Abstract
Physiological responses and control of body systems differ between women and men. Moreover, within women, female gonadal hormones have important influences on organs and systems outside of reproduction. Until the NIH Revitalization Act of 1993, laboratories focused physiological research primarily on men, and this focus placed limitations on women's health care. Thus, the NIH directive to include women required scientists and physicians studying humans to consider female reproductive physiology. Even though this directive was enacted over 20 years ago, there is still a great deal of misunderstanding as to the best methods to control hormones or account for changes in internal hormone exposure in women. This discussion describes common methods investigators use to include women in physiological studies and to examine the impact of female reproductive hormone exposure for research purposes. In some cases, the goal is to control for phase of the cycle, so women are studied when the endogenous hormones should be similar to each other. When the goal of the research is to examine the effects of hormones on a physiological response, it is important to use methods that will change hormone exposure in a controlled fashion. We recommend a method that employs gonadotropin-releasing hormone (GnRH) agonist or antagonist to suppress estrogens, gonadotropins, progesterone, and androgens followed by administration of these hormones. While this method is more invasive, it is safe and is the strongest research design to examine both hormone effects within women and between women and men.
Keywords: hormonal contraceptives, gonadotropin-releasing hormone agonist, GnRH antagonist, estrogens, progesterone, progestins
the nih revitalization act of 1993 (1) directed the NIH to ensure the inclusion of women in clinical research unless “a clear and compelling rationale and justification establishes to the satisfaction of the relevant Institute/Center Director that inclusion is inappropriate with respect to the health of the subjects or the purpose of the research” and that women of childbearing age cannot be routinely excluded from scientific research without a compelling reason. The aim of this mandate was to ensure that health recommendations as well as diagnosis and treatment of disease were relevant to women as well as men. Until this mandate, many laboratories had focused their research exclusively on men, so the challenge for scientists was to update their laboratories to accommodate women and to consider how female reproductive physiology might impact their findings. While the primary roles of estrogens and progestogens are to create an environment hospitable for conception and a developing fetus, largely because of the 1993 NIH directive, we now know that gonadal hormones have important influences on organs and systems outside of reproduction. Moreover, physiological systems function differently within women according to hormonal milieu. This discussion describes common methods investigators use to control female reproductive steroid hormone exposure for research purposes and to examine reproductive hormone effects on physiological systems within or outside of reproduction. We also describe a method of special value in which a gonadotropin-releasing hormone (GnRH) agonist or antagonist suppresses estrogen and progesterone followed by administration of these hormones. This method provides the most controlled environment to permit causal inferences about hormonal effects on the system of study.
This paper addresses challenges in testing young, healthy women, regularly menstruating women, and women who are not pregnant, have no chronic or acute disease, and are not medicated, much like the women (and men) recruited for physiological studies. Our focus is on young women in this paper, because the impact of aging is an important confounding variable, especially in investigations examining cardiovascular disease, metabolism, and neurological or endocrine function. Thus, comparing pre- and postmenopausal women cannot isolate differential effects of estradiol and progestogens. Our purpose in writing this paper is to emphasize that investigators need to take the same care in considering hormone milieu in both women and men as they do with any variable that can impact their findings.
Minimizing Hormonal Effects for Physiological Studies
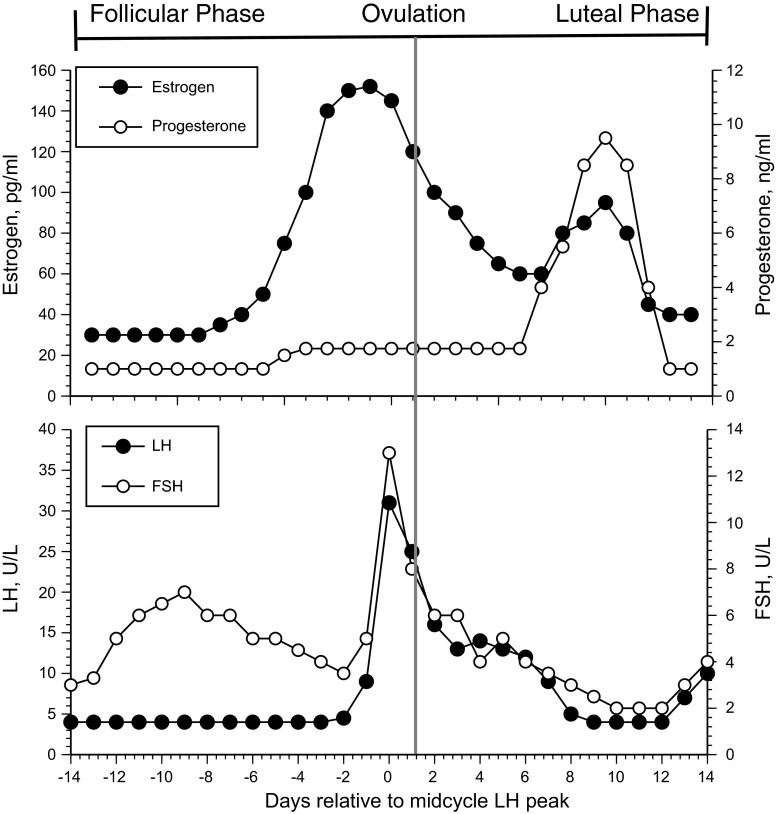
Estradiol is the predominant biologically active estrogen in young, healthy women and therefore has the greatest impact on physiological studies. While a number of estrogens [estradiol (15–350 pg/ml), estrone (17–200 pg/ml), estriol (<0.80 ng/ml)] (21) are present in young, healthy women, 17β-estradiol is the most abundant and has the greatest activity on estrogen receptors. It is important to note the wide ranges as estradiol and estrone reflect changes across the menstrual cycle (Fig. 1). Estriol is the most abundant estrogen in pregnant women, but it is also the weakest and has little impact on estrogen receptors. Estrone is less abundant than 17β-estradiol in young women; however, because of the cessation of 17β-estradiol by the postmenopausal ovary, it is the primary estrogen during menopause. Estrone is a weak estrogen with far less potency than 17β-estradiol and is not able to compensate for the loss of 17β-estradiol in menopausal women.
Fig. 1.
Hormonal changes across a normal menstrual cycle. LH, luteinizing hormone; FSH, follicle-stimulating hormone. Note that actual levels of estradiol, progesterone, LH, and FSH are approximate because these vary greatly across individuals.
The most common method to minimize the hormone effects that confound research findings is to study women in the same phase of the menstrual cycle across subjects or across study days in longitudinal studies. Investigators often test women in the early follicular phase (between days 1–7 following the onset of menses) when both estrogen and progesterone exposures are at their lowest levels (Fig. 1). Despite the convenience of studying women in this part of the menstrual cycle, the broader clinical relevance of the findings may be limited: women are in this part of their cycle for only ∼25% of their reproductive lives. Although estradiol and progesterone exposure is low relative to other phases of the cycle (Fig. 1), hormone levels are still considerably higher compared with those in men (∼14 pg/ml and < 0.9 ng/ml for estradiol and progesterone, respectively), so their impact when making comparisons between the sexes is not entirely eliminated. Moreover, focusing on hormone exposure to define hormonal impact ignores the potency of their associated receptors. Finally, while estrogens and progesterone are at their lowest phasic levels, the gonadotropin follicle-stimulating hormone (FSH) increases during the early follicular phase and may exert its own effect on the physiological outcome of study (Fig. 1). Thus, researchers can attempt to keep the cycle days consistent across women within a given study to study physiological effects independent of cycle phase, but they must take into account the variability of these hormones across cycles phases within women (see more on this below). Hormonal contraceptives resolve the problem of hormonal variability but do so by providing estradiol and progestins.
Examining Hormone Effects
Menstrual cycle phase.
For studies in which the goal is to evaluate the influence of reproductive hormones on physiological variables, a common method is to compare women across phases of the menstrual cycle, usually the early follicular, ovulatory and midluteal phases because these phases are associated with different hormonal levels in women (Fig. 1; in Fig. 1 the values along the ordinal axes are approximate, considering the variability among women, and even within women across different cycles. Fig. 1 is meant to show general changes of these hormones across a normal menstrual cycle.). Using menstrual cycle phase to keep hormone levels consistent within an investigation has the advantage of examining women during natural endogenous hormonal changes (5). The early follicular phase is the period within 7 days after the first day of menstrual bleeding. Ovulation occurs ∼12–14 days after the beginning of the menstrual cycle and within 24 h of the LH peak. Identification of this phase requires an ovulation prediction kit (available over the counter) because some women menstruate but do not ovulate. Estradiol peaks just prior to ovulation but is still somewhat elevated during the LH peak. The highest progesterone levels during the menstrual cycle occur 7 to 8 days after the LH peak, with little variability in the length of the luteal phase within or across women. However, without ovulation there will be no progesterone peak. Thus, it is important to ensure that subjects in the study ovulate and to measure serum 17β-estradiol and progesterone levels from blood samples on each study day. The primary limitation is that studies over the menstrual cycle do not isolate progesterone effects on outcome variables because there is no time in the menstrual cycle when progesterone is elevated without concomitant estrogen elevations.
Hormonal contraceptives.
Hormonal contraceptives (usually combinations of different types of estrogens and progestins) are also used to study hormonal effects on physiological responses. Many women take hormonal contraceptives, indicating high clinical applicability of findings. Hormonal contraceptives increase blood levels above endogenous estrogens and progesterone and provide a steady-state environment with which to compare with women not taking hormones. Progestins in hormonal contraceptives differ in some of their basic hormonal actions compared with endogenous progesterone. Progesterone is synthesized from pregnenolone, which like other steroid hormones is derived from cholesterol, whereas most progestins are synthesized using chemical processes. The resulting structural dissimilarities cause progestins and progesterone to impact physiological systems differently. For example, most progestins in hormonal contraceptives have mild androgenic properties relative to endogenous progesterone (22), and progesterone, progestins, and androgens can alter peripheral circulation (10, 33), blood pressure (18), aldosterone function (2, 3) and release (26), and temperature regulation (25). Moreover, the types of progestogens in hormonal contraceptives differ from one another in important ways (12). For example, levonorgestrel, the most widely used progestin, has greater progestational, androgenic, and antiestrogen effects relative to norethindrone acetate or desogestrel, which minimize both progestational and androgenic effects. Finally, drospirenone (Yaz) is the only progestin derived from 17α-spirolactone and therefore is an analog of spironolactone. Drospirenone has no androgenic effects and is even itself a weak androgen antagonist. Thus, because of the variable effects of the different progestogens, it is key that women participating in studies are all taking the same hormones, in the same way, and that the timing of the physiological studies is consistent across subjects.
An interesting example is the use of depot-medroxyprogesterone (MPA) to investigate progestin effects on endothelial function (14, 28). This progestin works by interfering with the hypothalamic-pituitary-ovarian axis and inhibiting pituitary gonadotropin release, which prevents ovulation. This progestin has limitations similar to those described above for contraceptives, but these studies have clinical relevance for the millions of women who use MPA. A recent elegant series of studies demonstrated an interaction between estradiol and progestin exposure on cardiovascular function such that estradiol administration improved endothelium-dependent flow-mediated dilation in women taking MPA (14, 28, 30). Estrogen induces liver production and release of sex hormone-binding globulin (SHBG), which binds to androgens, so it may attenuate a weak androgenic effect of MPA. Conversely, long-term (>1 yr) use of MPA can impair endothelial function, perhaps through estrogen suppression (20).
Investigators also use the “placebo” week of the hormone contraceptive cycle, labeling weeks with their subjects on contraceptives as “high” hormone and weeks on placebo as “low” hormone phases (6). For example, this design has been used to demonstrate that local warming-induced vasodilation of the skin was augmented while women were taking hormonal contraceptives compared with their placebo phase, an effect attributed to estradiol promoting vasodilation (6), although progestins can attenuate estradiol-mediated vasodilation (11, 13). Despite these findings, the placebo phase of the hormonal contraceptive pill cycle is not strictly a “low” hormonal phase. Immediately after stopping the hormonal contraceptives, blood or tissue levels of the exogenous estrogens or progestins or their metabolites are likely elevated. Moreover, by the end of that 7-day placebo period, endogenous estrogens are variable (7, 19), and there are no good data on the impact of progestin metabolites that may still be present in tissue. Thus, the placebo week during regular hormonal contraception is not a controlled period of low hormone exposure in women. A better method is to use a baseline test when women are not taking contraceptives and then begin controlled administration.
Hormone suppression-administration.
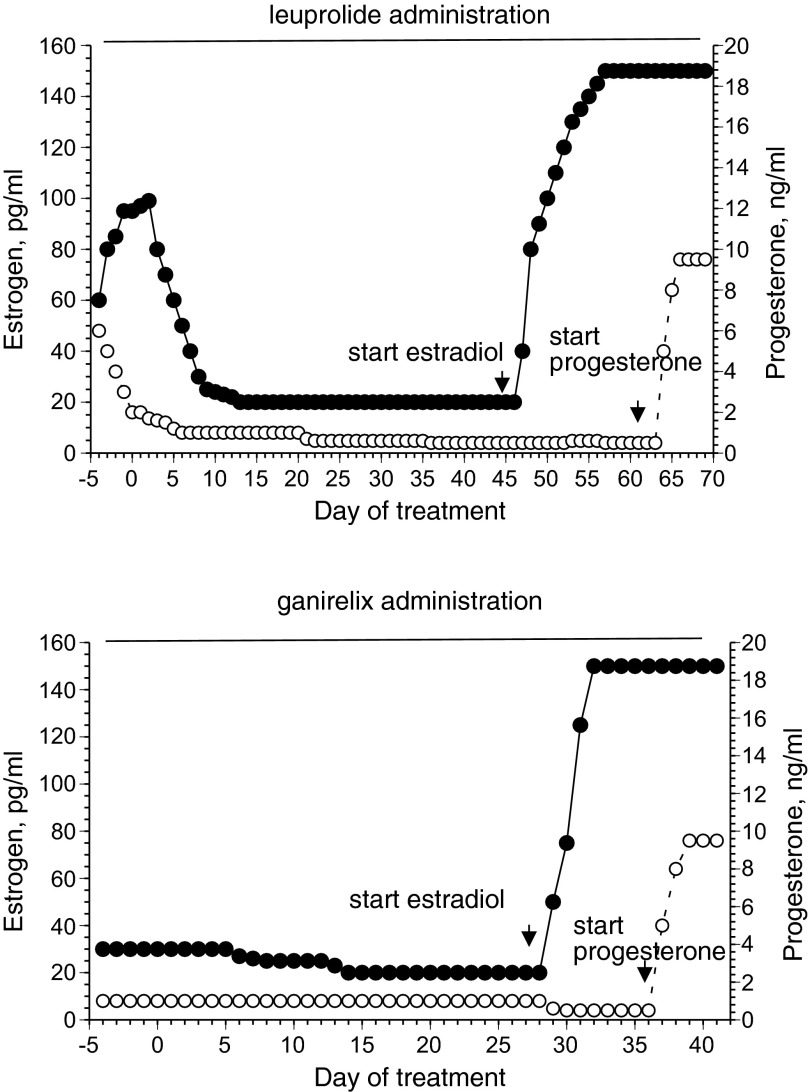
The most controlled method to isolate individual effects of estradiol or progesterone on physiological systems in young women is temporary suppression of the menstrual cycle with a GnRH agonist (leuprolide acetate) or antagonist (ganirelix and cetrorelix acetate). This method requires subjects to use a small needle for subcutaneous injections of the drugs and thus is more invasive than measuring changes in endogenous hormones over the course of the menstrual cycle or hormonal contraceptives. Leuprolide has greater GnRH receptor binding and decreased degradation than endogenous GnRH, so it is a potent inhibitor of gonadotropin secretion. Continuous leuprolide administration downregulates the hypothalamic-pituitary-ovarian axis, causing internalization and uncoupling of the GnRH receptors in the pituitary. Leuprolide administration leads to initial FSH stimulation and related steroidogenesis, followed by low or undetectable estrogen and progesterone concentrations within 7–14 days (23) (Fig. 2). Ganirelix and cetrorelix are synthetic decapeptides that compete with GnRH for receptor binding and so function as competitive receptor antagonists, inducing rapid, reversible suppression of gonadotropin secretion and suppressing estrogens and progesterone to postmenopausal levels after 36–48 h of administration(16, 17) (Fig. 2). When hormones are suppressed, estrogens or progestogens (or both) can be administered in a controlled fashion to test the hypothesis of interest (Fig. 2). This method is ideal to examine causal inferences about hormonal effects on the system targeted for study and has been used to study body fluid regulation (23, 24, 27), cardiovascular function (4, 15, 32, 33), and metabolism (8, 9). Subject recruitment extends to women with irregular menstrual cycles, women with reproductive dysfunction, and women who take contraceptives (they stop taking contraceptives during the hormonal study). Women can begin taking the GnRH agonist 2–7 days following the subject's LH peak, or day ∼12–14 of a 28-day menstrual cycle, determined individually by the use of urine ovulation prediction kits. Women begin using the GnRH antagonist on days 25–28 of their menstrual cycle. At this point of the menstrual cycle, the corpus luteum is almost completely involuted, and the endometrium is normally shed. In both cases, this specific timing minimizes or eliminates endometrial bleeding. The GnRH agonist/antagonist hormone suppression is quickly reversed upon cessation of drug administration and has no known long-term health risks (in fact lower risk than hormonal contraceptives in some cases), making the method ideal for research. Because in eumenorrheic women administration of leuprolide or ganirelix acetate leads to suppression of estrogens and progesterone to postmenopausal levels, women can experience vasomotor symptoms (“hot flashes”), breast tenderness, headaches, transient mood changes, or some temporary mild water retention. As with all hormone administration, leuprolide, ganirelix, estradiol, and progestogen administration require supervision by a physician trained in reproductive endocrinology to minimize risks or symptoms associated with these drugs. Of the two drugs, leuprolide vs. ganirelix, ganirelix holds the distinct advantage of rapid competitive antagonism, and thus the hormone suppression is faster (Fig. 2). Leuprolide can take up to 2 wk for complete suppression, while estradiol and progesterone are suppressed within 38–48 h. Thus, the subjects take it for a shorter time and experience fewer antagonist side effects. Finally, GnRH suppression (with either method) combined with hormone administration is also a viable method to examine androgens in men (29) and in women (31, 33). Important limitations of the GnRH agonist/antagonist protocols are that they do not mimic a natural hormone exposure period, are inconvenient for subjects because the GnRH agonist/antagonist is injected subcutaneously daily, and requires supervision of an experienced obstetrician/gynecologist and the expense of the drugs.
Fig. 2.
Estradiol and progesterone changes in response to GnRH agonist (leuprolide, left) and GnRH antagonist (ganirelix, right).
Conclusion
Including women in all appropriate physiological research studies is essential for women's health. The changing hormonal milieu in women across the menstrual cycle creates a challenge for designing controlled studies in women but can also be used to study the effects of estrogens and progestogens. Hormonal contraceptives can be used to increase estradiol and progestin exposure, but close attention to methodology and timing is essential. Gonadotropin agonist/antagonist administration represents the most effective, low-risk method to study reproductive hormone effects on physiological systems in women. As physiologists, we all know that environmental control is essential to interpreting outcome variables. The control of hormone exposure in women deserves the same attention to detail.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.S.S. prepared figures; N.S.S. drafted manuscript; N.S.S. and H.S.T. edited and revised manuscript; N.S.S. and H.S.T. approved final version of manuscript.
REFERENCES
- 1.Anonymous. NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research - October, 2001. Bethesda, MD: NIH, 2001 [Google Scholar]
- 2.Boschitsch E, Mayerhofer S, Magometschnigg D. Hypertension in women: the role of progesterone and aldosterone. Climacteric 13: 307–313, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Braley LM, Menachery AI, Yao T, Mortensen RM, Williams GH. Effect of progesterone on aldosterone secretion in rats. Endocrinology 137: 4773–4778, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Brunt VE, Miner JA, Meendering JR, Kaplan PF, Minson CT. 17beta-estradiol and progesterone independently augment cutaneous thermal hyperemia but not reactive hyperemia. Microcirculation 18: 347–355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension 61: 395–399, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charkoudian N, Johnson J. Altered reflex control of cutaneous circulation by female sex steroids is independent of prostaglandins. Am J Physiol Heart Circ Physiol 276: H1634–H1640, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Creinin MD, Lippman JS, Eder SE, Godwin AM, Olson W. The effect of extending the pill-free interval on follicular activity: triphasic norgestimate/35 μg ethinyl estradiol versus monophasic levonorgestrel/20 μg ethinyl estradiol. Contraception 66: 147–152, 2002 [DOI] [PubMed] [Google Scholar]
- 8.D'Eon TM, Sharoff C, Chipkin SR, Grow D, Ruby BC, Braun B. Regulation of exercise carbohydrate metabolism by estrogen and progesterone in women. Am J Physiol Endocrinol Metab 283: E1046–E1055, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Day DS, Gozansky WS, Van Pelt RE, Schwartz RS, Kohrt WM. Sex hormone suppression reduces resting energy expenditure and (beta)-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab 90: 3312–3317, 2005 [DOI] [PubMed] [Google Scholar]
- 10.El-Mas MM, Afify EA, Mohy El-Din MM, Omar AG, Sharabi FM. Testosterone facilitates the baroreceptor control of reflex bradycardia: role of cardiac sympathetic and parasympathetic components. J Cardiovasc Pharmacol 38: 754–763, 2001 [DOI] [PubMed] [Google Scholar]
- 11.English JL, Jacobs LO, Green G, Andrews TC. Effect of the menstrual cycle on endothelium-dependent vasodilation of the brachial artery in normal young women. Am J Cardiol 82: 256–258, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Hapgood JP, Africander D, Louw R, Ray RM, Rohwer JM. Potency of progestogens used in hormonal therapy: Toward understanding differential actions. J Steroid Biochem Mol Biol 2013. August 14 pii: S0960–0760(13)00144–1. 10.1016/j.jsbmb.2013.08.001 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Hirata K, Nagasaka T, Hirashita M, Takahata T, Nuriomura T. Effects of human menstrual cycle on thermoregulatory vasodilation during exercise. Eur J Appl Physiol 54: 559–565, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol 294: H1630–H1637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol 301: H1716–H1722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberye JJL, Mannaerts BMJL, Huisman JAM, Timmer CJ. Pharmacokinetic and Pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part I. Absolute bioavailability of 025 mg of ganirelix after a single subcutaneous injection in healthy female volunteers. Fertil Steril 72: 1001–1005, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Oberye JJL, Mannaerts BMJL, Huisman JAM, Timmer CJ. Pharmacokinetic and Pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril 72: 1006–1012, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exper Pharmacol Physiol 26: 127–131, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Schlaff WD, Lynch AM, Hughes HD, Cedars MI, Smith DL. Manipulation of the pill-free interval in oral conceptive pill users: the effect on follicular suppression. Am J Obstet Gynecol 190: 943–951, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Sorensen MB, Collins P, Ong PJ, Webb CM, Hayward CS, Asbury EA, Gatehouse PD, Elkington AG, Yang GZ, Kubba A, Pennell DJ. Long-term use of contraceptive depot medroxyprogesterone acetate in young women impairs arterial endothelial function assessed by cardiovascular magnetic resonance. Circulation 106: 1646–1651, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Speroff L, Glass RH, Kase NG. Clinical Gynecologic Endocrinol Infertility. Baltimore, MD: Lippincott Williams & Wilkins, 1999 [Google Scholar]
- 22.Speroff L, Glass RH, Kase NG. Steroid contraception. In: Clinical Gynecological Endocrinol Infertility. Baltimore, MD: Lippincott Williams & Wilkins, 1999, p. 873–879 [Google Scholar]
- 23.Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Metab 283: E711–E721, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Stachenfeld NS, Keefe DL, Palter SF. Effects of estrogen and progesterone on transcapillary fluid dynamics. Am J Physiol Regul Integr Comp Physiol 281: R1319–R1329, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. J Appl Physiol 88: 1643–1649, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Stachenfeld NS, Silva CS, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol 87: 1016–1025, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Stachenfeld NS, Taylor HS. Progesterone increases plasma and extracellular fluid volumes independent of estradiol. J Appl Physiol 98: 1991–1997, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Torgrimson BN, Meendering JR, Kaplan PF, Minson CT. Depot-medroxyprogesterone acetate and endothelial function before and after acute oral, vaginal, and transdermal estradiol treatment. Hypertension 57: 819–824, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veldhuis JD, Liu PY, Takahashi PY, Weist SM, Wigham JR. Analysis of the impact of intravenous LH pulses versus continuous LH infusion on testosterone secretion during GnRH-receptor blockade. Am J Physiol Regul Integr Comp Physiol 303: R994–R1002, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on endothelium-dependent vasodilation in postmenopausal women receiving estrogen. Circulation 104: 1773–1778, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Wenner MM, Taylor HS, Stachenfeld N. Endothelin B receptor contribution to peripheral microvascular function in women with polycystic ovary syndrome. J Physiol 589: 4671–4679, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab 305: E818–E825, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol 589: 975–986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]