Abstract
Although nicotine mediates its effects through several nicotinic acetylcholine receptor (nAChR) subtypes, it remains to be determined which nAChR subtypes directly mediate heightened anxiety during withdrawal. Relative success in abstinence has been found with the nAChR partial agonist varenicline (Chantix; Pfizer, Groton, CT); however, treatment with this drug fails to alleviate anxiety in individuals during nicotine withdrawal. Therefore, it is hypothesized that success can be found by the repurposing of other nAChR partial agonists for cessation therapies that target anxiety. It is noteworthy that the selective partial agonists for α4β2, ABT-089 [2-methyl-3-[2(S)-pyrrolidinylmethoxy]pyridine], and α7, ABT-107 [5-(6-[(3R)-1-azabicyclo[2.2.2]oct-3-yloxy] pyridazin-3-yl)-1H-indole] (AbbVie, North Chicago, IL), have not been evaluated as possible therapeutics for nicotine cessation. Therefore, we examined the effect of ABT-089 and ABT-107 on anxiety during withdrawal from nicotine in the novelty-induced hypophagia (NIH) paradigm. We found that short-term administration of ABT-089 and ABT-107 alleviate anxiety-like behavior during withdrawal from nicotine while long-term administration of ABT-089 but not ABT-107 reduces anxiety-like behavior during withdrawal. After behavioral testing, brains were harvested and β2-containing nAChRs were measured using [3H]epibaditine. ABT-089 and ABT-107 do not upregulate nAChRs, which is in contrast to the upregulation of nAChRs observed after nicotine. Furthermore, ABT-089 is anxiogenic in nicotine naive animals, suggesting that the effects on anxiety are specifically related to the nicotine-dependent state. Together, these studies identify additional nAChR partial agonists that may aid in the rational development of smoking cessation aids.
Introduction
Cigarette smoking is the leading cause of death in the United States each year [Centers for Disease Control and Prevention (CDC), 2002]. However, approximately 20% of the American population smokes despite the acknowledged health risks and socioeconomic costs [Centers for Disease Control and Prevention (CDC), 2011]. In addition, maintenance of smoking cessation is at best modest, with 80% of smokers relapsing within the first year of quitting (Polosa and Benowitz, 2011). It is projected that if prevalence of use does not decrease from present rates, cigarette smoking and tobacco use will result in 10 million deaths per year by 2020 (Adhikari et al., 2009). Thus, identification of effective smoking cessation therapies is urgently needed.
Nicotine, which is the major addictive component in tobacco, plays a critical role in initial tobacco reinforcement and dependence (Le Foll and Goldberg, 2006). Although many factors influence ongoing nicotine dependence, relapse to smoking is highly correlated with the severity of withdrawal symptoms present during abstinence (Ockene et al., 2000; Krall et al., 2002). These symptoms include difficulty concentrating, increased craving, depressed mood, and increased anxiety (Hughes, 1992, 2007). Varenicline (Chantix; Pfizer, Groton, CT), a partial agonist at α4β2 nicotinic acetylcholine receptors (nAChRs) and a full agonist at α7 and α3β4 nAChRs is currently the best in class treatment of smoking cessation (Coe et al., 2005; Garrison and Dugan, 2009). However, although varenicline has been shown to improve both concentration and depressed mood and mitigate craving, recent studies in mice and human subjects have shown treatment does not improve nicotine withdrawal-induced anxiety (Turner et al., 2013b; Cinciripini et al., 2013). This may be of special importance because anxiety arising due to nicotine withdrawal has been correlated with relapse rates (Zhou et al., 2009).
Nicotine acts at multiple nAChR subtypes and understanding which subtypes contribute to the detrimental side effects experienced during withdrawal is critical for identification of novel and improved therapeutics. More specifically, it is important to examine how targeting of the cholinergic system can promote abstinence by reducing withdrawal-induced anxiety. The various subtypes of nAChRs play different roles in anxiety and nicotine withdrawal. For example, alterations in the activation of the α4 nAChR subunit result in heightened anxiety (Ross et al., 2000; Labarca et al., 2001). Furthermore, although α7 nAChRs have not been implicated directly in anxiety (Paylor et al., 1998; Vicens et al., 2011) they are necessary in the ventral tegmental area for the expression of withdrawal from nicotine, suggesting that targeting of the α7 subtype may also relieve nicotine withdrawal symptoms (Nomikos et al., 1999).
Long-term nicotine exposure produces a region-specific upregulation of β2-containing nAChRs. This phenomenon is thought to contribute to nicotine addiction (Wonnacott, 1990; Marks et al., 1992; Buisson and Bertrand, 2002) and has been confirmed in several systems, including cultured cells and rodent and human tissues (Marks et al., 1983; Schwartz and Kellar, 1985; Benwell et al., 1988; Peng et al., 1994; Breese et al., 1997; Perry et al., 1999; Staley et al., 2006). Long-term exposure to varenicline upregulates nAChRs, which parallels its anxiolytic effects during cessation from nicotine (Turner et al., 2011). However, the more selective α4β2 nAChR compound, sazetidine-A, does not increase nAChRs despite behavioral anxiolytic effects (Turner et al., 2010, 2013b; Hussmann et al., 2012). Therefore, the role of nAChR upregulation in mediating withdrawal-induced anxiety is as yet unclear.
ABT-089 [2-methyl-3-[2(S)-pyrrolidinylmethoxy]pyridine; structure in Lin et al., 1997] is a selective partial agonist for α4β2* receptors with high selectivity for α4α5β2 and activity at α6β2* receptors (Rueter et al., 2004; Marks et al., 2009). ABT-089 has been rigorously studied as a treatment of cognitive disorders and has been used successfully in patients with Alzheimer’s disease and attention deficit disorder, with few unintended side effects (Lin et al., 1997; Rueter et al., 2004). ABT-107 [5-(6-[(3R)-1-azabicyclo[2.2.2]oct-3-yloxy] pyridazin-3-yl)-1H-indole; structure in Bitner et al., 2010] is a selective agonist with high affinity at α7 nAChRs that has been characterized as a cognitive enhancer in animal models of Alzheimer's disease and has low incidence of side effects at varying doses in patients (Bitner et al., 2010; Malysz et al., 2010; Othman et al., 2011). Both ABT-089 and ABT-107 enhance learning in naive animals (Decker et al., 1997; Bitner et al., 2010). However, ABT-089 and ABT-107 have not been evaluated for their role in reducing anxiety after nicotine withdrawal or regulation of nAChRs. Therefore, we tested ABT-089 and ABT-107 and the selective targeting of distinct nAChR subtypes to identify which subtype may mediate anxiety during withdrawal from nicotine.
Materials and Methods
Animals.
Male 129SvJ;C57Bl/6J F1 hybrid mice (6–12 weeks of age, 20–30 g) were purchased from Taconic Farms (Hudson, NY), double-housed, and maintained on a 12-hour light/dark cycle with food and water ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee. All experimental testing sessions were conducted between 9:00 AM and 1:00 PM, with animals randomly assigned to treatment conditions and tested in counterbalanced order. For all studies, mice were acclimated to the behavioral testing facility for 1 hour prior to testing.
Drugs.
Doses of nicotine tartrate (Sigma-Aldrich, St. Louis, MO), ABT-089, and ABT-107 (synthesized by AbbVie) are reported as free base weight. For short-term studies, all drugs were prepared immediately before use in 0.9% saline and injected intraperitoneally. Dose response curves indicated that 1.2 mg/kg for ABT-089 and 0.03 mg/kg for ABT-107 (data not shown) do not alter locomotor activity in male 129SvJ;C57Bl/6J F1 hybrid mice.
For long-term treatment studies, nicotine (18 mg/kg per day), ABT-089 (0.769 mg/kg per day), and ABT-107 (0.32 mg/kg per day) were dissolved in 0.9% saline solution and administered for 14 days subcutaneously via osmotic minipump (model 2002; Alzet, Cupertino, CA). Mice were anesthetized with an isoflurane/oxygen mixture (1–3%), and osmotic minipumps were inserted subcutaneously using aseptic surgery techniques. Minipumps were placed parallel to the spine at shoulder level with the flow moderator directed away from the surgical incision. The wound was closed with 7-mm stainless steel wound clips (Reflex; Cellpoint Scientific, Gaithersburg, MD).
Novelty-Induced Hypophagia.
One week prior to the start of training, mice were housed in groups of two. Training days consisted of daily sessions in which mice were exposed to a highly palatable food (peanut butter chips; Nestle, Glendale, CA) presented in a clear plastic dish. During training and home cage-testing sessions, a plastic insert (dividing the standard cage lengthwise) was used to separate mice in each cage. Mice were acclimated to the barriers 1 hour prior to presentation of the food. Food was placed in the cage for 15 minutes, and latency to consume was measured (seconds). Training criterion was met once a latency under 20 seconds to approach and consume the food with <20% variability existed between mice.
Experiment 1: Short-Term Administration.
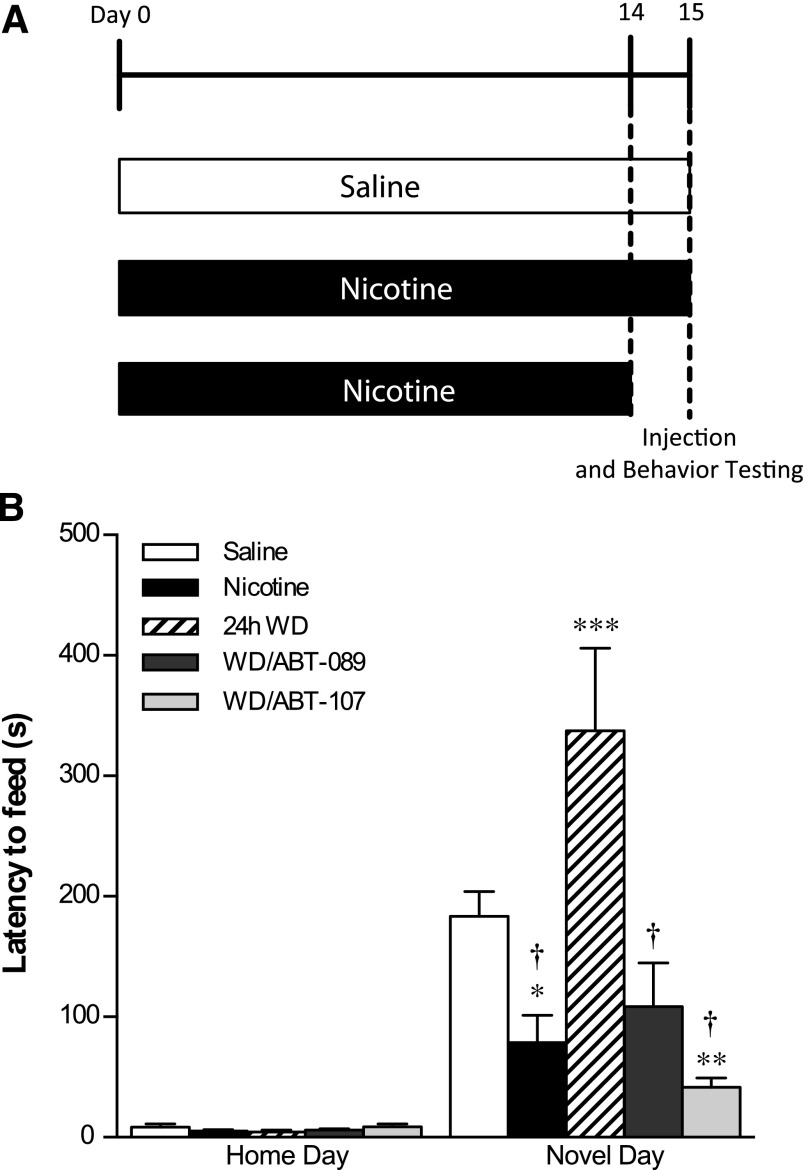
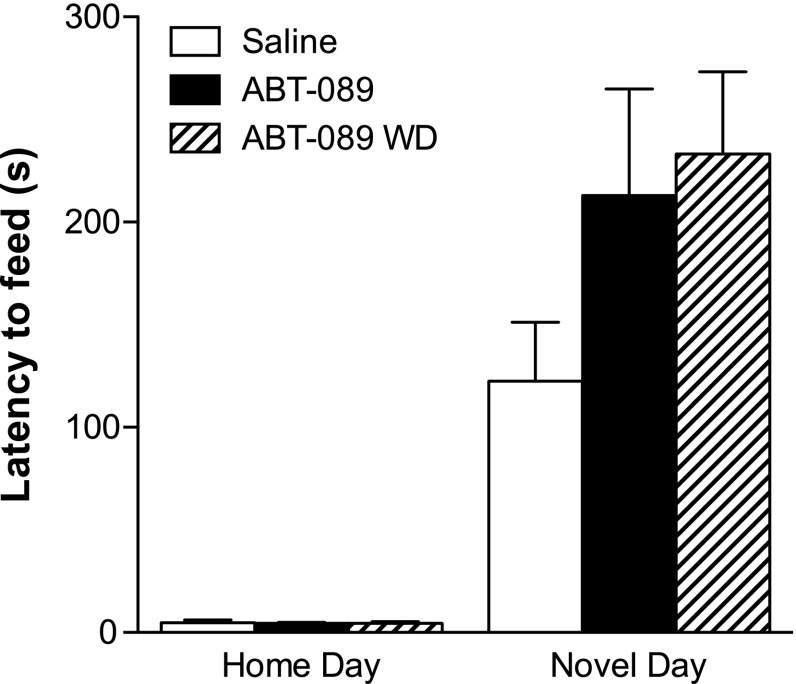
For short-term ABT-089/ABT-107 drug treatment studies, mice were implanted with 14-day osmotic minipumps filled with nicotine or saline (for experimental design schematic, see Fig. 1A). On the last day of minipump viability, animals were tested in the home cage environment 10 minutes after an injection of saline. After the home test occurred, the nicotine minipump was removed in three-fourths of the nicotine-treated animals and half of the saline-treated animals. Twenty-four hours later, animals were acclimated for a novel testing day and given an intraperitoneal injection of ABT-089/ABT-107 or saline 10 minutes prior to testing in the novel environment. The novel environment consisted of an empty standard cage with no bedding that was wiped with cleanser (1:10 Pine-Sol dilution; Clorox Co., Oakland, CA) to supply a novel odor, and placed in a white box with white light illumination (2150 lux). Latency to consume was recorded over 15 minutes. On both test days, the amount consumed (grams) of peanut butter chips was recorded.
Fig. 1.
The effect of short-term ABT-089 and ABT-107 on the behavior of mice during nicotine withdrawal in the NIH. (A) Mice were implanted with osmotic minipumps containing nicotine or saline. After 2 weeks of treatment, minipumps were removed from three-fourths of the nicotine animals to induce spontaneous nicotine withdrawal. ABT-089 and ABT-107 were administered intraperitoneally 10 minutes before NIH testing. (B) Latency to consume in the novel environment was measured over 15 minutes and is reported as mean latency ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 compared with saline on Novel Day. †P < 0.0001 compared with 24-hour withdrawal (WD) on Novel Day (n = 8–12).
Experiment 2: Long-Term Administration.
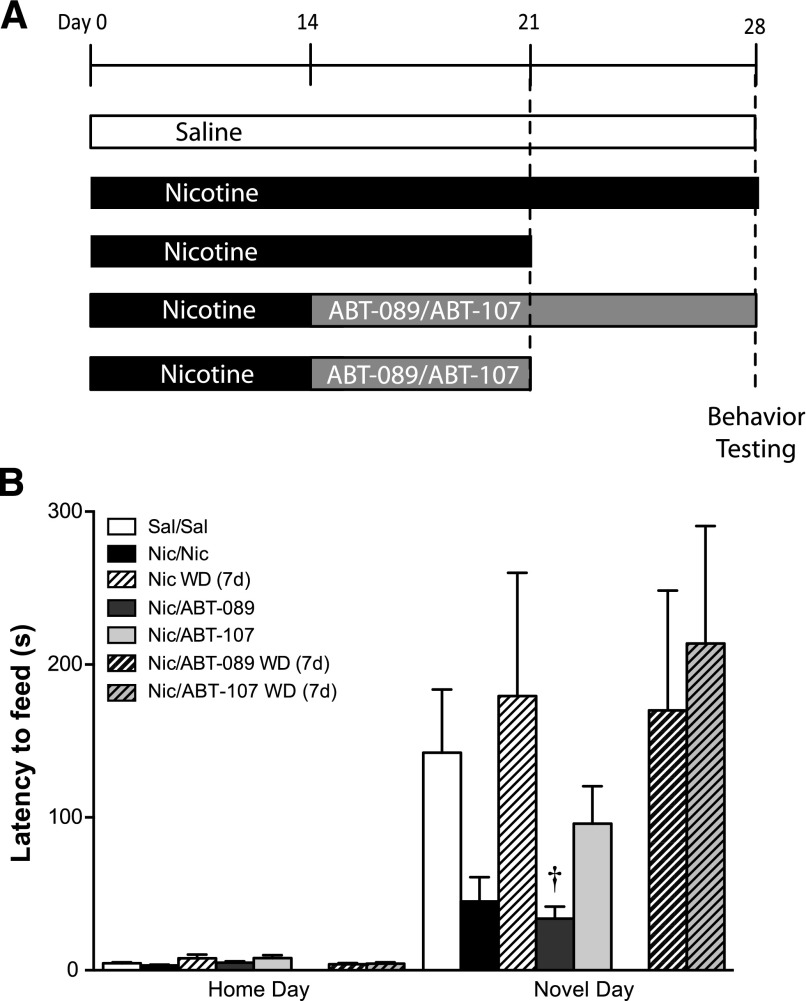
For long-term drug treatment studies, mice were implanted with 14-day osmotic minipumps filled with nicotine or saline. After 14 days, nicotine minipumps were removed and replaced with a 14-day osmotic minipump containing either nicotine, ABT-089, or ABT-107. After 7 days of the second treatment, osmotic minipumps were removed from half of the nicotine, ABT-089, and ABT-107 treatment groups to initiate spontaneous withdrawal. The remaining animals continued drug treatment for an additional 7 days or underwent 7 days of withdrawal from nicotine, ABT-089, or ABT-107. A home day was conducted, and novel day testing occurred 24 hours later. Animals were killed, and brains were harvested for receptor binding experiments (for experimental design schematic, see Fig. 2A).
Fig. 2.
The effect of long-term ABT-089 and ABT-107 on the behavior of mice during nicotine withdrawal in the NIH. Data are mean (± S.E.M.) of treatment groups composed of four to nine mice per group. (A) Mice were implanted with osmotic minipumps containing nicotine (Nic) or saline (Sal). After 2 weeks of treatment, minipumps were removed from half of the nicotine animals and replaced with a minipump containing ABT-089 or ABT-107. One week after minipump switch, minipumps were removed from half of the nicotine, ABT-089, and ABT-107 treatment groups to induce spontaneous withdrawal. (B) Animals were tested in the NIH and latency to consume in the novel environment 2 weeks after minipump switch and 1 week after removal of the second minipump was measured. Latency to consume in the novel environment 2 weeks after minipump exchange and 7-day withdrawal from nicotine is reported as mean latency ± S.E.M. †P < 0.05 compared with nicotine withdrawal on Novel Day. (n = 4–9).
Receptor Binding.
Mice used in the long-term ABT-089/ABT-107 experiment were killed and used for the receptor binding experiment. Brain regions examined were constrained by a minimal tissue amount required for homogenate-binding assays. Tissues were harvested from animals immediately after behavioral testing. The samples were homogenized in 50 mM Tris-HCl (Sigma-Aldrich) buffer, pH 7.4 at 24°C, and centrifuged twice at 35,000g for 10 minutes in fresh buffer. The membrane pellets were resuspended in fresh buffer and added to tubes containing a saturating concentration (2 nM) of [3H]epibaditine ([3H]EB; PerkinElmer Life and Analytical Sciences, Boston, MA). [3H]EB is a high-affinity ligand for all heteromeric nAChRs with low nonspecific binding (Badio and Daly, 1994). [3H]EB was incubated with tissue in Tris buffer, pH 7.4, for 2 hours at 24°C with [3H]EB. Bound receptors were separated from free ligand by vacuum filtration over GF/C glass fiber filters (Brandel, Gaithersburg, MD) that were pretreated with 0.5% polyethyleneimine (Sigma-Aldrich). The filters were then counted in a liquid scintillation counter. Nonspecific binding was determined in the presence of 300 μM nicotine, and specific binding was defined as the difference between total binding and nonspecific binding.
Statistical Analysis.
All data are presented as mean ± S.E.M. For experiment 1, latency served as a dependent variable in two-way analysis of variance (ANOVA) followed post hoc by Bonferonni multiple comparisons test to detect differences. In experiment 2 (Fig. 2), a repeated measures two-way ANOVA was used to determine significant differences between treatment groups with time (Home Day, Novel Day) as a repeated-measure (within) factor. A planned comparison (Bonferonni multiple comparison) was performed to test the hypothesis that long-term ABT-089 or ABT-107 administration during nicotine withdrawal shows decreased latency to consume in a novel environment, comparing the Nic/ABT-089 and Nic/ABT-107 group with all other groups. For receptor binding studies, an ANOVA followed post hoc by Bonferonni multiple comparisons was used to detect differences between treatment groups. Statistical analyses were carried out using the GraphPad Prism 5.0 software package (GraphPad Software, San Diego, CA).
Results
Short-Term Administration of ABT-089 and ABT-107 Is Anxiolytic during Nicotine Withdrawal In the NIH Test.
Withdrawal from long-term nicotine increases latency to consume in a novel environment and short-term administration of ABT-089 and ABT-107 significantly reduces this latency (Fig. 1B). There are significant differences between treatment groups on novel day [main effect of day, F(1,35) = 82.34, P < 0.0001; main effect of treatment, F(4,35) = 9.736, P < 0.0001; interaction, F(4,35) = 10.25, P < 0.0001]. Bonferonni post hoc analysis indicated that nicotine-treated mice had significantly lower latency to consume (P < 0.05), whereas mice experiencing 24-hour withdrawal from nicotine had significantly greater latency to consume (P < 0.001) compared with saline-treated controls. Short-term administration of ABT-107 significantly reduced latency to consume the food in the novel environment during 24-hour withdrawal from nicotine (P < 0.01) compared with saline controls. Administration of ABT-107 and ABT-089 significantly reduced latency to consume at 24-hour withdrawal compared with mice undergoing 24-hour withdrawal from nicotine (P < 0.0001). Likewise, animals maintained on nicotine showed a reduced latency to consume in the novel environment compared with animals experiencing 24-hour withdrawal from nicotine (P < 0.0001). There was no change in amount consumed between home day and novel test day (data not shown).
Long-Term Administration of ABT-089 Is Anxiolytic during Nicotine Withdrawal In the NIH Test.
To better model a therapeutic administration paradigm, we tested the impact of ABT-089 and ABT-107 on withdrawal-induced anxiety after long-term exposure of the drug. A schematic of the long-term administration paradigm is shown in Fig. 2A. Data in Fig. 2B demonstrate that 14-day administration of ABT-089 and ABT-107 during nicotine withdrawal reduced latency to consume compared with saline controls, although not significantly [main effect of day, F(1,24) = 18.22, P = 0.0003]. Planned comparison of Nic/ABT-089 and Nic/ABT-107 to other treatment groups revealed that compared with nicotine withdrawal (7 days), ABT-089 significantly reduced latency to consume (P < 0.05), whereas ABT-107 did not. Animals in which minipumps were removed to induce spontaneous withdrawal from ABT-089 or ABT-107 did not show reduced latency to consume in the novel environment compared with saline controls or nicotine withdrawal.
ABT-089 Does Not Upregulate nAChRs during Nicotine Withdrawal.
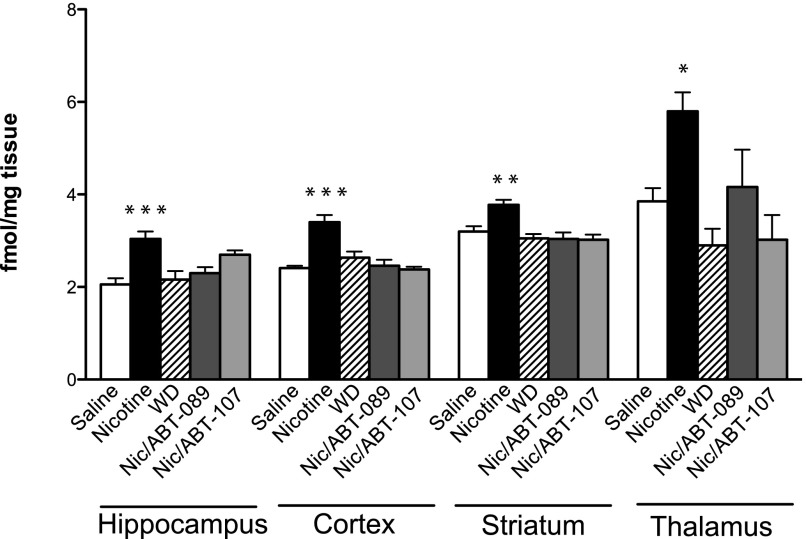
As previously demonstrated, nicotine upregulates nAChRs, and receptors return to basal levels as early as 24 hour into withdrawal from nicotine (Fig. 3 and Turner et al., 2011). Long-term nicotine increases nAChRs in the hippocampus, cortex, striatum, and thalamus (Fig. 3; main effect of treatment, hippocampus: [F(4,27) = 8.462; P = 0.0001], cortex: [F(4,29) = 15.91; P < 0.0001], striatum: [F(4,29) = 8.017; P = 0.0002], thalamus: [F(4,28) = 6.774; P = 0.0006]. However, after 7 days of withdrawal from nicotine, nAChRs are no longer upregulated compared with saline control animals. ABT-089 (Nic/ABT-089) and ABT-107 (Nic/ABT-107) administration during withdrawal from nicotine does not maintain upregulated nAChRs compared with saline control animals.
Fig. 3.
Nicotinic receptor regulation after nicotine withdrawal in the presence or absence of ABT-089 or ABT-107. Homogenate-binding experiments with a saturating concentration of [3H]EB were performed on hippocampal, cortical, striatal, and thalamic tissues from animals treated long term with nicotine for 2 weeks and then long term with ABT-089 or ABT-107 for 2 weeks during withdrawal from nicotine. Additional treatment groups include animals treated with long-term saline (4 weeks), long-term nicotine (4 weeks), and animals treated with nicotine for 3 weeks and then withdrawn from nicotine for 7 days (via nicotine minipump removal and spontaneous withdrawal). Data are mean (±S.E.M.) of treatment groups. *P < 0.01, **P < 0.001, ***P < 0.0001 compared with saline (n = 5–10).
ABT-089 Alone Does Not Upregulate nAChRs.
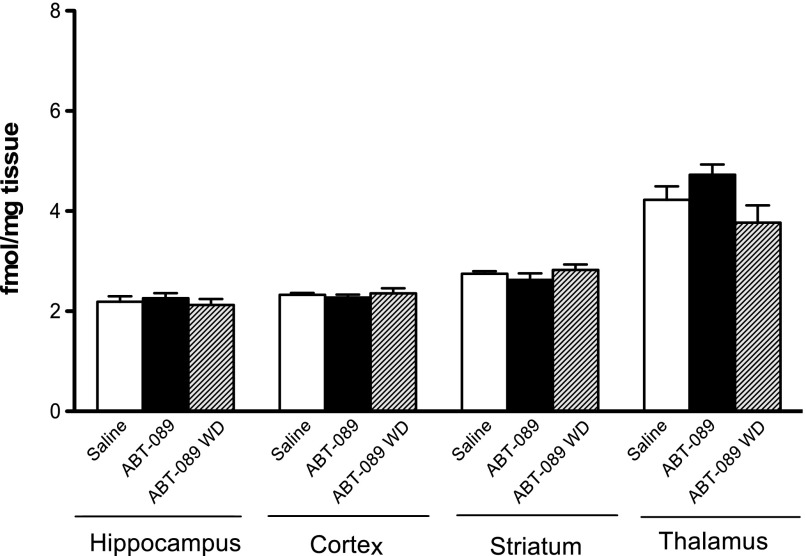
To determine whether long-term administration of ABT-089 alone can upregulate nAChRs, brain regions of interest were harvested from animals exposed to ABT-089 for 14 days. Long-term administration of ABT-089 in nicotine naive mice did not significantly upregulate nAChRs in the hippocampus, cortex, striatum, or thalamus of treated animals compared with saline controls: hippocampus: [F(2,21) = 0.3893 ;P = 0.6820), cortex: [F(2,19) = 0.3749; P = 0.6923], striatum: [F(2,21) = 0.9581; P = 0.3998], thalamus: [F(2,20) = 2.985; P = 0.0734]. Additionally, there is no up- or downregulation of receptors after 24-hour withdrawal from ABT-089 in brain regions chosen for analysis (Fig. 4).
Fig. 4.
Nicotinic receptor regulation after long-term treatment with ABT-089. Homogenate-binding experiments with a saturating concentration of [3H]EB were performed on hippocampal, cortical, striatal, and thalamic tissues from animals treated long term with ABT-089 or saline for 2 weeks. Minipumps were removed from half of the ABT-089 animals to induce spontaneous withdrawal for 24 hours. Data are mean (±S.E.M.) of treatment groups (n = 7–8).
ABT-089 Alone Is Anxiogenic in Naive Animals.
Because ABT-089 blocked nicotine-induced anxiety long term in nicotine-dependent mice, we tested the effects of long-term administration of ABT-089 in nicotine naive mice. An effect of day was revealed using two-way ANOVA [F(1,21) = 61.06; P < 0.0001]; however, there was no effect of treatment on latency to consume (Fig. 5). Although latency to consume in the novel environment appears greater in animals treated with long-term administration of ABT-089 as well as in animals undergoing 24-hour withdrawal from long-term ABT-089 at the time of testing, this increase in latency is nonsignificant
Fig. 5.
The effect of long-term ABT-089 and withdrawal from ABT-089 on the behavior of mice in the NIH. Data are mean (±S.E.M.) of treatment groups composed of eight mice per group. Mice were implanted with osmotic minipumps containing ABT-089 or saline. After 2 weeks of treatment, minipumps were removed from half of the ABT-089 animals to induce spontaneous nicotine withdrawal. Twenty-four hours later, latency to consume in a novel environment over 15 minutes was measured in all treatment groups. Data are presented as mean latency (±S.E.M.) of treatment groups (n = 8).
Discussion
Short-term administrations of ABT-089 and ABT-107 alleviate anxiety during nicotine withdrawal; however, only ABT-089 is effective in alleviating anxiety after long-term administration. Additionally, ABT-089 mediates its effects without upregulating nAChRs, a hallmark of sustained nAChR activation with nicotine. Therefore, ABT-089 may be an effective compound in treating individuals with heightened anxiety during nicotine abstinence through a different cellular mechanism than other cessation therapies.
Although administration of varenicline during smoking cessation results in the improvement of both positive affect and cognitive function (Patterson et al., 2009), efficacy in alleviating anxiety during nicotine withdrawal is more complicated (Cinciripini et al., 2013). Specifically, short-term but not long-term administration of varenicline reduces anxiety during withdrawal. For example, in naive animals, short-term and long-term varenicline administration is anxiolytic in the NIH test (Turner et al., 2010). However, in nicotine-experienced animals, short-term administration of varenicline during nicotine withdrawal fails to alleviate anxiety-like behavior (Turner et al., 2013b), suggesting a differential effect of varenicline based on the drug experienced state of the subject. Additionally, a recent study in smokers identified anxiety as one of the symptom domains in which varenicline treatment was ineffective (Cinciripini et al., 2013). Therefore, this lack of anxiolytic activity during nicotine withdrawal may underlie the low success rate of varenicline in a subset of smokers (Garrison and Dugan, 2009; Moore et al., 2010). Because varenicline acts at a number of different nAChR subtypes, identifying those subtypes that underlie the beneficial effects of the drug while avoiding subtypes responsible for negative side effects is necessary. Additionally, using a more selective ligand to produce desirable effects during nicotine withdrawal could provide tailored therapies to suit the individual needs of quitters.
Nicotine exerts its biologic effects through activation of central nAChRs that exist as subtypes determined by α and β subunit compositions (Gotti and Clementi, 2004). The heteromeric α4β2* subtype and the homomeric α7 are the most prevalent receptor subtypes in the central nervous system (Court et al., 2000). Varenicline, the best in class medication for smoking cessation, acts at both of these subtypes (Coe et al., 2005). ABT-089 is selective for the α4β2* and α6β2* (Marks et al., 2009) subtypes that have been implicated in anxiety (Ross et al., 2000; Labarca et al., 2001). ABT-107 is selective for the α7 subtype (Malysz et al., 2010) that has been implicated in generalized nicotine withdrawal syndrome (Nomikos et al., 1999). Therefore, we used these highly selective nicotinic compounds to determine the contribution of specific subtypes during nicotine withdrawal on anxiety using the NIH paradigm and further examined the effects of these drugs on receptor regulation with [3H]EB binding assay.
The NIH paradigm is a sensitive measure for potential anxiolytic drugs and anxiogenic effects of withdrawal on both acute and extended drug administration paradigms (Dulawa et al., 2004; Dulawa and Hen, 2005). The NIH test is sensitive to the anxiolytic effect of long-term nicotine (Turner et al., 2010, 2013b) and the anxiogenic effect of 24-hour withdrawal from nicotine (as shown in Fig. 1). Short-term administration of ABT-089 and ABT-107 during 24-hour withdrawal from long-term nicotine treatment reduces anxiety-like behavior in animals (Fig. 1B). However, long-term administration of ABT-089, but not ABT-107, during nicotine withdrawal showed a significant decrease in latency to consume compared with animals undergoing 7-day withdrawal from nicotine (Fig. 2B).
Nicotine withdrawal typically causes an increased latency to consume food in a novel environment above that of saline-treated animals (Fig. 1B and Turner et al., 2010, 2013a,b). However, these observations are generally evident during the first 24 to 72 hours of nicotine withdrawal. In Fig. 2B, the long-term effects of nicotine withdrawal are observed, and data demonstrate that increased anxiety occurs compared with long-term nicotine administration during nicotine withdrawal that persists beyond a 72-hour time point. Thus, long-term exposure of ABT-089 throughout the withdrawal period when individuals may be particularly vulnerable to withdrawal-induced anxiety is an important aspect of our study design. In humans, although most reports of anxiety occur during the first 24 hours, a return to baseline can take up to 4 weeks (Hughes, 1992). Therefore, the efficacy of ABT-089 to reduce anxiety within an extended time period suggests that it would be an effective treatment of the alleviation of both the initial anxiety experienced during withdrawal (Fig. 1) as well as anxiety over the course of abstinence. However, it should be noted that the anxiolytic effect of ABT-089 is only evident when drug is on board, because latency to consume in a novel environment is increased 7 days after withdrawal from ABT-089 (Fig. 2B).
Previous studies suggest that the upregulated pool of nAChRs arising from long-term exposure to nicotine may drive elements of the nicotine withdrawal syndrome (Turner et al., 2011; Gould et al., 2012), and this protracted upregulation of nAChRs has been correlated with reduced ability to maintain abstinence in the clinical population (Staley et al., 2006). Therefore, to determine whether ABT-089 reduced anxiety during nicotine withdrawal in the NIH via maintenance of upregulated nAChRs, we quantified [3H]EB binding density after long-term ABT-089 administration during nicotine withdrawal. Long-term ABT-089 does not upregulate heteromeric nAChRs during nicotine withdrawal (Fig. 3). However, it is effective in alleviating anxiety during withdrawal (Fig. 2B). Likewise, long-term ABT-107 does not upregulate heteromeric nAChRs (Fig. 3). This outcome was expected, because α7 nAChRs do not upregulate to the same degree as heteromeric nAChRs after long-term nicotine treatment (Mugnaini et al., 2002). These findings suggest that ABT-089 functionally reduces anxiety during nicotine withdrawal without maintaining upregulation of heteromeric nAChRs. Therefore, in contrast to varenicline, ABT-089 allows nAChRs to downregulate back to saline levels while providing an anxiolytic effect during withdrawal from nicotine.
Because of the efficacy of ABT-089 in alleviating nicotine withdrawal-induced anxiety, we sought to determine whether long-term ABT-089 alone could reduce anxiety in the NIH, thereby broadening the clinical applications of this compound. In addition, we evaluated whether abstinence from long-term administration of ABT-089 produces a withdrawal state that may affect anxiety-like behavior. We found that administration of ABT-089 for 2 weeks in drug-naive animals did not decrease anxiety in the NIH. In addition, 24-hour withdrawal from ABT-089 did not significantly increase latency compared with saline-treated animals (Fig. 5). Although a previous study showed ABT-089 decreased anxiety, this was after short-term rather than long-term administration (Lin et al., 1997). In addition, unlike the decrease in latency observed with long-term nicotine administration (Fig. 1B), ABT-089 administration fails to produce an anxiolytic effect. The vastly different behavioral effects of ABT-089 compared with nicotine suggest that the partial agonist activity produces different biochemical effects compared with the promiscuous activity of nicotine. Additionally, our findings from [3H]EB binding assays demonstrate that long-term ABT-089 administration in drug-naive animals does not upregulate nAChRs (Fig. 4), similar to its effects in nicotine-dependent animals.
Our findings suggest that partial activation of α4β2*/α6* and α7 nAChRs may have an effect on the initial anxiety experienced during nicotine withdrawal, and sustained activation of α4β2*/α6* nAChRs through long-term exposure to ABT-089 during nicotine withdrawal alleviates anxiety. This finding supports previous research implicating α4 in anxiety (Ross et al., 2000; Labarca et al., 2001). Additionally, β2 knockout mice and, therefore, bereft of α4β2* heterodimers have reduced anxiety during withdrawal from long-term nicotine and after mecamylamine precipitated withdrawal from nicotine (Jackson et al., 2008). Finally, a subset of α6-containing nAChRs (α6β2β3*) regulates sustained release on dopamine nerve terminals and, therefore, may provide the efficacy through which both short-term and long-term ABT-089 alleviate withdrawal symptoms (Marks et al., 2009). To date, α7 nAChRs have not been implicated in anxiety during nicotine withdrawal (Vicens et al., 2011), and they do not appear to be necessary for somatic signs of withdrawal (Markou and Paterson, 2001). However, as short-term ABT-107 reduced latency to consume during nicotine withdrawal, the targeting of α7 nAChRs may relieve withdrawal symptoms by activating nAChRs in the absence of nicotine.
Finally, findings from this study suggest a complex association between nAChR regulation and behavior. This study is the first to demonstrate that the selective partial nAChR agonist ABT-089 reduces anxiety during nicotine withdrawal without maintaining the upregulated pool of heteromeric nAChRs. However, although the ligand binding results are similar in drug-naive and drug-experienced animals exposed to long-term administration of ABT-089, their behavioral profiles are strikingly different. These findings highlight the importance of drug screening in nicotine-experienced animals. Interestingly, ABT-107, a selective partial agonist at α7 nAChRs is capable of alleviating anxiety at 24-hour withdrawal from nicotine, but not after extended administration, suggesting the role of several types of nAChRs in the initial withdrawal symptoms after nicotine cessation that may differ when withdrawal is extended. Therefore, the temporal specificity of the roles of discrete nAChR subtypes after the onset of nicotine withdrawal needs to be further studied to better identify improved therapeutics for nicotine cessation.
Acknowledgments
The authors thank AbbVie for generously providing ABT-089 and ABT-107 and Dr. Lynne Rueter (Associate Director II, Neuroscience Discovery, AbbVie) for helpful discussions. They also thank Emily Fernandez for experimental assistance.
Abbreviations
- ABT-089
2-methyl-3-[2(S)-pyrrolidinylmethoxy]pyridine
- ABT-107
5-(6-[(3R)-1-azabicyclo[2.2.2]oct-3-yloxy] pyridazin-3-yl)-1H-indole
- ANOVA
analysis of variance
- [3H]EB
[3H]epibaditine
- nAChR
nicotinic acetylcholine receptor
- NIH
novelty-induced hypophagia
Authorship Contributions
Participated in research design: Yohn, Turner, and Blendy.
Conducted experiments: Yohn.
Performed data analysis: Yohn and Turner.
Wrote or contributed to the writing of the manuscript: Yohn, Turner, and Blendy.
Footnotes
This work was supported by the National Institutes of Health National Cancer Institute [Grant P50-CA143187]; and the National Institutes of Health National Institute on Drug Abuse [Grants K99-DA032681, T32-DA28874].
This work was presented at the following meeting: Yohn NL, Turner JR, Klehm J, and Blendy JA (2013) Nicotinic acetylcholine receptor regulation during nicotine withdrawal in mice. Society for Research on Nicotine and Tobacco 19th Annual International Conference; 2013 Mar 16; Abstract No. P054-99, Boston, MA.
References
- Adhikari B, Kahende J, Malarcher A, Pechacek T, Tong V. (2009) Smoking-attributable mortality, years of potential life lost, and productivity losses. Oncology Times 31:40, 42, 43 [Google Scholar]
- Badio B, Daly JW. (1994) Epibatidine, a potent analgetic and nicotinic agonist. Mol Pharmacol 45:563–569 [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. (1988) Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem 50:1243–1247 [DOI] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Decker MW, Drescher KU, Kohlhaas KL, Markosyan S, Marsh KC, Nikkel AL, Browman K, Radek R, et al. (2010) In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer’s disease. J Pharmacol Exp Ther 334:875–886 [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. (1997) Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther 282:7–13 [PubMed] [Google Scholar]
- Buisson B, Bertrand D. (2002) Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci 23:130–136 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2002) Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995-1999. MMWR Morb Mortal Wkly Rep 51:300–303 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2011) Vital signs: current cigarette smoking among adults aged ≥18 years—United States, 2005-2010. MMWR Morb Mortal Wkly Rep 60:1207–1212 [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, Brown VL, Engelmann JM, Wetter DW. (2013) Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry 70:522–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, et al. (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477 [DOI] [PubMed] [Google Scholar]
- Court JA, Martin-Ruiz C, Graham A, Perry E. (2000) Nicotinic receptors in human brain: topography and pathology. J Chem Neuroanat 20:281–298 [DOI] [PubMed] [Google Scholar]
- Decker MW, Bannon AW, Curzon P, Gunther KL, Brioni JD, Holladay MW, Lin NH, Li Y, Daanen JF, Buccafusco JJ, et al. (1997) ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine dihydrochloride]: II. A novel cholinergic channel modulator with effects on cognitive performance in rats and monkeys. J Pharmacol Exp Ther 283:247–258 [PubMed] [Google Scholar]
- Dulawa SC, Hen R. (2005) Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev 29:771–783 [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. (2004) Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29:1321–1330 [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. (2006) Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology (Berl) 184:367–381 [DOI] [PubMed] [Google Scholar]
- Garrison GD, Dugan SE. (2009) Varenicline: a first-line treatment option for smoking cessation. Clin Ther 31:463–491 [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. (2004) Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol 74:363–396 [DOI] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, André JM, Tadman MP, Marks MJ, Kenney JW, Yildirim E, Adoff M. (2012) The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology 62:2118–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. (1992) Tobacco withdrawal in self-quitters. J Consult Clin Psychol 60:689–697 [DOI] [PubMed] [Google Scholar]
- Hughes JR. (2007) Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 9:315–327 [DOI] [PubMed] [Google Scholar]
- Hussmann GP, Turner JR, Lomazzo E, Venkatesh R, Cousins V, Xiao Y, Yasuda RP, Wolfe BB, Perry DC, Rezvani AH, et al. (2012) Chronic sazetidine-A at behaviorally active doses does not increase nicotinic cholinergic receptors in rodent brain. J Pharmacol Exp Ther 343:441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. (2008) Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325:302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall EA, Garvey AJ, Garcia RI. (2002) Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res 4:95–100 [DOI] [PubMed] [Google Scholar]
- Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, Nashmi R, Kofuji P, Dang H, Shi W, et al. (2001) Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A 98: 2786–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N-H, Gunn DE, Ryther KB, Garvey DS, Donnelly-Roberts DL, Decker MW, Brioni JD, Buckley MJ, Rodrigues AD, Marsh KG, et al. (1997) Structure-activity studies on 2-methyl-3-(2(S)-pyrrolidinylmethoxy) pyridine (ABT-089): an orally bioavailable 3-pyridyl ether nicotinic acetylcholine receptor ligand with cognition-enhancing properties. J Med Chem 40:385–390 [DOI] [PubMed] [Google Scholar]
- Malysz J, Anderson DJ, Grønlien JH, Ji J, Bunnelle WH, Håkerud M, Thorin-Hagene K, Ween H, Helfrich R, Hu M, et al. (2010) In vitro pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107. J Pharmacol Exp Ther 334:863–874 [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. (1983) Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther 226:291–302 [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. (1992) Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci 12:2765–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Wageman CR, Grady SR, Gopalakrishnan M, Briggs CA. (2009) Selectivity of ABT-089 for alpha4beta2* and alpha6beta2* nicotinic acetylcholine receptors in brain. Biochem Pharmacol 78:795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE. (2001) The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res 3:361–373 [DOI] [PubMed] [Google Scholar]
- Moore TJ, Glenmullen J, Furberg CD. (2010) Thoughts and acts of aggression/violence toward others reported in association with varenicline. Ann Pharmacother 44:1389–1394 [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Tessari M, Tarter G, Merlo Pich E, Chiamulera C, Bunnemann B. (2002) Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of alpha7 or alpha6 subunit mRNA: an autoradiography and in situ hybridization study in rat brain. Eur J Neurosci 16:1633–1646 [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Hildebrand BE, Panagis G, Svensson TH. (1999) Nicotine withdrawal in the rat: role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroreport 10:697–702 [DOI] [PubMed] [Google Scholar]
- Ockene JK, Emmons KM, Mermelstein RJ, Perkins KA, Bonollo DS, Voorhees CC, Hollis JF. (2000) Relapse and maintenance issues for smoking cessation. Health Psychol 19(Suppl. 1)17–31 [DOI] [PubMed] [Google Scholar]
- Othman AA, Lenz RA, Zhang J, Li J, Awni WM, Dutta S. (2011) Single- and multiple-dose pharmacokinetics, safety, and tolerability of the selective alpha7 neuronal nicotinic receptor agonist, ABT-107, in healthy human volunteers. J Clin Pharmacol 51:512–526 [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. (2009) Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry 65:144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. (1998) Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem 5:302–316 [PMC free article] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. (1994) Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol 46: 523–530 [PubMed] [Google Scholar]
- Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ. (1999) Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther 289:1545–1552 [PubMed] [Google Scholar]
- Polosa R, Benowitz NL. (2011) Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol Sci 32:281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, et al. (2000) Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci 20:6431–6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter LE, Anderson DJ, Briggs CA, Donnelly-Roberts DL, Gintant GA, Gopalakrishnan M, Lin N-H, Osinski MA, Reinhart GA, Buckley MJ, et al. (2004) ABT-089: pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Rev 10:167–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. (1985) In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem 45:427–433 [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, et al. (2006) Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci 26:8707–8714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. (2010) Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. J Pharmacol Exp Ther 334:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. (2011) Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res 13: 41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Ray R, Lee B, Everett L, Xiang J, Jepson C, Kaestner KH, Lerman C, Blendy JA. (2013a) Evidence from mouse and man for a role of neuregulin 3 in nicotine dependence. Mol Psychiatry DOI: 10.1038/mp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Wilkinson DS, Poole RL, Gould TJ, Carlson GC, Blendy JA. (2013b) Divergent functional effects of sazetidine-a and varenicline during nicotine withdrawal. Neuropsychopharmacology 38:2035–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicens P, Ribes D, Torrente M, Domingo JL. (2011) Behavioral effects of PNU-282987, an alpha7 nicotinic receptor agonist, in mice. Behav Brain Res 216:341–348 [DOI] [PubMed] [Google Scholar]
- Wonnacott S. (1990) The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci 11:216–219 [DOI] [PubMed] [Google Scholar]
- Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. (2009) Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addict Behav 34:365–373 [DOI] [PubMed] [Google Scholar]