Abstract
Development of 5-HT2C agonists for treatment of neuropsychiatric disorders, including psychoses, substance abuse, and obesity, has been fraught with difficulties, because the vast majority of reported 5-HT2C selective agonists also activate 5-HT2A and/or 5-HT2B receptors, potentially causing hallucinations and/or cardiac valvulopathy. Herein is described a novel, potent, and efficacious human 5-HT2C receptor agonist, (−)-trans-(2S,4R)-4-(3′[meta]-bromophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine (−)-MBP), that is a competitive antagonist and inverse agonist at human 5-HT2A and 5-HT2B receptors, respectively. (−)-MBP has efficacy comparable to the prototypical second-generation antipsychotic drug clozapine in three C57Bl/6 mouse models of drug-induced psychoses: the head-twitch response elicited by [2,5]-dimethoxy-4-iodoamphetamine; hyperlocomotion induced by MK-801 [(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (dizocilpine maleate)]; and hyperlocomotion induced by amphetamine. (−)-MBP, however, does not alter locomotion when administered alone, distinguishing it from clozapine, which suppresses locomotion. Finally, consumption of highly palatable food by mice was not increased by (−)-MBP at a dose that produced at least 50% maximal efficacy in the psychoses models. Compared with (−)-MBP, the enantiomer (+)-MBP was much less active across in vitro affinity and functional assays using mouse and human receptors and also translated in vivo with comparably lower potency and efficacy. Results indicate a 5-HT2C receptor-specific agonist, such as (−)-MBP, may be pharmacotherapeutic for psychoses, without liability for obesity, hallucinations, heart disease, sedation, or motoric disorders.
Introduction
Psychotic disorders, which affect approximately 3% of the population (Perala et al., 2007), are associated with an overactive striatal dopamine system (Abi-Dargham et al., 1998; Seeman and Seeman, 2014). Specifically, persons with schizophrenia are hypersensitive to psychostimulants (Curran et al., 2004), show elevated psychostimulant-induced dopamine release (Abi-Dargham et al., 1998), and display increased presynaptic dopamine synthesis in the striatum (Seeman and Seeman, 2014). Most existing antipsychotic medications interact primarily with dopamine D2 receptors to, theoretically, normalize dopamine signaling. Approximately two-thirds of patients, however, are noncompliant or cease taking their neuroleptic medication (Bellack, 2006), typically attributed to serious side effects that include weight gain, diabetes, high cholesterol, extrapyramidal symptoms, sedation, lethargy, and emotional dampening (NIMH, 2010; Moritz et al., 2013). Furthermore, extant antipsychotic drugs have limited efficacy in approximately one-third of patients (Lindenmayer, 2000), and so-called second-generation antipsychotic drugs do not have superior efficacy compared with their first-generation predecessors (Lieberman et al., 2005).
Targeting the serotonin (5-HT) system, and precisely the 5-HT2C receptor, represents an alternative approach to pharmacotherapy for psychoses. 5-HT2C receptors are expressed in several neural systems affected in schizophrenia, including the frontal cortex and the striatum (Lopez-Gimenez et al., 2001; Pandey et al., 2006), and a corpus of preclinical observations supports a role for 5-HT2C receptors in regulating the brain’s dopamine system. 5-HT2C agonists and inverse agonists modulate dopamine release (Di Giovanni et al., 2000; De Deurwaerdere et al., 2004; Alex et al., 2005). 5-HT2C receptor knockout mice possess enhanced baseline dopamine levels in the striatum and behavioral hypersensitivity to dopamine-releasing psychostimulants (Abdallah et al., 2009), and genetic manipulations that lead to overexpression of 5-HT2C receptors alter dopamine metabolism (Kimura et al., 2009; Olaghere da Silva et al., 2010). In addition, induced-overexpression of dopamine D2 receptors increases expression of 5-HT2C receptors (Simpson et al., 2011), and 5-HT2C receptor ligands modulate D2 receptor activity (Olijslagers et al., 2004), further corroborating a physiologic link between 5-HT2C receptors and central dopamine function. Finally, selective 5-HT2C receptor agonists show preclinical efficacy in animal models of psychoses (Rosenzweig-Lipson et al., 2012). In clinical trials, the novel 5-HT2C agonist vabicaserin showed proof-of-concept for treating schizophrenia (Shen et al., 2010), suggesting that activation of 5-HT2C receptors may be a novel approach to treating schizophrenia.
5-HT2C receptors are also localized on pro-opiomelanocortin neurons in the hypothalamus, a brain region involved in regulating metabolism, hunger, and satiety signals. 5-HT2C agonists stimulate the expression of anorexigenic pro-opiomelanocortin in the hypothalamus, resulting in decreased appetite (Lam et al., 2007; Xu et al., 2008). In clinical trials, lorcaserin, a 5-HT2 agonist with selectivity for the 5-HT2C subtype (Thomsen et al., 2008), significantly reduced weight relative to placebo (Smith et al., 2010). Lorcaserin (Belviq) recently was approved by the U.S. Food and Drug Administration for treatment of obesity (Arena Pharmaceuticals, 2012). Thus, 5-HT2C receptor agonists may reduce feeding and symptoms of psychoses by acting on independent neural systems. Furthermore, 5-HT2C receptor agonists may show an improved safety profile in humans relative to existing antipsychotic drugs. This is observed in the clinic with aripiprazole (Abilify), which possesses 5-HT2C receptor partial agonism and is associated with less weight gain compared with other antipsychotics drugs (Zhang et al., 2006; Leucht et al., 2013). Finally, because 5-HT2C receptors are expressed predominantly in the central nervous system (Molineaux et al., 1989), compounds that specifically target and activate 5-HT2C receptors should have limited impact on peripheral tissues, further decreasing the risk of side effects.
One common problem with most existing, selective 5-HT2C agonists, including lorcaserin, is that they also activate 5-HT2A and 5-HT2B receptors at higher concentrations, which can lead to hallucinations (Glennon et al., 1984; Nichols, 2009) and cardiac valvulopathy, respectively (Rothman and Baumann, 2009). Pharmacological and behavioral data obtained using the novel and potent 5-HT2C-specific agonist (−)-MBP are presented herein. (−)-MBP possesses high affinity at each of the 5-HT2 receptors radiolabeled with an antagonist but high affinity at only 5-HT2C receptors when 5-HT2 receptors are radiolabeled with an agonist. With regard to 5-HT2-Gq-mediated phosphoinositide hydrolysis signaling, (−)-MBP activates only the 5-HT2C receptor subtype from both mouse and human cDNA. In addition, (−)-MBP behaves as a competitive antagonist of 5-HT at 5-HT2A and 5-HT2B receptors and also as an inverse agonist at 5-HT2B receptors. In vivo, (−)-MBP displays anorexigenic and antipsychotic activity in mouse models but does not alter locomotion. The data suggest a 5-HT2C receptor-specific agonist such as (−)-MBP may be pharmacotherapeutic for psychoses, without liability for obesity, hallucinations, heart disease, sedation, or motoric disorders.
Materials and Methods
Compounds
The (+)-(2R, 4S)- and (−)-(2S, 4R)-trans enantiomers of 4-phenyl-3′-bromo-N,N-dimethyl-1,2,3,4-tetrahydronaphthalene-2-amine [(+)-MBP and (−)-MBP, respectively, Fig. 1; built using Benchware 3D Explorer 2.7; Tripos, St. Louis, MO] were synthesized in our laboratories as racemates that were resolved by a preparative chiral polysaccharide-based stationary-phase high-performance liquid chromatography system and converted to hydrochloride salts, as previously described elsewhere (Booth et al., 2009; Vincek and Booth, 2009). 5-HT hydrochloride was purchased from Alfa Aesar (Ward Hill, MA). (+)-MK-801 [(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (dizocilpine maleate)], d-amphetamine sulfate, clozapine hydrochloride, mianserin hydrochloride, and (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) were purchased from Sigma-Aldrich (St. Louis, MO). Compounds were weighed with accuracy ± 0.001 mg on a microanalytical balance (model XP26; Mettler-Toledo, Columbus, OH). Solutions of all compounds used for behavioral assays were made fresh on the day of testing. [3H]Mesulergine, [3H]ketanserin, [3H]5-HT, and [3H]myoinositol at commercially available specific activity were purchased from Perkin-Elmer Life and Analytical Sciences (Waltham, MA).
Fig. 1.
Structures of (+)-MBP and (−)-MBP.
In Vitro Pharmacology
Radioligand Binding and Phosphoinositide Hydrolysis Assays.
Antagonist radioligand receptor binding assays were performed in 96-well plates based on procedures previously described elsewhere (Canal et al., 2013). In brief, human embryonic kidney 293 (HEK293) cells were transfected with 10 μg of human 5-HT2A, 5-HT2B, or 5-HT2C-ini receptor cDNA or 10 μg of mouse 5-HT2A or 5-HT2C-vnv receptor cDNA using Lipofectamine 2000 reagent (Invitrogen/Life Technologies, Carlsbad, CA) per the manufacturer’s instructions (mouse 5-HT2B cDNA was not procured). Cell membranes were collected 48 hour later. (+)- or (−)-MBP, at increasing concentrations from 0.1 nM to 10 μM, was used to compete for receptor orthosteric binding sites labeled with 1 nM [3H]ketanserin (5-HT2A) or 2 nM [3H]mesulergine (5-HT2B, 5-HT2C), and 10 μM mianserin was used to define the nonspecific antagonist radioligand binding at all three 5-HT2 subtypes. Both enantiomers were tested for affinities at antagonist-labeled 5-HT2 subtypes. Only (−)-MBP was tested in agonist-labeled competition binding assays wherein [3H]5-HT at 3.7 nM (calculated) was used to label the 5-HT2 subtypes. 5-HT at 10 µM was used to define nonspecific agonist radioligand binding. The assay buffer for competition with [3H]5-HT contained 50 mM Tris-HCl, 3 mM CaCl2, 10 µM pargyline, and 0.1% ascorbic acid. After a 120-minute equilibration period at room temperature, the incubation mixtures were rapidly passed through GF/B filters using a Mach 2 cell harvester (Tomtec, Hamden, CT) and subsequently washed with 50 mM Tris–HCl. Filter disks were placed in vials containing 2 ml of scintillation cocktail (ScintiVerse; Thermo Fisher Scientific, Waltham, MA) and counted for 3H-induced scintillation using a Beckman-Coulter LS6500 counter (Indianapolis, IN).
5-HT2 receptor-mediated inositol phosphate hydrolysis assays to measure functional responses of (+)- and (−)-MBP and 5-HT (positive control agonist) were performed as previously described elsewhere (Canal et al., 2013). In brief, transiently transfected HEK293 cells were labeled with 1 μCi/ml [3H]myoinositol and seeded into 48-well plates. Cells were treated with test compounds for 30 minutes. The reaction was stopped by addition of 50 mM formic acid. Anion-exchange columns (Bio-Rad Laboratories, Hercules, CA) were used to bind and collect [3H]inositol phosphates. 3H-Induced scintillations then were measured. Competitive antagonism studies were performed with (−)-MBP only. In these studies, 0.1–10 µM (−)-MBP was used to compete with 0.0001–10 µM 5-HT for activation of 5-HT2A and 5-HT2B receptors. HEK293 cells transiently expressing each one of the 5-HT2 subtypes were treated with (−)-MBP and 5-HT simultaneously for 30 minutes before stopping the reaction, as noted previously. Each binding and function experiment included triplicate measurements for each concentration of test compound, and each experiment was performed a minimum of three times.
Statistics.
Binding data were analyzed using nonlinear regression, curve-fitting algorithms in GraphPad Prism version 6.00 for Microsoft Windows (San Diego, CA). Hill slopes were constrained to 1.0, consistent with the limited number of data points (Motulsky and Christopoulos, 2003). Ligand affinity is expressed as an approximation of Ki values by conversion of the IC50 data using the equation Ki = IC50/1 + L/KD, where L is the concentration of radioligand (Cheng and Prusoff, 1973). Data from phosphoinositide hydrolysis assays are presented as half-maximum (EC50), half-minimum (IC50), and maximum (EMAX) values, representing potency and efficacy, as computed using GraphPad nonlinear regression curve-fitting algorithms. Agonist efficacy is presented as a percentage of the maximum 5-HT response. Inverse agonist efficacy is presented as a percentage of the basal values (scintillation counts per minute).
In Vivo Behavioral Pharmacology
Subjects.
Male C57Bl/6 mice were obtained at ∼8 weeks of age from The Jackson Laboratory (Bar Harbor, ME) for head-twitch response (HTR) and locomotion studies or Harlan Laboratories (Indianapolis, IN) for the food studies; they were allowed to acclimate to the temperature (23°C) and humidity-controlled vivarium for at least 1 week before testing. The vivarium was illuminated from 7:00 AM to 7:00 PM. Mice were housed in pairs for HTR and locomotion studies and singly for food studies. Standard rodent pellets (Purina 5001; LabDiet, St. Louis, MO) were available ad libitum, along with drinking water. Experiments were conducted at approximately the middle of the light phase.
(+)- or (−)-MBP, clozapine, or DOI was dissolved in sterile 0.9% saline or Milli-Q water (Millipore Corp., Billerica, MA). Clozapine was used as the comparative antipsychotic drug and positive control in all psychoses behavioral models. All compounds were administered systemically (by intraperitoneal or subcutaneous injection) in a volume of 0.01–0.02 ml/g body weight. All behavioral procedures were approved by the University of Florida and Northeastern University Institutional Animal Care and Use Committee and were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
DOI-Elicited Head-Twitch Response and Locomotion.
Experimentally naïve mice were habituated to the testing room for approximately 30 minutes. Testing consisted of administration (subcutaneously) of Milli-Q water (vehicle), (+)- or (−)-MBP (3.0, 5.6, or 10.0 mg/kg), or clozapine (0.1 or 1.0 mg/kg) followed 10 minutes later by an injection of the 5-HT2 agonist DOI (1.0 mg/kg). Ten minutes later, mice were placed into a clear Plexiglas open-field chamber (43 × 43 cm; Med Associates, St. Albans, VT) for a 10-minute observation period. During this session, HTRs, defined as a clear, rapid, and discrete back-and-forth rotation of the head, were counted by a trained observer (D.M.) who had been blinded to the drug treatment conditions. A camera videotaped the session, and activity (distance traveled in cm) was calculated by Ethovision software (Noldus Information Technology, Leesburg, VA).
MK-801-Elicited Hyperlocomotion.
Experimentally naïve mice were habituated to the testing room for approximately 30 minutes. Locomotor activity testing consisted of administration (intraperitoneally) of saline (vehicle), clozapine (0.1 or 1.0 mg/kg), or (−)-MBP (3.0, 5.6, and 10.0 mg/kg), followed 10 minutes later by an injection of vehicle or the N-methyl-d-aspartate (NMDA) antagonist MK-801 (0.3 mg/kg). Mice were immediately placed into one of four opaque Plexiglas chambers (29.2 × 17.8 cm, 43.2 cm tall; Magnum Wood LLC, Gainesville, FL) for a 60-minute session. An overhead camera videotaped the session, and activity (distance traveled in cm) was calculated by Ethovision software (Noldus Information Technology). To examine the time course of behavioral activity, (−)-MBP (10.0 mg/kg) was administered 10 minutes, 1 hour, or 3 hours before MK-801 (0.3 mg/kg) administration, and locomotion was assessed for 60 minutes thereafter.
Amphetamine-Elicited Hyperlocomotion.
Experimentally naïve mice were habituated to the testing room for approximately 30 minutes. Locomotor activity testing consisted of administration (i.p.) of saline (vehicle), clozapine (0.1 and 1 mg/kg), or (−)-MBP (3.0, 5.6, and 10.0 mg/kg), followed 10 minutes later by an injection of vehicle or the dopamine and norepinephrine transporter inhibitor and the substrate amphetamine (3.0 mg/kg). Locomotion was assessed exactly as noted in the MK-801 experiment. The effects of clozapine (1 mg/kg) or (−)-MBP (10 mg/kg) alone on locomotion were also tested during these experiments; the timing of injections and behavioral testing remained consistent.
Palatable Meal Eating.
Mice were adapted to eating a supplemental treat of Fruit Crunchies (Bio-Serv, Frenchtown, NJ), which are 190-mg pellets of purified materials that contain a similar macronutrient balance and caloric density (3.45 kcal/g) as chow. Mice were presented 10 Crunchies, including at least three in each of the three flavors, in 10-ml glass jars suspended inside the cage via a metal stirrup. On the first day, access was for 24 hours, but thereafter daily access was rapidly tapered to 30 minutes, starting at ~14:00 hours. Crunchies were presented 5 days per week (Monday–Friday). After 30 minutes, uneaten Crunchies or halves were retrieved and the intake recorded. Each week, intakes on Tuesday through Thursday were used to compute a mean baseline for each mouse, and three groups were formed that were matched with this baseline. Friday was the test day on which animals were injected (intraperitoneally) with (−)-MBP, (+)-MBP (6 or 12 mg/kg), or saline. Crunchies were presented 15 minutes later, and intake was measured as before, expressed as a percentage of each individual’s baseline for that week. Mice were tested repeatedly with different drugs and doses at 1-week intervals. Testing occurred during 2 weeks that were not consecutive.
Statistics.
The dependent measures were analyzed by one- or two-way analysis of variance (ANOVA) with multiple comparisons (Newman-Keuls, Tukey’s, Dunnett’s test) or by unpaired two-tailed t tests, as appropriate, using commercially available statistical software (Sigmastat 3.1; Systat Software, San Jose, CA and GraphPad Prism 6.00; GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant. ED50 values and 95% confidence intervals (CI) were determined using log-linear interpolation from the descending limb of the dose-effect curves.
Results
In Vitro Pharmacology
Affinity (Ki) and function (EC50, EMAX, relative to 5-HT, and IC50, IMAX, relative to basal baseline) for (+)-MBP and (−)-MBP at each of the 5-HT2 receptors are shown in Table 1. At human 5-HT2A, 5-HT2B, and 5-HT2C receptors labeled with antagonist radioligand, (−)-MBP had 17-, 2-, and 18-fold higher affinity (Ki), respectively, in comparison with (+)-MBP. Likewise, at mouse 5-HT2A and 5-HT2C receptors labeled with antagonist radioligand, (−)-MBP had much higher affinity (20- and 88-fold, respectively) than (+)-MBP. At human 5-HT2 receptors labeled with agonist radioligand, (−)-MBP had much higher affinity for the 5-HT2C subtype, with greater than 8- and 20-fold binding selectivity for 5-HT2C over 5-HT2A and 5-HT2B receptors, respectively.
TABLE 1.
Pharmacology of (+) and (−)-MBP at human (h) and mouse (m) 5-HT2 receptors
Ki values (nM) were determined by displacement of [3H]5-HT (agonist labeled), [3H]ketanserin (5-HT2A), or [3H]mesulergine (5-HT2B, 5-HT2C) (antagonist labeled). Function values were determined by an inositol phosphate hydrolysis assay that measured the 5-HT2-mediated activation of phospholipase C. The pA2 value was determined from competitive antagonism functional assays with 5-HT. All data were from HEK cells transiently expressing one of the three 5-HT2 receptors. Data represent the mean (± S.E.M.) from at least three independent experiments.
| Compound | In Vitro Pharmacology | h5-HT2A | h5-HT2B | h5-HT2Ca | m5-HT2A | m5-HT2Ca |
|---|---|---|---|---|---|---|
| (−)-MBP | Ki antagonist labeled | 20 (4.5) | 13 (5.2) | 12 (2.8) | 26 (2.3) | 11 (2.5) |
| Ki agonist labeled | 77 (14) | 199 (35) | 9.1 (0.5) | Not tested | Not tested | |
| Function | pA2 = 2.64 (0.05) | IC50 = 112 (24) | EC50 = 19 (3) | No activation @ 10 μM | EC50 = 115 (4) | |
| Efficacy (%) | No activation @ 10 μM | 69 (5) (inverse agonist)b | 63 (13) (agonist)c | No activation @ 10 μM | 60 (1) (agonist)c | |
| (+)-MBP | Ki antagonist labeled | 332 (42.1) | 31 (7.1) | 200 (24.1) | 534 (63.0) | 969 (77.5) |
| Function | EC50 > 1000 | EC50 > 1000 | EC50 > 1000 | EC50 > 1000 | EC50 = 122 (9.0) | |
| Efficacy (%) | 34 (4) (agonist)c | 42 (6) (agonist)c | 81 (8) (agonist)c | 30 (5) (agonist)c | 57 (10) (agonist)c |
h5-HT2C, human 5-HT2C-ini isoform; m5-HT2C = mouse 5-HT2C-vnv isoform.
For efficacy, the percentage of basal signaling (inverse agonism).
For efficacy, the percentage of maximal 5-HT response (agonism).
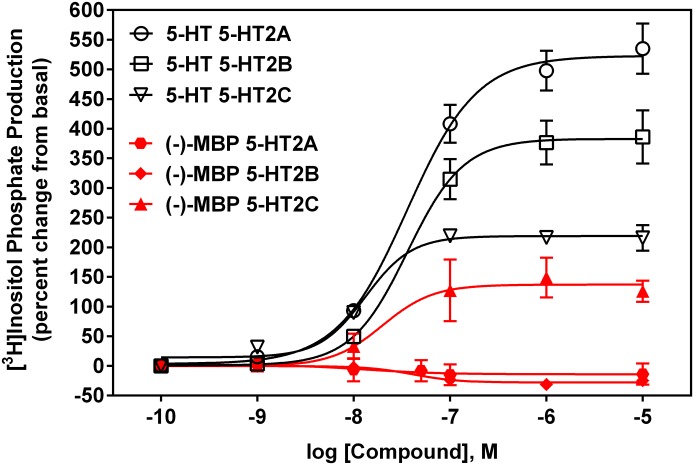
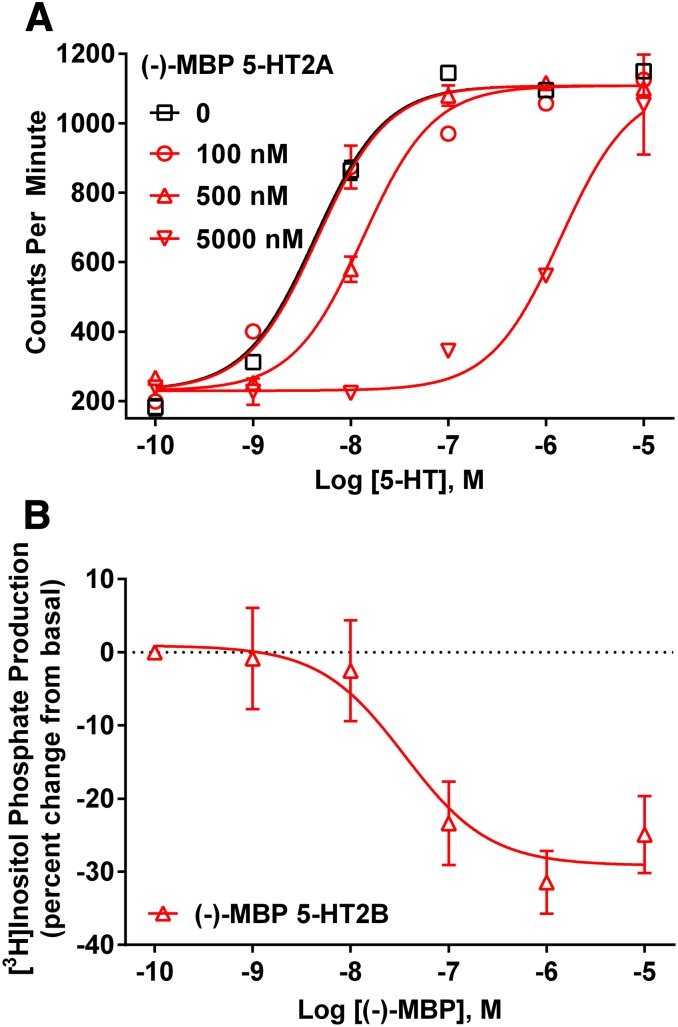
Results from functional assays (data summarized in Table 1) revealed that (−)-MBP exclusively activated human and mouse 5-HT2C receptors (Fig. 2). (−)-MBP agonist potency at human 5-HT2C receptors (EC50 = 19 nM) was 6-fold higher than its potency at mouse 5-HT2C receptors (EC50 = 115 nM), and in both cases, maximum efficacy was approximately 60% compared with 5-HT (Fig. 2). (−)-MBP did not activate human or mouse 5-HT2A receptors at concentrations up to 10 µM (Fig. 2) and was a competitive antagonist of 5-HT activation of human 5-HT2A receptors (Fig. 3A), with a mean (±S.E.M.) Kb value of 441 (45) nM and pA2 value of 2.64 (0.05). At human 5-HT2B receptors, (−)-MBP was an inverse agonist (Fig. 3B), with a mean (± S.E.M.) IC50 value of 112 (24) nM, and (−)-MBP also was a competitive antagonist of 5-HT activation of 5-HT2B receptors (data not shown), with a mean (S.E.M.) Kb value of 313 (118) nM and pA2 value of 2.43 (0.17). In contrast to the discriminating 5-HT2 functional pharmacology of (−)-MBP, (+)-MBP was a low potency, partial agonist at each of the human and mouse 5-HT2 receptor subtypes (Table 1). Accordingly, further molecular pharmacologic characterization of (+)-MBP was not pursued, and (−)-MBP was designated the lead stereoisomer in light of its higher affinity and specific agonist activity at 5-HT2C receptors.
Fig. 2.
Representative functional responses of (−)-MBP at human 5-HT2A, 5-HT2B, and 5-HT2C receptors compared with 5-HT.
Fig. 3.
(A) Representative competitive antagonism results of (−)-MBP at human 5-HT2A receptors. (B) Representative inverse agonist results of (−)-MBP at human 5-HT2B receptors.
In Vivo Pharmacology
(−)-MBP Reduces the DOI-Elicited Head-Twitch Response without Altering Locomotion.
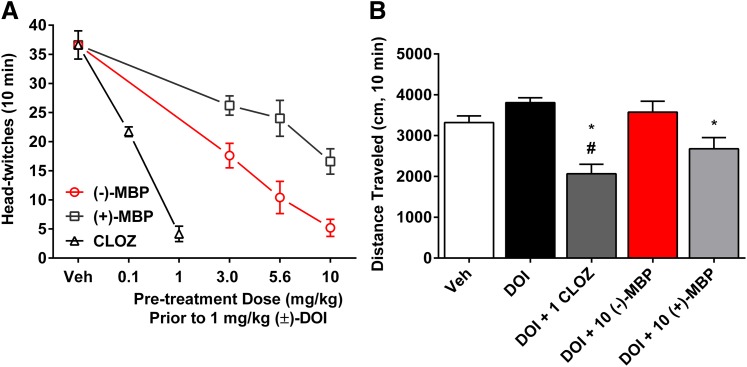
Administration of DOI (1.0 mg/kg) (preceded by a vehicle injection) resulted in 37.1 (± 1.4) HTRs during the 10-minute session (Fig. 4). All doses of both enantiomers of MBP attenuated this response (F6,30 = 28.1; P < 0.0001). This effect was dose dependent (F2,24 = 11.69; P < 0.0001), with the (−) and (+) enantiomers reducing the number of DOI-elicited HTRs by 86% and 55%, respectively, at doses of 10.0 mg/kg (Fig. 4). (−)-MBP was more potent (F1,24 = 26.1; P < 0.0001) and had an ED50 (± 95% CI) value of 2.67 (1.69–4.20) mg/kg compared with 8.80 (5.26–14.73) mg/kg for (+)-MBP. Clozapine also dose-dependently blocked the DOI-elicited HTR (P < 0.05) (Fig. 4). A linear regression analysis of data from Fig. 4A showed that the slopes of the lines from each group were statistically different (F2,52 = 28.7; P < 0.0001); rank order of potency was clozapine > (−)-MBP > (+)-MBP. During HTR sessions, locomotor activity was recorded. There was no combination of DOI and (−)-MBP doses that resulted in activity levels different from vehicle or DOI administration. In contrast, (+)-MBP at 10 mg/kg, in combination with DOI (1.0 mg/kg) resulted in decreased activity relative to vehicle plus DOI (P < 0.05), but not vehicle alone. Conversely, clozapine at 1 mg/kg alone (see below) or in combination with DOI (1.0 mg/kg) significantly decreased locomotion relative to vehicle (mean difference 1254 cm; 95% CI 50–2458; P < 0.05) and DOI alone (mean difference 1742 cm; 95% CI 857–2627; P < 0.05) (Fig. 4, inset).
Fig. 4.
(A) Both enantiomers of (−)-MBP dose-dependently attenuated the DOI-elicited HTR. (−)-MBP was more potent and efficacious than (+)-MBP, consistent with in vitro pharmacology data. Clozapine (CLOZ) also dose-dependently blocked the DOI-elicited HTR. Each data point represents the mean (± S.E.M.) of 5 to 7 subjects. All drug groups are significantly different from the DOI-only group (vehicle). (B) Pretreatment with (−)-MBP did not affect locomotion, but CLOZ and (+)-MBP significantly decreased locomotion relative to DOI (1 mg/kg). CLOZ also reduced locomotion compared with the group treated with vehicle (Veh) only; numbers on the x-axis refer to mg/kg dose. Bar graphs of locomotion (mean ± S.E.M.) are from representative groups shown in A. *Significantly different from DOI. #Significantly different from vehicle.
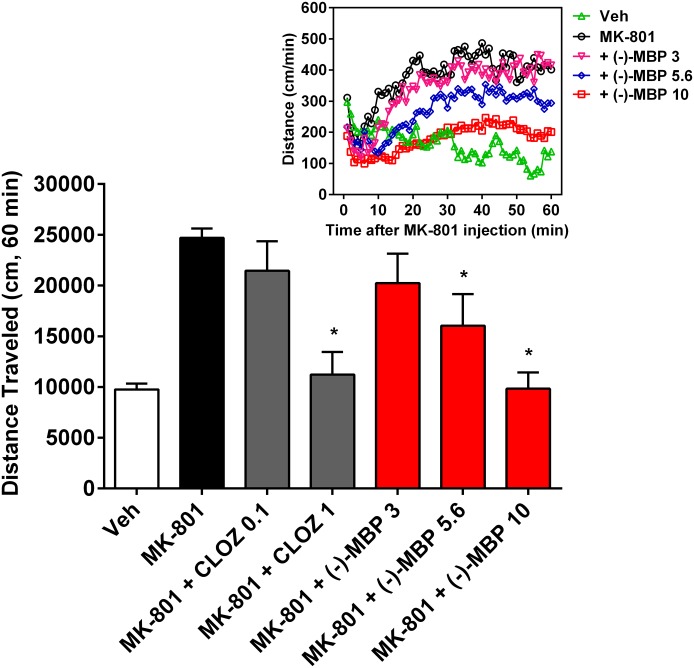
(−)-MBP Reduces MK-801-Elicited Hyperlocomotion, an Effect Lasting at Least 2 Hours.
MK-801 (0.3 mg/kg) administration resulted in increased levels of activity relative to vehicle administration (Fig. 5) that persisted for at least 60 minutes (Figs. 5, inset, and 6). (−)-MBP dose-dependently decreased MK-801 hyperlocomotion, which was significant at 5.6 mg/kg (mean difference 8654 cm; 95% CI 352–16,955; P < 0.05) and 10 mg/kg (mean difference 14,872 cm; 95% CI 6571–23,174; P < 0.005). The attenuation of MK-801-elicited activity was apparent throughout the entire 60-minute session (F236, 2242 = 4.43; P < 0.0001). The results with (−)-MBP were similar to clozapine, which also dose-dependently reduced MK-801-elicited hyperlocomotion (Fig. 5).
Fig. 5.
(−)-MBP dose-dependently attenuated MK-801-elicited hyperactivity, similar to clozapine (CLOZ). Effects are shown for the total 60-minute session (bar graphs), and numbers on the x-axis refer to dose in mg/kg. Bar graphs represent the mean (± S.E.M.) of 6 (CLOZ groups) to 10 subjects. *Significantly different from MK-801 alone. Inset: Effects are plotted in 1-minute bins for the primary comparisons. Error bars in inset are excluded for clarity.
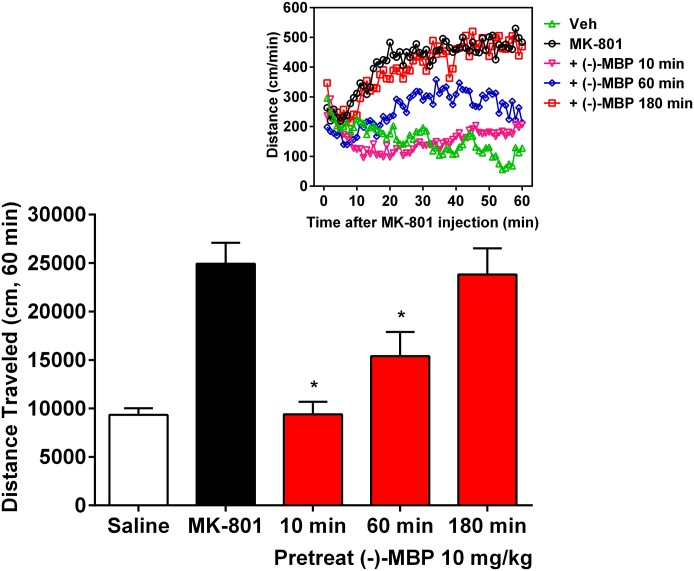
Fig. 6.
Time course analysis of (−)-MBP (10 mg/kg). (−)-MBP administered 10 or 60 minutes before MK-801 significantly reduced MK-801-elicited hyperactivity. Effects are shown for the total 60-minute session (bar graphs). Bar graphs represent the mean (± S.E.M.) of 11 (vehicle) and 6 (for each of the remaining groups) subjects. *Significantly lower activity relative to MK-801 alone. Inset: Effects are plotted in 1-minute bins for the primary comparisons. Error bars in inset are excluded for clarity.
To examine the time course of behavioral activity, (−)-MBP (10.0 mg/kg) was administered 10 minutes, 1 hour, or 3 hours before MK-801 administration, and locomotor activity was assessed for 60 minutes thereafter (Fig. 6). At a 10-minute pretreatment time, there was a complete attenuation of MK-801’s effects in which activity levels decreased from ∼25,000 cm to ∼10,000 cm (Fig. 6; mean difference 15,533 cm; 95% CI 7805–23,261; P < 0.005). When (−)-MBP was administered at a 1-hour pretreatment time (thus assessing behavioral activity from hours 1 to 2), attenuation of the MK-801 behavioral effects was still apparent throughout most of the session (mean difference 9540 cm; 95% CI 1813 to 17,268; P < 0.05). When administered 3 hours before the session, (−)-MBP had little effect on MK-801 elicited hyperactivity. (+)-MBP was not tested in this the MK-801 assay nor in the amphetamine-induced hyperactivity assay because of its relatively poor activity in the DOI-elicited-HTR model, which putatively reflects its low affinity and partial agonist functional activity at 5-HT2A and 5-HT2C receptors (Table 1).
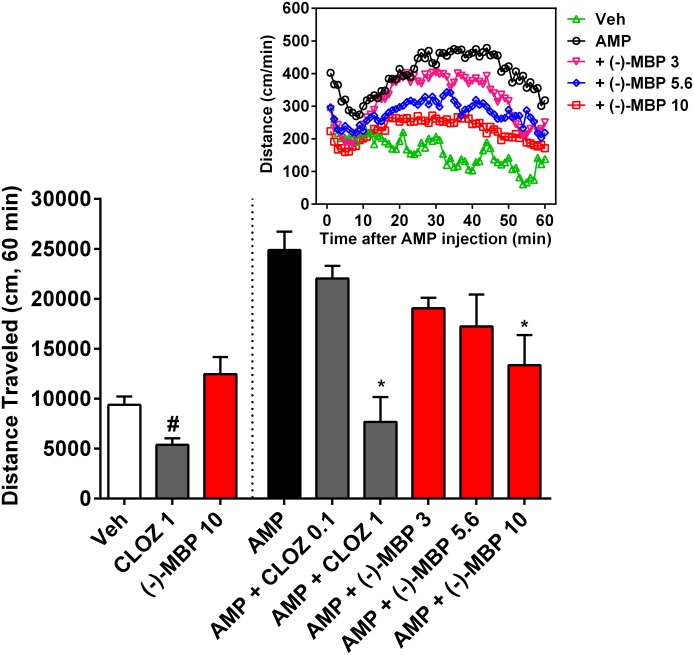
(−)-MBP Reduces Amphetamine-Elicited Hyperlocomotion but Does Not Alter Locomotion When Administered Alone.
Amphetamine (3.0 mg/kg) administration resulted in significantly increased levels of activity relative to saline administration, which lasted for at least 60 minutes (Fig. 7; P < 0.001). (−)-MBP at 10 mg/kg significantly decreased amphetamine-induced hyperactivity (mean difference 11,506 cm; 95% CI 4071–18,942; P < 0.001). The attenuation of amphetamine-elicited activity was apparent throughout the entire 60-minute session (F236,2832 = 3.46; P < 0.0001). Also, clozapine at 1 mg/kg (mean difference 17,187 cm; 95% CI 8874–25,500; P < 0.001) but not 0.1 mg/kg significantly decreased amphetamine-elicited hyperactivity (Fig. 7). Clozapine also significantly reduced locomotor activity when administered alone (P < 0.005), but even the highest dose of (−)-MBP (10 mg/kg) did not significantly alter locomotor activity when administered alone (P = 0.14). (+)-MBP was not tested in this assay because of its relatively poor activity in the DOI-elicited-HTR model.
Fig. 7.
(−)-MBP attenuated amphetamine (AMP)-elicited increases in locomotion, similar to clozapine (CLOZ) but with less potency (groups right of dashed line). Note, however, that CLOZ (1 mg/kg) alone significantly decreased locomotion, but (−)-MBP (10 mg/kg) alone did not alter locomotion, relative to vehicle (Veh) (groups left of dashed line). Effects are shown for the total 60-minute session (bar graphs), and numbers on the x-axis refer to dose in mg/kg. Bar graphs represent the mean (± S.E.M.) of six (CLOZ groups) to nine subjects. *Combination of CLOZ or (−)-MBP with AMP was significantly lower relative to AMP alone. #Significantly different from vehicle group. Inset: Effects are plotted in 1-minute bins for the primary comparisons. Error bars in inset are excluded for clarity.
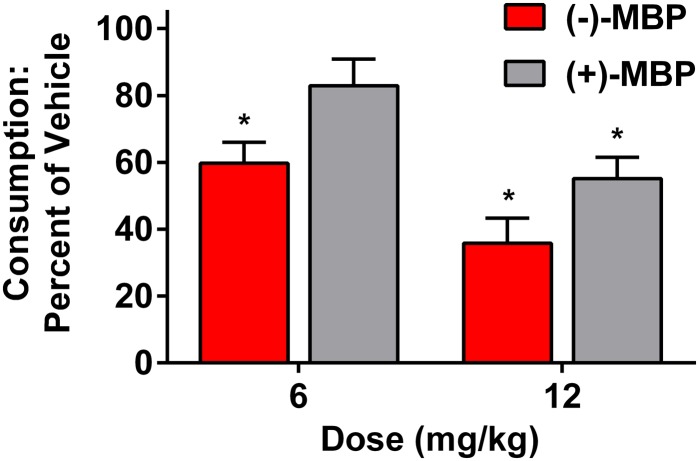
(−)-MBP Reduces Palatable Food Eating.
Both (−)-MBP and (+)-MBP produced a dose-related suppression of intake of Crunchies (Fig. 8). (−)-MBP was significantly more potent and efficacious than (+)-MBP, similar to the effects seen in the DOI-elicited HTR tests. The main effect of dose was statistically significant (P < 0.001), the difference between (−)- and (+)-MBP was marginally significant (P = 0.054), and the dose × drug interaction was not significant. At 6 and 12 mg/kg, (−)-MBP reduced feeding to a mean (± S.E.M.) of 59.7% (6.3) and 35.8% (7.5), respectively, below the vehicle-treated group (both doses, P < 0.05). At 6 and 12 mg/kg, (+)-MBP reduced feeding to a mean (± S.E.M.) of 82.9% (8.0) and 55.1% (6.4), respectively, below the vehicle-treated group (6 mg/kg, not significant; 12 mg/kg, P < 0.05). From linear regressions (r2 = 0.59, 0.69, P < 0.01), the estimated ED50 values for (−)-MBP and (+)-MBP were 9.6 and 13.6 mg/kg, respectively.
Fig. 8.
Both (+) and (−)-MBP decreased consumption of “Crunchies,” a highly palatable treat, in non-food-deprived mice. Bar graphs represent the mean (± S.E.M.) of eight subjects. Both doses of (−)-MBP (outlined, red bars), and the highest dose of the (+)-MBP (gray bars) decreased consumption. *Significantly lower levels of consumption relative to vehicle administration.
Discussion
Drugs that activate 5-HT2C receptors hold promise for the treatment of psychoses and psychostimulant abuse, in part because of their ability to modulate central dopamine signaling and their effectiveness in preclinical models and at least one clinical study (Di Matteo et al., 2004; Shen et al., 2010; Higgins et al., 2012; Cunningham et al., 2013). Herein is described (−)-MBP, a novel and potent 5-HT2C receptor-specific agonist with 5-HT2A and 5-HT2B competitive antagonist and inverse agonist properties that is effective in preclinical mouse models of psychoses, does not affect locomotion on its own, and reduces palatable food intake, important properties distinguishing it from available antipsychotic drugs that suppress locomotion and increase appetite, leading to obesity (Stip et al., 2012). In our present studies, (−)-MBP was compared directly with its enantiomer (+)-MBP, which has identical physiochemical properties but with a mirror image three-dimensional arrangement of atoms, to provide molecular support of successful 5-HT2 receptor-mediated translation from cellular to behavioral potency and efficacy.
Relative to (+)-MBP, (−)-MBP showed considerably higher affinity at each of the [3H]antagonist-labeled 5-HT2 receptor subtypes in vitro, which paralleled its significantly enhanced behavioral potency and efficacy in vivo in the DOI-elicited HTR assay. Moreover, molecular determinants for function were found to differ between 5-HT2 subtypes: (−)-MBP activated 5-HT2C receptors exclusively, but (+)-MBP activated 5-HT2A and 5-HT2B as well as 5-HT2C receptors. The affinity of (−)-MBP at [3H]agonist-labeled human 5-HT2C receptors (9 nM Ki) was more than 9- and 20-fold higher than its affinity at [3H]agonist-labeled 5-HT2A and 5-HT2B receptors, respectively, providing evidence that (−)-MBP selectively stabilizes a high-affinity agonist conformation of the 5-HT2C receptor but not of the 5-HT2A or 5-HT2B receptors. Thus, a molecular basis for 5-HT2C-specific activation was established despite the relatively high (∼75%) transmembrane sequence homology between 5-HT2 subtypes; the risks associated with 5-HT2A and/or 5-HT2B receptor activation can and should be avoided with regard to 5-HT2C-activating drugs.
(−)-MBP was a competitive antagonist of 5-HT activation of human 5-HT2A and 5-HT2B signaling and did not activate either receptor, even at 10 µM, which is 50- to 800-fold higher than its 5-HT2A/2B affinity values, depending on whether an agonist or antagonist is used to label the receptors. It is noteworthy that (−)-MBP was an inverse agonist at human 5-HT2B receptors, prospectively eliminating the possibility of 5-HT2B-mediated cardiac valvulopathy. Inverse agonism, however, was not observed consistently at human 5-HT2A receptors, suggesting that (−)-MBP may be a 5-HT2A neutral antagonist. In summary, (−)-MBP is a potent 5-HT2C receptor-specific partial agonist that does not activate 5-HT2A or 5-HT2B receptors, setting it apart from all other reported selective 5-HT2C agonists, including the novel antiobesity drug Belviq, the widely used research agonist Ro60-0175 [(S)-2(6-chloro-5-fluorindol-1-yl)-1-methylethylamine], and the prototypical agonist m-chlorophenylpiperazine (mCPP), all of which also activate 5-HT2A and 5-HT2B receptors.
(−)-MBP was effective in several preclinical animal models of psychoses, including a model of 5-HT2-mediated hallucinations (DOI-elicited HTR), a model of dopamine hyperactivity (amphetamine-elicited hyperlocomotion), and a model of glutamate hypofunction (MK-801-elicited hyperlocomotion). Each of the targeted neurotransmitter systems associated with the animal models (i.e., 5-HT2A receptors, dopamine and norepinephrine transporter, and glutamate NMDA receptors, respectively) has been implicated in psychoses and schizophrenia, and drugs within each of these classes can mimic psychosis in humans (Aghajanian and Marek, 2000; Gonzalez-Maeso and Sealfon, 2009; Coyle et al., 2012; Masana et al., 2012), providing the models with some etiological validity. In these animal models, (−)-MBP was compared directly to the prototypical second-generation antipsychotic drug clozapine, which previously had been reported to attenuate the DOI-elicited HTR, and also amphetamine- and NMDA antagonist-induced hyperlocomotion (Corbett et al., 1995; Gleason and Shannon, 1997; Rojas-Corrales et al., 2007). (−)-MBP demonstrated similar efficacy as clozapine, although it was less potent. It is noteworthy that in contrast to clozapine, (−)-MBP did not compromise locomotion when administered alone, suggesting promise as an antipsychotic drug without liability for motoric disorders or sedation. However, we acknowledge that the aforementioned behavioral models are likely permissive and could lead to false-positive results; compounds effective in these models could potentially fail to ameliorate psychotic symptoms in humans, indicative that improved animal models for the core symptoms of schizophrenia are necessary (Brown et al., 2013).
All other reported 5-HT2C agonists that are effective as antipsychotic drugs, either in preclinical animal models or in clinical trials, also have 5-HT2A and/or 5-HT2B receptor agonist properties (Dunlop et al., 2005; Marquis et al., 2007; Siuciak et al., 2007; Rosenzweig-Lipson et al., 2012), raising the possibility that their therapeutic effects could be due to some combination of 5-HT2 subtype activation. However, we are not aware of any studies documenting antipsychotic activity of lorcaserin, the only FDA-approved 5-HT2C agonist that also activates 5-HT2A and 5-HT2B receptors (Thomsen et al., 2008). Meanwhile, (−)-MBP did not activate 5-HT2A or 5-HT2B receptors, which were expressed at relatively high densities in the transiently transfected HEK cells here and elsewhere (Booth et al., 2009); thus, the efficacy of (−)-MBP demonstrated in the rodent models of psychoses supports the assertion that 5-HT2C receptor activation alone or in combination with 5-HT2A and/or 5-HT2B antagonism or inverse agonism may negatively modulate psychotic behaviors. Finally, the results that (−)-MBP negatively modulates DOI-, amphetamine-, and MK-801-induced behaviors suggest that 5-HT2C agonism together with 5-HT2A/5-HT2B antagonism/inverse agonism may translate to optimal 5-HT2-based pharmacotherapy for behaviors associated with substance abuse (Cunningham et al., 2013).
Importantly, (−)-MBP did not alter locomotion when administered alone or in combination with DOI, MK-801, or amphetamine at behaviorally active doses (up to 10 mg/kg), indicating that its modulation of MK-801 and amphetamine-induced locomotion was not due to primary motor deficits. This effect has been noted for related trans-4-phenyl-2-dimethylaminotetralins (Canal et al., 2013; Morgan et al., 2013). In contrast, clozapine substantially decreased locomotion below levels of vehicle-treated animals when administered alone or in combination with DOI, mirroring its sedative effects in humans, a side effect that may translate to the oft-reported “empty-headed” sensation caused by available antipsychotic drugs (Moritz et al., 2013). It is noteworthy that a recent paper reports that the hypolocomotion effect of clozapine, which is a 5-HT2A receptor inverse agonist of the canonical 5-HT2-Gq signaling pathway (Vanover et al., 2004), is mediated by 5-HT2A receptors (Williams et al., 2012). The affinity of clozapine and (−)-MBP at rodent 5-HT2A receptors is very similar, suggesting that the inverse-agonist effects of clozapine and neutral-antagonist effects of (−)-MBP at 5-HT2A receptors may translate to different behavioral outcomes or that the compounds are functionally selective regarding 5-HT2A signaling that affects locomotion. Alternatively, there may be an as yet undiscovered target(s) of (−)-MBP that counterweighs the hypolocomotion effect mediated by 5-HT2A receptors.
Also interesting regarding (−)-MBP's lack of effect on locomotion is that most 5-HT2C receptor agonists decrease locomotion in rodents (Fletcher et al., 2009; Halberstadt et al., 2009; Canal et al., 2013). Several reports show that 5-HT2C receptor-targeting compounds modulate the release of central dopamine, with agonists decreasing and antagonists or inverse agonists increasing dopamine release in an apparently neural system-dependent manner (Di Giovanni et al., 2011). The lack of effect on locomotor behavior with (−)-MBP may be due to partial agonism of 5-HT2C receptors, which may dampen, for example, amphetamine-stimulated dopamine release but not cause a reduction in dopamine levels on its own. This phenomenon has been described with reference to dopamine D2 partial agonists, including the antipsychotic drug aripiprazole (Strange, 2008). Collectively, results indicate that (−)-MBP may selectively negatively modulate psychostimulant-induced behaviors and not affect vigilance potentially translating to a drug that lacks sedative effects. Furthermore, the lack of effect of (−)-MBP on locomotion in rodents suggests that it may treat psychoses without causing extrapyramidal side effects or catalepsy.
Other serious side effects of especially second-generation antipsychotic drugs include metabolic syndrome—specifically high glucose and cholesterol (Pramyothin and Khaodhiar, 2010)—as well as increased appetite and weight gain leading to obesity (Stip et al., 2012). (−)-MBP, in contrast, suppressed feeding in a mouse model of compulsive binge-eating/snack-food intake, suggestive of 5-HT2C agonism, which is known to decrease feeding and reduce weight in rodents and humans (Smith et al., 2010).
In summary, the novel 5-HT2C receptor-specific partial agonist (−)-MBP displayed clear, favorable activity in animal models predictive of neuropsychiatric symptomology without possessing deleterious side effects associated with the administration of currently available antipsychotic medications, including alterations in motor activity and increased appetite. These results support further development of (−)-MBP and other drug candidates with similar 5-HT2C agonism together with 5-HT2A/2B antagonism/inverse agonism for the treatment of psychoses and compulsive behavioral disorders involving substance (including food) abuse and addiction.
Acknowledgments
The authors thank Dr. Yue Liu (Northeastern University Center for Drug Discovery) for performing radioligand binding assays to corroborate Ki values.
Abbreviations
- AMP
amphetamine
- CLOZ
clozapine
- DOI
(±)-(2,5)-dimethoxy-4-iodoamphetamine
- GPCR
G protein-coupled receptor
- HEK293
human embryonic kidney 293 cells
- 5-HT
serotonin
- HTR
head-twitch response
- (+)-MBP
(+)-trans-(2R,4S)-(3′[meta]-bromophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine
- (−)-MBP
(−)-trans-(2S,4R)-4-(3′[meta]-bromophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine
- MK-801
(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (dizocilpine maleate)
- NMDA
N-methyl-d-aspartate
- PAT
4-phenyl-2-dimethylaminotetralin (4-phenyl-N,N-dimethyl-1,2,3,4-tetrahydronaphthalene-2-amine)
- Ro60-0175
(S)-2(6-chloro-5-fluorindol-1-yl)-1-methylethylamine
Authorship Contributions
Participated in research design: Canal, Morgan, Rowland, Robertson, Sakhuja, Booth.
Conducted experiments: Canal, Morgan, Felsing, Kondabolu, Robertson, Sakhuja.
Performed data analyses: Canal, Morgan, Rowland, Robertson.
Wrote or contributed to the writing of the manuscript: Canal, Morgan, Booth.
Footnotes
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant R01-MH081193]; and National Institutes of Health National Institute on Drug Abuse [Grants R01-DA023928, R01-DA030989] (to R.G.B. and D.M.).
References
- Abdallah L, Bonasera SJ, Hopf FW, O’Dell L, Giorgetti M, Jongsma M, Carra S, Pierucci M, Di Giovanni G, Esposito E, et al. (2009) Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. J Neurosci 29:8156–8165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. (1998) Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 155:761–767 [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. (2000) Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev 31:302–312 [DOI] [PubMed] [Google Scholar]
- Alex KD, Yavanian GJ, McFarlane HG, Pluto CP, Pehek EA. (2005) Modulation of dopamine release by striatal 5-HT2C receptors. Synapse 55:242–251 [DOI] [PubMed] [Google Scholar]
- Arena Pharmaceuticals (2012) Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQ® (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182 ed).
- Bellack AS. (2006) Scientific and consumer models of recovery in schizophrenia: concordance, contrasts, and implications. Schizophr Bull 32:432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth RG, Fang L, Huang Y, Wilczynski A, Sivendran S. (2009) (1R, 3S)-(−)-trans-PAT: a novel full-efficacy serotonin 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist/antagonist activity. Eur J Pharmacol 615:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Rueter LE, Zhang M. (2013) Predictive validity of a MK-801-induced cognitive impairment model in mice: implications on the potential limitations and challenges of modeling cognitive impairment associated with schizophrenia preclinically. Prog Neuropsychopharmacol Biol Psychiatry 49:53–62 [DOI] [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D. (2013) Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology 70:112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Corbett R, Camacho F, Woods AT, Kerman LL, Fishkin RJ, Brooks K, Dunn RW. (1995) Antipsychotic agents antagonize non-competitive N-methyl-d-aspartate antagonist-induced behaviors. Psychopharmacology (Berl) 120:67–74 [DOI] [PubMed] [Google Scholar]
- 213.Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. (2012) Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol 267–295 DOI: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, et al. (2013) Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci 4:110–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran C, Byrappa N, McBride A. (2004) Stimulant psychosis: systematic review. Br J Psychiatry 185:196–204 [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U. (2004) Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24:3235–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Di Mascio M, Esposito E. (2000) Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin(2C/2B) receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse 35:53–61 [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Esposito E, Di Matteo V. (2011) The 5-HT2C receptor subtype controls central dopaminergic systems: evidence from electrophysiological and neurochemical studies in 5-HT2C Receptors in the Pathophysiology of CNS Disease (Di Giovanni G, Esposito E, Di Matteo V, eds) pp 215–247, Humana Press, New York [Google Scholar]
- Di Matteo V, Pierucci M, Esposito E. (2004) Selective stimulation of serotonin2c receptors blocks the enhancement of striatal and accumbal dopamine release induced by nicotine administration. J Neurochem 89:418–429 [DOI] [PubMed] [Google Scholar]
- Dunlop J, Sabb AL, Mazandarani H, Zhang J, Kalgaonker S, Shukhina E, Sukoff S, Vogel RL, Stack G, Schechter L, et al. (2005) WAY-163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole], a novel 5-hydroxytryptamine 2C receptor-selective agonist with anorectic activity. J Pharmacol Exp Ther 313:862–869 [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. (2009) Characterizing the effects of 5-HT2C receptor ligands on motor activity and feeding behaviour in 5-HT2C receptor knockout mice. Neuropharmacology 57:259–267 [DOI] [PubMed] [Google Scholar]
- Gleason SD, Shannon HE. (1997) Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 129:79–84 [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. (1984) Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 35:2505–2511 [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Sealfon SC. (2009) Psychedelics and schizophrenia. Trends Neurosci 32:225–232 [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. (2009) 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 34:1958–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. (2012) The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37:1177–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Stevenson PL, Carter RN, Maccoll G, French KL, Simons JP, Al-Shawi R, Kelly V, Chapman KE, Holmes MC. (2009) Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity. Eur J Neurosci 30:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O'Rahilly S, Heisler LK. (2007) Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 149:1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, et al. (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962 [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223 [DOI] [PubMed] [Google Scholar]
- Lindenmayer J-P. (2000) Treatment refractory schizophrenia. Psychiatr Q 71:373–384 [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Mengod G, Palacios JM, Vilaró MT. (2001) Regional distribution and cellular localization of 5-HT2C receptor mRNA in monkey brain: comparison with [3H]mesulergine binding sites and choline acetyltransferase mRNA. Synapse 42:12–26 [DOI] [PubMed] [Google Scholar]
- Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA, Grauer SM, Ashby CR, Jr, Nguyen HQ, Dawson LA, et al. (2007) WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole]: A novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther 320:486–496 [DOI] [PubMed] [Google Scholar]
- Masana M, Santana N, Artigas F, Bortolozzi A. (2012) Dopamine neurotransmission and atypical antipsychotics in prefrontal cortex: a critical review. Curr Top Med Chem 12:2357–2374 [DOI] [PubMed] [Google Scholar]
- Molineaux SM, Jessell TM, Axel R, Julius D. (1989) 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci USA 86:6793–6797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Kondabolu K, Kuipers A, Sakhuja R, Robertson KL, Rowland NE, Booth RG. (2013) Molecular and behavioral pharmacology of two novel orally-active 5HT2 modulators: potential utility as antipsychotic medications. Neuropharmacology 72:274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz S, Andreou C, Klingberg S, Thoering T, Peters MJ. (2013) Assessment of subjective cognitive and emotional effects of antipsychotic drugs. Effect by defect? Neuropharmacology 72:179–186 [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Christopoulos A. (2003) Fitting Models to Biological Data Using Linear and Non-linear Regression: A Practical Guide to Curve-Fitting, GraphPad Software, San Diego, CA [Google Scholar]
- Nichols CD. (2009) Serotonin 5-HT2A receptor function as a contributing factor to both neuropsychiatric and cardiovascular diseases. Cardiovasc Psychiatry Neurol 2009:475108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health (NIMH) (2010) Mental Health Medications, U.S. Department of Health and Human Services, Bethesda, MD: http://www.nimh.nih.gov/health/publications/mental-health-medications/nimh-mental-health-medications.pdf [Google Scholar]
- Olaghere da Silva UB, Morabito MV, Canal CE, Airey DC, Emeson RB, Sanders-Bush E. (2010) Impact of RNA editing on functions of the serotonin 2C receptor in vivo. Front Neurosci 4:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olijslagers JE, Werkman TR, McCreary AC, Siarey R, Kruse CG, Wadman WJ. (2004) 5-HT2 receptors differentially modulate dopamine-mediated auto-inhibition in A9 and A10 midbrain areas of the rat. Neuropharmacology 46:504–510 [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Faludi G, Sarosi A, Palkovits M. (2006) Regional distribution and relative abundance of serotonin(2c) receptors in human brain: effect of suicide. Neurochem Res 31:167–176 [DOI] [PubMed] [Google Scholar]
- Perälä J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsä E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppä T, et al. (2007) Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry 64:19–28 [DOI] [PubMed] [Google Scholar]
- Pramyothin P, Khaodhiar L. (2010) Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes 17:460–466 [DOI] [PubMed] [Google Scholar]
- Rojas-Corrales MO, Gibert-Rahola J, Mico JA. (2007) Role of atypical opiates in OCD. Experimental approach through the study of 5-HT2A/C receptor-mediated behavior. Psychopharmacology (Berl) 190:221–231 [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Comery TA, Marquis KL, Gross J, Dunlop J. (2012) 5-HT2C Agonists as Therapeutics for the Treatment of Schizophrenia, in Novel Antischizophrenia Treatments (Geyer MA, Gross G, eds) pp 147–165, Springer, New York: [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. (2009) Serotonergic drugs and valvular heart disease. Expert Opin Drug Saf 8:317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman MV, Seeman P. (2014) Is schizophrenia a dopamine supersensitivity psychotic reaction? Prog Neuropsychopharmacol Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zhao Y, Rosenzweig-Lipson S, Popp D, Williams J, Giller E, Detke M, Kane J. (2010) A 6-Week Randomized, Double-Blind, Placebo-controlled, Comparator Referenced, Multicenter Trial of Vabicaserin in Subjects with Acute Exacerbation of Schizophrenia, ACNP, Waikoloa Beach, HI: [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, Kandel ER, Balsam PD. (2011) Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry 69:928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, et al. (2007) CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 52:279–290 [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR, Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group (2010) Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 363:245–256 [DOI] [PubMed] [Google Scholar]
- Stip E, Lungu OV, Anselmo K, Letourneau G, Mendrek A, Stip B, Lipp O, Lalonde P, Bentaleb LA. (2012) Neural changes associated with appetite information processing in schizophrenic patients after 16 weeks of olanzapine treatment. Transl Psychiatry 2:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange PG. (2008) Antipsychotic drug action: antagonism, inverse agonism or partial agonism. Trends Pharmacol Sci 29:314–321 [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, et al. (2008) Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325:577–587 [DOI] [PubMed] [Google Scholar]
- Vanover KE, Harvey SC, Son T, Bradley SR, Kold H, Makhay M, Veinbergs I, Spalding TA, Weiner DM, Andersson CM, et al. (2004) Pharmacological characterization of AC-90179 [2-(4-methoxyphenyl)-N-(4-methyl-benzyl)-N-(1-methyl-piperidin-4-yl)-acetamide hydrochloride]: a selective serotonin 2A receptor inverse agonist. J Pharmacol Exp Ther 310:943–951 [DOI] [PubMed] [Google Scholar]
- Vincek AS, Booth RG. (2009) New approach to 4-phenyl-β-aminotetralin from 4-(3-halophenyl)tetralen-2-ol phenylacetate. Tetrahedron Lett 50:5107–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AA, Ingram WM, Levine S, Resnik J, Kamel CM, Lish JR, Elizalde DI, Janowski SA, Shoker J, Kozlenkov A, et al. (2012) Reduced levels of serotonin 2A receptors underlie resistance of Egr3-deficient mice to locomotor suppression by clozapine. Neuropsychopharmacology 37:2285–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, et al. (2008) 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Kowal DM, Nawoschik SP, Lou Z, Dunlop J. (2006) Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms. Biochem Pharmacol 71:521–529 [DOI] [PubMed] [Google Scholar]