL-6 mRNA was detected when whole FDB muscles were incubated with 500 μM ATP for 4 h (Fig. 2E). These results reinforce the role of extracellular ATP as a trigger for IL-6-expression in adult muscle.
Fig. 2.
Extracellular ATP induced IL-6 expression in newborn-derived myotubes, adult muscle fibers, and whole adult skeletal muscle. Rat myotubes were stimulated with exogenous nucleotides, as indicated. Total RNA was isolated and IL-6 mRNA expression assessed by conventional semiquantitative RT-PCR (A–C) or by real-time quantitative PCR (D and E). A: exogenous ATP (100 μM) induced IL-6 expression in a time-dependent manner. Myotubes were incubated with 100 μM ATP for different times before total RNA extraction. B: ATP and ADP, but not pyrimidine-derived nucleotides, evoked IL-6 expression in a concentration-dependent manner. Myotubes were incubated for 30 min with increasing concentrations (0.1–500 μM) of ATP, ADP, UTP, or UDP. Representative agarose gels for RT-PCR products from IL-6 mRNA amplifications with their corresponding GAPDH control are shown. C: intensity quantization of IL-6 bands obtained in B, normalized to GAPDH expression and presented as fold increase of untreated control cells (n = 3–4). D: incubation of isolated mice flexor digitorum brevis (FDB) fibers with 100 μM ATP for 4 h evoked a 10-fold increase in IL-6 expression. E: incubation of mouse whole FDB muscles with 500 μM ATP for 4 h evoked a 2-fold increase in IL-6 expression. In D and E, data were normalized to GAPDH mRNA levels and expressed as fold increase related to nonstimulated conditions (control) (n = 6). Values are expressed as means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001, analysis of variance followed by Dunnett's multiple comparison test.
ES or Exogenous ATP Activates the Canonical IL-6 Signaling Pathway Through IP3-Dependent Calcium Transients and an Autocrine IL-6-Positive Loop
ATP evokes IL-6 secretion that can activate a positive IL-6 loop through the canonical IL-6 receptor pathway.
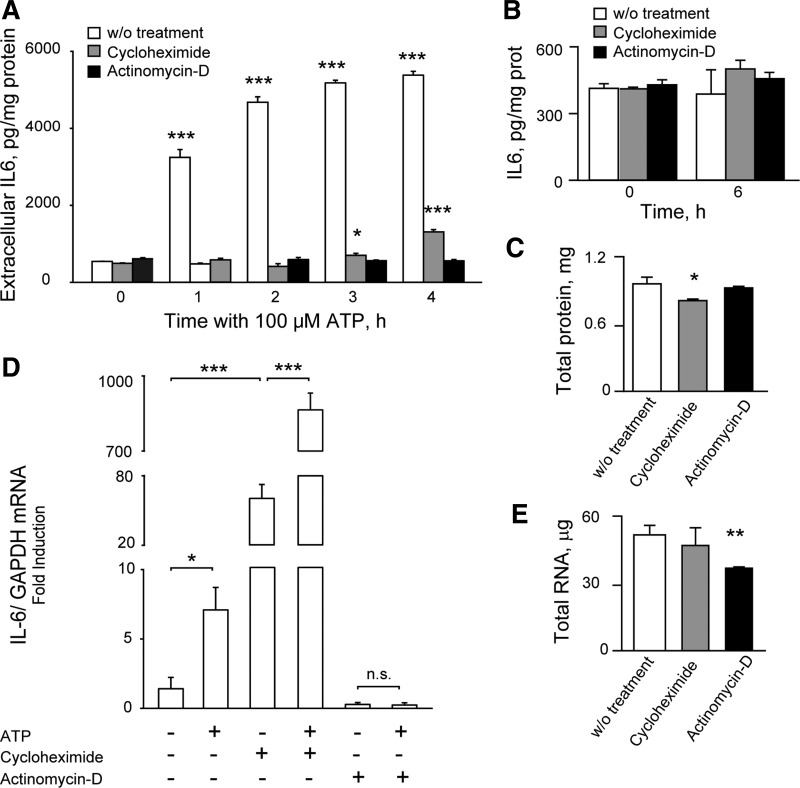
The canonical IL-6 signaling pathway considers sequential activation of plasma membrane IL-6 receptors, JAK2 kinase, and the transcription factor STAT3 (69, 74). An autocrine-positive loop of IL-6 has been widely described in several cell types, where released IL-6 controls their own expression by activation of its canonical pathway (30, 48, 73, 87). In our model, we demonstrated that 100 μM ATP evoked a strong increase in extracellular IL-6 in 1 h, reaching a 10-fold peak after 3 h of incubation (Fig. 3A). To assess whether released IL-6 involves de novo synthesis or a preformed pool, we measured ATP-evoked IL-6 release after 2 h of preincubation with 30 μM cycloheximide (translation inhibitor) or 0.5 μM actinomycin D (transcription inhibitor). Actinomycin D abolished ATP-evoked IL-6 release (from 1 to 4 h); cycloheximide abolished ATP-induced IL-6 at 1 and 2 h, maintaining just a 15–25% release at longer times (Fig. 3A). It can be seen that neither cycloheximide nor actinomycin D altered the extracellular IL-6 content at rest (Fig. 3B) at the maximal incubation time (6 h, corresponding to 2-h preincubation and 4-h treatment). Although 6-h actinomycin D did not modify total protein content of skeletal myotubes, 6-h incubation with cycloheximide reduced it by 15% (Fig. 3C). Four-hour ATP evoked a fivefold increase in IL-6 mRNA, as measured by qPCR, that was totally abolished by actinomicyn D (Fig. 4D). Actinomycin D also reduced by 80% the IL-6 mRNA content at rest (Fig. 4D). Surprisingly, 6-h cycloheximide increased ≤40-fold IL-6 mRNA levels at rest and rose 120-fold ATP-evoked IL-6 mRNA expression (Fig. 4D), suggesting that translation blockade triggers a compensatory response by increasing IL-6 expression levels. Total RNA content of rat myotubes was unaffected by 6-h cycloheximide but reduced by 30% after 6 h with actinomycin D (Fig. 4E).
Fig. 3.
Extracellular ATP evoked IL-6 de novo synthesis and secretion. A: ATP increased IL-6 extracellular levels ≤10-fold, depending on transcription and translation processes. Rat myotubes were stimulated with 100 μM ATP after 2-h preincubation, with 30 μM cycloheximide (translation blocker), with 0.5 μM actinomycin D (transcription blocker), or without (w/o) treatment. At different times, 50 μl of supernatant was removed, and IL-6 was quantified by ELISA. B: resting levels of extracellular IL-6 were not affected by 6-h treatment with either cyclohemimide or actinomycin D. C: total protein levels from myotube lysates were significantly reduced by 6-h treatment with cycloheximide but not actinomycin D. D: IL-6 mRNA increase evoked by 100 μM ATP (4 h) was abolished by actinomycin D treatment but largely increased after cycloheximide. Cycloheximide treatment increased IL-6 mRNA levels at rest by 40-fold, suggesting that translation blockade activates a positive loop of IL-6 transcription. IL-6 mRNA was measured by quantitative PCR, normalized to GAPDH, and expressed as fold increase related to nonstimulated condition. E: total mRNA levels form myotube extracts were significantly reduced by 6-h actinomycin D treatment but unaffected by 6-h cycloheximide. Values (n = 3) are expressed as means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001, analysis of variance followed by Dunnett's multiple comparison test against each “without treatment” value (A–C and E) or Bonferroni's test (D). NS, not significant.
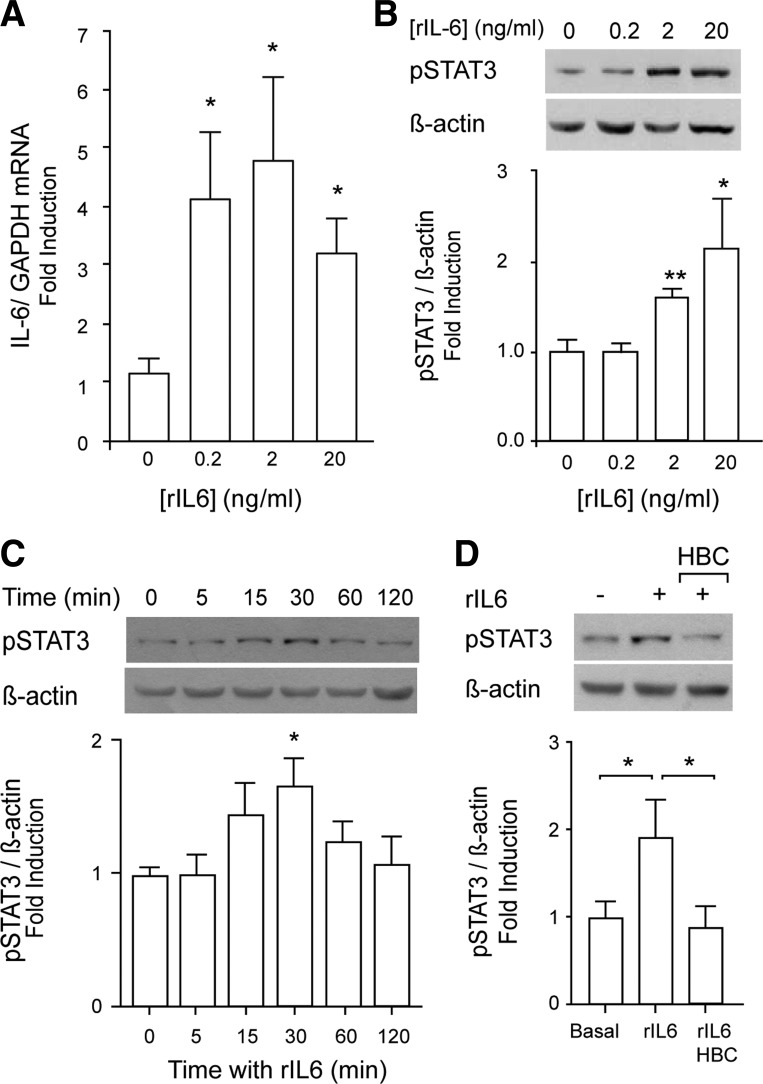
Fig. 4.
Recombinant IL-6 (rIL-6) increased IL-6 expression and evoked STAT3 phosphorylation through JAK2 activity. A positive IL-6 loop is suggested. A: incubation of isolated mouse FDB fibers with 0.2–20 ng/ml rIL-6 for 2 h increased IL-6 mRNA expression. Total RNA extraction, reverse transcription, and quantitative PCR were used for IL-6 mRNA detection. Data were normalized to GAPDH mRNA level and expressed as fold increase related to unstimulated conditions (means ± SE; n = 5–6). B: rIL-6 induced a concentration-dependent STAT3 phosphorylation. Rat myotubes were stimulated with 0.2–20 ng/ml rIL-6 for 0-120 min, and the effects over p-STAT3 levels were assessed by Western blot (n = 4–6). C: rIL-6 induced a time-dependent STAT3 phosphorylation. Rat myotubes were stimulated with 20 ng/ml rIL-6 for 0–120 min, and the effects over p-STAT3 levels were assessed by Western blot (n = 6). D: JAK2 mediates STAT3 phosphorylation evoked by rIL-6. Rat myotubes were incubated overnight with 50 μM of the JAK2 inhibitor 1,2,3,4,5,6-hexabromocyclehexane (HBC) or the respective vehicle control. After that, myotubes were stimulated with 20 ng/ml rIL-6 in the presence of HBC for 30 min (n = 3). The values are expressed as means ± SE. *P < 0.05 and **P < 0.01, analysis of variance followed by Dunnett's multiple comparison test.
Considering the IL-6 release, we explored whether a positive IL-6-loop could be controlling IL-6 expression in skeletal muscle cells. In adult mouse isolated skeletal fibers, 0.2–2 ng/ml recombinant IL-6 (rIL-6) increased IL-6 mRNA levels significantly ≤4.5-fold, as measured by qPCR assays (Fig. 4A). To study the canonical pathway involved in IL-6-evoked IL-6 expression in our model, we first assessed the activation of the transcription factor STAT3 by detecting its phosphorylated form (p-Tyr705). Addition of rIL-6 to rat myotubes evoked a transient and concentration-dependent STAT3 phosphorylation, reaching a 70% increase after 30 min with 20 ng/ml rIL-6 (Fig. 4, B and C). STAT3 activation evoked by rIL-6 was abolished when myotubes were preincubated with the JAK2 inhibitor HBC (50 μM, overnight), demonstrating a role of this kinase in the signaling pathway activated by this stimulus (Fig. 4D). Control of HBC alone did not change STAT3 phosphoryation levels (not shown).
These results suggest that ATP stimulation evokes de novo synthesis and release of IL6, which could control its own expression, by activating the canonical IL-6 receptor signaling pathway.
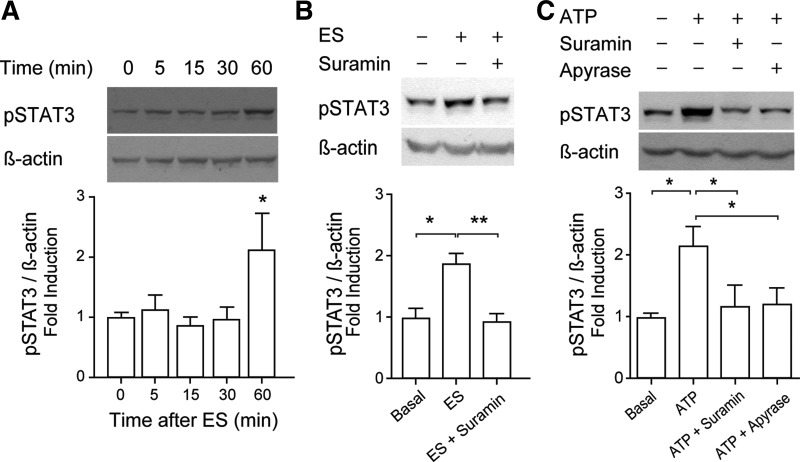
ES evokes canonical IL-6 pathway activation mediated by extracellular ATP.
ES doubled the STAT3 phosphorylation level after 60 min (Fig. 5A). One-hundred micromolars of suramin abolished STAT3 activation evoked by ES (Fig. 5B), reinforcing the role of extracellular ATP as a mediator between ES and cell signaling related to IL-6 expression. Exogenous ATP (100 μM, 60 min) also doubled the STAT3 phosphorylation level (Fig. 5C). Either 100 μM suramin or 2 U/ml apyrase (ATP metabolizing enzyme) abolished STAT3 activation evoked by 100 μM ATP, confirming that extracellular ATP/ADP is required and that nucleotide receptors are involved in the signaling (Fig. 5C). Neither suramin nor apyrase modified STAT3 basal phosphorylation level (not shown). It is important to note that none of the treatments or blockers assessed along this work altered total STAT3 levels (not shown); p-STAT3 was normalized against β-actin due to interference between p-STAT3 and total STAT3 antibodies.
Fig. 5.
Phosphorylation of STAT3 evoked by ES depends on extracellular ATP signaling. To assess the effect of ATP or ES over STAT3 phosphorylation, total protein extracts were obtained from stimulated myotubes at the indicated times, and Western blot was performed. A–C, top: representative Western blots of p-Tyr705-STAT3 and β-actin used as loading control. A–C, bottom: bar graphs showing normalized levels of phosphorylated STAT3 compared with untreated control. A: STAT3 phosphorylation was induced by ES (n = 7). B: STAT3 phosphorylation evoked by ES was dependent on nucleotide receptor activation. Rat myotubes were incubated or not with 100 μM suramin for 30 min before ES; 1 h later, protein extracts were obtained to perform Western blot analyses (n = 3). C: exogenous ATP induced STAT3 phosphorylation. Rat myotubes were incubated for 1 h with 100 μM ATP in the presence or not of 100 μM suramin or 2 U/ml apyrase (n = 3). Values are expressed as means ± SE. *P < 0.05 and **P < 0.01, analysis of variance followed by Dunnett's multiple comparison test.
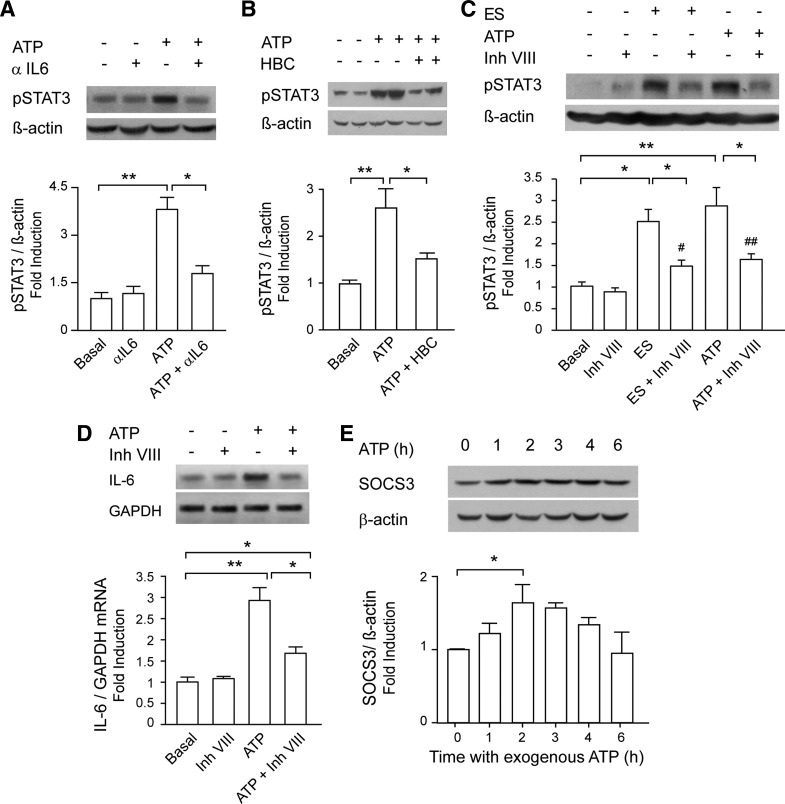
STAT3 activation evoked by 100 μM ATP was totally abolished when myotubes were preincubated with a neutralizing antibody against IL-6 (Fig. 6A) or with the JAK2 inhibitor HBC (Fig. 6B). STAT3 inhibitor VIII, which selectively prevents STAT3 dimerization and ligand binding without modifying its phosphorylation (84), reduced STAT3 phosphorylation evoked by ES and ATP by 70 and 65%, respectively (Fig. 6C). Interestingly, inhibitor VIII reduced by 60% the induction of IL-6 mRNA levels evoked by 100 μM ATP (Fig. 6D).
Fig. 6.
Extracellular ATP activated STAT3 and increased IL-6 expression partially through a positive IL-6 loop. A: blockade of released IL-6 abolishes STAT3 activation evoked by ATP. Myotubes were incubated with 100 μM ATP for 1 h in the absence or presence of an anti-rat IL-6-neutralizing antibody (aIL6, 1 μg/ml, 30 min before and during the ATP stimuli; n = 4). B: ATP induced STAT3 phosphorylation via JAK2. Rat myotubes were incubated overnight with 50 μM HBC or vehicle. Stimulation with 100 μM ATP for 1 h was performed in the presence of HBC. After that, total protein extracts were obtained (n = 3). C: STAT3 inhibitor VIII reduced STAT3 phosphorylation evoked by ES or ATP. Rat myotubes were incubated overnight with 50 μM inhibitor VIII or vehicle; 1 h after ES or 100 μM ATP addition, total protein extracts were obtained, and phosphorylated STAT3 was detected by WB (n = 4). D: STAT3 inhibitor VIII reduced IL-6 expression evoked by ATP. Myotubes were stimulated as in C but processed for total RNA extraction. IL-6 mRNA expression was assessed by conventional semiquantitative RT-PCR, normalized to GAPDH expression, and presented as fold increase of untreated control cells (n = 3). E: ATP evoked expression of suppressor of cytokine signaling 3 (SOCS3), a negative regulator of the JAK/STAT3 pathway. Myotubes were incubated with 100 μM ATP for different times; total protein extracts were obtained, and SOCS3 expression was assessed by WB (n = 4). Values are expressed as means ± SE. *P < 0.05 and **P < 0.01 (as indicated); #P < 0.05 and ##P < 0.01 (compared against condition with only inhibitor VIII), analysis of variance followed by Dunnett's multiple comparison test.
It has been described that the final step of the IL-6 signaling pathway is the STAT3-dependent increase in SOCS3 expression, which acts as a negative regulator of the IL-6 pathway and stops the signaling, avoiding further JAK2 activation (38, 74). We assessed the protein levels of SOCS3 in skeletal muscle cells after stimulation with exogenous ATP at different times. Rat myotubes incubated with 100 μM ATP increased SOCS3 levels in a time-dependent manner, with a peak of 60% increase at 2 h and a complete return to basal levels after 6 h (Fig. 6E).
All of these results suggest that ES, through extracellular ATP, increases IL-6 expression at least in part by activating a positive IL-6 loop.
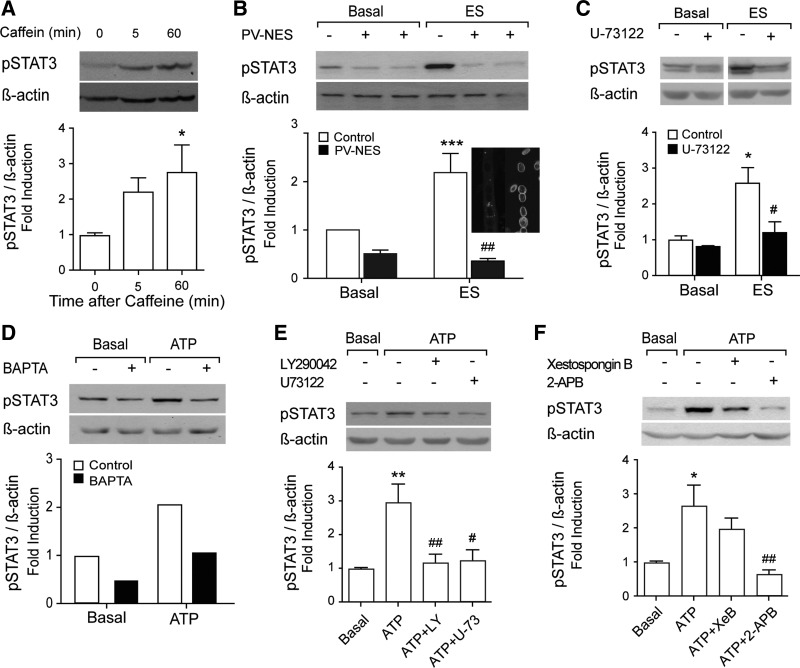
IP3-dependent calcium transients are relevant for STAT3 activation promoted by ATP or ES.
Previously, we have demonstrated the relevance of the slow IP3-dependent calcium signal in gene expression evoked by ES in rat myotubes and adult skeletal fibers (16, 43, 45). In the current work, we studied the dependence of STAT3 activation on these calcium signals using molecular and pharmacological approaches. The rise in free intracellular Ca2+ evoked by 40 mM caffeine activated STAT3 at levels similar to those observed previously with tetanic stimulation or ATP (Fig. 7A). To assess whether free intracellular Ca2+ signals were required for STAT3 phosphorylation, myotubes were transfected with a plasmid coding for parvalbumin protein with cytosolic localization (PV-NES-DsRed; Fig. 7B). This tool, used as Ca2+ chelator, strongly reduced basal STAT3 phosphorylation levels as well as ES-evoked STAT3 activation (Fig. 7B). We also tested the blockade of other critical mediators of the slow calcium signal such as PLC, PI3K, and IP3R (11, 16, 23, 24, 39). Ten micromolars of U-73122, a PLC inhibitor, abolished STAT3 activation evoked by ES (Fig. 7C). On the other hand, STAT3 activation evoked directly by exogenous ATP was abolished when free intracellular Ca2+ was chelated with 50 μM BAPTA-AM (Fig. 7D). Either 10 μM U-73122 or 40 μM LY-290042 (PI3K inhibitor) totally blocked ATP-evoked STAT3 phosphorylation (Fig. 7E). STAT3 activation induced by extracellular ATP was also abolished when myotubes were preincubated with 50 μM 2-APB (Fig. 7F), which has been used in previous studies as a blocker of the IP3-dependent slow calcium signal and gene expression in skeletal myotubes (15, 43, 45, 62). The specific blocker of IP3R xestospongin B (5 μM) reduced by 40% the STAT3 phosphorylation evoked by ATP (Fig. 7F). All of these data suggest that the IP3-dependent calcium signal is a critical step in the pathway for STAT3 activation promoted by either ATP or ES.
Fig. 7.
Inositol trisphosphate (IP3)-related calcium signaling mediates STAT3 phosphorylation by ES and ATP. To determine the participation of intracellular Ca2+ signaling on phosphorylation of STAT3, myotubes were incubated with 40 mM caffeine (A) and 100 μM ATP (D–F), or electrically stimulated, as described in experimental procedures (B and C) and different inhibitors or blockers of the IP3-dependent Ca2+ pathway were used. A–F, top: representative Western blot of p-STAT3 and β-actin used as loading control. A–F, bottom: bar graph showing normalized levels of phophorylated STAT3 compared with basal. A: myotubes were preincubated in Krebs buffer for 30 min. The cells were stimulated with 40 mM caffeine by 9 s in the same buffer. After the times indicated, total protein extracts were obtained and Western blot performed (n = 3–4). B: myotubes expressing PV-NES-DsRed plasmid or a control DsRed plasmid were electrically stimulated, and total protein extracts were obtained after 60 min to perform Western blot (n = 3). Cell micrograph shows staining of PV-NES-DsRed (left) or LAP2 immunofluorescence (right) in transfected myotubes. C: STAT3 activation by electrical stimulation is dependent on IP3-signaling pathway. Myotubes were preincubated with 10 μM U-73122 for 20 min, and ES was performed. After 60 min, total protein extracts were obtained to perform Western blot (n = 3). D: myotubes were preincubated in Krebs buffer containing 50 μM BAPTA-AM. The cells were washed twice in the same buffer, and 100 μM ATP was added. After 1 h, the proteins were obtained and Western blot performed. The values are the mean of 2 different experiments. E: to further investigate the participation of IP3 pathway on STAT3 activation by ATP, phosphatidylinositol 3-kinase and phospholipase C inhibitors were used. Cells were preincubated with 40 μM LY-290042 for 30 min or with 10 μM U-73122 for 20 min. Then, the cells were stimulated with 100 μM ATP. The analysis of STAT3 phosphorylation was carried out by Western blot (n = 3–4). F: to see whether STAT3 activation by ATP was also dependent on IP3-Ca2+ pool, cultured myotubes were preincubated or not with the IP3 receptor inhibitor xestospongin B (5 μM, 30 min) or with the IP3 pathway inhibitor 2-aminoethoxydiphenyl borate (2-APB; 50 μM, 30 min). After that, 100 μM ATP was added, and total protein extracts were obtained after 60 min (n = 4). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. basal nonstimulated cells; #P < 0.05 and ##P < 0.01 vs. ATP stimulated without pharmacological inhibitors. Analysis of variance followed by Dunnett's multiple comparison test (A, E, and F) or Bonferroni's test (B and C).
DISCUSSION
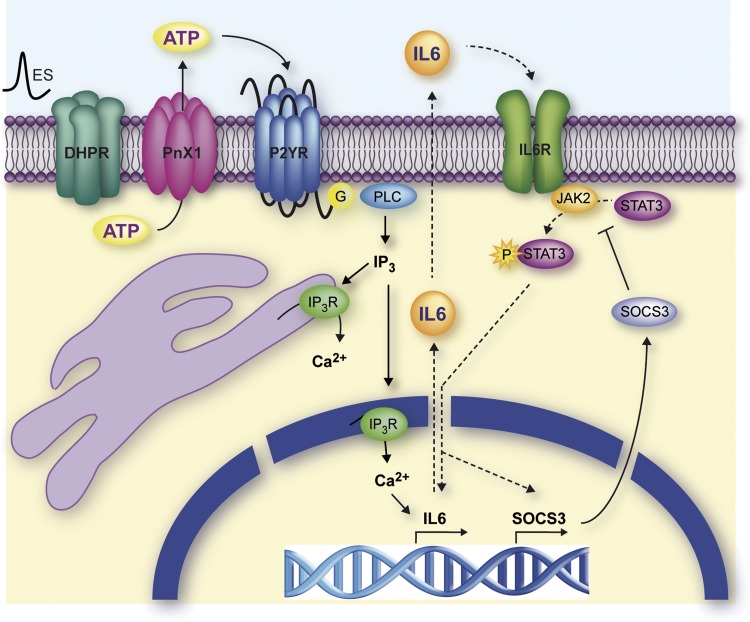
In this work, as schematically depicted in Fig. 8, we have demonstrated that electrical stimulation of cultured myotubes induces both expression and secretion of IL-6 mediated by extracellular ATP and the consequent IP3-dependent intracellular Ca2+ signal. IL-6 released into the culture medium would induce the activation of the IL-6 receptor-α (IL-6Rα) through an autocrine mechanism, leading to the activation of transcription factor STAT3. In turn, STAT3 would modulate IL-6 signaling at the transcriptional level either by increasing the synthesis of the cytokine or by promoting the expression of the negative regulator of IL-6 SOCS3.
Fig. 8.
Proposed model for IL-6 expression in skeletal muscle cells. Membrane depolarization of skeletal muscle induces ATP secretion through pannexin 1 (Pnx1) hemichannels. After binding to P2Y purinergic receptors (P2YR), ATP provokes an increase in intracellular free Ca2+ concentration by activating Gβγ-PI3K-PLC signaling cascade. IL-6 expression increases after free Ca2+ rise mediated by AP-1 and NF-κB and possibly through CREB activity (not shown). The newly formed IL-6 is stored in vesicles and released to extracellular media. Now, IL-6 binds to its own receptor (IL6R) on skeletal muscle cells membrane, activating the canonical IL-6 signaling cascade, i.e., JAK2 and STAT3 phosphorylation. p-STAT3 would have a partial role on IL-6 expression, whereas it would directly influence SOCS3 expression. IP3R, IP3 receptor; G, G trimeric protein. DHPR, dihydropyridine receptor.
Muscle contraction depends on the depolarization of the plasma membrane (T-tubules), where Cav1.1 acts as a voltage sensor (63). Membrane depolarization is the primary event for excitation-transcription coupling (9, 22) that triggers Cav1.1 activation and the IP3-dependent Ca2+ pathway (6, 24). Previous results from our laboratory have placed the activation of gene expression, particularly of IL-6, as an event downstream of the myotube membrane depolarization (15, 45, 46). Depolarization of cultured myotubes using a high K+ solution induces the expression of IL-6 (45). By using a more physiological stimulus, we now show expression of IL-6 induced by tetanic electrical stimulation. The kinetics of activation of IL-6 mRNA expression after this stimulus is similar to that obtained with K+ (45). The expression of IL-6 in response to membrane electrical activity has also been studied in other cell types (47, 67, 79). Depolarization of neurons in culture using an extracellular solution of 45 mM K+ evokes an increment in IL-6 expression. The same effect was observed in neuronal-type PC12 cells (67). Furthermore, it has been observed that ischemia-reperfusion injury of rat brain induces expression of IL-6 via activation of voltage-dependent Ca2+ channels (79). The expression of IL-6 in neurons and glia would have a neuroprotective function against damage caused by hypoxia or inflammation (31, 79). All these data suggest that the expression of IL-6 appears to be a general physiological phenomenon in excitable cells, responding to specific demands of each tissue such as the energy stress in skeletal muscle (17, 77) or the need for neuronal protection against ischemia-reperfusion injury, epilepsy, or other brain diseases (47).
Our laboratory has described that electrical stimulation of skeletal muscle cells evokes the release of ATP, subsequent activation of metabotropic purinergic receptors, and the generation of IP3-dependent Ca2+ transients (6, 11, 23, 40). In this work, we have determined that this pathway is required for IL-6 expression. Blockade of the purinergic receptors with the general antagonist suramin, as well as extracellular nucleotide metabolization using apyrase, abolished the increase in IL-6 mRNA induced by depolarization. These results convincingly suggest that the cellular signaling that leads to the expression of IL-6 in electrically stimulated myotubes is mediated by extracellular ATP, indicating that membrane depolarization, ATP signaling, and the expression of IL-6 are part of the same signaling pathway. Numerous reports have related the expression and secretion of IL-6 with extracellular nucleotides (10, 31, 60). Previously, we have reported the expression of IL-6 in skeletal myotubes after 30-min incubation with 500 μM ATP (11). Our results indicate that ATP-induced IL-6 expression is concentration dependent in rat myotubes. In addition, we know that the release of ATP depends on the activity of pannexin-1 channels, suggesting a regulated process of ATP release (11). The results obtained in this study reinforce the idea of a specific and physiological response to ATP in myotubes to promote the expression of IL-6. In this work we did not address the question of which purinergic receptor subtype commands IL-6 expression; however, pharmacological analysis and the fact that the expression of IL-6 and STAT3 phosphorylation depends on IP3/Ca2+ signaling strongly suggest that a P2Y receptor subtype is involved. Four P2Y receptors, P2Y1, P2Y2, P2Y4, and P2Y11, are expressed mainly in myotubes, with P2Y1 and P2Y2 being expressed in higher amounts (11). In addition, recent findings in our laboratory showed that P2Y1 and P2Y2 are also the predominant P2Y receptor subtypes in skeletal fibers isolated from adult mice FDB; both of them are putatively activated by ATP and ADP (28).
We demonstrated an increase in IL-6 mRNA after 100–500 μM ATP in either adult FDB-isolated fibers or adult whole FDB muscle. ATP concentrations at the micromolar range could appear too high considering the P2Y receptor's affinity at the nanomolar range (1) and the measured interstitial ATP concentration of contracting muscles (2 μM) (36). However, it is relevant to note that released ATP plays a role in autocrine signaling, acting very locally inside the T-tubule space and at the pericellular space, being rapidly metabolized by ectonucleotidases, which precludes a significant convection into the bulk milieu (44, 90). So the ATP locally released at the T-tubules is probably much higher than we could measure. For the same reason, to allow ATP diffusion to the T-tubules and surpass ectonucleotidases metabolization, we need to use higher ATP concentrations and time exposures for whole muscle experiments (500 μM, 4 h) than for newborn-derived myotubes (10–100 μM for 15–60 min). All the studies relating P2Y receptor activity with skeletal muscle physiology, using varying experimental models, have used high μM ATP concentrations (50–180 μM) (18, 20, 21, 56, 57, 80). It is relevant to note that, considering that ATP release is a local event, only ≤1% intracellular ATP pool needs to be released to maximally activate all receptors. Thus, extracellular ATP signaling can occur without compromising cell metabolism or essential enzyme reactions (reviewed in Ref. 70).
It has been described that IL-6 plasma levels range between 1 pg/ml at rest and ≤120 pg/ml after strenuous physical performance such as a marathon (27, 29, 49, 76). However, interstitial IL-6 concentration in skeletal muscle is 100-fold higher than plasma levels, ranging between 300 pg/ml at rest and 2,000 pg/ml during and after exercise (49, 65). Very interestingly, concentrations of recombinant IL-6 widely described for in vitro assays are even higher, between 20 ng/ml in C2C12 and L6 myotubes and 120 ng/ml in skeletal muscle strips from patients (14, 32, 88). Considering that in vivo IL-6 is probably secreted from skeletal fibers to the highly packed T-tubule, it is possible that the local IL-6 concentration for autocrine signaling is higher than that measured by microdialysis in muscle interstitium. We assessed from 0.2 to 20 ng/ml rIL6 in our systems and obtained STAT3 phosphorylation starting from 2 ng/ml and IL-6 mRNA expression from 0.2 ng/ml.
From the results obtained in this work, it appears that STAT3 activation evoked by myotube depolarization is totally secondary to IP3-dependent Ca2+ transients. Previous studies have indirectly shown cytosolic Ca2+-induced phosphorylation of STAT3. Gong et al. (34) suggest a link between impaired levels of intracellular Ca2+, caused by a protein from hepatitis C virus, and the activity of STAT3. In another report, the activation of L-type Ca2+ channels by ischemia in rat hippocampus results in the activation of STAT3 (52). Finally, Shi and Kehrl (72) stated the involvement of Ca2+-dependent tyrosine kinase Pyk2 in potentiating the activity of STAT3 induced by epidermal growth factor. However, in none of these works did the authors identify the Ca2+ pool that would participate in the activation of STAT3. Here we show that intracellular free Ca2+ is required for STAT3 activation by either ATP or tetanic stimulation. Considering recent reports suggesting that IL-6 release evoked by skeletal muscle contraction is independent of calcium transients (33, 50), in our system Ca2+ would probably be required for an initial step in IL-6 expression but not for the IL-6 release mechanism. Our results suggest that STAT3 activation mediated by Ca2+ transients is an indirect effect; 1) both electrical stimulation and stimulation with ATP evoke transient IP3-dependent Ca2+ signals (11, 24, 40), 2) both depolarization and ATP induce the expression of IL-6 in skeletal muscle (this work and Refs. 11 and 45), 3) the increased expression of IL-6 by depolarization is dependent on IP3-dependent Ca2+ transients (45), and 4) inhibition of IL-6 signaling cascade blocks STAT3 phosphorylation. Together, these results suggest that intracellular free Ca2+ would be necessary for the expression of IL-6 and that the cytokine could act as a mediator between cell stimulation and STAT3 phosphorylation.
The fact that STAT3 activation is a late event (1 h post-stimulation) also suggests that there should be a secondary mediator between stimulation of the myotubes and activation of the transcription factor, which is probably IL-6. In other cell types, a late activation of STAT3 as a result of stimuli as varied as the epidermal growth factor (EGF)-like growth factor binding to heparin, isoproterenol, or angiotensin II (51, 68, 91) has been observed. In each of these cases, STAT3 activation was due to prior expression and release of IL-6 to the culture medium and the establishment of an autocrine signaling pathway for the cytokine. It is interesting to note that, in our experiments, when the autocrine IL-6 loop or the canonical IL-6 pathway was blocked using neutralizing IL-6 antibodies or a STAT3 dimerization inhibitor, a 30% residual-activated STAT3 or IL-6 expression evoked by ATP was maintained. These results reinforce the idea of a parallel signaling pathway, most probably Ca2+ dependent, promoting IL-6-expression as a first step to evoke the autocrine IL-6 loop. Indeed, we have demonstrated that IL-6 release evoked by ATP involves de novo synthesis. In our case, the time course of events ending in STAT3 phosphorylation would be as follows: 1) the depolarization of cultured myotubes induces the release of ATP into the culture medium reaching a maximum extracellular ATP 15 s after depolarization (11); 2) the ATP released by depolarization activates P2Y receptors and induces a transient IP3-dependent Ca2+ signal, which remains high for seconds to minutes in the cytoplasm (11); 3) these Ca2+ signals induce the activation of transcription factors like AP-1 and NF-κB (15–90 min), which participate in the expression of IL-6 (10 min to 2 h) (15, 85); 4) IL-6 is released into the extracellular medium (hours), reaching an increase of ∼4.5-fold over baseline 1 h after stimulation with ATP; and 5) finally, IL-6 activates IL-6Rα, triggering JAK2 phosphorylation, which induces STAT3 phosphorylation and IL-6 expression (hours post-stimulus).
Very interestingly, we noted that cycloheximide treatment intended for protein translation blockade increased resting levels of IL-6 mRNA ≤40-fold, and ATP-evoked IL-6 expression ≤120-fold. Previously, some authors have reported IL-6 superinduction evoked by blockade of protein synthesis in epithelial cells through increases in both IL-6 mRNA synthesis and stability (26, 37, 64). In the light of our results, considering that actinomycin D totally blocked ATP-evoked IL-6 expression and secretion, the effect over mRNA synthesis is probably more important than that over mRNA stability. This mechanism has never been described in skeletal muscle cells, and it could be a very important control point for IL-6 expression regulation in physiopathological conditions.
We also demonstrated that stimulation with ATP induced a 65% increase in expression of SOCS3 protein. The expression of SOCS3 in response to exercise has been shown previously (82, 83). Analysis performed on young people and older adults shows that the expression of SOCS3 is also much higher in young people; this correlates well with the fact that phosphorylation of STAT3 is higher in older adults (83). Those authors suggest that these alterations in cytokine signaling are explained by reduced expression of SOCS3 in older people. Furthermore, those authors speculate that this signaling potentiation by IL-6 would be involved in impaired muscle regenerative capacity observed in older adults through the establishment of a proinflammatory state. In our case, the expression of SOCS3 reaches the peak after 3 h in stimulated cells, which temporally coincides with the decrease in IL-6 present in the environment, suggesting that SOCS3 is negatively regulating the expression of IL-6. Thus, the negative regulator could have a function of terminating signal to avoid a chronic IL-6 response to exercise.
It is worth noting the fact that myotube depolarization evokes a late (2 h) expression of IL-6 compared with that obtained with ATP (30 min), whereas STAT3 phosphorylation by electrical stimulation occurs at the same time with both stimuli. This point that appears as a discrepancy could be explained by the fact that although we know that electrical stimulation of myotubes, as well as exercise or the activity of motor neurons, causes ATP release from the muscle cells (11, 53, 54, 75), we do not know the effective amount of ATP reaching purinergic receptors on muscle. This is very important considering the different affinity (EC50) that the purinergic receptor subtypes have for ATP and ADP (78, 81). One possibility then is that the purinergic receptors that are activated by exogenous ATP (100 μM) are different (in expression levels or receptor subtype) from the ones activated by ATP released from the depolarized myotubes, thus generating different responses. Another possibility is that exogenous ATP activates purinergic receptors located both at the sarcolemma and the T-tubules, triggering signaling pathways parallel to those evoked by endogenous ATP released at the T-tubules during depolarization. STAT3 phosphorylation prior to increased expression of IL-6 by depolarization could correspond to preformed IL-6, which is stored in vesicles within the cytoplasm of muscle cells, since these vesicles merge with plasma membrane and release their contents upon muscular contraction.
The results presented here contribute to a wider understanding of the ways by which electrical activity of skeletal muscle cells could drive gene expression. IL-6 is a good model to study ETC not only because it responds to membrane depolarization but also because it does in response to IP3-dependent calcium signals that we have related previously to gene expression. IL-6 has a major role in exercise physiology, acting as an energy sensor for skeletal muscle and also acting systemically, helping to avoid metabolic syndrome (66). Future research will shed light on the role of IL-6 in energetic and metabolic balance of skeletal muscle and further unravel details of its regulation.
GRANTS
This work was funded by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) nos. 11100454 (S. Buvinic), 1110467 (E. Jaimovich and S. Buvinic), and ACT1111 (E. Jaimovich and S. Buvinic), Comisión Nacional de Ciencia y Tecnología (CONICYT) no. 79090021 (E. Jaimovich and S. Buvinic), Fondo Nacional de Áreas Prioritarias FONDAP no. 15010006 (E. Jaimovich, S. Buvinic, R. Fernández-Verdejo, and M. Bustamante), and MECESUP UCH306 (M. Bustamante).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.B., E.J., and S.B. conception and design of research; M.B., R.F.-V., and S.B. performed experiments; M.B., R.F.-V., E.J., and S.B. analyzed data; M.B., R.F.-V., E.J., and S.B. interpreted results of experiments; M.B., R.F.-V., and S.B. prepared figures; M.B., E.J., and S.B. drafted manuscript; M.B., E.J., and S.B. edited and revised manuscript; M.B., R.F.-V., E.J., and S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mónica Silva for cell cultures.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J 9: 1897–1906, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Altamirano F, Lopez JR, Henriquez C, Molinski T, Allen PD, Jaimovich E. Increased resting intracellular calcium modulates NF-kappaB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. J Biol Chem 287: 20876–20887, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen DL, Uyenishi JJ, Cleary AS, Mehan RS, Lindsay SF, Reed JM. Calcineurin activates interleukin-6 transcription in mouse skeletal muscle in vivo and in C2C12 myotubes in vitro. Am J Physiol Regul Integr Comp Physiol 298: R198–R210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araya R, Liberona JL, Cardenas JC, Riveros N, Estrada M, Powell JA, Carrasco MA, Jaimovich E. Dihydropyridine receptors as voltage sensors for a depolarization-evoked, IP3R-mediated, slow calcium signal in skeletal muscle cells. J Gen Physiol 121: 3–16, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeza-Raja B, Muñoz-Cánoves P. p38 MAPK-induced nuclear factor-kappaB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol Biol Cell 15: 2013–2026, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banzet S, Koulmann N, Simler N, Birot O, Sanchez H, Chapot R, Peinnequin A, Bigard X. Fibre-type specificity of interleukin-6 gene transcription during muscle contraction in rat: association with calcineurin activity. J Physiol 566: 839–847, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbado M, Fablet K, Ronjat M, De Waard M. Gene regulation by voltage-dependent calcium channels. Biochim Biophys Acta 1793: 1096–1104, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Bulanova E, Budagian V, Orinska Z, Koch-Nolte F, Haag F, Bulfone-Paus S. ATP induces P2X7 receptor-independent cytokine and chemokine expression through P2X1 and P2X3 receptors in murine mast cells. J Leukoc Biol 85: 692–702, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Buvinic S, Almarza G, Bustamante M, Casas M, Lopez J, Riquelme M, Saez JC, Huidobro-Toro JP, Jaimovich E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem 284: 34490–34505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardenas C, Liberona JL, Molgo J, Colasante C, Mignery GA, Jaimovich E. Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J Cell Sci 118: 3131–3140, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Cárdenas C, Müller M, Jaimovich E, Pérez F, Buchuk D, Quest AF, Carrasco MA. Depolarization of skeletal muscle cells induces phosphorylation of cAMP response element binding protein via calcium and protein kinase Calpha. J Biol Chem 279: 39122–39131, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55: 2688–2697, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Carrasco MA, Riveros N, Rios J, Muller M, Torres F, Pineda J, Lantadilla S, Jaimovich E. Depolarization-induced slow calcium transients activate early genes in skeletal muscle cells. Am J Physiol Cell Physiol 284: C1438–C1447, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Casas M, Figueroa R, Jorquera G, Escobar M, Molgo J, Jaimovich E. IP(3)-dependent, post-tetanic calcium transients induced by electrostimulation of adult skeletal muscle fibers. J Gen Physiol 136: 455–467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan MH, McGee SL, Watt MJ, Hargreaves M, Febbraio MA. Altering dietary nutrient intake that reduces glycogen content leads to phosphorylation of nuclear p38 MAP kinase in human skeletal muscle: association with IL-6 gene transcription during contraction. FASEB J 18: 1785–1787, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Choi RC, Siow NL, Cheng AW, Ling KK, Tung EK, Simon J, Barnard EA, Tsim KW. ATP acts via P2Y1 receptors to stimulate acetylcholinesterase and acetylcholine receptor expression: transduction and transcription control. J Neurosci 23: 4445–4456, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Deli T, Szappanos H, Szigeti GP, Cseri J, Kovacs L, Csernoch L. Contribution from P2X and P2Y purinoreceptors to ATP-evoked changes in intracellular calcium concentration on cultured myotubes. Pflugers Arch 453: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Deli T, Toth BI, Czifra G, Szappanos H, Biro T, Csernoch L. Differences in purinergic and voltage-dependent signalling during protein kinase Calpha overexpression- and culturing-induced differentiation of C2C12 myoblasts. J Muscle Res Cell Motil 27: 617–630, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Dolmetsch R. Excitation-transcription coupling: signaling by ion channels to the nucleus. Sci STKE 2003: PE4, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Eltit JM, Garcia AA, Hidalgo J, Liberona JL, Chiong M, Lavandero S, Maldonado E, Jaimovich E. Membrane electrical activity elicits inositol 1,4,5-trisphosphate-dependent slow Ca2+ signals through a Gbetagamma/phosphatidylinositol 3-kinase gamma pathway in skeletal myotubes. J Biol Chem 281: 12143–12154, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Eltit JM, Hidalgo J, Liberona JL, Jaimovich E. Slow calcium signals after tetanic electrical stimulation in skeletal myotubes. Biophys J 86: 3042–3051, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J Cell Sci 119: 733–743, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Faggioli L, Costanzo C, Merola M, Furia A, Palmieri M. Protein synthesis inhibitors cycloheximide and anisomycin induce interleukin-6 gene expression and activate transcription factor NF-kappaB. Biochem Biophys Res Commun 233: 507–513, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53: 1643–1648, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Verdejo R, Casas M, Galgani JE, Jaimovich E, Buvinic S. Exercise Sensitizes Skeletal Muscle to Extracellular ATP for IL-6 Expression in Mice. Int J Sports Med. In press. [DOI] [PubMed] [Google Scholar]
- 29.Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12: 6–33, 2006 [PubMed] [Google Scholar]
- 30.Franchimont N, Rydziel S, Canalis E. Interleukin 6 is autoregulated by transcriptional mechanisms in cultures of rat osteoblastic cells. J Clin Invest 100: 1797–1803, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita T, Tozaki-Saitoh H, Inoue K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia 57: 244–257, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Glund S, Deshmukh A, Long YC, Moller T, Koistinen HA, Caidahl K, Zierath JR, Krook A. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes 56: 1630–1637, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Glund S, Treebak JT, Long YC, Barres R, Viollet B, Wojtaszewski JF, Zierath JR. Role of adenosine 5′-monophosphate-activated protein kinase in interleukin-6 release from isolated mouse skeletal muscle. Endocrinology 150: 600–606, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA 98: 9599–9604, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation 98: 6–8, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Hershko DD, Robb BW, Wray CJ, Luo GJ, Hasselgren PO. Superinduction of IL-6 by cycloheximide is associated with mRNA stabilization and sustained activation of p38 map kinase and NF-kappaB in cultured caco-2 cells. J Cell Biochem 91: 951–961, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Ilangumaran S, Ramanathan S, Rottapel R. Regulation of the immune system by SOCS family adaptor proteins. Semin Immunol 16: 351–365, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Jaimovich E, Mattei C, Liberona JL, Cardenas C, Estrada M, Barbier J, Debitus C, Laurent D, Molgo J. Xestospongin B, a competitive inhibitor of IP3-mediated Ca2+ signalling in cultured rat myotubes, isolated myonuclei, and neuroblastoma (NG108–15) cells. FEBS Lett 579: 2051–2057, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Jaimovich E, Reyes R, Liberona JL, Powell JA. IP3 receptors, IP3 transients, and nucleus-associated Ca2+ signals in cultured skeletal muscle. Am J Physiol Cell Physiol 278: C998–C1010, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol 528: 157–163, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jorquera G, Altamirano F, Contreras-Ferrat A, Almarza G, Buvinic S, Jacquemond V, Jaimovich E, Casas M. Cav1.1 controls frequency-dependent events regulating adult skeletal muscle plasticity. J Cell Sci 126: 1189–1198, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Jorquera G, Juretic N, Jaimovich E, Riveros N. Membrane depolarization induces calcium-dependent upregulation of Hsp70 and Hmox-1 in skeletal muscle cells. Am J Physiol Cell Physiol 297: C581–C590, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem 278: 23331–23342, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Juretić N, García-Huidobro P, Iturrieta JA, Jaimovich E, Riveros N. Depolarization-induced slow Ca2+ transients stimulate transcription of IL-6 gene in skeletal muscle cells. Am J Physiol Cell Physiol 290: C1428–C1436, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Juretic N, Urzua U, Munroe DJ, Jaimovich E, Riveros N. Differential gene expression in skeletal muscle cells after membrane depolarization. J Cell Physiol 210: 819–830, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Juttler E, Tarabin V, Schwaninger M. Interleukin-6 (IL-6): a possible neuromodulator induced by neuronal activity. Neuroscientist 8: 268–275, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Keller P, Keller C, Carey AL, Jauffred S, Fischer CP, Steensberg A, Pedersen BK. Interleukin-6 production by contracting human skeletal muscle: autocrine regulation by IL-6. Biochem Biophys Res Commun 310: 550–554, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Kreiner F, Langberg H, Galbo H. Increased muscle interstitial levels of inflammatory cytokines in polymyalgia rheumatica. Arthritis Rheum 62: 3768–3775, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Lauritzen HP, Brandauer J, Schjerling P, Koh HJ, Treebak JT, Hirshman MF, Galbo H, Goodyear LJ. Contraction and AICAR stimulate IL-6 vesicle depletion from skeletal muscle fibers in vivo. Diabetes 62: 3081–3092, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KS, Park JH, Lee S, Lim HJ, Choi HE, Park HY. HB-EGF induces delayed STAT3 activation via NF-kappaB mediated IL-6 secretion in vascular smooth muscle cell. Biochim Biophys Acta 1773: 1637–1644, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Li H, Zhang Q, Zhang G. Signal transducer and activator of transcription-3 activation is mediated by N-methyl-d-aspartate receptor and L-type voltage-gated Ca2+ channel during cerebral ischemia in rat hippocampus. Neurosci Lett 345: 61–64, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 95: 577–583, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Li J, King NC, Sinoway LI. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation 111: 2748–2751, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Martinello T, Baldoin MC, Morbiato L, Paganin M, Tarricone E, Schiavo G, Bianchini E, Sandona D, Betto R. Extracellular ATP signaling during differentiation of C2C12 skeletal muscle cells: role in proliferation. Mol Cell Biochem 351: 183–196, 2011 [DOI] [PubMed] [Google Scholar]
- 57.May C, Weigl L, Karel A, Hohenegger M. Extracellular ATP activates ERK1/ERK2 via a metabotropic P2Y1 receptor in a Ca2+ independent manner in differentiated human skeletal muscle cells. Biochem Pharmacol 71: 1497–1509, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 82: 4196–4200, 1997 [DOI] [PubMed] [Google Scholar]
- 59.Murgia M, Serrano AL, Calabria E, Pallafacchina G, Lomo T, Schiaffino S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol 2: 142–147, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Myrtek D, Müller T, Geyer V, Derr N, Ferrari D, Zissel G, Dürk T, Sorichter S, Luttmann W, Kuepper M, Norgauer J, Di Virgilio F, Virchow JC, Jr, Idzko M. Activation of human alveolar macrophages via P2 receptors: coupling to intracellular Ca2+ increases and cytokine secretion. J Immunol 181: 2181–2188, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Pedersen BK, Fischer CP. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care 10: 265–271, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Powell JA, Carrasco MA, Adams DS, Drouet B, Rios J, Muller M, Estrada M, Jaimovich E. IP(3) receptor function and localization in myotubes: an unexplored Ca(2+) signaling pathway in skeletal muscle. J Cell Sci 114: 3673–3683, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Rios E, Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev 71: 849–908, 1991 [DOI] [PubMed] [Google Scholar]
- 64.Roger T, Out TA, Jansen HM, Lutter R. Superinduction of interleukin-6 mRNA in lung epithelial H292 cells depends on transiently increased C/EBP activity and durable increased mRNA stability. Biochim Biophys Acta 1398: 275–284, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Rosendal L, Sogaard K, Kjaer M, Sjogaard G, Langberg H, Kristiansen J. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol 98: 477–481, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes 55, Suppl 2: S48–S54, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Sallmann S, Juttler E, Prinz S, Petersen N, Knopf U, Weiser T, Schwaninger M. Induction of interleukin-6 by depolarization of neurons. J Neurosci 20: 8637–8642, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sano M, Fukuda K, Kodama H, Takahashi T, Kato T, Hakuno D, Sato T, Manabe T, Tahara S, Ogawa S. Autocrine/Paracrine secretion of IL-6 family cytokines causes angiotensin II-induced delayed STAT3 activation. Biochem Biophys Res Commun 269: 798–802, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem 282: 20059–20063, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 1615: 7–32, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Shi CS, Kehrl JH. Pyk2 amplifies epidermal growth factor and c-Src-induced Stat3 activation. J Biol Chem 279: 17224–17231, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Shouda T, Hiraoka K, Komiya S, Hamada T, Zenmyo M, Iwasaki H, Isayama T, Fukushima N, Nagata K, Yoshimura A. Suppression of IL-6 production and proliferation by blocking STAT3 activation in malignant soft tissue tumor cells. Cancer Lett 231: 176–184, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3: 900–911, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Silinsky EM. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol 247: 145–162, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-α levels after prolonged running. Am J Physiol Cell Physiol 280: C769–C774, 2001 [DOI] [PubMed] [Google Scholar]
- 77.Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol 537: 633–639, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stojilkovic SS. Ca2+-regulated exocytosis and SNARE function. Trends Endocrinol Metab 16: 81–83, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Suzuki S, Tanaka K, Nogawa S, Dembo T, Kosakai A, Fukuuchi Y. Expression of interleukin-6 is suppressed by inhibition of voltage-sensitive Na+/Ca2+ channels after cerebral ischemia. Neuroreport 11: 2565–2569, 2000 [DOI] [PubMed] [Google Scholar]
- 80.Szigeti GP, Szappanos H, Deli T, Cseri J, Kovacs L, Csernoch L. Differentiation-dependent alterations in the extracellular ATP-evoked calcium fluxes of cultured skeletal muscle cells from mice. Pflugers Arch 453: 509–518, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Trautwein C, Caelles C, van der Geer P, Hunter T, Karin M, Chojkier M. Transactivation by NF-IL6/LAP is enhanced by phosphorylation of its activation domain. Nature 364: 544–547, 1993 [DOI] [PubMed] [Google Scholar]
- 82.Trenerry MK, Carey KA, Ward AC, Cameron-Smith D. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol 102: 1483–1489, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Trenerry MK, Carey KA, Ward AC, Farnfield MM, Cameron-Smith D. Exercise-induced activation of STAT3 signaling is increased with age. Rejuvenation Res 11: 717–724, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Uehara Y, Mochizuki M, Matsuno K, Haino T, Asai A. Novel high-throughput screening system for identifying STAT3-SH2 antagonists. Biochem Biophys Res Commun 380: 627–631, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Valdés JA, Hidalgo J, Galaz JL, Puentes N, Silva M, Jaimovich E, Carrasco MA. NF-κB activation by depolarization of skeletal muscle cells depends on ryanodine and IP3 receptor-mediated calcium signals. Am J Physiol Cell Physiol 292: C1960–C1970, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Wu H, Zhang Z, Liu S, Yang J, Chen X, Fan M. Effects of interleukin-6, leukemia inhibitory factor, and ciliary neurotrophic factor on the proliferation and differentiation of adult human myoblasts. Cell Mol Neurobiol 28: 113–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weigert C, Dufer M, Simon P, Debre E, Runge H, Brodbeck K, Haring HU, Schleicher ED. Upregulation of IL-6 mRNA by IL-6 in skeletal muscle cells: role of IL-6 mRNA stabilization and Ca2+-dependent mechanisms. Am J Physiol Cell Physiol 293: C1139–C1147, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Weigert C, Hennige AM, Lehmann R, Brodbeck K, Baumgartner F, Schauble M, Haring HU, Schleicher ED. Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J Biol Chem 281: 7060–7067, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Whitham M, Chan MH, Pal M, Matthews VB, Prelovsek O, Lunke S, El-Osta A, Broenneke H, Alber J, Brüning JC, Wunderlich FT, Lancaster GI, Febbraio MA. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem 287: 10771–10779, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673–694, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Yin F, Li P, Zheng M, Chen L, Xu Q, Chen K, Wang YY, Zhang YY, Han C. Interleukin-6 family of cytokines mediates isoproterenol-induced delayed STAT3 activation in mouse heart. J Biol Chem 278: 21070–21075, 2003 [DOI] [PubMed] [Google Scholar]