Abstract
Clinically used calcineurin inhibitors, including tacrolimus (FK506) and cyclosporine A, can induce calcineurin inhibitor-induced pain syndrome (CIPS), which is characterized as severe pain and pain hypersensitivity. Increased synaptic N-methyl-d-aspartate receptor (NMDAR) activity in the spinal dorsal horn plays a critical role in the development of CIPS. Casein kinase II (CK2), a serine/threonine protein kinase, can regulate synaptic NMDAR activity in the brain. In this study, we determined whether spinal CK2 is involved in increased NMDAR activity and pain hypersensitivity caused by systemic administration of FK506 in rats. FK506 treatment caused a large increase in the amplitude of NMDAR-mediated excitatory postsynaptic currents (EPSCs) evoked by primary afferent stimulation and in the frequency of miniature EPSCs of spinal dorsal horn neurons. CK2 inhibition with either 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) or 4,5,6,7-tetrabromobenzotriazole (TBB) completely normalized the amplitude of evoked NMDAR-EPSCs of dorsal horn neurons in FK506-treated rats. In addition, DRB or TBB significantly attenuated the amplitude of NMDAR currents elicited by puff application of N-methyl-D-aspartate to dorsal horn neurons in FK506-treated rats. Furthermore, treatment with DRB or TBB significantly reduced the frequency of miniature EPSCs of spinal dorsal horn neurons increased by FK506 treatment. In addition, intrathecal injection of DRB or TBB dose-dependently reversed tactile allodynia and mechanical hyperalgesia in FK506-treated rats. Collectively, our findings indicate that CK2 inhibition abrogates pain hypersensitivity and increased pre- and postsynaptic NMDAR activity in the spinal cord caused by calcineurin inhibitors. CK2 inhibitors may represent a new therapeutic option for the treatment of CIPS.
Introduction
Calcineurin is a Ca2+/calmodulin-dependent serine/threonine protein phosphatase, which is present in the T cells of the immune system and in the nervous system, including the dorsal root ganglion and spinal cord (Strack et al., 1996; Wu et al., 2005, 2006). Calcineurin inhibitors, such as tacrolimus (FK506) and cyclosporine A, are commonly used immunosuppressants and have greatly improved the long-term survival of patients after organ and tissue transplantation. However, these drugs can result in unexplained severe pain and pain hypersensitivity, clinically known as calcineurin inhibitor-induced pain syndrome (CIPS) (Grotz et al., 2001; Collini et al., 2006; Noda et al., 2008; Kakihana et al., 2012). CIPS predominantly affects the lower limbs, and the pain is particularly excruciating during standing and walking (Grotz et al., 2001; Fujii et al., 2006; Noda et al., 2008). Because the underlying mechanisms of CIPS are not fully known, effective treatments for CIPS are still limited. We recently developed a rat model of CIPS in which systemic administration of FK506 causes a long-lasting pain hypersensitivity (Chen et al., 2014). We have shown that repeated FK506 treatment in rats increases pre- and postsynaptic N-methyl-d-aspartate receptor (NMDAR) activity in the spinal dorsal horn and that blocking spinal NMDARs can effectively attenuate the pain hypersensitivity caused by FK506 (Chen et al., 2014). Nevertheless, little is known about how spinal NMDAR activity is potentiated by calcineurin inhibitors. Furthermore, because directly blocking NMDARs can produce intolerable side effects in patients, identifying new therapeutic targets involved in NMDAR regulation in CIPS could lead to improved treatment options for patients with CIPS.
NMDAR phosphorylation by the coordinated activities of protein kinases and phosphatases is central to many physiologic functions (Lieberman and Mody, 1994, 1999; Tong et al., 1995; MacDonald et al., 1998). Because synaptic NMDARs can fluctuate between phosphorylated and dephosphorylated forms, normal NMDAR function may be controlled by a delicate balance between the activities of protein kinases and protein phosphatases. Calcineurin inhibition may augment NMDAR activity by causing excessive phosphorylation of NMDARs and/or NMDAR-interacting proteins (Tong et al., 1995; Chen et al., 2014). On the other hand, inhibition of protein kinase CK2 (formerly known as casein kinase II) can attenuate NMDAR activity in the brain, possibly by reducing NMDAR phosphorylation (Lieberman and Mody, 1994, 1999; Tong et al., 1995; Ye et al., 2012). CK2 is present in the spinal cord and plays a role in inflammatory pain (Li et al., 2005). We reasoned that if calcineurin and CK2 reciprocally control NMDAR activity through phosphorylation, inhibition of endogenous CK2 might reverse calcineurin inhibitor-induced potentiation of the NMDAR activity in the spinal cord. In the present study, we used a rat model of CIPS to test the hypothesis that CK2 contributes to increased NMDAR activity in the spinal cord and persistent pain hypersensitivity caused by calcineurin inhibitors.
Materials and Methods
Animal Model of CIPS.
Eighty-seven adult male Sprague-Dawley rats (280–320 g; Harlan, Indianapolis, IN) were used for the study. The specific calcineurin inhibitor FK506 was used to induce CIPS in the rats, as we previously described (Chen et al., 2014). FK506 (1.5 mg/kg) was dissolved in dimethylsulfoxide (DMSO) and injected intraperitoneally once a day for 7 days. Rats in the control group received daily intraperitoneal injections of the vehicle (DMSO). The electrophysiological and behavioral experiments were performed 3–5 days after the last FK506 or vehicle injection. The surgical procedures and experimental protocols were approved by the Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and conformed to the National Institutes of Health guidelines for the ethical use of animals.
Intrathecal Catheter Cannulation.
In some rats, intrathecal catheters were implanted after the animals were anesthetized with 2–3% isoflurane. In brief, each animal was placed prone on a stereotactic frame, and a small incision was made at the back of the neck of the animal. A small puncture was made in the atlanto-occipital membrane of the cisterna magna, and a catheter was then inserted such that the tip of the catheter reached the lumbar enlargement of the spinal cord (Chen and Pan, 2001, 2006). The animals were allowed to recover for 3–5 days before intrathecal injections. Rats displaying signs of motor or neurologic dysfunction were killed immediately with an overdose of phenobarbital (200 mg/kg i.p.).
Behavioral Assessments of Mechanical Nociception.
To assess the mechanical nociceptive threshold of the hindpaw, we used an Ugo Basile analgesimeter (Varese, Italy). A noxious pressure stimulus was gradually applied to the hindpaws by pressing the device pedal to increase the force at a constant rate. The pedal was immediately released when the animal displayed pain by withdrawing the paw or vocalizing, and the nociceptive threshold of the animal was read on the scale. A maximum of 400 g of pressure was used as a cutoff to avoid potential tissue injury to the rats (Chen and Pan, 2006). Both hindpaws were tested in each rat, and the mean value was used as the mechanical nociceptive withdrawal threshold.
To measure tactile hypersensitivity, each animal was placed in an individual plastic box on a mesh floor and allowed to acclimate for 30 minutes. A series of calibrated von Frey filaments were applied perpendicularly to the plantar surface of the hindpaw with sufficient force to bend the filaments for 6 seconds. Brisk paw withdrawal or flinching was considered a positive response. The tactile stimulus producing a 50% likelihood of withdrawal was determined by using the “up-down” method (Chaplan et al., 1994; Chen et al., 2000).
Spinal Cord Slice Preparation and Electrophysiological Recordings.
The lumbar segment of the spinal cord at the L4–L6 level was rapidly removed through laminectomy after the rats were anesthetized with the use of 2–3% isoflurane. The spinal cord tissues were immediately placed in ice-cold artificial cerebrospinal fluid containing (in mM) 234 sucrose, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 25.0 NaHCO3, and 12.0 glucose. The spinal cord was glued onto the stage of a vibratome. Transverse spinal cord slices were cut (400 μm in thickness) in ice-cold sucrose artificial cerebrospinal fluid and then incubated in Krebs solution presaturated with 95% O2 and 5% CO2 at 34°C for at least 1 hour before being transferred to the recording chamber.
The spinal slice was continuously perfused with Krebs solution at 5.0 ml/min at 34°C, and neurons were visualized on a fixed-stage microscope with differential interference contrast/infrared illumination. Lamina II outer zone neurons were selected for electrophysiological recordings because they are the central site of termination for the majority of unmyelinated C-fibers carrying nociceptive information (Cervero and Iggo, 1980; Pan et al., 2003). It has been demonstrated that most neurons in lamina II are glutamate-releasing excitatory interneurons (Santos et al., 2007). Excitatory postsynaptic currents (EPSCs) were recorded using whole-cell voltage-clamp techniques (Pan and Pan, 2004; Li et al., 2008). The impedance of the glass electrode was 5–10 MΩ when the pipette was filled with the internal solution containing (in mM) 135 potassium gluconate, 5 KCl, 2.0 MgCl2, 0.5 CaCl2, 5.0 HEPES, 5.0 EGTA, 5.0 ATP-Mg, 0.5 Na-GTP, and 10 QX314 (adjusted to pH 7.25 with 1.0 M CsOH, 280–300 mOsm). EPSCs were evoked from the dorsal root using a bipolar tungsten electrode connected to a stimulator (0.6 mA, 0.2 ms, and 0.1 Hz; Grass Instruments, Quincy, MA). α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated EPSCs (AMPAR-EPSCs) were recorded in the presence of 2 μM strychnine and 10 μM bicuculline. NMDAR-mediated EPSCs (NMDAR-EPSCs) were recorded in the presence of 2 μM strychnine, 10 μM bicuculline, and 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione at a holding potential of +40 mV (Li et al., 2008; Zhou et al., 2012). In addition, miniature EPSCs (mEPSCs) were recorded at a holding potential of −60 mV in the presence of 1 μM tetrodotoxin, 10 μM bicuculline, and 2 μM strychnine. The input resistance was continuously monitored, and the recording was abandoned if the resistance changed by more than 15%. EPSCs were recorded using an amplifier (MultiClamp 700A, Axon Instruments, Foster City, CA), filtered at 1–2 kHz, and digitized at 10 kHz.
To directly determine postsynaptic NMDAR activity, currents were elicited by puff application of 100 μM NMDA to the recorded neuron in the extracellular solution containing a low concentration of Mg2+ (0.1 mM), 10 μM glycine, and 1 μM tetrodotoxin at a holding potential of −60 mV (Li et al., 2008; Zhou et al., 2012). The pipette internal solution contained (in mM) 110.0 Cs2SO4, 2.0 MgCl2, 0.1 CaCl2, 1.1 EGTA, 10.0 HEPES, 2.0 MgATP, and 0.3 Na2GTP (pH was adjusted to 7.25 with 1.0 M CsOH (280–300 mOsm) (Li et al., 2008; Zhou et al., 2012). The puff electrode was placed approximately 150 μm away from the neuron being recorded. Puff application of NMDA was performed using a Pressure System IIe (4 p.s.i., 15 ms; Toohey Co., Fairfield, NJ). For each protocol, four to six rats were used (and two or three neurons were generally recorded from each rat). All drugs were freshly prepared in artificial cerebrospinal fluid before the experiments and delivered via syringe pumps to reach their final concentrations.
FK506 and d-2-amino-5-phosphonopentanoate (AP5) were purchased from Tocris Bioscience (Ellisville, MO). 5,6-Dichloro-1-β-d-ribofuranosylbenzimidazole (DRB), 4,5,6,7-tetrabromobenzotriazole (TBB), and NMDA were purchased from Sigma-Aldrich. 6-Cyano-7-nitroquinoxaline-2,3-dione, bicuculline, and tetrodotoxin were purchased from Ascent Scientific (Princeton, NJ).
Statistical Analysis.
All the data are expressed as means ± S.E.M. To determine the amplitude of the evoked EPSCs, 6–10 consecutive EPSCs were averaged and analyzed offline with Clampfit 9.2 software (Axon Instruments). The mEPSCs were analyzed offline with a peak detection program (MiniAnalysis, Synaptosoft Inc., Decatur, GA). For the statistical analysis of electrophysiological data, one-way analysis of variance followed by Dunnett’s or Tukey’s post hoc test was used. The effects of CK2 inhibitors on the paw withdrawal thresholds were determined by one-way analysis of variance followed by Dunnett's post hoc test. A P value of < 0.05 was considered statistically significant.
Results
CK2 Contributes to Potentiated Synaptic NMDAR Currents of Spinal Dorsal Horn Neurons in FK506-Treated Rats.
To determine the contribution of CK2 to increased synaptic NMDAR activity of spinal dorsal horn neurons in FK506-treated rats, we used two selective and structurally dissimilar CK2 inhibitors, DRB (100 μM) and TBB (2 μM), which competitively inhibit the binding of the phosphate donors ATP and GTP to CK2 (Sarno et al., 2001, 2002). It is noteworthy that DRB is a highly specific CK2 inhibitor, and it does not affect protein kinase C, protein kinase A, calmodulin-dependent protein kinase II, or the Src tyrosine kinase (Pinna, 1990). The concentrations of DRB and TBB selected for our study have been shown to inhibit CK2 activity in rat brain slices (Lieberman and Mody, 1999; Ye et al., 2011). We recorded AMPAR-EPSCs and NMDAR-EPSCs of lamina II neurons monosynaptically evoked from dorsal root stimulation in spinal cord slices obtained from vehicle-treated and FK506-treated rats. Monosynaptic EPSCs were identified on the basis of the constant latency of evoked EPSCs and the absence of conduction failure of evoked EPSCs in response to a brief 20-Hz electrical stimulation (Li et al., 2002; Zhou et al., 2010).
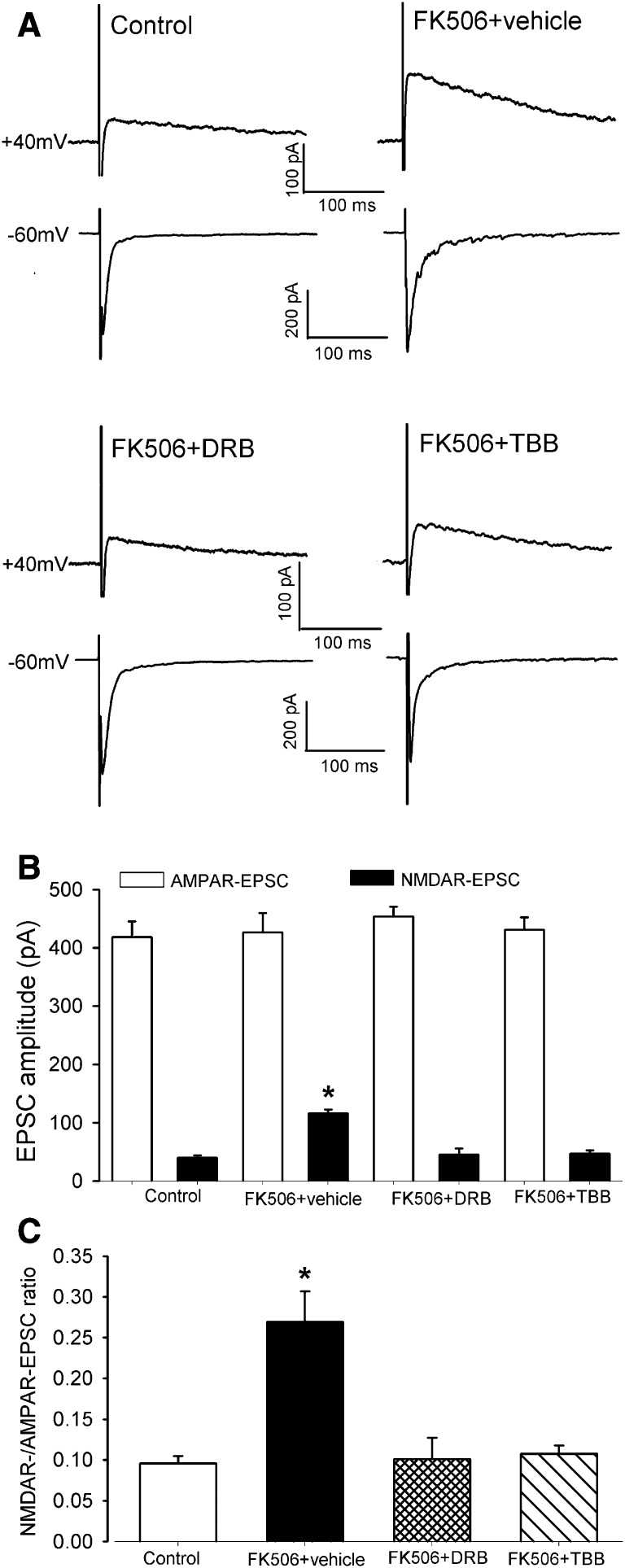
Although the amplitude of evoked AMPAR-EPSCs of spinal dorsal horn neurons did not differ significantly between vehicle-treated control rats (n = 11 neurons) and FK506-treated rats (n = 10 neurons), the amplitude of NMDAR-EPSCs was much larger in FK506-treated rats than in vehicle-treated rats (115.71 ± 6.86 versus 40.02 ± 4.17 pA, P < 0.05; Fig. 1, A and B). Furthermore, the ratio of NMDAR-EPSCs to AMPAR-EPSCs in these neurons was significantly greater in FK506-treated rats than in vehicle-treated rats (Fig. 1C). Treatment of spinal cord slices with either 100 μM DRB (n = 11 neurons) or 2 μM TBB (n = 10 neurons) for approximately 2 hours completely normalized the amplitude of evoked NMDAR-EPSCs and the ratio of NMDAR-EPSCs to AMPAR-EPSCs in FK506-treated rats (Fig. 1, A–C).
Fig. 1.
CK2 inhibition normalizes synaptic NMDAR activity in the spinal dorsal horn increased by FK506 treatment. (A) Original current traces (averaged responses from 6 EPSCs) show that NMDAR-EPSCs (at the holding potential of +40 mV) and AMPAR-EPSCs (at the holding potential of −60 mV) recorded from lamina II neurons in spinal cord slices obtained from one control rat, one rat treated with systemic injection of FK506, one FK506-treated rat plus DRB treatment (100 μM for ∼2 hours), and one FK506-treated rat plus TBB treatment (2 μM for ∼2 hours). (B and C) Summary data show the effect of DRB and TBB on the amplitude of NMDAR-EPSCs and the ratio of NMDAR-EPSCs to AMPAR-EPSCs of lamina II neurons potentiated by FK506 treatment. *P < 0.05 compared with the value in the vehicle control group.
CK2 Inhibition Normalizes Postsynaptic NMDAR Currents of Spinal Dorsal Horn Neurons in FK506-Treated Rats.
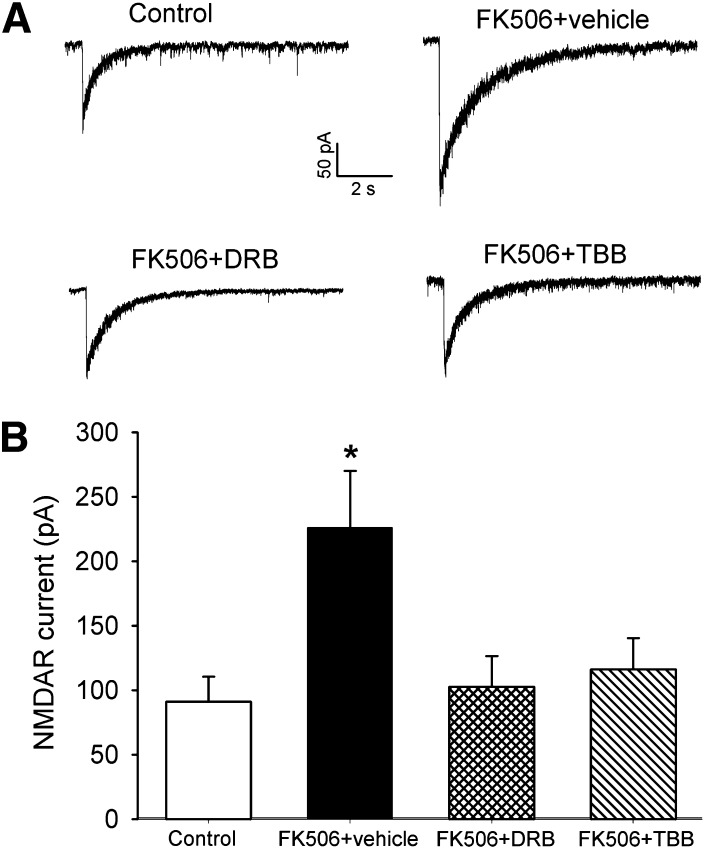
To directly determine whether CK2 plays a role in increased postsynaptic NMDAR activity in the spinal cord of FK506-treated rats, we determined the effect of DRB or TBB on NMDAR currents induced by puff application of 100 μM NMDA to the recorded lamina II neurons of FK506-treated rats. The amplitude of puff NMDA-elicited NMDAR currents of lamina II neurons in FK506-treated rats (n = 13 neurons) was significantly larger than that in vehicle-treated control rats (n = 13 neurons) (Fig. 2, A and B). Treatment of spinal cord slices from FK506-treated rats with DRB (100 μM, n = 13 neurons) or TBB (2 μM, n = 11 neurons) for approximately 2 hours profoundly decreased the amplitude of puff NMDAR currents of lamina II neurons (Fig. 2, A and B). In fact, DRB or TBB treatment normalized the amplitude of puff NMDAR currents of FK506-treated rats to that of control rats. These data suggest that CK2 contributes to the increased postsynaptic NMDAR activity of spinal dorsal horn neurons induced by the calcineurin inhibitor.
Fig. 2.
CK2 Inhibition reduces postsynaptic NMDAR currents of spinal dorsal horn neurons potentiated by FK506 treatment. (A) Representative traces show NMDAR currents elicited by puff application of 100 μM NMDA to the lamina II neuron in spinal cord slices obtained from one control rat, one rat treated with systemic injection of FK506, one FK506-treated rat plus DRB treatment (100 μM for ∼2 hours), and one FK506-treated rat plus TBB treatment (2 μM for ∼2 hours). (B) Group data show the effect of DRB and TBB on the amplitude of puff NMDA-elicited NMDAR currents of lamina II neurons. *P < 0.05 compared with the value in the vehicle-treated control group.
CK2 Is Involved in Increased Presynaptic NMDAR Activity of Spinal Dorsal Horn Neurons in FK506-Treated Rats.
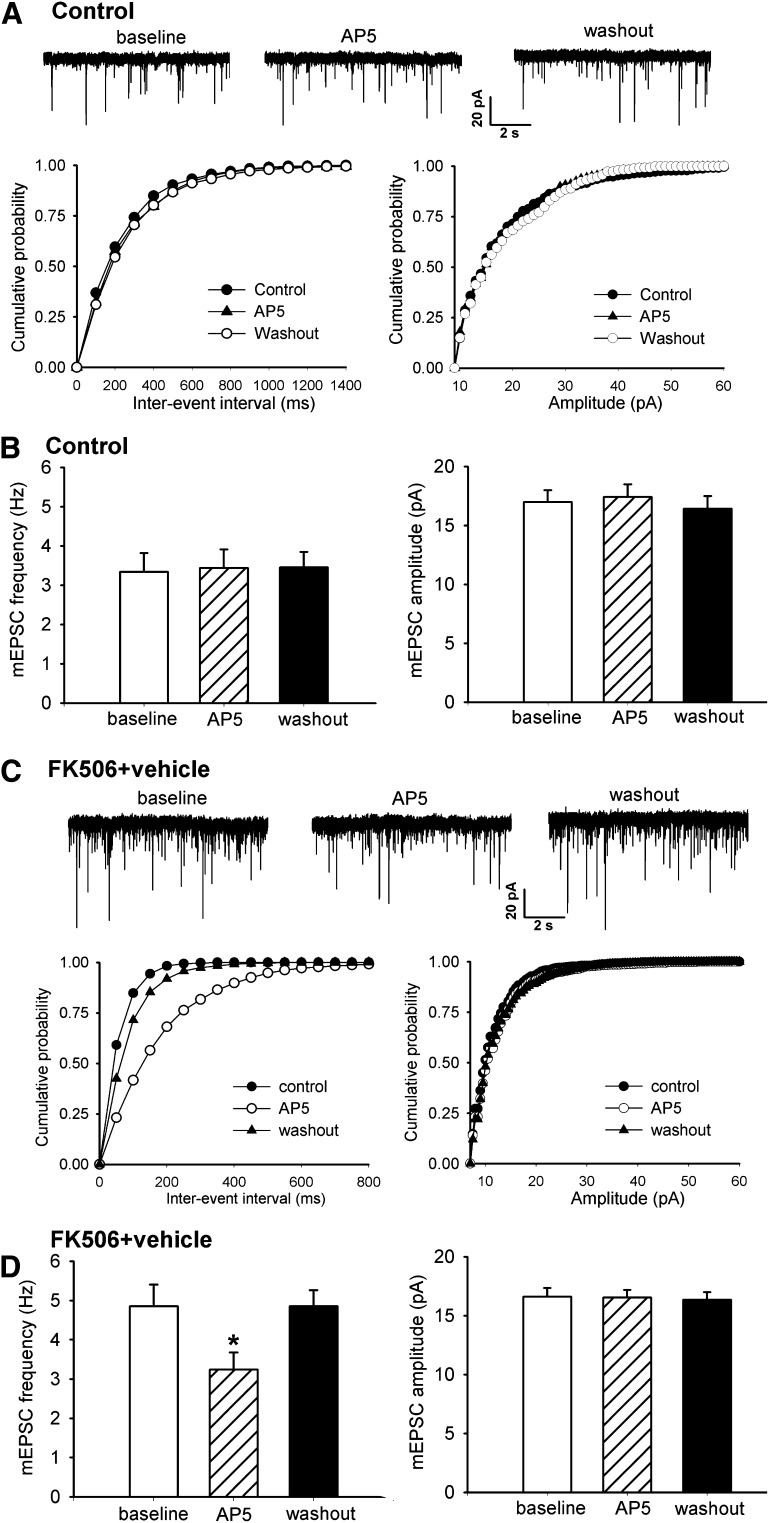
Presynaptic NMDARs regulate synaptic glutamate release in the spinal cord (Zhao et al., 2012). Systemic treatment with FK506 increases synaptic glutamate release through stimulation of presynaptic NMDARs in the spinal cord (Chen et al., 2014). To determine whether CK2 plays a role in FK506 treatment-induced increases in presynaptic NMDAR activity in the spinal cord, we tested the effect of DRB and TBB on glutamatergic mEPSCs (reflecting presynaptic quantal release of glutamate) of lamina II neurons in FK506-treated rats. The baseline frequency (4.85 ± 0.55 versus 3.34 ± 0.47 Hz, P < 0.05), but not the amplitude, of mEPSCs in lamina II neurons of FK506-treated rats (n = 15 neurons) was significantly higher than that in control rats (n = 16 neurons) (Fig. 3, A–D). Furthermore, bath application of the specific NMDAR antagonist AP5 (50 μM) significantly reduced the frequency of mEPSCs in FK506-treated rats but not in control rats (Fig. 3, A–D).
Fig. 3.
Inhibition of calcineurin augments presynaptic NMDAR activity of spinal dorsal horn neurons. (A and C) Original recordings and cumulative plots show changes in the frequency of glutamatergic mEPSCs in a lamina II neuron and the effect of bath application of 50 μM AP5 on mEPSCs in one vehicle-treated control rat (A) and one FK506-treated rat (C). (B and D) Summary data show changes in the baseline frequency and amplitude of mEPSCs and the effect of AP5 on mEPSCs in lamina II neurons recorded from vehicle-treated control (B) and FK506-treated (D) rats. *P < 0.05 compared with the respective baseline value.
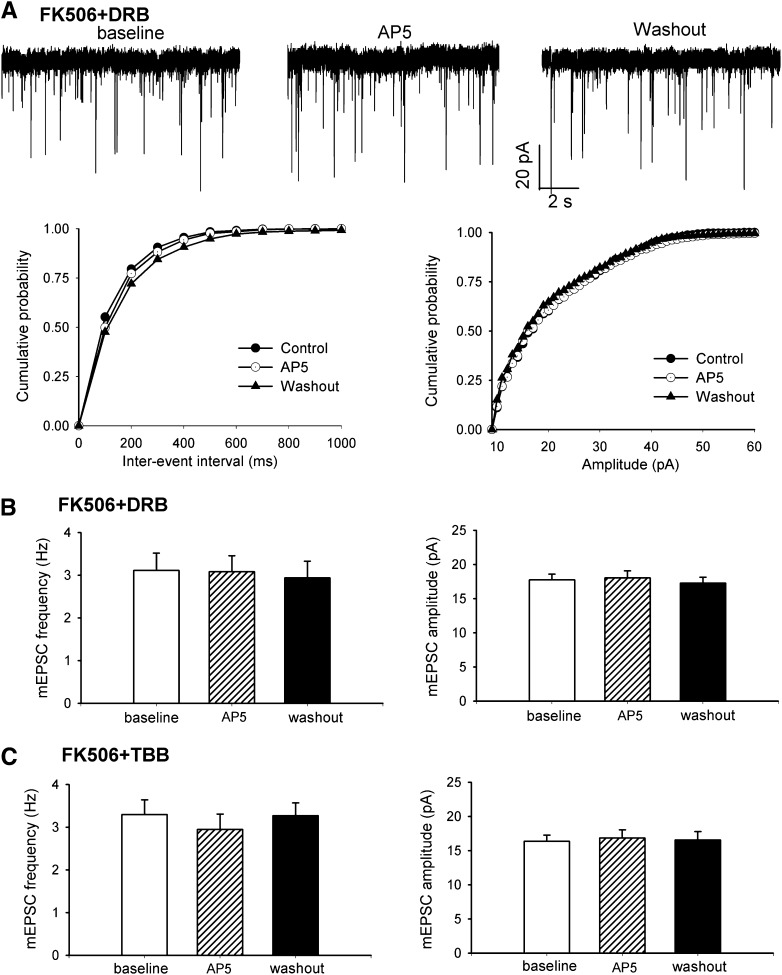
Treatment of spinal cord slices from FK506-treated rats with DRB (100 μM, n = 19 neurons) or TBB (2 μM, n = 18 neurons) for approximately 2 hours significantly reduced the baseline frequency of mEPSCs in lamina II neurons, but it had no significant effect on the amplitude of mEPSCs (Fig. 4, A–C). The baseline frequency of mEPSCs in lamina II neurons in DRB- or TBB-treated spinal cord slices in FK506-treated rats was similar to that in control rats. In addition, bath application of AP5 (50 μM) had no significant effect on the frequency of mEPSCs in FK506-treated rats after treatment with DRB or TBB (Fig. 4, A–C). These results suggest that CK2 contributes to the increased presynaptic NMDAR activity and synaptic glutamate release to spinal dorsal horn neurons caused by calcineurin inhibitors.
Fig. 4.
CK2 inhibition normalizes increased presynaptic NMDAR activity of spinal dorsal horn neurons by FK506 treatment. (A) Representative recordings and cumulative plots show that glutamatergic mEPSCs of one lamina II neuron recorded from spinal cord slice of an FK506-treated rat after incubation with 100 μM DRB for 2 hours during baseline control, bath application of 50 μM AP5, and washout. (B and C) Summary data show the reduced baseline frequency of mEPSCs and the lack of effect of bath application of AP5 on mEPSCs in lamina II neurons of FK506-treated rats after incubation with DRB or TBB.
CK2 Inhibition at the Spinal Cord Level Reverses Tactile Allodynia and Mechanical Hyperalgesia in FK506-Treated Rats.
To determine whether inhibition of CK2 activity at the spinal cord level reduces pain hypersensitivity caused by long-term administration of FK506, we tested the effect of intrathecal injection of DRB or TBB on tactile allodynia and mechanical hyperalgesia 3–5 days after the last FK506 administration. Systemic administration of FK506 (1.5 mg/kg per day i.p. for 7 days) profoundly reduced the tactile (18.62 ± 2.16 g of pretreatment control versus 2.38 ± 0.21 g 3 days after the last FK506 injection, P < 0.05) and pressure (155.27 ± 6.81 g of pretreatment control versus 96.86 ± 3.11 g 3 days after the last FK506 injection, P < 0.05) withdrawal thresholds in all 28 rats tested.
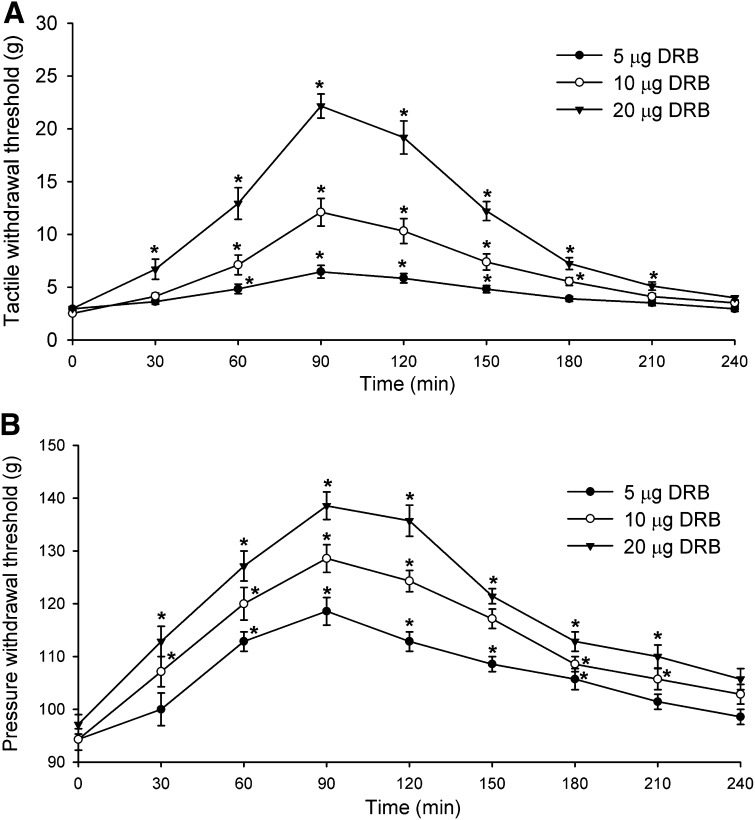
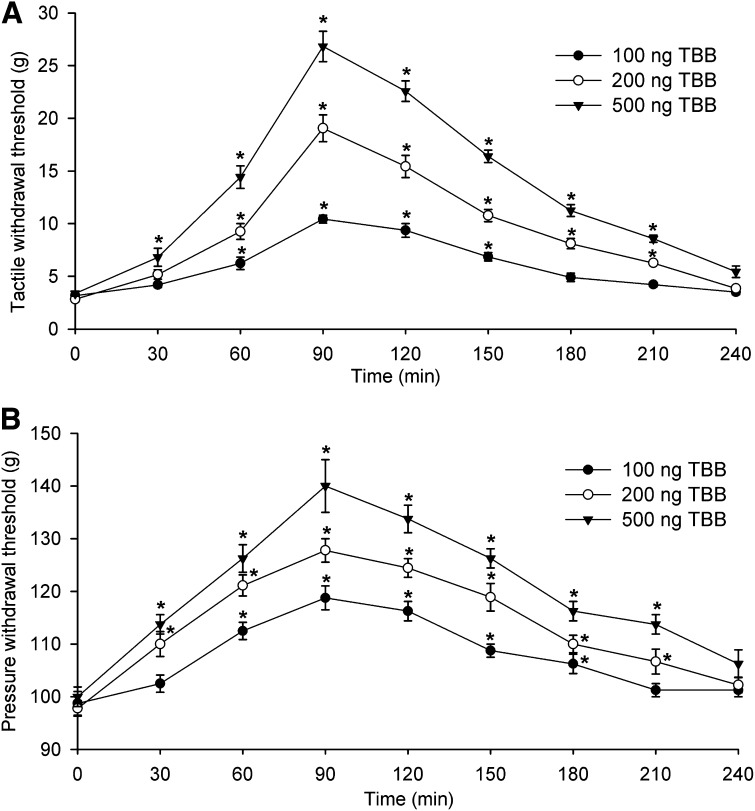
DRB or TBB was injected intrathecally in a volume of 5 μl followed by a 10-μl flush with normal saline. We previously showed that intrathecal injection of the vehicle (30% DMSO) had no significant effects on the paw-withdrawal thresholds in rats (Jin et al., 2011; Zhou et al., 2012). Intrathecal injection of DRB (5, 10, or 20 μg) produced a dose-dependent reversal of the reduced tactile and pressure withdrawal thresholds in FK506-treated rats (n = 8 rats in each dose group, Fig. 5). Likewise, intrathecal injection of TBB (100, 200, or 500 ng) significantly attenuated tactile allodynia and mechanical hyperalgesia in FK506-treated rats (n = 9 rats in each dose group, Fig. 6). At all of the doses tested, the effect of DRB and TBB was evident within 60 minutes and reached maximal at around 90 minutes. The effect of DRB and TBB gradually subsidized to baseline by 3.5 hours after injection (Figs. 5 and 6).
Fig. 5.
Inhibition of CK2 at the spinal level with DRB reverses pain hypersensitivity of rats caused by systemic administration of FK506. (A) Time course of the effect of intrathecal injection of 5–20 μg of DRB on the paw withdrawal threshold measured with von Frey filaments in rats treated with FK506 (1.5 mg/kg per day for 7 days). (B) Time course of the effect of intrathecal injection of 5–20 μg of DRB on the paw withdrawal threshold measured with a pressure stimulus in rats treated with FK506. *P < 0.05 compared with the respective baseline value (time 0).
Fig. 6.
Intrathecal injection of TBB attenuates mechanical hypersensitivity of rats induced by systemic administration of FK506. (A) Time course of the effect of intrathecal injection of 100–500 ng of TBB on the paw withdrawal threshold measured with von Frey filaments in rats treated with FK506 (1.5 mg/kg per day for 7 days). (B) Time course of the effect of intrathecal injection of 100–500 ng of TBB on the paw withdrawal threshold measured with a pressure stimulus in rats treated with FK506. *P < 0.05 compared with the respective baseline value (time 0).
Discussion
Calcineurin inhibitors can cause pain and pain hypersensitivity in patients receiving organ and tissue transplants (Grotz et al., 2001; Fujii et al., 2006; Noda et al., 2008; Kakihana et al., 2012). The primary treatment of CIPS is discontinuation of these agents, which could endanger the transplanted organs and tissues. We demonstrated recently that systemic administration of FK506 can lead to unrestrained nociceptive input by potentiating NMDARs at the spinal cord level (Chen et al., 2014). NMDAR activity and phosphorylation state are dynamically controlled by a balance between the activity of protein kinases and protein phosphatases. For example, CK2 activation increases the phosphorylation and activity of NMDARs, whereas calcineurin can negatively regulate the phosphorylation and function of NMDARs in the brain (Tong et al., 1995; Lieberman and Mody, 1999; Ye et al., 2011). CK2 is an endogenous serine/threonine protein kinase widely expressed in many types of cells and is distributed in the central nervous system (Blanquet, 2000; Litchfield, 2003). CK2 is specifically distributed in postsynaptic densities, which are crucial for modulating synaptic NMDAR function (Chung et al., 2004; Soto et al., 2004). Although the mechanisms responsible for increased spinal NMDAR activity by calcineurin inhibitors are not fully known, it is possible that in CIPS the phosphorylation/dephosphorylation cycle of NMDARs in the spinal dorsal horn is shifted to a predominantly phosphorylated state through reduced calcineurin activity.
In this study, we used whole-cell patch-clamp recordings of glutamatergic EPSCs in spinal cord slices to determine whether inhibition of CK2 can lead to a “rebalancing” of spinal NMDAR activity potentiated by a calcineurin inhibitor. We observed that blocking NMDARs with AP5 significantly reduced the basal frequency of mEPSCs only in FK506-treated rats. These results suggest that presynaptic NMDAR activity is augmented by calcineurin inhibition, resulting in increased synaptic glutamate release to spinal dorsal horn neurons. It is noteworthy that we found that treatment of spinal cord slices with either of two structurally distinct CK2 inhibitors, DRB and TBB, significantly reduced the baseline frequency of mEPSCs of dorsal horn neurons in FK506-treated rats. Furthermore, AP5 no longer had any effect on the frequency of mEPSCs in dorsal horn neurons of FK506-treated rats after treatment with DRB or TBB. Our data indicate that CK2 inhibition normalizes synaptic glutamate release to spinal dorsal horn neurons by reducing presynaptic NMDAR activity potentiated by the calcineurin inhibitor.
Increased postsynaptic NMDAR activity in the spinal dorsal horn by a calcineurin inhibitor may facilitate nociceptive transmission by amplifying excitatory input from primary sensory nerves and by diminishing synaptic GABA/glycine inhibition (Leem et al., 1996; Chen et al., 2000; Zhou et al., 2012). We found that inhibiting CK2 with DRB or TBB completely normalized the amplitude of evoked NMDAR-EPSCs and the ratio of evoked NMDAR-EPSCs to AMPAR-EPSCs in spinal dorsal horn neurons increased by FK506 treatment. On the other hand, neither DRB nor TBB had any significant effects on the amplitude of evoked AMPAR-EPSCs in spinal dorsal horn neurons of FK506-treated rats. Furthermore, DRB or TBB significantly inhibited NMDAR currents of dorsal horn neurons elicited by direct NMDA puff application in FK506-treated rats. Our results strongly suggest that CK2 inhibition normalizes the increased postsynaptic NMDAR activity in the spinal dorsal horn caused by the calcineurin inhibitor. Our findings support the notion that spinal NMDAR activity potentiated by the calcineurin inhibitor probably results from an imbalance between the activity of CK2 and calcineurin. The increased presynaptic and postsynaptic NMDAR activity in the spinal dorsal horn caused by calcineurin inhibition can cause long-lasting tactile allodynia and mechanical hyperalgesia (Chen et al., 2014). To determine whether CK2 inhibition at the spinal cord level can reduce pain hypersensitivity in our rat model of CIPS, we tested the effect of intrathecal injection of CK2 inhibitors on the mechanical hypersensitivity of FK506-treated rats. We found that intrathecal injection of DRB or TBB produced dose-dependent and long-lasting reductions in tactile allodynia and mechanical hyperalgesia induced by systemic FK506 administration in rats. These data indicate that inhibition of CK2 at the spinal level effectively reduces calcineurin inhibitor-induced pain hypersensitivity.
It is not clear how CK2 inhibition normalizes spinal NMDAR activity potentiated by calcineurin inhibition. Our study suggests that the imbalance between the levels of CK2 and calcineurin activity could play a critical role in increased spinal NMDAR activity and pain hypersensitivity induced by calcineurin inhibitors. It is possible that CK2 inhibition may reduce the increased phosphorylation levels of NMDARs and/or NMDAR-interacting proteins brought about by the calcineurin inhibitor. It has been reported that CK2 may increase NMDAR activity through the phosphorylation of the NR2B subunit (Chung et al., 2004) or through indirect mechanisms, possibly involving the NMDAR scaffolding proteins, such as postsynaptic density-95/synapse-associated 90 (Soto et al., 2004). In addition, calmodulin can inhibit NMDAR activity through binding to the NR1 subunit in a Ca2+-dependent manner (Ehlers et al., 1996; Krupp et al., 1999). Thus, CK2 inhibition may reduce the phosphorylation level of calmodulin (Sacks et al., 1992), resulting in the inhibition of NMDAR activity by promoting calmodulin binding to the NR1 subunit. Nevertheless, the precise mechanisms through which calcineurin and CK2 interact to alter the phosphorylation of NMDARs and/or their interacting proteins remain unclear and need to be delineated in future studies.
In summary, our study provides important new information that CK2 critically contributes to pain hypersensitivity and increased pre- and postsynaptic NMDAR activity in the spinal dorsal horn caused by calcineurin inhibitors. This new information advances our understanding of the reciprocal relationship between CK2 and calcineurin in the regulation of NMDARs and nociceptive transmission at the spinal cord level. Because directly blocking NMDARs can cause serious side effects in patients (Zhou et al., 2011), NMDAR antagonists may have limited clinical use. CK2 inhibitors, such as CX-4945, have been recently developed for clinical use and seem to be well tolerated in humans. Thus, CK2 could be a promising therapeutic target for the treatment of patients with CIPS. There are no studies showing that inhibition of CK2 interferes with calcineurin inhibitor-induced immunosuppression. Because CK2 inhibition can also reduce nephrotoxicity induced by calcineurin inhibitors (Son et al., 2013), CK2 inhibitors may provide additional benefits for treating various side effects produced by calcineurin inhibitors.
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- AP5
d-2-amino-5-phosphonopentanoate
- CIPS
calcineurin inhibitor–induced pain syndrome
- CK2
casein kinase II
- CX-4945
5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid
- DMSO
dimethylsulfoxide
- DRB
5,6-dichloro-1-β-d-ribofuranosylbenzimidazole
- EPSCs
excitatory postsynaptic currents
- FK506
tacrolimus
- mEPSCs
miniature excitatory postsynaptic currents
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-d-aspartate receptor
- TBB
4,5,6,7-tetrabromobenzotriazole
Authorship Contributions
Participated in research design: Pan.
Conducted experiments: Hu, S.R. Chen, and H. Chen.
Performed data analysis: Hu, S.R. Chen, H. Chen, and Pan.
Wrote or contributed to the writing of the manuscript: Hu, S.R. Chen, and Pan.
Footnotes
This work was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS073935] and National Institutes of Health National Institute of Dental and Craniofacial Research [Grant DE022015]. This work was also supported by the N.G. and Helen T. Hawkins Endowment (to H.-L. P.).
References
- Blanquet PR. (2000) Casein kinase 2 as a potentially important enzyme in the nervous system. Prog Neurobiol 60:211–246 [DOI] [PubMed] [Google Scholar]
- Cervero F, Iggo A. (1980) The substantia gelatinosa of the spinal cord: a critical review. Brain 103:717–772 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63 [DOI] [PubMed] [Google Scholar]
- Chen SR, Eisenach JC, McCaslin PP, Pan HL. (2000) Synergistic effect between intrathecal non-NMDA antagonist and gabapentin on allodynia induced by spinal nerve ligation in rats. Anesthesiology 92:500–506 [DOI] [PubMed] [Google Scholar]
- Chen SR, Hu YM, Chen H, Pan HL. (2014) Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. J Physiol 592:215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Pan HL. (2001) Spinal endogenous acetylcholine contributes to the analgesic effect of systemic morphine in rats. Anesthesiology 95:525–530 [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. (2006) Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol 95:3086–3096 [DOI] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. (2004) Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci 24:10248–10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collini A, De Bartolomeis C, Barni R, Ruggieri G, Bernini M, Carmellini M. (2006) Calcineurin-inhibitor induced pain syndrome after organ transplantation. Kidney Int 70:1367–1370 [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. (1996) Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84:745–755 [DOI] [PubMed] [Google Scholar]
- Fujii N, Ikeda K, Koyama M, Aoyama K, Masunari T, Kondo E, Matsuzaki T, Mizobuchi S, Hiraki A, Teshima T, et al. (2006) Calcineurin inhibitor-induced irreversible neuropathic pain after allogeneic hematopoietic stem cell transplantation. Int J Hematol 83:459–461 [DOI] [PubMed] [Google Scholar]
- Grotz WH, Breitenfeldt MK, Braune SW, Allmann KH, Krause TM, Rump JA, Schollmeyer PJ. (2001) Calcineurin-inhibitor induced pain syndrome (CIPS): a severe disabling complication after organ transplantation. Transpl Int 14:16–23 [DOI] [PubMed] [Google Scholar]
- Jin XG, Chen SR, Cao XH, Li L, Pan HL. (2011) Nitric oxide inhibits nociceptive transmission by differentially regulating glutamate and glycine release to spinal dorsal horn neurons. J Biol Chem 286:33190–33202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakihana K, Ohashi K, Murata Y, Tsubokura M, Kobayashi T, Yamashita T, Sakamaki H, Akiyama H. (2012) Clinical features of calcineurin inhibitor-induced pain syndrome after allo-SCT. Bone Marrow Transplant 47:593–595 [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. (1999) Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci 19:1165–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Choi EJ, Park ES, Paik KS. (1996) N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor antagonists differentially suppress dorsal horn neuron responses to mechanical stimuli in rats with peripheral nerve injury. Neurosci Lett 211:37–40 [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan YZ, Levey AI, Pan HL. (2002) Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol 543:807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL. (2008) Plasticity of pre- and postsynaptic GABAB receptor function in the paraventricular nucleus in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295:H807–H815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shi X, Liang DY, Clark JD. (2005) Spinal CK2 regulates nociceptive signaling in models of inflammatory pain. Pain 115:182–190 [DOI] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. (1994) Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature 369:235–239 [DOI] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. (1999) Casein kinase-II regulates NMDA channel function in hippocampal neurons. Nat Neurosci 2:125–132 [DOI] [PubMed] [Google Scholar]
- Litchfield DW. (2003) Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J 369:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Xiong XG, Lu WY, Raouf R, Orser BA. (1998) Modulation of NMDA receptors. Prog Brain Res 116:191–208 [DOI] [PubMed] [Google Scholar]
- Noda Y, Kodama K, Yasuda T, Takahashi S. (2008) Calcineurin-inhibitor-induced pain syndrome after bone marrow transplantation. J Anesth 22:61–63 [DOI] [PubMed] [Google Scholar]
- Pan HL, Khan GM, Alloway KD, Chen SR. (2003) Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci 23:2911–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, Pan HL. (2004) Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J Neurophysiol 91:2413–2421 [DOI] [PubMed] [Google Scholar]
- Pinna LA. (1990) Casein kinase 2: an ‘eminence grise’ in cellular regulation? Biochim Biophys Acta 1054:267–284 [DOI] [PubMed] [Google Scholar]
- Sacks DB, Davis HW, Williams JP, Sheehan EL, Garcia JG, McDonald JM. (1992) Phosphorylation by casein kinase II alters the biological activity of calmodulin. Biochem J 283:21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SF, Rebelo S, Derkach VA, Safronov BV. (2007) Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol 581:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno S, Moro S, Meggio F, Zagotto G, Dal Ben D, Ghisellini P, Battistutta R, Zanotti G, Pinna LA. (2002) Toward the rational design of protein kinase casein kinase-2 inhibitors. Pharmacol Ther 93:159–168 [DOI] [PubMed] [Google Scholar]
- Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. (2001) Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’). FEBS Lett 496:44–48 [DOI] [PubMed] [Google Scholar]
- Son YK, Lee SM, An WS, Kim KH, Rha SH, Rho JH, Kim SE. (2013) The role of protein kinase CK2 in cyclosporine-induced nephropathy in rats. Transplant Proc 45:756–762 [DOI] [PubMed] [Google Scholar]
- Soto D, Pancetti F, Marengo JJ, Sandoval M, Sandoval R, Orrego F, Wyneken U. (2004) Protein kinase CK2 in postsynaptic densities: phosphorylation of PSD-95/SAP90 and NMDA receptor regulation. Biochem Biophys Res Commun 322:542–550 [DOI] [PubMed] [Google Scholar]
- Strack S, Wadzinski BE, Ebner FF. (1996) Localization of the calcium/calmodulin-dependent protein phosphatase, calcineurin, in the hindbrain and spinal cord of the rat. J Comp Neurol 375:66–76 [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. (1995) Synaptic desensitization of NMDA receptors by calcineurin. Science 267:1510–1512 [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. (2005) Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J Biol Chem 280:18142–18151 [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. (2006) Signaling mechanisms of down-regulation of voltage-activated Ca2+ channels by transient receptor potential vanilloid type 1 stimulation with olvanil in primary sensory neurons. Neuroscience 141:407–419 [DOI] [PubMed] [Google Scholar]
- Ye ZY, Li DP, Li L, Pan HL. (2011) Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci 31:8271–8279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Li L, Li DP, Pan HL. (2012) Casein kinase 2-mediated synaptic GluN2A up-regulation increases N-methyl-d-aspartate receptor activity and excitability of hypothalamic neurons in hypertension. J Biol Chem 287:17438–17446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YL, Chen SR, Chen H, Pan HL. (2012) Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-d-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J Biol Chem 287:25073–25085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Byun HS, Chen H, Li L, Han HD, Lopez-Berestein G, Sood AK, Pan HL. (2012) N-Methyl-d-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J Biol Chem 287:33853–33864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. (2010) Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci 30:4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Pan HL. (2011) Targeting N-methyl-d-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol 4:379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]