Abstract
Sulfotransferase (SULT) 4A1 is an orphan enzyme that shares distinct structure and sequence similarities with other cytosolic SULTs. SULT4A1 is primarily expressed in neuronal tissue and is also the most conserved SULT, having been identified in every vertebrate investigated to date. Certain haplotypes of the SULT4A1 gene are correlated with higher baseline psychopathology in schizophrenic patients, but no substrate or function for SULT4A1 has yet been identified despite its high level of sequence conservation. In this study, deep RNA sequencing was used to search for alterations in gene expression in 72-hour postfertilization zebrafish larvae following transient SULT4A1 knockdown (KD) utilizing splice blocking morpholino oligonucleotides. This study demonstrates that transient inhibition of SULT4A1 expression in developing zebrafish larvae results in the up-regulation of several genes involved in phototransduction. SULT4A1 KD was verified by immunoblot analysis and quantitative real-time polymerase chain reaction (qPCR). Gene regulation changes identified by deep RNA sequencing were validated by qPCR. This study is the first identification of a cellular process whose regulation appears to be associated with SULT4A1 expression.
Introduction
Sulfotransferase (SULT) 4A1 was first identified, molecularly cloned, and expressed in 2000 by Falany et al. from the brains of humans and rats (Falany et al., 2000). The protein was initially named brain sulfotransferase-like protein because of sequence similarity to the cytosolic SULTs and a lack of identifiable SULT activity. However, the protein was later renamed SULT4A1 based on sequence and structural homologies to other cytosolic SULTs (Liyou et al., 2003; Blanchard et al., 2004). SULTs are a superfamily of enzymes responsible for the sulfonation of a wide variety of compounds, both endogenous and exogenous. The SULTs catalyze a phase 2 conjugation reaction in which a sulfonate group is transferred from the obligate donor, 3′-phosphoadenosine-5′-phosphosulfate (PAPS), onto the hydroxyl group of an acceptor substrate to form a sulfate. Addition of the charged sulfonate group generally renders the substrate more water-soluble and increases excretion. Key structural features shared between SULT4A1 and other SULTs include the KXXFTVXXXE dimerization domain, active site histidine, and PAPS binding site (Falany et al., 2000).
SULT4A1 is the most conserved of all of the SULTs, suggesting an important and conserved function. That function or enzymatic activity, however, has yet to be elucidated. Unlike other SULTs, SULT4A1 is exclusively expressed in neural tissue (Falany et al., 2000; Liyou et al., 2003). Moreover, recent evidence suggests that a specific SNP haplotype in the 5′ untranslated region of the SULT4A1 gene is correlated with higher baseline psychopathology in patients with schizophrenia and is predictive of those patients’ responses to treatment with olanzapine (Ramsey et al., 2011). However, a mechanism for the role of SULT4A1 in the pathology of schizophrenia has not been described.
The use of zebrafish (Danio rerio) as a model organism offers a unique opportunity to gain insight into the activity or function of SULT4A1. To date, 16 SULT genes have been identified in and cloned from zebrafish (Liu et al., 2010). Like all other vertebrate forms of SULT4A1, zebrafish SULT4A1 (zfSULT4A1) and human SULT4A1 (hSULT4A1) exhibit extensive amino acid sequence homology (86.97% identity and 91.9% similarity). SULT4A1 is expressed almost exclusively in neuronal tissue, and the nervous system develops early in zebrafish larvae. In light of these facts, D. rerio is an excellent organism for the investigation of the regulation, localization, and function of SULT4A1. Furthermore, the ability to knockdown (KD) SULT4A1 expression in the developing zebrafish embryo using splice-blocking antisense morpholino oligonucleotides (MOs) may provide key insights into the function of this protein.
Previous studies have demonstrated the practicality of MOs as a means to knockdown gene expression in zebrafish larvae during the first few days of development (Draper et al., 2001). Given the rapid development of the zebrafish nervous system and the anticipated rapid onset of SULT4A1 expression, this ability provides an excellent opportunity to investigate a developmental response to the loss of SULT4A1 expression. This report describes the effect of SULT4A1 KD on the transcriptome of 72-hour postfertilization (hpf) zebrafish larvae as assessed by deep RNA-sequencing (RNA-seq) analysis.
Materials and Methods
Zebrafish Lines and Maintenance.
Tubingen and type AB zebrafish strains were housed in a recirculating aquaria system (Aquaneering Inc., San Diego, CA) in the University of Alabama at Birmingham Zebrafish Research Facility and cared for in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (IACUC APN: 09641).
Morpholino (MO) KD.
MOs (Gene Tools, Philomath, OR) were designed to target the splice donor sites of exon 1 of the SULT4A1 transcript (SULT4A1 MO, 5′-TAATGCACGCGATTGAATACCTGAT-3′). This results in the inclusion of intron 1 in the transcript and an in-frame premature stop codon 382 bases downstream from the translation start site. MOs were reconstituted in deionized water and diluted to a working concentration of 1.64 mM. Embryos were collected from natural matings and injected using a Harvard Apparatus PLI-100 injection system at the one- or two-cell stage with 0.82 pmol of either SULT4A1 MO or a standard control MO (SCM) (Gene Tools). Effectiveness of KD was verified by quantitative polymerase chain reaction (qPCR) using TaqMan Gene Expression Assays (Life Technologies, Carlsbad, CA). Zebrafish embryos injected with SULT4A1 MO and SCM were observed for gross morphologic phenotype changes at 48, 72, and 120 hpf. At each time point, 10 SCM and 10 SULT4A1 MO embryos were selected at random and assessed for the development of heart, ears, eyes, circulatory system, and swim bladder.
Sample Preparation and RNA-seq Data Analysis.
Embryos injected with either SCM or SULT4A1 MO were separated into four groups of 15 embryos (two SCM and two SULT4A1 MO). At 72 hpf, all four groups were sacrificed, and total RNA was isolated using STAT-60 (Tel-Test, Friendswood, TX). mRNA-sequencing was performed on an Illumina HiSeq2000 (Illumina, San Diego, CA) in the University of Alabama at Birmingham Heflin Center for Genomic Sciences. Briefly, the quality of the total RNA was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) followed by two rounds of polyA+ selection and conversion to cDNA. TruSeq library generation kits were used as per the manufacturer’s instructions (Illumina). Library construction consisted of random fragmentation of the polyA+ mRNA followed by cDNA production using random primers. The ends of the resulting double stranded cDNA were made blunt by using a combination of T4 DNA Polymerase, Klenow fragment and T4 Polynucleotide Kinase under standard conditions. Addition of an Adenosine was done using exo- Klenow fragment of DNA polymerase I in the presence of 10mM ATP. Finally, we performed a ligation reaction to add standard Illumina adaptors necessary for cluster generation on the flow cell to the cDNA library and including an adaptor with an individual 6 base pair barcode to allow for mixing several samples per lane of the HiSeq flow cell and for demultiplexing after completion of sequencing. The cDNA libraries were quantitated using qPCR in a Roche LightCycler 480 with the Kapa Biosystems kit for library quantitation (Kapa Biosystems, Woburn, MA) prior to cluster generation. Clusters were generated to yield approximately 725K–825K clusters/mm2. Cluster density and quality were determined during the run after the first base-addition parameters were assessed.
For each sample, the sequences (in FASTQ format) were aligned to the zebrafish reference genome (Zv9, Sanger Institute, Cambridge, UK). This was done using software Galaxy that is hosted in University of Alabama at Birmingham (http://galaxy.uabgrid.uab.edu). Prealignment was conducted to determine if trimming was needed based on reads quality score. The BAM files were generated following RNA-seq data analysis workflow of Tophat (Trapnell et al., 2009), Cufflinks, and Cuffcompare (Trapnell et al., 2010). These BAM files were loaded into Partek Genomics Suite 6.6 (Partek, Inc., Saint Louis, MO) for further statistical and functional analysis. Briefly, the reads per kilobase of exon model per million mapped reads (RPKM)-normalized reads (Mortazavi et al., 2008) were calculated and the expression levels of genes were estimated (Xing et al., 2006; Mortazavi et al., 2008; Wang et al., 2008). The differential expressions were determined by analysis of variance as described in the vender user manual. A gene list was then created after false-discovery-rate P-value correction using the Benjamini and Hochberg method (Benjamini and Hochberg, 1995). Further functional analysis was conducted using Ingenuity Pathway Analysis (IPA, Redwood City, CA).
Quantitative PCR.
Embryos injected with either SCM or SULT4A1 MO were separated into six groups of 30 embryos (three SCM and three SULT4A1 MO). At 72 hpf, all six groups were sacrificed, and total RNA was isolated using STAT-60. RNA concentration was determined using a NanoDrop ND-1000 spectrophotometer, and SuperScript III (Invitrogen, Carlsbad, California) was used to generate cDNA using 200-ng RNA from each sample. qPCR experiments comparing the following genes were carried out using predesigned TaqMan gene expression assays from Life Technologies with an Applied Biosystems 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA)—SULT4A1, assay ID: Dr03078008_g1; retinal pigment epithelium–derived rhodopsin homolog (rrh), assay ID: Dr03108770_m1; G protein–coupled receptor kinase 1b (grk1b), assay ID: Dr03128502_m1; guanylate cyclase activator 1e (guca1e), assay ID: Dr03094375_m1; and opsin 1, medium-wave-sensitive, 1 (opn1mw1), assay ID: Dr03079939_g1. Samples were compared using the ΔCt method, and P values were determined using a one-tailed t test. Statistical significance was assumed if the P value was less than 0.05.
Adult type AB zebrafish were sacrificed; brain, eye, intestine, liver, and testes were dissected and flash-frozen in liquid nitrogen. RNA was isolated, and cDNA was generated as described above. Relative SULT4A1 expression levels were determined for each tissue as described above (n = 3). Conventional PCR using REDTaq DNA Polymerase (Sigma-Aldrich, St. Louis, MO) and primers to generate full-length SULT4A1 (forward: 5′-atggcggaaagcgaggtgga-3′; reverse: 5′-ctgctttacaggataaagtc-3′) was used in these tissues to verify the presence of full-length SULT4A1 mRNA.
Immunoblot Analysis.
Lysate was prepared from 72-hpf zebrafish embryos (KD and control) and adult zebrafish brain, eye, intestine, liver, and testes. Samples were dissected and placed in sterile phosphate-buffered saline with Complete Mini EDTA-free Protease Inhibitor Cocktail Tablets (Roche, Indianapolis, IN) and Phosphatase Inhibitor Cocktail 2 (Sigma-Aldrich, Minneapolis, MN). Samples were disrupted by pipetting, vortexed for 5 minutes at 4°C, and sonicated twice for 10 seconds with 30-second cooling on ice between sonications. The cycle of pipetting, vortexing, and sonicating was repeated, and lysate was collected by centrifugation at 15,000 × g for 20 minutes at 4°C. Immunoblot analyses were carried out using a goat polyclonal antibody raised to human SULT4A1 (R&D Systems, Minneapolis, MN). Lysate protein concentration was determined by Bradford analysis (Bradford 1976), and 396-µg total protein was loaded from each sample. SULT4A1 specificity was corroborated in each blot using an anti-human SULT4A1 mAb (R&D Systems). The polyclonal antibody was detected using a donkey anti-goat horseradish peroxidase-conjugated secondary (Santa Cruz Biotechnology; Santa Cruz, CA). The monoclonal antibody was detected using a goat anti-mouse horseradish peroxidase-conjugated secondary antibody (Southern Biotech, Birmingham, AL). Both were developed with SuperSignal West Chemiluminescent Substrate (Thermo Scientific, Waltham, MA) and exposed to autoradiograph film.
Results
Sequence Conservation of SULT4A1 in D. rerio.
As observed with the other vertebrate SULT4A1 isoforms, zfSULT4A1 shares extensive homology with hSULT4A1. The two sequences are 87% identical and 92% similar. Although this is slightly lower conservation than is observed between hSULT4A1 and other terrestrial vertebrates (Table 1), it is still very high compared with the conservation between other cytosolic SULTs. Key conserved structural features that have resulted in the inclusion of SULT4A1 in the SULT gene family include the active site histidine (residue 111), the KXXFTVXXXE dimerization domain (residues 254–263), and the TYPKSGT sequence involved with PAPS binding (residues 52–58), which are all conserved in both hSULT4A1 and zSULT4A1 (Fig. 1).
TABLE 1.
Sequence homology of SULT4A1 across different vertebrate species
“AA Changed” denotes the number of amino acid residues that differ from the human isoform of SULT4A1. “Similar AA” denotes the number of amino acid residues that differ from the human isoform of SULT4A1 but maintain the same electrochemical properties. Parentheses indicate GenBank accession numbers.
| Species | Total AA | AA Changed | % Identical | Similar AA | % Similar |
|---|---|---|---|---|---|
| Human (CAG30474) | 284 | ||||
| Rabbit (NP_001076173) | 284 | 4 | 98.59 | 2 | 99.3 |
| Rat (NP_113829) | 284 | 6 | 97.89 | 4 | 98.59 |
| Finch (NP_001232743) | 284 | 16 | 94.37 | 7 | 97.54 |
| Frog (NP_001087553) | 284 | 31 | 89.08 | 18 | 93.66 |
| Zebrafish (NP_001035334) | 284 | 37 | 86.97 | 23 | 91.9 |
AA, amino acid.
Fig. 1.
Amino acid sequence homology between human and zebrafish SULT4A1. Sequences are 86.9% identical and 91.9% similar. Asterisks indicate conserved amino acids. Periods indicate a changed residue that maintains the same electrochemical properties. Key conserved features are highlighted in black and include the active site His (residue 111), the KXXFTVXXXE dimerization domain (residues 254–263), and the TYPKSGT PAPS binding domain (residues 52–58).
SULT4A1 Expression in Brain and Eye of Adult Zebrafish.
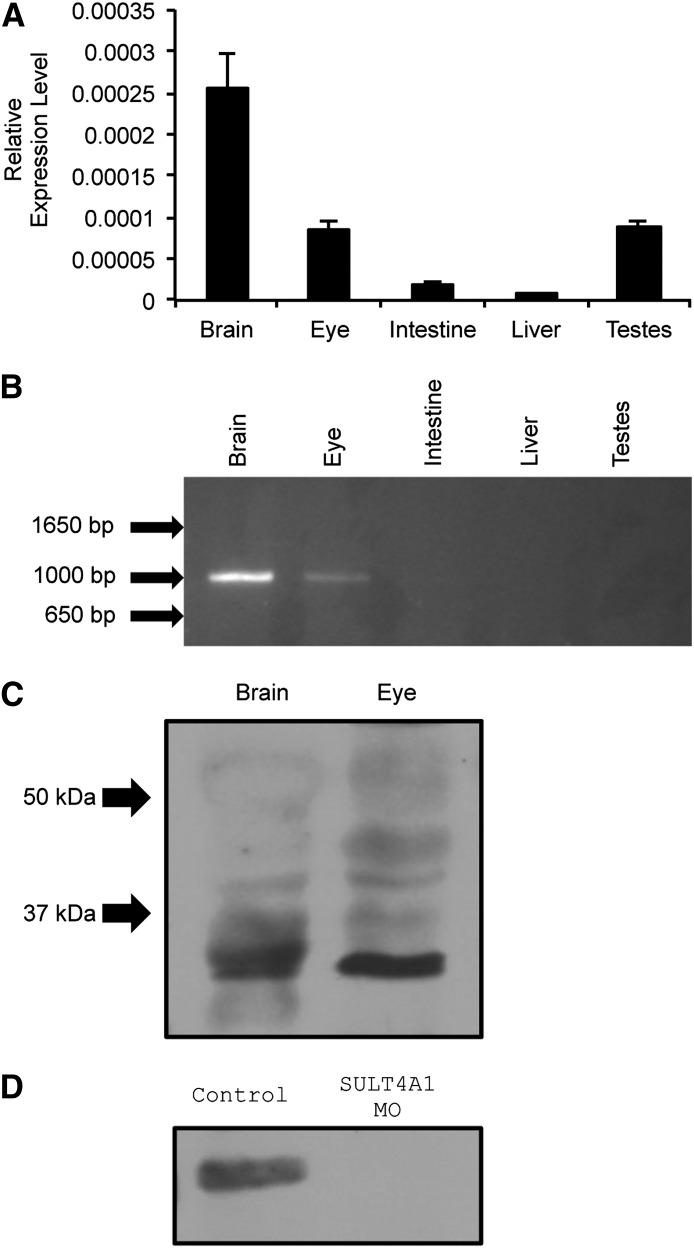
The zfSULT4A1 gene is located on chromosome nine and consists of seven exons separated by six introns (ZDB-GENE-060421-2705). When qPCR was performed on cDNA from the brain, eye, intestine, liver, and testes of adult fish using a TaqMan assay that spans the junction of exons 2 and 3, SULT4A1 mRNA was detected in the brain, eye, and testes (Fig. 2A). The findings in the brain and eye were corroborated by conventional PCR using primers to generate full-length SULT4A1. However, no SULT4A1 message was detected in cDNA of the testes when analyzed by conventional PCR to generate the SULT4A1 coding region and agarose gel separation (Fig. 2B).
Fig. 2.
SULT4A1 expression and MO knockdown in zebrafish. (A) Quantitative real-time PCR. Relative expression levels were analyzed using the ΔCt method and normalized to endogenous expression of 18S RNA. Error bars indicate standard error of the mean. Brain (2.54e−4 +/− 4.24e−5); eye (8.45e−5 +/− 1.01e−5); intestine (1.75e−5 +/− 5.35e−6); liver (7.7e−6 +/− 1.48e−6); and testes (8.69e−5 +/− 8.09e−6). (B) Qualitative PCR of SULT4A1 message in adult AB zebrafish brain, eye, intestine, liver, and testes using full-length primers (forward: 5′-atggcggaaagcgaggtgga-3′; reverse: 5′-ctgctttacaggataaagtc-3′). (C) Immunoblot of zebrafish brain and eye lysate using anti-human SULT4A1 polyclonal antibody. Each lane was loaded with 396-µg lysate. Arrows indicate molecular weight. (D) Immunoblot of SULT4A1 in control and knockdown embryos at 72 hpf using an anti-human SULT4A1 polyclonal antibody. Each lane was loaded with 173 µg.
After establishing SULT4A1 mRNA expression in the zebrafish brain and eye, immunoblot analyses were used to demonstrate that the SULT4A1 protein is detectable in these tissues. Immunoblot analysis using a goat polyclonal antibody of human SULT4A1 detected a band at approximately 33 kDa in lysate of both the brain and eye (Fig. 2C) corresponding to zSULT4A1 (mol. wt. = 33 kDa). As expected, no SULT4A1 was detected via immunoblot analysis in the testes lysate. Immunoblot analysis with the polyclonal antibody to human SULT4A1 did detect SULT4A1 protein in control 72 hpf larvae lysate but not in SULT4A1 MO larvae (Fig. 2D).
SULT4A1 KD Induced Up-Regulation of Phototransduction Genes.
To investigate the effects of the inhibited expression of SULT4A1, the gene expression profile of 72 hpf embryos injected with either SCM or SULT4A1 MO was assessed using RNA-seq. RNA-seq detects transcripts over a much larger dynamic range of expression compared with microarray technology (Wang et al., 2009). Evaluation of the RNA-seq data using Ingenuity Pathway Analysis revealed a number of cellular processes to be significantly affected in the SULT4A1 KD larvae as compared with control larvae. A total of 135 messages were shown to be significantly dysregulated by SULT4A1 KD. Of these, 47 genes were down-regulated, and 88 were up-regulated. A total of 13 genes known to be involved in phototransduction were dysregulated by SULT4A1 KD, and all 13 of these affected genes were shown to be up-regulated (Table 2). Other pathways, including LXR/RXR activation, circadian rhythm signaling, and CREB signaling in neurons were also affected by SULT4A1 KD, but none to the extent or significance of phototransduction (Table 3).
TABLE 2.
Summary of affected genes involved in phototransduction
Embryos injected with SCM or SULT4A1 MO were subjected to gene expression profiling using RNA-seq. P values were determined using analysis of variance.
| Protein | Gene | Fold Change | P value | Localization |
|---|---|---|---|---|
| Sulfotransferase family 4A member 1 | SULT4A1 | −2.15 | <0.0001 | |
| Rhodopsin | RHO | 1.17 | 0.8233 | Rod |
| G protein, α-transducing activity polypeptide 1 | GNAT1 | 2.09 | <0.0001 | Rod |
| G protein, α-transducing activity polypeptide 2 | GNAT2 | 3.35 | <0.0001 | Cone |
| G protein–coupled receptor kinase 7a | grk7a | 3.33 | <0.0001 | Cone |
| Opsin 1, long-wave-sensitive, 2 | OPN1LW2 | 2.31 | <0.0001 | Cone |
| Opsin 1, medium-wave-sensitive, 2 | OPN1MW1 | 4.56 | <0.0001 | Cone |
| Opsin 1, short-wave-sensitive, 1 | OPN1SW1 | 3.62 | <0.0001 | Cone |
| Opsin 1, short-wave-sensitive, 2 | OPN1SW2 | 3.29 | <0.0001 | Cone |
| G protein–coupled receptor kinase 1b | grk1b | 4.27 | <0.0001 | Cone |
| Arrestin 3, retinal | ARR3 | 1.87 | <0.0001 | Cone |
| Guanine nucleotide binding protein (G protein) γ-transducing activity polypeptide 2b | gngt2b | 3.24 | <0.0001 | Both |
| Phosducin b | pdcb | 2.76 | <0.0001 | Both |
| Guanylate cyclase activator 1e | guca1e | 4.39 | 0.00018 | Both |
| Cyclic nucleotide gated channel β 1a | cngb1a | 3.51 | <0.0001 | Both |
TABLE 3.
Gene ontology of transcripts affected by SULT4A1 knockdown
Following collection of RNA-seq data, Ingenuity Pathway Analysis was used to group affected genes into functional pathways and generate P values.
| Pathway | P value | Genes |
|---|---|---|
| Phototransduction pathway | 2.48E-16 | ARR3a, cngb1a, guca1e, GNAT1, GNAT2, gngt2b, OPN1LW2, OPN1MW1, OPN1SW1, OPN1SW2, grk1b, grk7a, pdcb |
| LXR/RXR activation | 4.19E-3 | APOA4, CYP7A1, MMP9, CETP |
| Circadian rhythm signaling | 1.42E-2 | PER1, NR1D1 |
| CREB signaling in neurons | 1.43E-2 | GNAT1, GRM1, NAT2, OPN1SW |
APOA4, apolipoprotein A-IV; CETP, cholesteryl ester transfer protein; CYP7A1, cytochrome P450, family 7, subfamily A, polypeptide 1; GRM1, glutamate receptor, metabotropic 1; MMP9, matrix metalloproteinase 9; NAT2, N-Acetyltransferase 2; NR1D1, nuclear receptor subfamily 1, group d, member 1; PER1, period homolog 1.
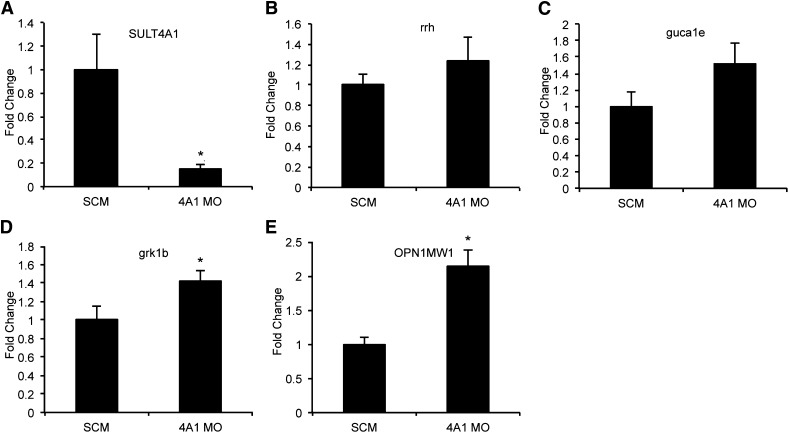
To validate the changes observed in the RNA-seq data, three up-regulated phototransduction genes were selected for further analysis by qPCR. OPN1MW1, guca1e, and grk1b were chosen based on their relative abundance, high observed fold-change, and P value. Predesigned TaqMan gene expression assays were used to verify the observed up-regulation of OPN1MW1 (P = 0.0047) and grk1b (P = 0.0392). Guca1e showed an absolute increase of 1.52-fold when analyzed by qPCR, but this change was not statistically significant (P = 0.0822). As expected, SULT4A1 showed a significant 7-fold decrease in expression when analyzed by qPCR (P = 0.0253). Rrh was used as a negative control since it is expressed in the retina and no change in expression levels was observed in the RNA-seq data. As expected, analysis by qPCR showed no significant change in rrh expression (P = 0.3835) (Fig. 3).
Fig. 3.
qPCR verification of differentially expressed phototransduction genes observed in RNA-seq data at 72 hpf. SuperScript III was used to generate cDNA from total RNA. Message level was determined on a 7900HT Sequence Detection System using predesigned TaqMan gene expression assays. Values represent average relative mRNA expression (n = 3) +/− the standard error of the mean. (A) SULT4A1: SCM (1.00 +/− 0.31); SULT4A1 MO (0.15 +/− 0.04); P = 0.0253. (B) rrh: SCM (1.00 +/− 0.12); SULT4A1 MO (1.24 +/− 0.23); P = 0.1917. (C) guca1e: SCM (1.00 +/− 0.17); SULT4A1 MO (1.52 +/− 0.26); P = 0.0822. (D) grk1b: SCM (1.00 +/− 0.14); SULT4A1 MO (1.43 +/− 0.11); P = 0.0392. (E) OPN1MW1: SCM (1.00 +/− 0.10); SULT4A1 MO (2.15 +/− 0.22); P = 0.0047. *P < 0.05 as compared with SCM-injected embryos.
MO KD of SULT4A1 Expression in the Developing Zebrafish Embryo.
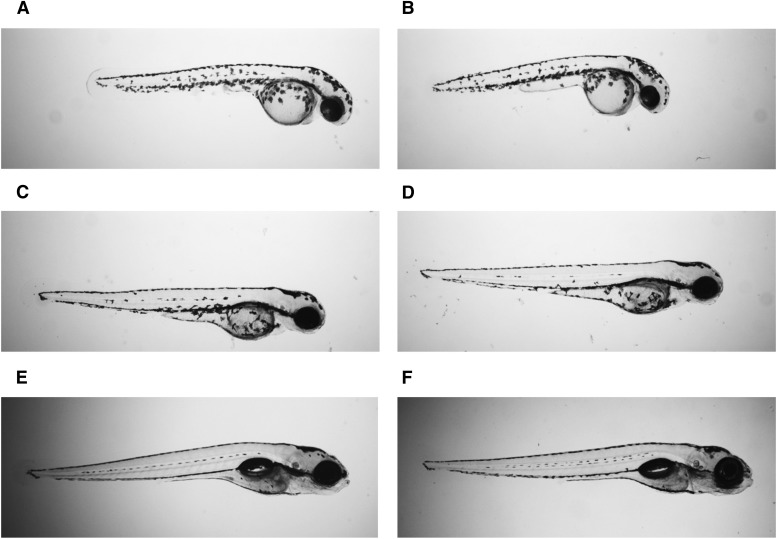
Although the SULT4A1 MO was effective at delaying SULT4A1 expression, no gross morphologic phenotype was observed in SULT4A1 MO versus SCM-injected embryos (Fig. 4). At 48 hpf (Fig. 4, A and B), all observed SCM and KD embryos had a functional beating heart. At 72 hpf (Fig. 4, C and D), all observed SCM and KD embryos displayed adequate blood flow throughout the body. At 120 hpf (Fig. 4, E and F), all observed SCM and KD embryos had morphologically normal ears, eyes, and swim bladder.
Fig. 4.
Normal development of WT and KD zebrafish embryos. Larvae were immobilized in 2% methyl cellulose and visualized using a Nikon AZ100 microscope. (A) Control, 48 hpf. (B) SULT4A1 MO, 48 hpf. (C) Control, 72 hpf. (D) SULT4A1 MO, 72 hpf. (E) Control, 120 hpf. (F) SULT4A1 MO, 120 hpf.
Discussion
The use of zebrafish as a model organism provides a unique opportunity to investigate the function of SULT4A1. Advantages of the zebrafish model include the fact that large numbers of KD larvae can be generated in a very short amount of time, and the rapid onset of SULT4A1 expression and nervous system development allows generation of specimens for in vivo study of SULT4A1 more rapidly than would be possible in other vertebrate model systems. Typically, the use of zebrafish to investigate the function of human proteins would carry the disadvantage that zebrafish are more distantly related to humans than other common model organisms. However, considering the highly conserved nature of SULT4A1’s sequence and neural localization, results from zebrafish studies may have greater relevance to the analysis of hSULT4A1 function than with less conserved proteins. Because zfSULT4A1 and hSULT4A1 are highly conserved, it is anticipated that they have an equally conserved function. That function, once elucidated, will no doubt prove to generate novel insights into SULT function in nervous tissue.
qRT-PCR (Fig. 3A), immunoblot analysis (Fig. 2D), and RNA-seq (Table 2) showed an effective KD of SULT4A1 expression at 72 hpf. However, both the control and KD larvae developed normally, and no gross phenotypic changes could be observed. There are a number of possible explanations. If SULT4A1 is in fact an enzyme, then an incomplete KD, as is typically achieved with MOs, may leave sufficient levels of the protein to retain its function. Given the primarily neuronal expression of SULT4A1, it is also possible that a KD phenotype may manifest itself in the form of abnormal behavior patterns or neural activity rather than developmental pathogenesis. The correlation established by Ramsey et al. (2011) between baseline schizophrenic psychopathology and a specific SULT4A1 haplotype lends credence to this possibility.
One unexpected finding of this study was the detection of SULT4A1 mRNA in the testes via qPCR (Fig. 2A). When full-length primers for SULT4A1 were used with cDNA from the testes, however, no amplification product was observed. Likewise, no translated protein was detected in the testis by immunoblot analysis. A similar phenomenon was observed in human liver by Falany et al. (2000). Hepatic expression of an incorrectly spliced and untranslatable form of hSULT4A1 mRNA was described. This type of aberrant splicing may also be occurring in the testes of zebrafish. If the included intron was of sufficient length, that could explain why no amplicon was observed after conventional PCR using full-length SULT4A1 primers (Fig. 2B). If intron 1 were included in the transcript, then the amplicon would increase from 860 to 6,239 bases. That would be too long for amplification under the PCR conditions used.
Previous studies have shown the brain to be the primary site of SULT4A1 expression in humans and rats (Falany et al., 2000; Liyou et al., 2003). As expected, SULT4A1 expression was observed at high levels in the brain of adult zebrafish. Interestingly, SULT4A1 expression was also observed in the adult zebrafish eye (Fig. 2, A and B). This represents a previously unreported finding that may provide key insights into the function of SULT4A1. If SULT4A1 does indeed prove to play an important role in vertebrate vision, then this finding further solidifies the zebrafish as a model organism to study its function. Zebrafish have the ability to respond to light as early as 68 hpf and can track movement as early as 73 hpf (Easter and Nicola, 1996). The strong ocular expression of SULT4A1 may also account for its relatively rapid onset of expression in zebrafish embryos, where protein was detectable by immunoblotting as early as 72 hpf (Fig. 2D). This stands in contrast to rats, where significant levels of brain SULT4A1 mRNA were not detectable by Northern-blot analysis until postnatal day 21 (Falany et al., 2000). Significant retinal development occurs in rats postnatally, especially with regard to photoreceptors. Cones do not reach peak density until postnatal day 10 (Arango-Gonzalez et al., 2010). This stands in contrast to larval zebrafish vision, in which cones develop early on and are the primary photoreceptor type during the first few days of eye development (Fadool and Dowling, 2008).
The ocular expression of SULT4A1 may help explain the observed up-regulation of 13 genes known to be involved in phototransduction. Although adult zebrafish express higher levels of SULT4A1 in the brain than in the eye, at 72 hpf the eyes are the largest neuronal structure in the developing larvae. The immediate cause of this increase in expression remains unclear. Either SULT4A1 KD leads to an increase in the overall number of photoreceptors in the retina, or the number of photoreceptors remains the same while phototransduction protein expression increases. Given the lack of any discernible change in gross eye morphology of SULT4A1 KD larvae at 72 hpf, the latter seems more likely. Further investigation of the retinal histology of SULT4A1 KD larvae is needed to address these possibilities.
An interesting aspect of the phototransduction up-regulation in the SULT4A1 KD larvae is that it appears to be cone-specific. Of the 13 up-regulated genes, eight are either exclusively or primarily expressed in cone photoreceptors as compared with rod photoreceptors. Four are expressed in both cones and rods, and only one is primarily expressed in rods. OPN1LW2, OPN1MW1, OPN1SW1, and OPN1SW2 encode the cone-specific opsin photopigments and displayed the most statistically significant up-regulation in the RNA-seq analysis. In contrast, the rod-specific photopigment rhodopsin was not significantly dysregulated. Arr3, grk1b, and grk7a are also primarily cone genes whose expression was shown by RNA-seq to be up-regulated (Wada et al., 2006; Renninger et al., 2011). The only rod-specific gene shown to be up-regulated was the transducin α subunit gnat1 (Nelson et al., 2008). The cone counterpart of gnat1, gnat2, was also up-regulated (Table 2). Larval zebrafish vision is dominated by cones (Fadool and Dowling, 2008), which may account for the disproportionate number of cone genes shown to be up-regulated by SULT4A1 KD.
Previous studies have shown that SULT4A1 does not bind the SULT cofactor product, 3′,5′-phosphoadenosine, the same way as other SULTs (Allali-Hassani et al., 2007). Combined with the lack of detectable sulfation activity with a wide range of known SULT substrates, it is possible that SULT4A1 may act as an allosteric regulator of another protein rather than as a catalytically active SULT (Falany et al., 2000; Allali-Hassani et al., 2007). Therefore, deficits in SULT4A1 expression as seen in KD embryos could lead to dramatic changes in a cellular pathway whose activity is modulated by SULT4A1. Whether SULT4A1 is directly involved in phototransduction or some other neuronal process less specific to retinal and pineal tissue remains to be determined. However, given SULT4A1’s wide distribution in the rat central nervous system (Liyou et al., 2003), the latter seems more likely. It is also possible that SULT4A1 may be expressed in the retinal ganglion cells, whose axons make up the optic nerve. While phototransduction was by far the most significantly affected cellular process in this study, this may be due to the rapid development of vision in the zebrafish larvae. Vision and the ability to react to visual stimuli play a prominent role in the development of zebrafish larvae (Easter and Nicola, 1996). Consequently, the eyes of a 72-hpf larva are disproportionately large compared with the rest of the body than those of an adult zebrafish. In contrast to KD larvae, adult SULT4A1-deficient zebrafish may present with more significant changes in different pathways such as circadian rhythm or CREB signaling in neurons (Table 3). To investigate the effects of SULT4A1 knockout in adult zebrafish, a mutant zebrafish line with inheritable deficits in SULT4A1 expression/function is under development. This study is the first to identify a cellular process whose regulation appears to be associated with SULT4A1 expression. The observation that KD of SULT4A1 expression in zebrafish larvae affects expression of genes in phototransduction represents the first possible function of this conserved orphan SULT. Characterization of visual responses and cone function in the KD larvae may identify the affected downstream visual signaling pathways.
Abbreviations
- grk1b
G protein–coupled receptor kinase 1b
- guca1e
guanylate cyclase activator 1e
- KD
knockdown
- MO
morpholino oligonucleotide
- OPN1MW1
opsin 1, medium-wave-sensitive, 1
- PAP
3′,5′-phosphoadenosine
- PAPS
3′-phosphoadenosine-5′-phosphosulfate
- qPCR
quantitative polymerase chain reaction
- RNA-seq
deep RNA sequencing
- RPKM
reads per kilobase of exon model per million mapped reads
- rrh
retinal pigment epithelium–derived rhodopsin homologue
- SCM
standard control morpholino
- SULT
sulfotransferase
- WT
wild-type
Authorship Contributions
Participated in research design: Crittenden, Kraft, Parant, Falany.
Conducted experiments: Crittenden, Thomas.
Contributed new reagents or analytic tools: Ethen, Wu, Parant, Falany.
Performed data analysis: Crittenden, Chen, Kraft, Parant, Falany.
Wrote or contributed to the writing of the manuscript: Crittenden, Chen, Falany.
Footnotes
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant R21MH095946].
References
- Allali-Hassani A, Pan PW, Dombrovski L, Najmanovich R, Tempel W, Dong A, Loppnau P, Martin F, Thornton J, Edwards AM, et al. (2007) Structural and chemical profiling of the human cytosolic sulfotransferases. PLoS Biol 5:1063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango-Gonzalez B, Szabó A, Pinzon-Duarte G, Lukáts A, Guenther E, Kohler K. (2010) In vivo and in vitro development of S- and M-cones in rat retina. Invest Ophthalmol Vis Sci 51:5320–5327 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc, B 57:289–300 [Google Scholar]
- Blanchard RL, Freimuth RR, Buck J, Weinshilboum RM, Coughtrie MW. (2004) A proposed nomenclature system for the cytosolic sulfotransferase (SULT) superfamily. Pharmacogenetics 14:199–211 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Draper BW, Morcos PA, Kimmel CB. (2001) Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis 30:154–156 [DOI] [PubMed] [Google Scholar]
- Easter Jr SS, Nicola GN. (1996) The development of vision in the zebrafish (Danio rerio). Dev Biol 180:646–663 [DOI] [PubMed] [Google Scholar]
- Fadool JM, Dowling JE. (2008) Zebrafish: a model system for the study of eye genetics. Prog Retin Eye Res 27:89–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falany CN, Xie X, Wang J, Ferrer J, Falany JL. (2000) Molecular cloning and expression of novel sulphotransferase-like cDNAs from human and rat brain. Biochem J 346:857–864 [PMC free article] [PubMed] [Google Scholar]
- Liu TA, Bhuiyan S, Liu MY, Sugahara T, Sakakibara Y, Suiko M, Yasuda S, Kakuta Y, Kimura M, Williams FE, et al. (2010) Zebrafish as a model for the study of the phase II cytosolic sulfotransferases. Curr Drug Metab 11:538–546 [DOI] [PubMed] [Google Scholar]
- Liyou NE, Buller KM, Tresillian MJ, Elvin CM, Scott HL, Dodd PR, Tannenberg AEG, McManus ME. (2003) Localization of a brain sulfotransferase, SULT4A1, in the human and rat brain: an immunohistochemical study. J Histochem Cytochem 51:1655–1664 [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628 [DOI] [PubMed] [Google Scholar]
- Nelson SM, Frey RA, Wardwell SL, Stenkamp DL. (2008) The developmental sequence of gene expression within the rod photoreceptor lineage in embryonic zebrafish. Dev Dyn 237:2903–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey TL, Meltzer HY, Brock GN, Mehrotra B, Jayathilake K, Bobo WV, Brennan MD. (2011) Evidence for a SULT4A1 haplotype correlating with baseline psychopathology and atypical antipsychotic response. Pharmacogenomics 12:471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renninger SL, Gesemann M, Neuhauss SCF. (2011) Cone arrestin confers cone vision of high temporal resolution in zebrafish larvae. Eur J Neurosci 33:658–667 [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi AM, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Sugiyama J, Okano T, Fukada Y. (2006) GRK1 and GRK7: unique cellular distribution and widely different activities of opsin phosphorylation in the zebrafish rods and cones. J Neurochem 98:824–837 [DOI] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. (2008) Alternative isoform regulation in human tissue transcriptomes. Nature 456:470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Yu T, Wu YN, Roy M, Kim J, Lee C. (2006) An expectation-maximization algorithm for probabilistic reconstructions of full-length isoforms from splice graphs. Nucleic Acids Res 34:3150–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]