Abstract
Increased osteopontin (OPN) expression associates with increased myocyte apoptosis and myocardial dysfunction. The objective of this study was to identify the receptor for OPN and get insight into the mechanism by which OPN induces cardiac myocyte apoptosis. Adult rat ventricular myocytes (ARVMs) and transgenic mice expressing OPN in a myocyte-specific manner were used for in vitro and in vivo studies. Treatment with purified OPN (20 nM) protein or adenoviral-mediated OPN expression induced apoptosis in ARVMs. OPN co-immunoprecipitated with CD44 receptors, not with β1 or β3 integrins. Proximity ligation assay confirmed interaction of OPN with CD44 receptors. Neutralizing anti-CD44 antibodies inhibited OPN-stimulated apoptosis. OPN activated JNKs and increased expression of Bax and levels of cytosolic cytochrome c, suggesting involvement of mitochondrial death pathway. OPN increased endoplasmic reticulum (ER) stress, as evidenced by increased expression of Gadd153 and activation of caspase-12. Inhibition of JNKs using SP600125 or ER stress using salubrinal or caspase-12 inhibitor significantly reduced OPN-stimulated apoptosis. Expression of OPN in adult mouse heart in myocyte-specific manner associated with decreased left ventricular function and increased myocyte apoptosis. In the heart, OPN expression increased JNKs and caspase-12 activities, and expression of Bax and Gadd153. Thus, OPN, acting via CD44 receptors, induces apoptosis in myocytes via the involvement of mitochondrial death pathway and ER stress.
Keywords: osteopontin, ER stress, apoptosis, CD44, JNKs, myocyte
heart failure represents a major cause of morbidity and mortality in the Western country. Myocyte apoptosis is recognized as an important determinant of structure and function of the heart (15, 25). Myocyte apoptosis occurs in the myocardium of patients during heart failure and in animal models of myocardial hypertrophy and failure (11, 15, 56).
The extracellular matrix (ECM) of the heart is considered to play an important role in left ventricular remodeling. Matricellular proteins, a class of nonstructural ECM proteins, play a significant role in ECM homeostasis and intracellular signaling via their interactions with cell surface receptors, structural proteins, and/or soluble extracellular factors such as growth factors and cytokines (10). Osteopontin (OPN), also called cytokine Eta-1, is a member of matricellular protein family. OPN has Arg-Gly-Asp (RGD) cell-binding sequence. In cells of noncardiac origin, OPN is suggested to interact with various integrins in RGD-dependent and non-RGD-dependent manner and CD44 receptor (16, 40).
Heart expresses OPN at low levels under basal conditions. OPN expression increases markedly under a variety of pathophysiological conditions of the heart in animal models (13, 43, 47, 49, 52, 57) and in humans (39, 46, 48, 51). Myocytes are identified as source of OPN in mice during streptozotocin-induced diabetic cardiomyopathy (47) and in humans during ischemic, idiopathic, and dilated cardiomyopathy (13, 46). Increased OPN expression associates with increased myocyte apoptosis in different models of heart disease (21, 38, 47). Cardiac myocyte-specific expression of OPN in the mouse heart during the first 11 wk of life led to increased myocyte apoptosis and myocardial dysfunction (34). Increased myocyte apoptosis occurs in the myocardium of patients with dilated cardiomyopathy (8). In patients with dilated cardiomyopathy, increased OPN expression in myocytes negatively correlated with left ventricular (LV) ejection fraction (46). Furthermore, elevated plasma OPN levels correlated with the severity and risk of subsequent death in patients with chronic heart failure due to dilated or ischemic cardiomyopathy (35). These studies suggest that increased OPN expression plays a deleterious role, at least in part, by increasing myocyte apoptosis. However, the receptor/s for OPN in cardiac myocytes and molecular mechanisms involved in OPN-stimulated cardiac apoptosis are not yet understood.
Mitochondria are accepted as the key organelles that play a crucial role in determination of cell fate with respect to survival and apoptosis in the heart (2). Endoplasmic reticulum (ER; sarcoplasmic reticulum in myocytes) stress is suggested to play a role in myocyte apoptosis and pathogenesis of heart failure (14, 28, 29). The objective of this study was to investigate the role of OPN in the induction of apoptosis and then to identify the receptor/s for OPN in adult cardiac myocytes. To get an insight into the mechanism, we investigated the involvement of mitochondrial death pathway and ER stress in OPN-stimulated cardiac myocyte apoptosis. The data presented here suggest that OPN, acting via CD44 receptors, induces apoptosis in adult rat ventricular myocytes (ARVMs). OPN-stimulated apoptosis occurs via the induction of mitochondrial death pathway and ER stress. Cardiac myocyte-specific expression of OPN in adult mouse heart deteriorated heart function, which associated with increased cardiac myocyte apoptosis, mitochondrial death pathway, and ER stress.
MATERIALS AND METHODS
Experimental animals.
The study used adult male Sprague-Dawley rats and transgenic mice expressing OPN in a cardiac myocyte-specific manner. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Revised 1996), and the animal protocol was approved by the East Tennessee State University Committee on Animal Care.
Cell isolation, culture, and treatment.
Molecular signaling with respect to apoptosis appears to be the same between adult rat and mouse myocytes, e.g., stimulation of β-adrenergic receptor (β-AR) induces apoptosis in isolated adult rat cardiac myocytes (3, 22–24) and in vivo in mouse cardiac myocytes (19). β1-integrins play an anti-apoptotic role in β-AR-stimulated apoptosis in vitro in isolated adult rat cardiac myocytes (4, 23) and in vivo in mouse cardiac myocytes (19). Because the yield of myocytes isolated from rat heart is significantly greater compared with that of the mouse heart, rat heart were used for in vitro experiments. Calcium-tolerant ARVMs were isolated from the hearts of adult male Sprague-Dawley rats (150–200 g) as described previously (23). ARVMs were plated in DMEM (Mediatech) supplemented with 25 mM HEPES, 0.2% albumin, 5 mM creatine, 2 mM L-carnitine, 5 mM taurine, and 0.1% penicillin-streptomycin at a density of 30–50 cells/mm2 on 100-mm tissue culture dishes or coverslips (Fisher Scientific) precoated with laminin (1 μg/cm2). ARVMs, cultured for 24 h, were treated with purified mouse OPN protein (2 or 20 nM; R&D system) for various time points. SP600125 (10 μM; Calbiochem), salubrinal (1 μM; Calbiochem), z-ATAD-FMK (10 μM; SM Biochemicals), neutralizing CD44 (IM7, 5 μg/ml; BD Biosciences) antibodies, or control IgG (5 μg/ml; BD Biosciences) were added 30 min before OPN treatment. The inhibitors or the antibodies were maintained in the medium during the treatment period with OPN.
OPN transgenic mice.
Two mouse lines were used to obtain cardiac myocyte-targeted expression of OPN. In one line, the α-myosin heavy-chain (α-MHC) promoter drives the expression of a tetracycline transactivator (tTA) in myocytes (α-MHC-tTA; obtained from Dr. Anthony Baker, Veterans Affairs Medical Center, San Francisco, CA). In the second line, the tTA-responsive promoter, tetO, is linked to the OPN transgene (TetO-OPN; obtained from Dr Alain-Pierre Gadeau, University of Bordeaux Victor Segalen, Pessac, France). The α-MHC-tTA mice were cross-bred with TetO-OPN mice (34). Transactivation by tTA is inhibited by doxycycline (Dox; analog of tetracycline), which prevents the tTA binding to DNA (32, 58). The cross-breeding between α-MHC-tTA (MHC) and TetO-OPN mice produced four genotypes: wild-type (WT), α-MHC-tTA, TetO-OPN, and dual transgenic (MHC-OPN) mice. Genotyping was performed by PCR using experimental conditions and primers as suggested (34). The MHC-OPN mice have Dox-repressed expression of OPN in the heart. The MHC-OPN mice were bred and maintained on a diet containing Dox (200 mg/kg, no. S3888; Bio-serve, Frenchtown, NJ). To express OPN in adult heart, Dox was removed from the diet when the mice were ∼4 mo old.
Echocardiography.
Transthoracic two-dimensional M-mode echocardiography was performed using a Toshiba Aplio 80 Imaging System (Tochigi, Japan) equipped with a 12 MHz linear transducer (6, 17). Mice were anesthetized using a mixture of isoflurane (1.5%) and oxygen (0.5 l/min). The body temperature was maintained at ∼37°C using a heating pad. Measurements were averaged from nine different readings per mouse. M-mode tracings were used to calculate percent fractional shortening (%FS) (19). Doppler tracings of mitral and aortic flow were acquired from apical four-chamber view and used to measure heart rate (HR), ejection time (ET), isovolumic relaxation time (IVRT), and isovolumic contraction time (IVCT). All echocardiographic assessments and measurements were initially performed by the same investigator. A second person also performed measurements on a separate occasion using the same recordings with no significant differences in interobserver variability.
Adenovirus infection.
Adenoviruses expressing mouse OPN (courtesy of Dr. Toshimitsu Uede, Hokkaido University, Japan) were propagated using HEK-293 cells. The adenoviral titer was determined using the end-point dilution method. ARVMs plated on laminin-coated dishes were infected with the adenoviruses expressing OPN or green fluorescent protein (GFP) at multiplicity of infection of 50–100/cell for 48 h. Cells infected with adenoviruses expressing GFP served as controls.
RT-PCR.
Total RNA was isolated as described previously (42). Total RNA (1 μg) was reverse transcribed using SuperScript III RT kit (Invitrogen, Carlsbad, CA). Primer sequences for RT-PCR amplification were as follows: OPN, 5′-GCTTGGCTTATGGACTGAGG-3′ and 5′-GGCTTTGGAACTTGCTTGAC-3′; and GAPDH, 5′-CTCATGACCACAGTCCATGC-3′ and 5′-TTCAGCTCTGGGATGACCTT-3′. The PCR products were analyzed by 2% agarose gel electrophoresis stained with ethidium bromide.
Apoptosis.
To detect apoptosis, TUNEL staining assay was performed on 4 μm thick myocardial sections or ARVMs plated on Thermanox coverslips as per manufacturer's instructions (cell death detection assay kit; Roche). To identify apoptosis associated with cardiac myocytes, the sections were immunostained with α-sarcomeric actin antibodies (1:50; 5C5 clone; Sigma, St Louis, MO). Hoechst 33258 (10 μM; Sigma) staining was used to count the total number of nuclei (18). TUNEL-positive nuclei that were clearly seen within cardiac myocytes were counted. The number of apoptotic cardiac myocyte nuclei in 10 fields was averaged, and the index of apoptosis was calculated as the percentage of apoptotic myocyte nuclei/total number of nuclei. In ARVMs, the percentage of TUNEL-positive cells (relative to total ARVMs) was determined by counting ∼200 cells in 10 randomly chosen fields per coverslip for each experiment.
Analysis of cytosolic cytochrome c.
To prepare cytosolic fraction, cells were washed with PBS, scraped into the lysis buffer containing 250 mM sucrose, 20 mM HEPES, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 17 μg/ml PMSF, 8 μg/ml aprotinin, and 2 μg/ml leupeptin, and homogenized gently using a glass homogenizer. The cell suspensions were centrifuged at 3,500 g for 5 min to pellet nuclei and other cell debris. The supernatants were centrifuged at 14,000 g for 10 min to pellet mitochondrial fraction. The collected supernatants were again centrifuged at 100,000 g for 30 min at 4°C. The supernatants (cytosolic fractions) were examined by Western blots using anti-cytochrome c antibodies (Santa Cruz).
Western blot analysis.
ARVMs were lysed in cell lysis buffer containing 10 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 0.2 mM sodium orthovanadate, 0.5% Nonidet P-40, 1% Triton X-100, and 1 mM PMSF. LV lysates were prepared in RIPA buffer containing 158 mM NaCl, 10 mM Tris·HCl (pH 7.2), 1 mM EGTA, 1 mM sodium orthovanadate, 0.1% sodium dodecyl sulphate, 1% Triton X-100, 1% sodium deoxycholate, and 1 mM PMSF. Equal amounts of tissue (50 μg) or cell (75 μg) lysates were analyzed by Western blots (23). The primary antibodies used were p-JNK, Gadd153, cytochrome c, Bax, OPN (Santa Cruz), Bcl2 (Cell signaling), and caspase-12 (Sigma). The membranes were stripped and probed for JNKs (Cell signaling) or GAPDH (Santa Cruz) as protein loading controls. Band intensities were quantified using Kodak photodocumentation system (Eastman Kodak).
Co-immunoprecipitation assay.
Total cell lysates were prepared in TNT buffer containing 20 mM Tris·HCl (pH 7.5), 137 mM NaCl, 20 mM NaF, 5 mM EDTA, 1 mM PMSF, 10 mM sodium pyrophosphate, 0.2 M sodium orthovanadate, digitonin 0.05%, and 1% Triton X-100, 1 mM PMSF, 8 μg/ml aprotinin, and 2 μg/ml leupeptin. Cell lysates (300 μg proteins) were incubated with 3 μg of anti-CD44 (Santa Cruz), anti-β1-integrin (Transduction Laboratory), or anti-β3-integrin (Santa Cruz) antibodies overnight at 4°C. Protein A/G beads (60 μl; Thermo Scientific, Rockford, IL) were then added to the mixture and incubated for an additional hour. The beads were washed six times with TNT buffer and resuspended in sample buffer. The samples were separated by 10% SDS-PAGE and transferred to PVDF membranes. The membranes were then probed with anti-OPN (Santa Cruz) antibodies.
Proximity ligation assay.
The Duolink mouse/rabbit red starter kit was used to detect the interaction of OPN and CD44 as per manufacturer's instructions (Olink Bioscience, Uppsala, Sweden). After fixing and permeabilization, the cells were incubated overnight with primary antibodies (monoclonal anti-OPN, Santa Cruz; and polyclonal anti-CD44 antibodies; IM7; Abcam). This was followed by a 37°C incubation with the proximity ligation assay (PLA) probe plus and the PLA probe minus against the different species. After a ligation reaction at 37°C of the DNA chains directly coupled to the PLA probes, the cells were mounted and detection of the amplified probe was done using fluorescent microscopy.
Statistical analyses.
All data are expressed as means ± SE. Statistical analysis was performed using Student's t-test or one-way ANOVA and a post hoc Tukey's test. Probability (p) values <0.05 were considered to be significant.
RESULTS
OPN induces apoptosis in ARVMs.
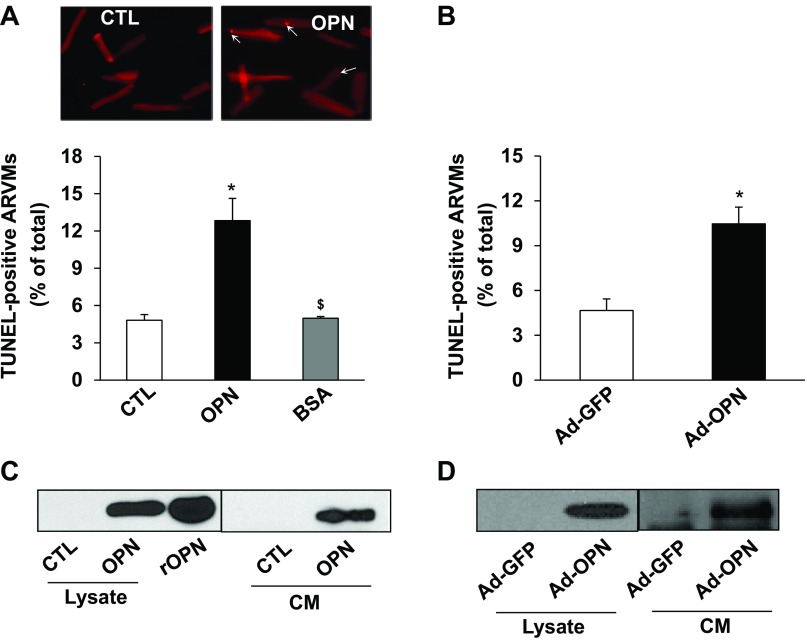
Patients with cardiac dysfunction display increased plasma OPN levels up to 1,145 ng/ml (7, 35, 51). This amounts to plasma concentration of OPN as ∼16.6 nM assuming molecular weight of OPN as 69 kDa. To investigate if OPN induces apoptosis, ARVMs were treated with 2.0 or 20 nM of purified OPN protein for 24 h. Analysis of apoptosis using TUNEL-assay showed approximately two- and threefold increase in apoptosis using 2.0 and 20 nM of OPN, respectively, compared with control (data not shown). Because the magnitude of apoptosis was higher with 20 nM OPN, this concentration was used for further experimentation. Treatment with BSA (20 nM) served as a negative control. OPN, not BSA, significantly increased the number of apoptotic ARVMs compared with control (CTL; 4.8 ± 0.4; OPN, 12.8 ± 1.77*; BSA, 4.9 ± 0.1; *P < 0.05 vs. CTL; n = 3–7; Fig. 1A). To confirm the effects of purified OPN on apoptosis, ARVMs were infected with adenoviruses expressing OPN (Ad-OPN) or GFP (Ad-GFP) for 48 h. TUNEL-assay showed that adenoviral-mediated OPN expression, not GFP, also significantly increases the number of apoptotic ARVMs (Fig. 1B). Western blot analyses showed presence of OPN in the conditioned media (CM) as well as cell lysates (Fig. 1C) 24 h after OPN treatment. Increased expression and secretion of OPN was also observed 48 h after infection of ARVMs with Ad-OPN (Fig. 1D).
Fig. 1.
Osteopontin (OPN) induces apoptosis in adult rat ventricular myocytes (ARVMs). ARVMs were treated with OPN (20 nM) and BSA (20 nM) for 24 h (A), or infected with adenoviruses expressing OPN or green fluorescent protein (GFP) for 48 h (B). Apoptosis was measured using TUNEL assay. A, top: TUNEL images of ARVMs. *P < 0.05 vs. control (CTL) or GFP; $P < 0.05 vs. OPN; n = 3–7. Cell lysates and conditioned media (CM) were analyzed by Western using anti-OPN antibodies following treatment with OPN (C) or infection with adenoviruses (D). Recombinant OPN (rOPN) served as a positive control.
OPN interacts with CD44 receptors.
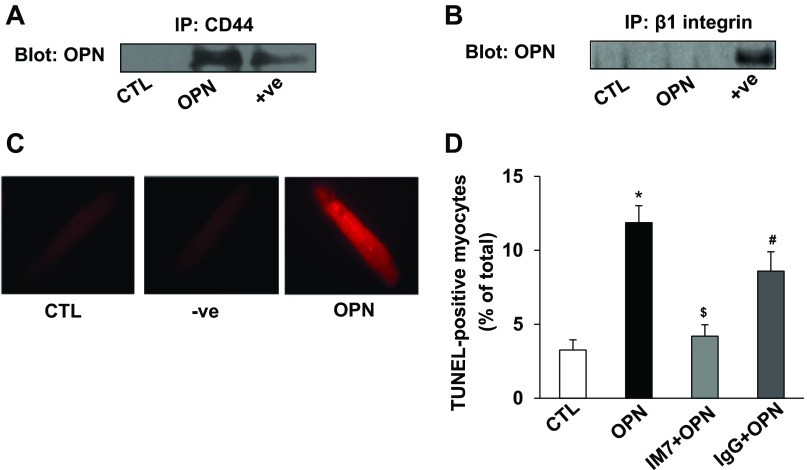
In cells of noncardiac origin, various integrins and CD44 are identified as receptor/s for OPN (44). Cardiac myocytes predominantly express β1-integrins (36). Adult feline cardiac myocytes are suggested to express β3-integrins (26). To investigate the interaction of OPN with its receptor, we first performed co-immunoprecipitation assays. Co-immunprecipitation is considered as a standard method to determine whether two proteins of interest form a complex. For this, cell lysates were immonoprecipitated with anti-CD44 or anti-β1-integrin or anti-β3-integrin antibodies. The resultant immunoprecipitates were analyzed by Western blots using anti-OPN antibodies. This analysis showed the presence of OPN in cell lysates immunoprecipitated with anti-CD44 antibodies (Fig. 2A), not with anti-β1-integrin (Fig. 2B) or anti-β3-integrin (data not shown) antibodies. To confirm interaction of OPN with CD44, we used a recently developed commercial assay (Duolink) called PLA. The PLA identifies interactions between two proteins in their native form. This assay results in a fluorescent signal in the form of a spot when the two proteins of interest are closer than 40 nm. The specificity of the assay was tested using single antibody directed against CD44. In our assay, no spots were observed using the single antibody. However, clear positive signal for the interaction of OPN with CD44 was detected in OPN-treated ARVMs as indicated by bright red fluorescence (Fig. 2C).
Fig. 2.
OPN interacts with CD44, and neutralization of CD44 inhibits OPN-stimulated apoptosis. ARVMs were treated with OPN (20 nM) for 24 h. Cell lysates were immunoprecipitated with anti-CD44 or anti-β1-integrin antibodies. Immunoprecipitates were analyzed by Western blot using anti-OPN (A and B) antibodies. Total ARVMs lysate treated with OPN served as positive (+ve) control. C: ARVMs were treated with OPN for 24 h. The cells were then used for proximity ligation assay (PLA) using anti-OPN and/or anti-CD44 antibodies. Middle: (−ve) PLA-staining using single (anti-CD44) antibody in OPN-treated cells and serves as a negative control. Fluorescent red staining in OPN-treated sample indicates interaction of OPN and CD44. D: ARVMs were pretreated with neutralizing anti-CD44 (IM7) or control IgG for 30 min followed by treatment with OPN for 24 h. Apoptosis was measured using TUNEL assay. *P < 0.05 vs. control (CTL); $P < 0.05 vs. OPN; #P < 0.05 vs. IM7 + OPN; n = 6.
OPN induces apoptosis via the involvement of CD44 receptor.
To investigate the involvement of CD44 receptors in OPN-stimulated apoptosis, ARVMs were pretreated with neutralizing CD44 (IM7) antibodies followed by treatment with OPN. IgG pretreated cells in the presence of OPN served as controls. Analysis of apoptosis using TUNEL-assay showed that IM7, not IgG, significantly inhibits OPN-stimulated apoptosis (CTL, 3.25 ± 0.7; OPN, 11.9 ± 1.2*; IM7 + OPN, 4.2 ± 0.8$; IgG + OPN, 8.6 ± 1.3#; *P < 0.05 vs CTL; $P < 0.05 vs. OPN; #P < 0.05 vs IM7 + OPN; Fig. 2D).
OPN induces mitochondrial death pathway and ER stress.
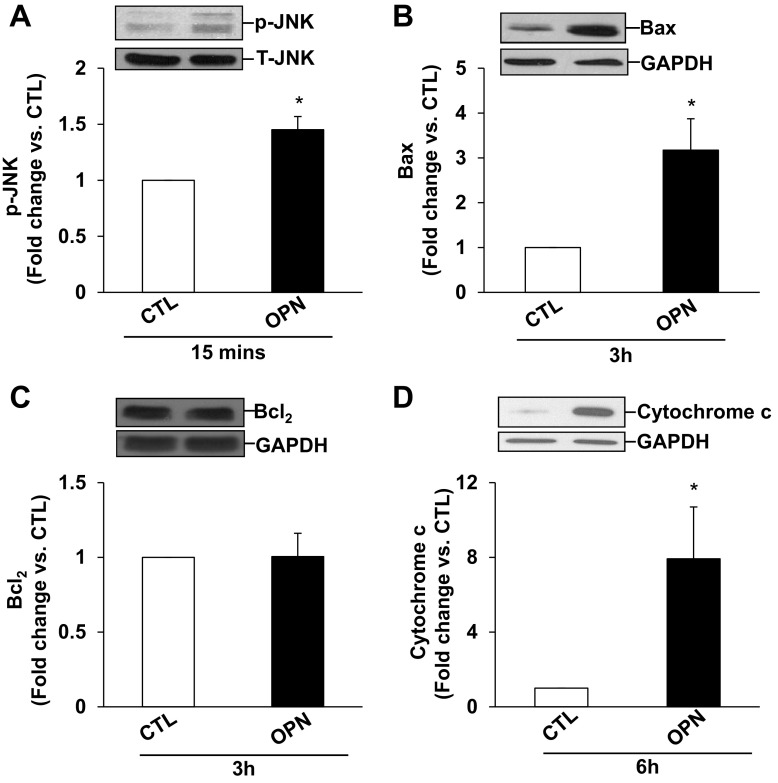
In ARVMs, β-adrenergic receptor-stimulated apoptosis is shown to occur via the involvement of mitochondrial death pathway and ER stress (5, 33). To assess induction of mitochondrial death pathway, we measured activation of JNKs, expression of Bax and Bcl2, and levels of cytosolic cytochrome c. For this, ARVMs were treated with OPN for indicated time points. Western blot analysis of cell lysates using phospho-specific antibodies showed that OPN activates JNKs (46 and 54 kDa; Fig. 3A). OPN treatment increased p-JNKs levels by ∼1.5-fold compared with CTL. OPN treatment significantly increased Bax expression (∼3.2-fold; Fig. 3B) compared with CTL. However, it had no effect on Bcl2 protein levels (Fig. 3C). Bcl2/Bax ratio was significantly lower in OPN-treated cells (CTL, 1.95 ± 0.38; OPN, 0.80 ± 0.11*; P < 0.05 vs. CTL). Cytosolic cytochrome c levels were significantly higher in OPN-treated (∼7.9-fold; Fig. 3D) cells.
Fig. 3.
OPN activates JNKs and increases Bax expression and levels of cytosolic cytochrome c. ARVMs were treated with OPN for 15 min (A), 3 h (B and C), or 6 h (D). Cell lysates were analyzed by Western blots using anti p-JNKs (A), anti-Bax (B), anti-Bcl2 (C), or anti-cyotchrome c (D) antibodies. Protein loading was normalized using total JNKs or GAPDH immunostaining. Bottom: mean data normalized to JNKs or GAPDH. *P < 0.05 vs. CTL; n = 3 to 4.
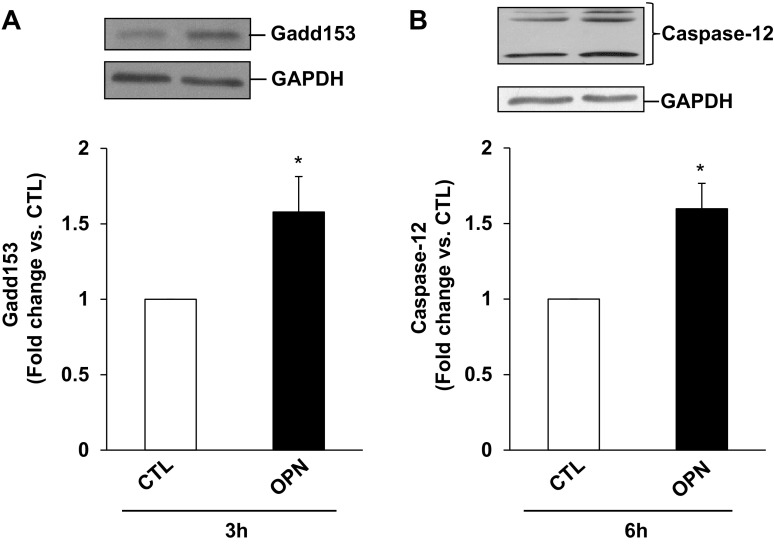
Increased Gadd153 expression is considered as a hallmark of ER stress, whereas caspase-12 is considered as the main caspase associated with the ER stress-induced apoptosis (27). Previously, we have shown that β-AR stimulation increases Gadd-153 expression within 3 h (5), whereas increased levels of cytosolic cytochrome c are observed 6 h after β-AR stimulation (45). To investigate whether OPN induces ER stress, ARVMs were treated with OPN for 3 or 6 h. Western blot analyses of cell lysates showed that OPN significantly increases Gadd-153 expression and activates caspase-12 (Fig. 4, A and B). Cleavage products of <45 kDa correspond to active caspase-12.
Fig. 4.
OPN induces endoplasmic reticulum (ER) stress. ARVMs were treated with OPN for 6 h. Cell lysates were analyzed by Western blot using anti-Gadd153 (A) or anti-caspase-12 (B) antibodies. Protein loading was normalized using GAPDH immunostaining. Bottom: mean data normalized to GAPDH; *P < 0.05 vs. CTL; n = 4.
Inhibition of JNKs inhibits OPN-stimulated apoptosis.
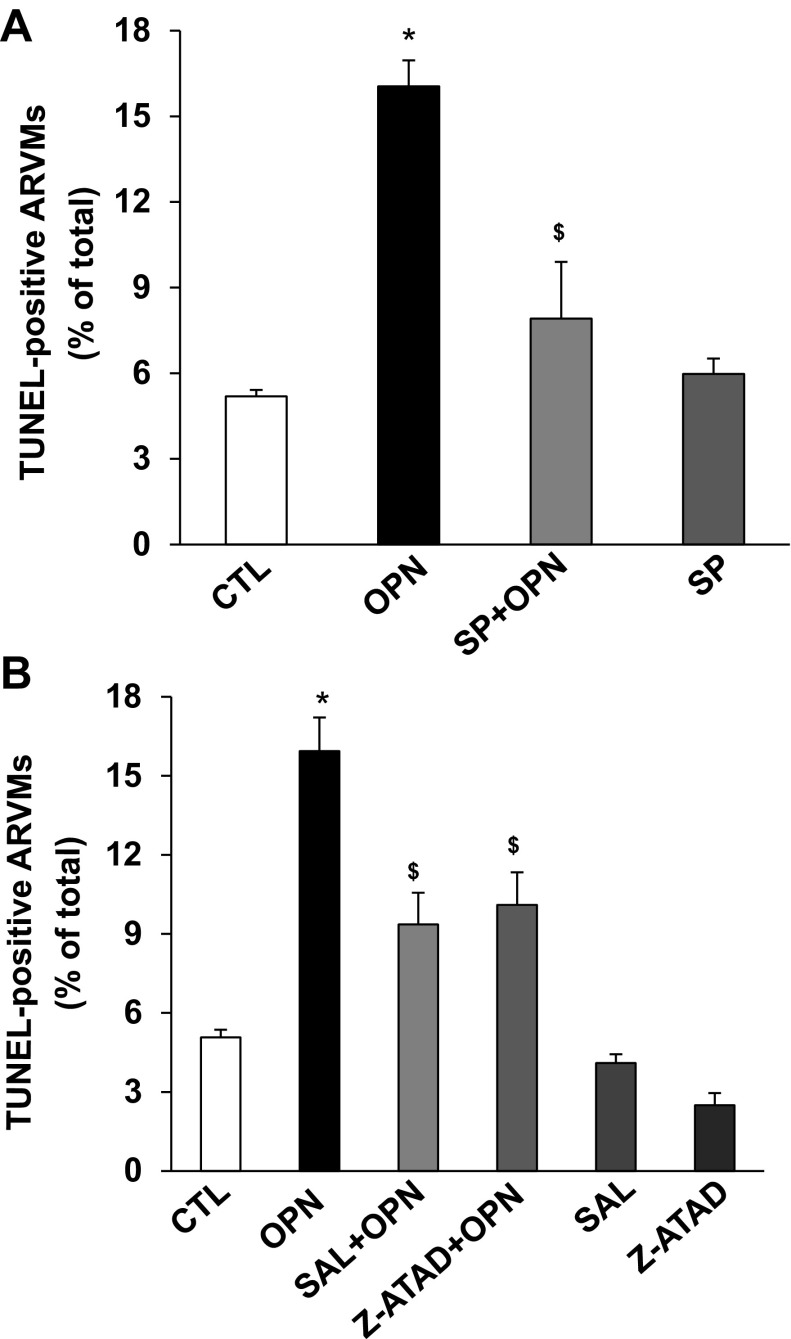
To investigate the involvement of JNKs in OPN-stimulated apoptosis, ARVMs were pretreated with SP6001 (SP; 10 μM; JNKs inhibitor) for 30 min followed by treatment with OPN for 24 h. Analysis of apoptosis using TUNEL-assay showed that inhibition of JNKs significantly inhibits OPN-stimulated apoptosis (CTL, 5.19 ± 0.22; OPN, 16.04 ± 0.91*; SP + OPN, 7.9 ± 1.98$; SP, 5.97 ± 0.53; *P < 0.05 vs. CTL; $P < 0.05 vs. OPN, n = 3–7, Fig. 5A). SP alone had no effect on apoptosis.
Fig. 5.
Inhibition of JNKs or ER stress pathway inhibits OPN-stimulated apoptosis. A: ARVMs were pretreated with SP600125 (SP; JNKs inhibitor) for 30 min followed by treatment with OPN for 24 h. Apoptosis was measured using TUNEL assay. *P < 0.05 vs. CTL; $P < 0.05 vs. OPN; n = 3–7. B: ARVMs were pretreated with salubrinal (SAL; ER stress inhibitor) or z-ATAD-FMK (z-ATAD; caspase-12 inhibitor) for 30 min followed by treatment with OPN for 24 h. Apoptosis was measured using TUNEL assay. *P < 0.05 vs. CTL; $P < 0.05 vs. OPN; n = 3–5.
Alleviation of ER stress or inhibition of caspase-12 inhibits OPN-stimulated apoptosis.
To investigate the involvement of ER stress in OPN-stimulated apoptosis, ARVMs were pretreated with salubrinal (SAL; 1 μM; ER stress inhibitor) or z-ATAD-FMK (z-ATAD; 10 μM; caspase-12 inhibitor) followed by treatment with OPN for 24 h. Analysis of apoptosis using TUNEL-assay showed that alleviation of ER stress or inhibition of caspase-12 significantly inhibits OPN-stimulated apoptosis (CTL, 5.07 ± 0.29; OPN, 15.94 ± 1.27*; SAL + OPN, 9.36 ± 1.2$; z-ATAD + OPN, 10.1 ± 1.24$ SAL, 4.1 ± 0.33; z-ATAD, 2.5 ± 0.46; *P < 0.05 vs. CTL; $P < 0.05 vs. OPN, n = 3–5; Fig. 5B). SAL or z-ATAD alone had no effect on apoptosis.
Increased OPN expression in the adult heart induces LV dysfunction and increases myocyte apoptosis.
Increased OPN expression in the heart in a cardiac myocyte-specific manner during the first few weeks of age is shown to associate with increased cardiac myocyte apoptosis and dilated cardiomyopathy (34). To investigate the effects of OPN in adult heart, doxycycline (Dox) was removed from the diet when mice were ∼4 mo old. RT-PCR and Western blot analyses confirmed increased OPN expression in the heart 28 days after Dox withdrawal (data not shown). The mortality rate before and 8 wk after the Dox withdrawal was same between the MHC-OPN and littermate controls. One mouse in each group died during the 8 wk of Dox withdrawal.
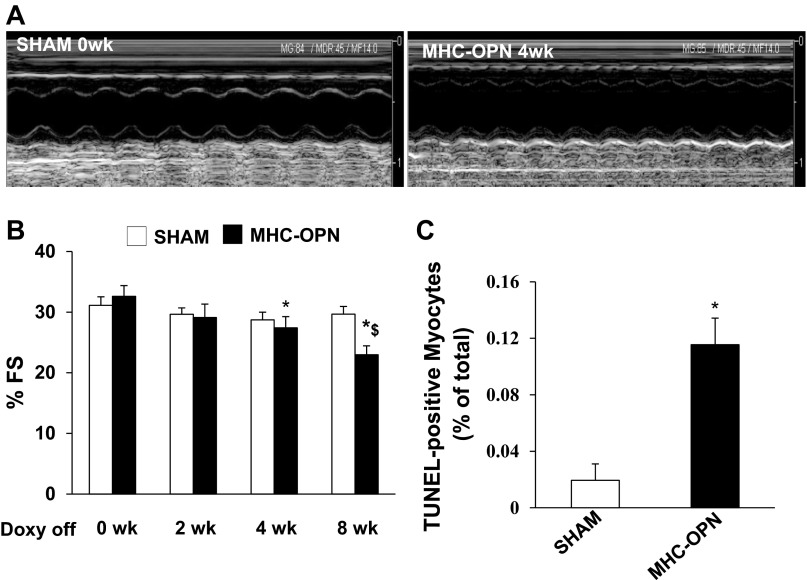
To measure heart function, echocardiography was performed in MHC-OPN and their littermate controls at baseline (0 wk), 2, 4, and 8 wk after Dox withdrawal. No differences in the echocardiographic parameters were observed among the littermate controls, WT, α-MHC-tTA, and TetO-OPN. Therefore, they were pooled as sham. Figure 6A depicts M-mode echocardiography images of sham (0 wk) and MHC-OPN mice (4 wk after Dox withdrawal). M-mode echocardiography showed no significant decline in %FS in sham animals at 2, 4, and 8 wk vs. 0 wk. In MHC-OPN group, no significant decline in %FS was observed at 2 wk after Dox withdrawal vs. 0 wk. However, a significant decrease in %FS was observed at 4 and 8 wk after Dox withdrawal vs. 0 wk (Fig. 6B). In fact, %FS was significantly lower in MHC-OPN mice at 8 wk after Dox withdrawal vs. at 4 wk after Dox withdrawal. Doppler echocardiography showed no change in HR, ET, IVRT, and IVCT in sham animals at 0, 4, and 8 wk after Dox withdrawal. In MHC-OPN group, HR and ET also remained unchanged during the observation period. However, a significant decline in IVRT and IVCT was observed at 4 and 8 wk vs. 0 wk (Table 1).
Fig. 6.
Increased OPN expression associates with left ventricular dysfunction and increased myocyte apoptosis. Doxycycline (DOX) was removed from the diet when the mice were 4 mo old. A: M-mode echocardiographic images obtained from sham (0 wk) and dual transgenic (MHC-OPN) mice 4 wk after DOX withdrawal (Doxy off). Percent fractional shortening (%FS) was measured using echocardiography at 0, 2, 4, and 8 wk after DOX withdrawal (B). *P < 0.05 vs. 0 wk MHC-OPN; $P < 0.05 vs. 4 wk MHC-OPN; n = 4–10. C: quantitative analysis of myocyte apoptosis using TUNEL assay. *P < 0.05 vs. sham; n = 4.
Table 1.
Doppler echocardiographic measurements
| Sham, wk |
Dual Transgenic, wk |
|||||
|---|---|---|---|---|---|---|
| Parameters | 0 | 4 | 8 | 0 | 4 | 8 |
| n | 16 | 16 | 13 | 8 | 8 | 5 |
| Heart rate, beats/min | 344.3 ± 8.2 | 367.1 ± 14.8 | 369.3 ± 12.3 | 311.5 ± 15.3 | 336.0 ± 12.8 | 367.4 ± 28.6 |
| Ejection time, ms | 54.2 ± 3.1 | 56.4 ± 3.5 | 48.8 ± 1.7 | 56.3 ± 2.4 | 51.3 ± 1.6 | 49.3 ± 4.7 |
| Isovolumetric relaxation time, ms | 29.2 ± 1.0 | 26.3 ± 1.6 | 25.8 ± 0.4 | 31.9 ± 3.7 | 26.0 ± 1.2 | 21.2 ± 1.6* |
| Isovolumetric contraction time, ms | 26.8 ± 1.2 | 26.3 ± 1.4 | 23.9 ± 1.0 | 30.2 ± 1.7 | 30.3 ± 1.9 | 19.4 ± 3.9* |
Values are means ± SE.
Comparison vs. 0 wk dual transgenic, P < 0.05.
Analysis of apoptosis using TUNEL-assay revealed increased myocyte apoptosis in OPN-expressing mice compared with sham (% apoptosis; sham, 0.02 ± 0.01; OPN, 0.12 ± .02*; *P < 0.05 vs. sham; n = 4; Fig. 6C).
Increased OPN expression in the heart associates with mitochondrial death pathway and ER stress.
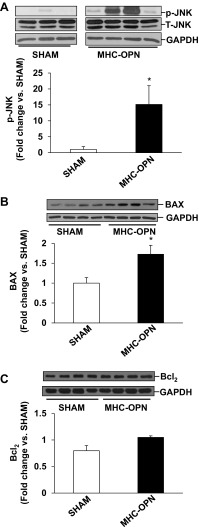
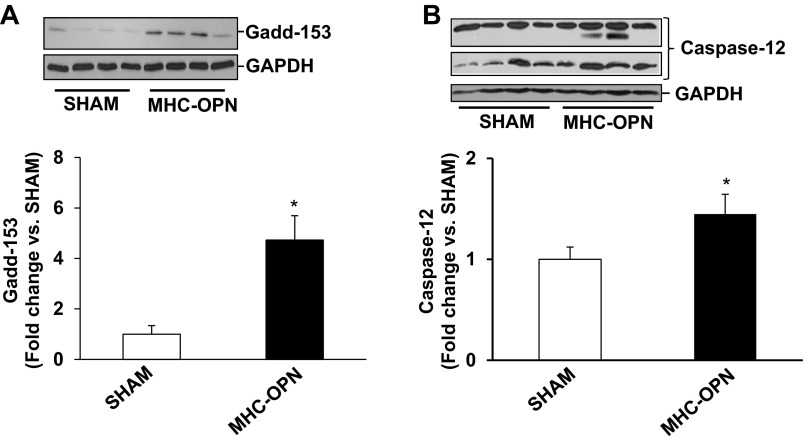
We next analyzed whether increased OPN expression in the heart in myocyte-specific manner induces mitochondrial death pathway and ER stress in the heart. Western blot analyses of LV lysates 4 wk after Dox withdrawal showed little or no phosphorylation of JNKs in the sham group. Phosphorylation of JNKs was significantly higher in MHC-OPN group versus sham (Fig. 7A). Bax protein levels were significantly higher in MHC-OPN group versus sham (Fig. 7B). There was no change in BCl2 protein levels (Fig. 7C). Bcl2/Bax ratio was significantly lower in OPN-treated cells (sham, 0.98 ± 0.07; MHC-OPN, 0.60 ± 0.07*; *P < 0.05 vs sham; n = 4). Expression of Gadd153 was greater than threefold higher in MHC-OPN group versus sham (Fig. 8A). The levels of cleavage products of caspase-12, representing caspase-12 activation, were also significantly higher in MHC-OPN group (Fig. 8B).
Fig. 7.
Increased OPN expression in the heart activates JNKs, increases Bax expression, and decreases Bcl2/Bax ratio. Left ventricular lysates were analyzed by Western using anti-p-JNKs (A), anti-Bax (B), and anti-Bcl2 (C) antibodies. Protein loading was normalized using total JNKs or GAPDH immunostaining. Bottom: mean data normalized with JNK or GAPDH. *P < 0.05 vs. sham; n = 3 to 4.
Fig. 8.
Increased OPN expression in the heart induces ER stress. Left ventricular lysates were analyzed by Western using anti-Gadd153 (A) and anti-caspase-12 (B) antibodies. Protein loading was normalized using GAPDH immunostaining. Bottom: mean data normalized to GAPDH. *P < 0.05 vs. sham; n = 3 to 4.
DISCUSSION
Increased OPN expression associates with increased myocyte apoptosis in different models of heart disease (21, 38, 47). Here we provide evidence that 1) OPN induces apoptosis in ARVMs; 2) OPN interacts with CD44 receptors, and CD44 receptor plays an important role in OPN-stimulated apoptosis; 3) OPN activates mitochondrial death pathway as evidenced by activation of JNKs, increased expression of Bax, and increased levels of cytosolic cytochrome c; 4) OPN induces ER stress as evidenced by increased expression of Gadd153 and activation of caspase-12; 5) inhibition of JNKs or alleviation of ER stress inhibits OPN-stimulated apoptosis; 6) cardiac myocyte-specific expression of OPN in the adult mouse heart induces LV dysfunction and increases myocyte apoptosis; and 7) in vivo, increased OPN expression in myocytes associates with the induction of mitochondrial death pathway and ER stress.
In tumor cells, OPN is accepted as an activator of anti-apoptotic and pro-survival pathways (1, 53). Lack of OPN associates with higher apoptosis in tubular epithelium and interstitium during the injury phase of post-ischemic acute renal failure (30). In contrast, lack of OPN associated with lower chondrocyte apoptosis in a model of rheumatoid arthritis (59). Hindlimb-unloading led to increased apoptosis in the spleen and thymus of WT, not OPN knockout, mice (55). In the heart, increased OPN expression associates with increased myocyte apoptosis in different models of heart disease (21, 38, 47). Cardiac myocyte-specific expression of OPN in the mouse heart during the first 11 wk of life led to increased myocyte apoptosis and myocardial dysfunction (34). Using isolated adult rat cardiac myocytes, we first provide evidence that OPN induces apoptosis in cardiac myocytes. Treatment with purified OPN induced apoptosis in a concentration-dependent manner. Adenoviral-mediated expression of OPN confirmed OPN-stimulated apoptosis in myocytes. In vascular smooth muscle cells, OPN is shown to induce autophagy (60). However, treatment of ARVMs with OPN for 24 h failed to increase beclin-1 and cathepsin-D protein levels (data not shown), suggesting that OPN induces apoptosis, not autophagy, in myocytes.
In cells of noncardiac origin, OPN is shown to interact with αvβ1-, αvβ3-, αvβ5-, and α8β1-integrins in an RGD-dependent manner. Evidence has been provided for the interaction of OPN with α4β1- and α9β1-integrins in a non-RGD-dependent manner. CD44 is also identified as a receptor for OPN (44, 54). Cardiac myocytes predominantly express β1-integrins (36). Feline myocytes are suggested to express β3-integrins (26). Most of the effects of OPN are attributed to the interaction of secreted OPN and its receptors on the target cells. However, alternate translation of OPN is shown to generate intracellular isoforms capable of distinct biological activities in dendritic cells (41). Using co-immunoprecipitation assays, we provide evidence that OPN interacts with CD44 receptors, not with β1- or β3-integrins, in myocytes. This was confirmed by visualization of a positive signal in OPN-treated samples using PLA. Furthermore, neutralizing anti-CD44 antibodies inhibited OPN-stimulated apoptosis. Collectively, these data suggest that OPN, most likely acting via CD44 receptors, induces apoptosis in myocytes. The observation that neutralization of CD44 receptor inhibits OPN-stimulated apoptosis suggests that OPN, at least in part, acting via a receptor-mediated process induces apoptosis in myocytes. However, involvement of intracellular isoforms of OPN cannot be ruled out.
Mitochondria play a central role in cellular metabolism, energy production, and determination of cell fate with respect to survival and apoptosis (2). Cardiac mitochondrial dysfunction is suggested to associate with the progression of heart failure. Translocation of Bax to mitochondria and release of cytochrome c (i.e., increased levels of cytosolic cytochrome c) are considered as major events of mitochondrial dysfunction. In cardiac myocytes, β-AR-stimulated apoptosis is mediated by JNK-dependent activation of the mitochondrial death pathway (33). Here we observed that OPN activates JNKs, increases Bax expression and levels of cytosolic cytochrome c, and decreases Bcl2/Bax ratio. Inhibition of JNKs inhibited OPN-stimulated apoptosis. These observations suggest OPN-mediated activation of mitochondrial death pathway.
The ER regulates protein synthesis, protein folding and trafficking, cellular responses to stress, and intracellular Ca++ levels (31, 37, 50). Alterations in Ca++ homeostasis and accumulation of misfolded proteins initiate an adaptive response in the cell, termed the unfolded protein response (UPR; ER stress response). As a result, ER localized chaperones are induced, protein synthesis is slowed down and a protein degrading system is initiated. However, prolonged ER stress triggers apoptosis in various cell types (50). ER stress is suggested to play a role in myocyte apoptosis and pathogenesis of heart failure (14, 28, 29). Previously, we have shown that β-AR (β-AR) stimulation induces ER stress in ARVMs, and induction of ER stress plays a pro-apoptotic role in β-AR-stimulated apoptosis (5). Here we provide evidence that OPN induces ER stress in ARVMs. Treatment of ARVMs with purified OPN increased Gadd153 expression and activated caspase-12. Alleviation of ER stress using salubrinal or inhibition of caspase-12 significantly inhibited OPN-stimulated apoptosis. Suggestions have been made that ER stress may act upstream in the induction of mitochondrial death pathway (12). Activation of p38 kinase and PKC, specifically PKCϵ, is suggested to play a role in the induction of ER stress, and ER-mitochondria crosstalk in myocytes (9, 20). Further investigations are needed to explore the signaling pathway/s, and the cross-talk between ER and mitochondria during OPN-stimulated myocyte apoptosis.
Myocyte-specific expression of OPN in the myocardium of mice during first few weeks of life is shown to associate with premature death. A significant increase in mortality was observed in MHC-OPN mice between the 8th and 15th wk after birth with a half-life of 12 wk (34). In our study, Dox was withdrawn from the diet when mice were ∼4 mo old. We did not observe any change in the mortality rates between MHC-OPN and control mice with or without DOX. However, similar to the studies of Renault et al. (34), OPN expression in myocyte-specific manner in adult heart associated with increased myocyte apoptosis and myocardial dysfunction. Myocyte-specific expression of OPN associated with mitochondrial death pathway as evidenced by activation of JNKs, increased Bax expression, and decreased Bcl2/Bax ratio. It also induced ER stress as evidenced by increased expression of Gadd153 and activation of caspase-12.
The data presented here are of clinical significance since OPN expression increases markedly under a variety of pathophysiological conditions of the heart in animal models (13, 43, 47, 49, 52, 57) and in humans (39, 46, 48, 51). Myocytes are identified as source of OPN in mice (47) and in humans during various pathologies of the heart (13, 46). Furthermore, elevated plasma OPN levels correlated with the severity and risk of subsequent death due to dilated or ischemic cardiomyopathy (35). Here we show that OPN, most likely acting via CD44 receptors, induces apoptosis in adult cardiac myocytes. OPN-mediated apoptosis occurs via the involvement of mitochondrial death pathway and ER stress. Future studies directed toward understanding the signaling pathways from CD44 receptors to the initiation of mitochondrial death pathway and ER stress may help uncover novel strategies for the treatment of myocardial dysfunction.
GRANTS
This work is supported by Merit Review award BX000640 from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development, National Heart, Lung, and Blood Institute Grants R21HL-091405 and R21HL-092459, and institutional research and improvement funds (to K. Singh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.D., Q.Z., C.R.D., and R.J.S. performed experiments; S.D., Q.Z., C.R.D., and R.J.S. analyzed data; S.D., C.R.D., M.S., and K.S. interpreted results of experiments; S.D. prepared figures; S.D. and K.S. drafted manuscript; S.D., W.L.J., A.-P.G., M.S., and K.S. edited and revised manuscript; S.D., Q.Z., C.R.D., R.J.S., W.L.J., A.-P.G., M.S., and K.S. approved final version of manuscript; A.-P.G., M.S., and K.S. conception and design of research.
REFERENCES
- 1.Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E, Lee NP, Wu GH, Luk JM. Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers. World J Gastroenterol 18: 3923–3930, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Knowlton AA. Mitochondria and heart failure: new insights into an energetic problem. Minerva Cardioangiol 58: 213–229, 2010 [PMC free article] [PubMed] [Google Scholar]
- 3.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation 98: 1329–1334, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Communal C, Singh M, Menon B, Xie Z, Colucci WS, Singh K. Beta1 integrins expression in adult rat ventricular myocytes and its role in the regulation of beta-adrenergic receptor-stimulated apoptosis. J Cell Biochem 89: 381–388, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Dalal S, Foster CR, Das BC, Singh M, Singh K. Beta-adrenergic receptor stimulation induces endoplasmic reticulum stress in adult cardiac myocytes: role in apoptosis. Mol Cell Biochem 364: 59–70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels CR, Foster CR, Yakoob S, Dalal S, Joyner WL, Singh M, Singh K. Exogenous ubiquitin modulates chronic β-adrenergic receptor-stimulated myocardial remodeling: role in Akt activity and matrix metalloproteinase expression. Am J Physiol Heart Circ Physiol 303: H1459–H1468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Ry S, Giannessi D, Maltinti M, Cabiati M, Prontera C, Iervasi A, Caselli C, Mazzone AM, Neglia D. Increased plasma levels of osteopontin are associated with activation of the renin-aldosterone system and with myocardial and coronary microvascular damage in dilated cardiomyopathy. Cytokine 49: 325–330, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Di Napoli P, Taccardi AA, Grilli A, Felaco M, Balbone A, Angelucci D, Gallina S, Calafiore AM, De Caterina R, Barsotti A. Left ventricular wall stress as a direct correlate of cardiomyocyte apoptosis in patients with severe dilated cardiomyopathy. Am Heart J 146: 1105–1111, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Dong S, Teng Z, Lu FH, Zhao YJ, Li H, Ren H, Chen H, Pan ZW, Lv YJ, Yang BF, Tian Y, Xu CQ, Zhang WH. Post-conditioning protects cardiomyocytes from apoptosis via PKCϵ-interacting with calcium-sensing receptors to inhibit endo(sarco)plasmic reticulum-mitochondria crosstalk. Mol Cell Biochem 341: 195–206, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 92: 635–688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg S, Narula J, Chandrashekhar Y. Apoptosis and heart failure: clinical relevance and therapeutic target. J Mol Cell Cardiol 38: 73–79, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Germain M, Mathai JP, Shore GC. BH-3-only BIK functions at the endoplasmic reticulum to stimulate cytochrome c release from mitochondria. J Biol Chem 277: 18053–18060, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Graf K, Do YS, Ashizawa N, Meehan WP, Giachelli CM, Marboe CC, Fleck E, Hsueh WA. Myocardial osteopontin expression is associated with left ventricular hypertrophy. Circulation 96: 3063–3071, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Suzuki M, Nishino T, Nakaya H, Koseki H, Aoe T. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol 24: 8007–8017, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajstura J, Bolli R, Sonnenblick EH, Anversa P, Leri A. Cause of death: suicide. J Mol Cell Cardiol 40: 425–437, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem 102: 912–924, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Krishnamurthy P, Peterson JT, Subramanian V, Singh M, Singh K. Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol Cell Biochem 322: 53–62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnamurthy P, Subramanian V, Singh M, Singh K. Deficiency of beta1 integrins results in increased myocardial dysfunction after myocardial infarction. Heart 92: 1309–1315, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnamurthy P, Subramanian V, Singh M, Singh K. Beta1 integrins modulate beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension 49: 865–872, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Mao W, Fukuoka S, Iwai C, Liu J, Sharma VK, Sheu SS, Fu M, Liang CS. Cardiomyocyte apoptosis in autoimmune cardiomyopathy: mediated via endoplasmic reticulum stress and exaggerated by norepinephrine. Am J Physiol Heart Circ Physiol 293: H1636–H1645, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, Liu L, Morimoto J, Rittling SR, Denhardt D, Kitabatake A, Uede T. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension 43: 1195–1201, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Menon B, Johnson JN, Ross RS, Singh M, Singh K. Glycogen synthase kinase-3beta plays a pro-apoptotic role in beta-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes: role of beta1 integrins. J Mol Cell Cardiol 42: 653–661, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon B, Singh M, Ross RS, Johnson JN, Singh K. β-Adrenergic receptor-stimulated apoptosis in adult cardiac myocytes involves MMP-2-mediated disruption of β1 integrin signaling and mitochondrial pathway. Am J Physiol Cell Physiol 290: C254–C261, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Menon B, Singh M, Singh K. Matrix metalloproteinases mediate β-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes. Am J Physiol Cell Physiol 289: C168–C176, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Nadal-Ginard B, Kajstura J, Anversa P, Leri A. A matter of life and death: cardiac myocyte apoptosis and regeneration. J Clin Invest 111: 1457–1459, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai T, Laser M, Baicu CF, Zile MR, Cooper G, Kuppuswamy D. Beta3-integrin-mediated focal adhesion complex formation: adult cardiocytes embedded in three-dimensional polymer matrices. Am J Cardiol 83: 38H–43H, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403: 98–103, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Nickson P, Toth A, Erhardt P. PUMA is critical for neonatal cardiomyocyte apoptosis induced by endoplasmic reticulum stress. Cardiovasc Res 73: 48–56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110: 705–712, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int 63: 543–553, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11: 372–380, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Redfern CH, Coward P, Degtyarev MY, Lee EK, Kwa AT, Hennighausen L, Bujard H, Fishman GI, Conklin BR. Conditional expression and signaling of a specifically designed Gi-coupled receptor in transgenic mice. Nat Biotechnol 17: 165–169, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res 92: 136–138, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Renault MA, Robbesyn F, Reant P, Douin V, Daret D, Allieres C, Belloc I, Couffinhal T, Arnal JF, Klingel K, Desgranges C, Dos SP, Charpentier F, Gadeau AP. Osteopontin expression in cardiomyocytes induces dilated cardiomyopathy. Circ Heart Fail 3: 431–439, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg M, Zugck C, Nelles M, Juenger C, Frank D, Remppis A, Giannitsis E, Katus HA, Frey N. Osteopontin, a new prognostic biomarker in patients with chronic heart failure. Circ Heart Fail 1: 43–49, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Ross RS. Cardiac remodeling: is 8 the heart's lucky number? J Mol Cell Cardiol 36: 323–326, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol 14: 20–28, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Sam F, Xie Z, Ooi H, Kerstetter DL, Colucci WS, Singh M, Singh K. Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am J Hypertens 17: 188–193, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Satoh M, Nakamura M, Akatsu T, Shimoda Y, Segawa I, Hiramori K. Myocardial osteopontin expression is associated with collagen fibrillogenesis in human dilated cardiomyopathy. Eur J Heart Fail 7: 755–762, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 27: 2302–2309, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci USA 105: 7235–7239, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh K, Balligand JL, Fischer TA, Smith TW, Kelly RA. Glucocorticoids increase osteopontin expression in cardiac myocytes and microvascular endothelial cells. Role in regulation of inducible nitric oxide synthase. J Biol Chem 270: 28471–28478, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Singh K, Sirokman G, Communal C, Robinson KG, Conrad CH, Brooks WW, Bing OH, Colucci WS. Myocardial osteopontin expression coincides with the development of heart failure. Hypertension 33: 663–670, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Singh M, Foster CR, Dalal S, Singh K. Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol 48: 538–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh M, Roginskaya M, Dalal S, Menon B, Kaverina E, Boluyt MO, Singh K. Extracellular ubiquitin inhibits beta-AR-stimulated apoptosis in cardiac myocytes: role of GSK-3beta and mitochondrial pathways. Cardiovasc Res 86: 20–28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stawowy P, Blaschke F, Pfautsch P, Goetze S, Lippek F, Wollert-Wulf B, Fleck E, Graf K. Increased myocardial expression of osteopontin in patients with advanced heart failure. Eur J Heart Fail 4: 139–146, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Subramanian V, Krishnamurthy P, Singh K, Singh M. Lack of osteopontin improves cardiac function in streptozotocin-induced diabetic mice. Am J Physiol Heart Circ Physiol 292: H673–H683, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Suezawa C, Kusachi S, Murakami T, Toeda K, Hirohata S, Nakamura K, Yamamoto K, Koten K, Miyoshi T, Shiratori Y. Time-dependent changes in plasma osteopontin levels in patients with anterior-wall acute myocardial infarction after successful reperfusion: correlation with left-ventricular volume and function. J Lab Clin Med 145: 33–40, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Szalay G, Sauter M, Haberland M, Zuegel U, Steinmeyer A, Kandolf R, Klingel K. Osteopontin: a fibrosis-related marker molecule in cardiac remodeling of enterovirus myocarditis in the susceptible host. Circ Res 104: 851–859, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Szegezdi E, FitzGerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci 1010: 186–194, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Tamura A, Shingai M, Aso N, Hazuku T, Nasu M. Osteopontin is released from the heart into the coronary circulation in patients with a previous anterior wall myocardial infarction. Circ J 67: 742–744, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, Apstein CS, Colucci WS, Singh K. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res 88: 1080–1087, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev 27: 103–118, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev 19: 333–345, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Wang KX, Shi Y, Denhardt DT. Osteopontin regulates hindlimb-unloading-induced lymphoid organ atrophy and weight loss by modulating corticosteroid production. Proc Natl Acad Sci USA 104: 14777–14782, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72: 19–44, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Williams EB, Halpert I, Wickline S, Davison G, Parks WC, Rottman JN. Osteopontin expression is increased in the heritable cardiomyopathy of Syrian hamsters. Circulation 92: 705–709, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Yu Z, Redfern CS, Fishman GI. Conditional transgene expression in the heart. Circ Res 79: 691–697, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Yumoto K, Ishijima M, Rittling SR, Tsuji K, Tsuchiya Y, Kon S, Nifuji A, Uede T, Denhardt DT, Noda M. Osteopontin deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice. Proc Natl Acad Sci USA 99: 4556–4561, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng YH, Tian C, Meng Y, Qin YW, Du YH, Du J, Li HH. Osteopontin stimulates autophagy via integrin/CD44 and p38 MAPK signaling pathways in vascular smooth muscle cells. J Cell Physiol 227:127–135, 2012 [DOI] [PubMed] [Google Scholar]