Abstract
Previous literature investigating neurobiological adaptations following cocaine self-administration has shown that high, continuous levels of cocaine intake (long access; LgA) results in reduced potency of cocaine at the dopamine transporter (DAT), whereas an intermittent pattern of cocaine administration (intermittent access; IntA) results in sensitization of cocaine potency at the DAT. Here, we aimed to determine whether these changes are specific to cocaine or translate to other psychostimulants. Psychostimulant potency was assessed by fast-scan cyclic voltammetry in brain slices containing the nucleus accumbens following IntA, short access, and LgA cocaine self-administration, as well as in brain slices from naive animals. We assessed the potency of amphetamine (a releaser), and methylphenidate (a DAT blocker, MPH). MPH was selected because it is functionally similar to cocaine and structurally related to amphetamine. We found that MPH and amphetamine potencies were increased following IntA, whereas neither was changed following LgA or short access cocaine self-administration. Therefore, whereas LgA-induced tolerance at the DAT is specific to cocaine as shown in previous work, the sensitizing effects of IntA apply to cocaine, MPH, and amphetamine. This demonstrates that the pattern with which cocaine is administered is important in determining the neurochemical consequences of not only cocaine effects but potential cross-sensitization/cross-tolerance effects of other psychostimulants as well.
Introduction
The rewarding effects of stimulants have been directly linked to their actions at the dopamine transporter (DAT) in experiments that show abolished cocaine-induced conditioned place preference in transgenic mice with cocaine-insensitive DATs (Tilley et al., 2009). Further, the DAT is a critical mediator of cocaine reinforcement and is necessary for self-administration behaviors (Roberts et al., 1977; Ritz and Kuhar, 1989). Thus, differences in the potency of cocaine and other stimulants to inhibit the DAT and elevate dopamine levels are particularly relevant for understanding the reinforcing and rewarding effects of the compound. Changes in cocaine potency at the DAT (Calipari et al., 2013c) and concomitant behavioral changes (Zimmer et al., 2012) following a history of cocaine self-administration depend on the specific self-administration paradigm being used.
For example, we have previously demonstrated that the development of cocaine tolerance or sensitization at the DAT is a function of temporal pattern of administration (Calipari et al., 2013c). Long-access (LgA) self-administration results in high and sustained cocaine levels over daily 6-hour self-administration sessions, and it is well documented that this pattern of cocaine exposure results in reduced potency of cocaine at the DAT (Mateo et al., 2005; Ferris et al., 2011, 2012; Calipari et al., 2012, 2013c, 2014a) and concomitant reductions in cocaine-induced increases in extracellular dopamine levels (Hurd et al., 1989; Ferris et al., 2011; Calipari et al., 2014a). Conversely, intermittent-access (IntA) self-administration, where animals are given time-outs to force self-administration patterns that result in sharp increases in cocaine levels followed by rapid decreases, results in sensitized cocaine potency at the DAT. Sensitization and tolerance of cocaine potency following intermittent and continuous administration have been demonstrated using self-administration (Calipari et al., 2013c) and with noncontingent administration of intermittent (intraperitoneal injections) and continuous (mini-pumps) regimens of cocaine exposure (Addy et al., 2010). Although the effects of cocaine at the DAT are well established following a number of paradigms, how these cocaine self-administration protocols affect the potency of other psychostimulants, and what factors dictate the expression of tolerance/sensitization, remains to be determined.
Therefore, we determined how the potencies of methylphenidate (MPH; a dopamine uptake inhibitor) and amphetamine (a dopamine releaser) were affected by IntA, short-access (ShA), and LgA cocaine self-administration. Previously published work from our laboratory has shown that, although MPH is a DAT blocker, it is affected by changes at the DAT that alter releaser potency, possibly due to the amphetamine-like structure of the compound (Calipari et al., 2012, 2013b, 2014b, Ferris et al., 2012). DAT binding is highly dependent on structural components; accordingly, work has shown that MPH binds to the DAT in a fashion that is similar to amphetamine (Wayment et al., 1999; Dar et al., 2005). Thus, MPH shares functional properties with cocaine but some structural properties with amphetamine and therefore can give insight into whether changes in potency at the DAT are due to a compound’s function or due to other factors, such as specific DAT-stimulant structure interactions. If the compensatory effects of cocaine self-administration are conferred to compounds of the same dopamine uptake inhibitor class, MPH potency will change in a fashion similar to cocaine; however, if the changes are specific to some shared aspect of MPH and amphetamine structure, then outcome for MPH will be similar to amphetamine. To be consistent with previous cocaine self-administration studies using extended-access paradigms (Ferris et al., 2012; Calipari et al., 2013c), we hypothesized that MPH and amphetamine potency would be unaffected by LgA compared with ShA controls. Alternatively, although IntA self-administration effects on drugs other than cocaine have not been studied previously, noncontingent administration of cocaine has been demonstrated to result in cross-sensitization for the releasers methamphetamine (Hirabayashi et al., 1991) and amphetamine (Lett, 1989). Thus, we hypothesized that the IntA cocaine self-administration would result in the sensitization of MPH and amphetamine.
Materials and Methods
Animals.
Male Sprague-Dawley rats (375–400 g; Harlan Laboratories, Frederick, MD) were maintained on a 12/12-hour reverse light/dark cycle (3:00 AM lights off; 3:00 PM lights on) with food and water ad libitum. All animals were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care–accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the Wake Forest School of Medicine.
Self-Administration.
Rats were anesthetized and implanted with long-term indwelling jugular catheters as previously described (Calipari et al., 2013a). Animals were singly housed, and all sessions took place in the home cage during the active/dark cycle (9:00 AM to 3:00 PM). After a 2-day recovery period from surgery, animals underwent a training paradigm within which animals were given access on a fixed-ratio 1 (FR1) schedule to a cocaine-paired lever, which, upon responding, initiated an intravenous injection of cocaine (0.75 mg/kg, infused over 4 seconds). After each response/infusion, the lever was retracted and a stimulus light was illuminated for a 20-second time-out period. Training sessions were terminated after a maximum of 20 infusions or 6 hours, whichever occurred first. Acquisition occurred when an animal responded for 20 injections for 2 consecutive days and a stable pattern of infusion intervals was present. Following training, animals were assigned to IntA, LgA, or ShA groups. All self-administration took place over 14 consecutive sessions, after which animals were sacrificed and brains were prepared for voltametric recordings.
LgA Group.
After training, subjects completed daily 6-hour sessions, during which they had unlimited access to cocaine (0.75 mg/kg, infused over 4 seconds; for structure, see Kinsey et al., 2010) on an FR1 schedule during 6-hour daily sessions for 14 consecutive days. At the start of each infusion, a stimulus light signaled a 20-second time-out period, during which the lever was retracted.
ShA Group.
After training, subjects were given unlimited access to cocaine (0.75 mg/kg, infused over 4 seconds) on an FR1 schedule during 2-hour daily sessions for 14 consecutive days. At the start of each infusion, a stimulus light signaled a 20-second time-out period, during which the lever was retracted.
IntA Group.
A separate group was given access to cocaine on an intermittent schedule of administration described previously (Zimmer et al., 2012). During each 6-hour session, animals had access to cocaine for twelve 5-minute trials separated by 25-minute time-out periods. Within each 5-minute session, there were no time-outs other than during each infusion, and the animal could press the lever on an FR1 schedule to receive a 1-second infusion of cocaine (0.375 mg/kg per infusion).
Calculating Brain Concentrations.
Brain-cocaine concentrations were estimated using equations used by Pan et al. (1991). The equation used was
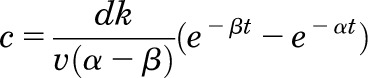 |
which calculates the brain-cocaine concentration in the brain compartment at time (t). The variables account for the dose of cocaine (d), the transfer of drug between the blood and brain compartments (k = 0.233 minute−1), the apparent volume of the brain compartment (v = 0.15 l/kg), and the removal of cocaine from the system via redistribution (α = 0.642 minute−1) and elimination (β = 0.097 minute−1). This equation has been widely used to correlate estimated brain-cocaine levels with behavioral (Ahmed and Koob, 2005), electrophysiological (Peoples et al., 2004, 2007), microdialysis (Wise et al., 1995), and voltammetric measures (Stuber et al., 2005a,b; Hermans et al., 2008). Estimates in brain-cocaine levels in the literature are highly variable, spanning from the nanomolar to the micromolar range. The aim of this study was not to determine brain levels of cocaine, which we did not directly measure, but rather to show the relative brain level fluctuations over time within a session. Therefore, we chose to present these data as arbitrary units.
Ex Vivo Voltammetry.
Fast-scan cyclic voltammetry was used to characterize the potency of psychostimulants to inhibit dopamine uptake in the nucleus accumbens (NAc) core. Voltammetry experiments were conducted during the dark phase of the light cycle 18 hours after commencement of the final self-administration session. A vibrating tissue slicer was used to prepare 400-µm-thick coronal brain sections containing the NAc. The tissue was immersed in oxygenated artificial cerebrospinal fluid containing the following: NaCl (126 mM), KCl (2.5 mM), NaH2PO4 (1.2 mM), CaCl2 (2.4 mM), MgCl2 (1.2 mM), NaHCO3 (25 mM), glucose (11 mM), and l-ascorbic acid (0.4 mM); pH was adjusted to 7.4. Once sliced, the tissue was transferred to the testing chambers containing bath artificial cerebrospinal fluid (32°C), which flowed at 1 ml/min. A carbon fiber microelectrode (100–200 μM length, 7 μM radius) and bipolar stimulating electrode were placed into the core of the NAc, which was selected because of its role in the reinforcing and rewarding actions of cocaine. Dopamine release was evoked by a single electrical pulse (300 μA, 4 ms, monophasic) applied to the tissue every 5 minutes. Extracellular dopamine was recorded by applying a triangular wave form (−0.4 to +1.2 to −0.4V vs. Ag/AgCl, 400 V/s). Once the extracellular dopamine response was stable, MPH (0.03–30 μM; for structure, see Froimowitz et al., 1995) or amphetamine (0.1–10 μM; for structure, see Santagati et al., 2002) was applied cumulatively to the brain slice.
Data Modeling and Analysis.
Demon Voltammetry and Analysis software was used for all acquisition and modeling of fast-scan cyclic voltammetry data (Yorgason et al., 2011). To evaluate drug potency, evoked levels of dopamine were modeled using Michaelis-Menten kinetics. Recording electrodes were calibrated by recording responses (in electrical current; nanoamp) to a known concentration of dopamine (3 μM) using a flow-injection system. This was used to convert electrical current to dopamine concentration. For MPH and amphetamine dose-response curves, a measure of apparent affinity (app. Km) for the DAT was used to determine changes in the potency of the psychostimulants to inhibit dopamine uptake. As app. Km increases, the affinity of dopamine for the DAT decreases. Increasing concentrations of MPH or amphetamine decrease the affinity of dopamine for the DAT, such that shifts in app. Km across treatment groups indicate shifts in the potency of the drug directly at the DAT.
Calculating Ki Values.
Inhibition constants (Ki) were determined by plotting the linear concentration-effect profiles and determining the slope of the linear regression (Jones et al., 1995; Calipari et al., 2013c). The Ki was calculated by the equation Km/slope. Ki values are reported in micromolars and are a measure of the drug concentration that is necessary to produce 50% uptake inhibition.
Statistics.
GraphPad Prism (version 5; La Jolla, CA) was used to statistically analyze data sets and create graphs. Release data and data obtained after perfusion of MPH or amphetamine were subjected to a two-way analysis of variance (ANOVA) with experimental group and concentration of drug as the factors. When main effects were obtained (P < 0.05), differences between groups were tested using a Bonferroni post-hoc test.
Results
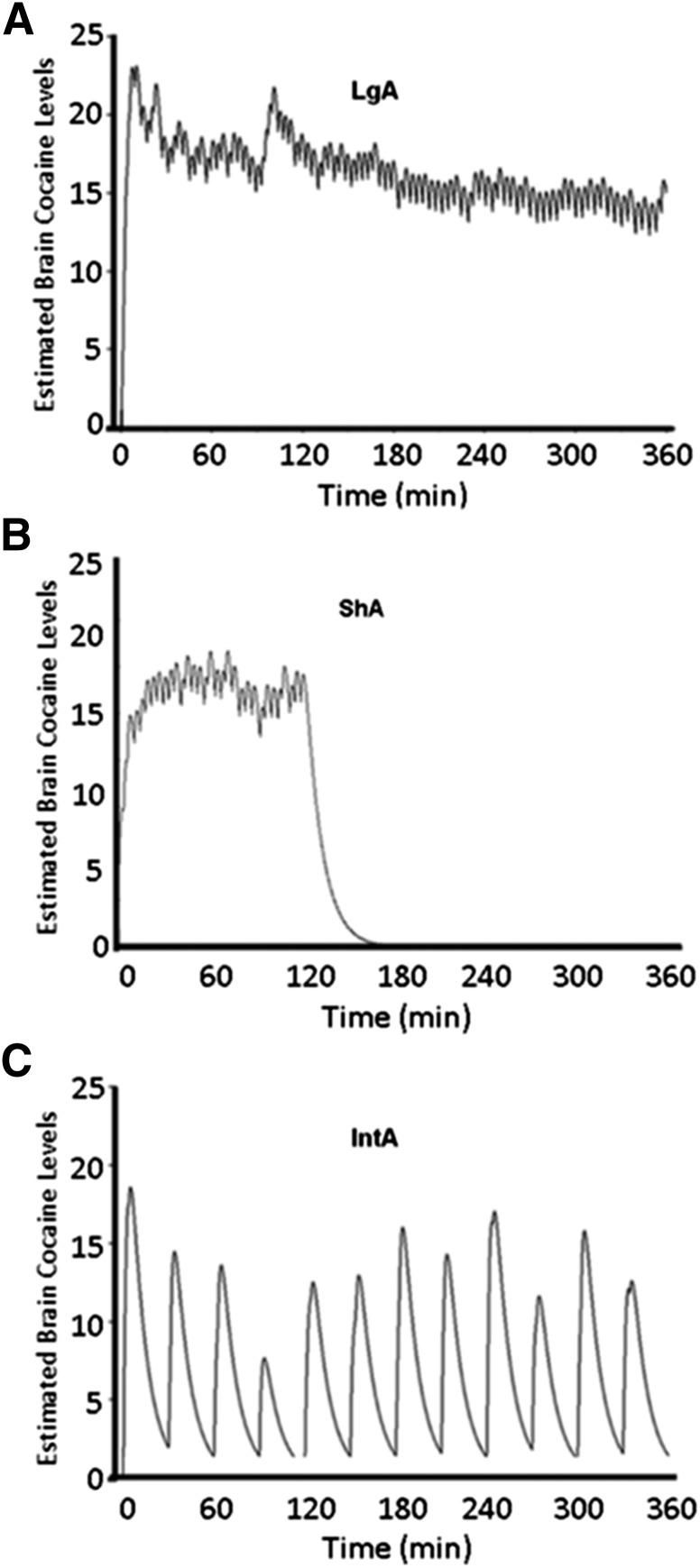
IntA Cocaine Self-Administration Resulted in “Spiking” Brain-Cocaine Levels, Whereas ShA and LgA Resulted in Sustained Brain-Cocaine Levels.
To determine the factors that influence psychostimulant potency, we manipulated temporal pattern of cocaine administration and daily cocaine intake (Fig. 1). LgA (Fig. 1A) and ShA (Fig. 1B) resulted in a similar pattern of self-administration, which is characterized by sustained brain-cocaine levels over a 6-hour and 2-hour session, respectively. Given the shorter session length, ShA rats administer significantly less cocaine per session (Calipari et al., 2013c). Thus, the comparison of ShA versus LgA was used to determine whether the neurochemical adaptations that occurred were attributed to differences in total daily intake. IntA animals were given 5-minute unlimited access periods to a cocaine-paired lever followed by 25-minute time-out periods. This resulted in a “spiking” pattern of brain-cocaine levels characterized by rapid increases, which return near baseline during the time-out period (Fig. 1C). Although the temporal profile of brain-cocaine levels differs between ShA and IntA, both groups have comparable levels of cocaine intake (Calipari et al., 2013c). Thus, ShA versus IntA was used to determine the effect of different patterns of consumption on psychostimulant potency, given the same amount of daily cocaine intake.
Fig. 1.
Differential effects of IntA, ShA, and LgA self-administration on presynaptic dopamine system kinetics. Work from Pan et al. (1991) was used to model brain levels of cocaine from representative animals within a self-administration session over time. LgA, IntA, and ShA paradigms, expressed as arbitrary units. Each panel shows the modeled brain levels of cocaine (y-axis) versus time (x-axis) throughout the entire session for individual rats self-administering cocaine to highlight the fluctuation of cocaine levels within the brain over each representative session. (A) LgA results in high, sustained brain cocaine over the 6-hour session. (B) ShA results in high, sustained brain cocaine over the 2-hour session. (C) IntA is achieved by giving 5 minutes of access followed by 25-minute forced timeouts. This results in “spiking” brain levels where animals load up to reach levels equivalent to LgA but cannot maintain; thus, cocaine is cleared from the brain, and levels return near baseline. Spacing between clusters of tick marks is attributed to a forced time-out period.
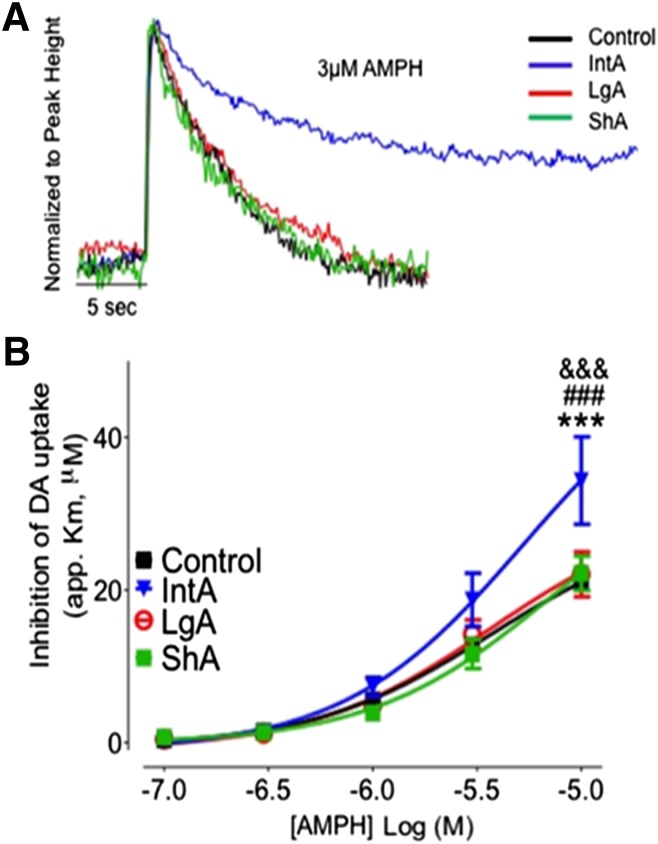
Amphetamine Potency Was Increased following IntA, but Not LgA or ShA Cocaine Self-Administration.
ANOVA revealed a main effect of self-administration paradigm on amphetamine potency (app. Km) (F3,84 = 6.213, P < 0.001; Fig. 2, A and B). Bonferroni post-hoc analysis revealed that there was a significant increase in amphetamine’s effect on the DAT (P < 0.001) at the highest dose of the dose-response curve following IntA. Ki values for amphetamine (t9 = 2.765, P < 0.05; Table 1) were reduced in the IntA group as compared with naïve control animals, indicating increased potency.
Fig. 2.
IntA self-administration results in sensitization to the neurochemical effects of amphetamine. (A) Representative traces from control (black), IntA (blue), LgA (red), and ShA (green) animals. Traces are represented as concentration of dopamine over time and are normalized to peak height. (B) Cumulative amphetamine (0.1–10 µM) dose-response curves in slices containing the nucleus accumbens core. Amphetamine potency is unchanged following LgA and ShA and increased following IntA. *P < 0.05 versus control; ***P < 0.001 versus control. AMPH, amphetamine; DA, dopamine.
TABLE 1.
Summary of Ki values across drugs and paradigms
| Treatment | Drug | Ki | P < 0.05 vs. Control |
|---|---|---|---|
| µM | |||
| Control | Cocaine | 0.397 | —a |
| Methylphenidate | 0.258 | — | |
| Amphetamine | 0.080 | — | |
| Intermittent-Access | Cocaine | 0.312 | ↑**,a |
| Methylphenidate | 0.217 | ↑* | |
| Amphetamine | 0.048 | ↑* | |
| Long-Access | Cocaine | 0.501 | ↓* |
| Methylphenidate | 0.271 | ↔ | |
| Amphetamine | 0.076 | ↔ | |
| Short-Access | Cocaine | 0.392 | ↔a |
| Methylphenidate | 0.245 | ↔ | |
| Amphetamine | 0.077 | ↔ |
↑, increase; ↓, decrease; ↔, no change.
P < 0.05 vs. control; **P < 0.01 vs. control.
Data from Calipari et al., 2013c.
ShA resulted in no change in amphetamine potency as measured by app. Km (Fig. 2) or Ki (Table 1) compared with control animals. LgA, which also resulted in sustained cocaine-brain levels, but with higher daily intake levels, resulted in no change in amphetamine potency as measured by app. Km or Ki (Fig. 2; Table 1) compared with naïve control animals or animals with a history of ShA cocaine self-administration.
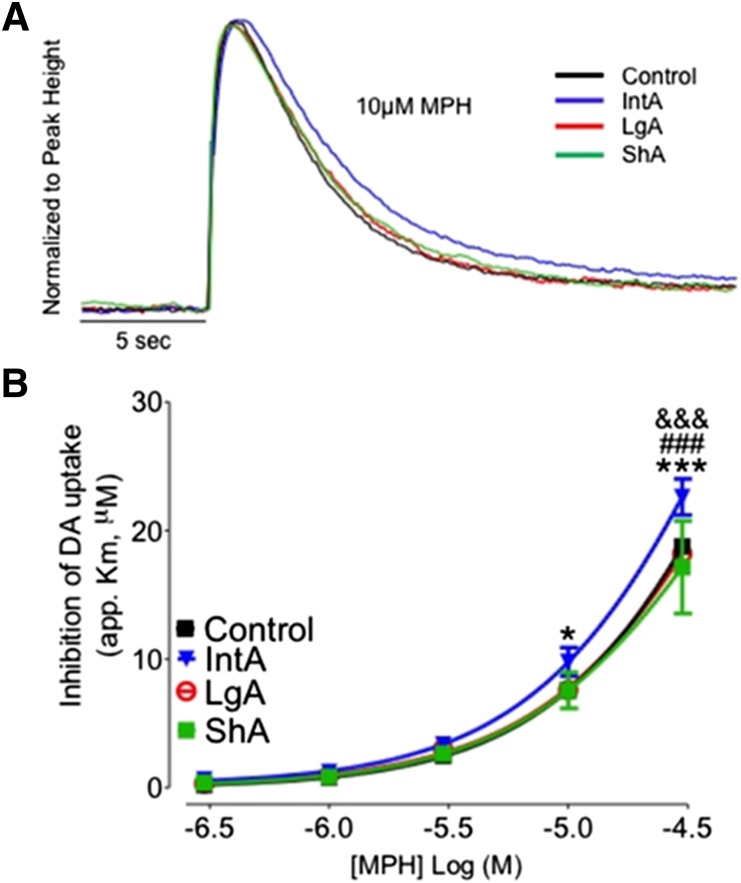
MPH Potency Was Increased following IntA, but Not LgA or ShA Cocaine Self-Administration.
For the IntA group, ANOVA revealed a main effect of paradigm on app. Km for MPH (F3,86 = 6.408, P < 0.001; Fig. 3, A and B). Bonferroni post-hoc analysis revealed that there was an increase in MPH potency at the highest dose of the dose-response curve (P < 0.001) as well as the 10 µM dose (P < 0.05) following IntA. In addition, Ki values for MPH (t10 = 2.474, P < 0.05; Table 1) were reduced in the IntA group, indicating an increased potency. ShA, which results in sustained cocaine levels at the DAT, resulted in no change in MPH potency as measured by app. Km (Fig. 3) or Ki (Table 1) compared with control animals. LgA resulted in no change in MPH potency as measured by app. Km or Ki (Fig. 3; Table 1) compared with naïve control animals or animals that had undergone ShA cocaine self-administration.
Fig. 3.
IntA self-administration results in sensitization to the neurochemical effects of MPH, whereas LgA results in change. (A) Representative traces from control (black), IntA (blue), LgA (red), and ShA (green) animals. Traces are represented as concentration of dopamine (DA) over time and are normalized to peak height. (B) Cumulative MPH (0.3–30 µM) dose-response curves in slices containing the nucleus accumbens core. MPH potency is unchanged following LgA and ShA and increased following IntA. *P < 0.05 versus control; ***P < 0.001 versus control.
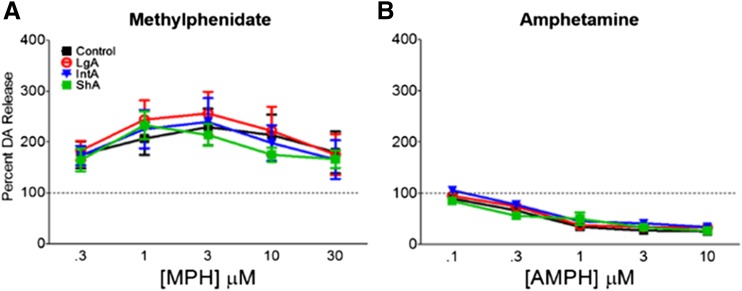
MPH- and Amphetamine-Induced Dopamine Alterations in Peak Amplitude of Dopamine Release Is Not Determined by Amount or Pattern of Cocaine Intake.
To determine how LgA, ShA, and IntA affected dopamine release in the presence of drug, we measured the effects of MPH and amphetamine on the peak height of evoked dopamine release across a dose-response curve for each of these drugs. First, we assessed the release profile of MPH to determine how it was affected by a prior cocaine self-administration history. Dopamine uptake occurs continuously in the presence of dopamine; thus, the peak height of evoked dopamine release is a balance between vesicular release and dopamine uptake via the DAT. Because of their ability to inhibit the DAT and slow uptake, blockers result in inverted “U”-shaped dose-response curves, where they increase peak height at lower doses, due to their uptake-inhibition effects. However, at the higher doses, peak height is reduced, likely attributed to the increased uptake inhibition resulting in an inability of the terminal to effectively repackage dopamine into vesicles. MPH exhibits an inverted “U”-shaped profile that is indicative of a dopamine transporter blocker (Fig. 4A; Ferris et al., 2012; Calipari et al., 2014b). ANOVA revealed a main effect of MPH on dopamine release (F3,80 = 11.89, P < 0.05). However, there were no differences in stimulated dopamine release in the presence of MPH between naïve control, LgA, ShA, and IntA (Fig. 4A).
Fig. 4.
LgA and IntA cocaine self-administration have no effect on dopamine release in the presence of amphetamine or MPH. There were no differences in MPH- or amphetamine-induced dopamine elevations between control, IntA, LgA, or ShA cocaine self-administration groups. (A) Normalized stimulated dopamine release measured across a dose-response curve for MPH. (B) Normalized stimulated dopamine release measured across a dose-response curve for amphetamine. AMPH, amphetamine; DA, dopamine.
Amphetamine has a different profile than MPH due to its mechanism of action as a dopamine releaser. Because amphetamine is releasing dopamine at all times, independent of stimulated release, it dose-dependently depletes dopamine releasable pools leading to decreased evoked dopamine release over the dose-response curve (Fig. 4B; Ferris et al., 2012; Calipari et al., 2013b). ANOVA revealed a main effect of amphetamine on dopamine release (F3,75 = 78.36, P < 0.0001). There were no differences in stimulated dopamine release in the presence of amphetamine between naïve control, LgA, ShA, and IntA (Fig. 4B).
Discussion
The present results show that the temporal pattern of cocaine intake during self-administration determines the changes in stimulant potency that occur following repeated administration of cocaine. Here, we propose that the changes in psychostimulant potency following LgA are dictated by psychostimulant interactions with altered motifs on the DAT protein that only affect the potency of a select group of stimulants, whereas IntA induces nonspecific increases in the potency of all psychostimulants. This is supported by the fact that LgA affects some drugs (cocaine) and not others (amphetamine, MPH), whereas IntA increases the potency of all of the compounds that were tested (cocaine, amphetamine, MPH). Previous work has demonstrated that LgA cocaine self-administration results in decreased cocaine potency; however, here we show no change in MPH or amphetamine potency, indicating that LgA-induced DAT changes do not affect the function of amphetamine-like compounds. This is consistent with work using a limited-intake, extended-access self-administration paradigm (5 days; 40 injections; 1.5 mg/kg per injection), which showed reduced potency of cocaine and other blockers (nomifensine, bupropion) at the DAT but no change in the potency of any releasers (amphetamine, phentermine, methamphetamine, benzypiperidine; methylenedioxymethamphetamine) or MPH (Ferris et al., 2011, 2012, 2013a,b; Calipari et al., 2012, 2014a). In addition, we show that IntA causes a sensitization of amphetamine and MPH, which is consistent with previous studies examining cocaine potency following IntA (Calipari et al., 2013c). Thus, unlike LgA, the effects of IntA cocaine self-administration on psychostimulant potency generalized to all stimulants tested, whereby we observed increases in the potency of amphetamine (releaser) and MPH (amphetamine-like blocker) as compared with both ShA and naïve control groups.
The unique properties of MPH may give insight into the factors driving the changes in drug potency following LgA and IntA. Although MPH is classified as a blocker, binding studies show that MPH binds to the DAT in a fashion that is similar to amphetamine, probably because of certain structural similarities between the two compounds (Wayment et al., 1999; Dar et al., 2005). Studies using DAT mutants that are stabilized in either an outward-facing or inward-facing conformation have elucidated two distinct classes of psychostimulant compounds that are not dictated by their function as a “blocker” or “releaser.” Some compounds preferentially interact with outward-facing conformations compared with inward-facing conformations. Outward-facing mutants increase the affinity for both MPH and cocaine, while leaving the affinity of the blockers benztropine, GBR12909 (1-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride), bupropion, or releasers such as amphetamine unaffected (Schmitt et al., 2008, 2013). However, changes in the inward-/outward-facing selectivity of the DAT are likely not the mechanism for the changes observed following LgA within the current study, because these changes would likely affect cocaine and MPH in a similar fashion. Previous work has shown that cocaine potency is reduced following LgA (Calipari et al., 2013c), whereas the current study shows that MPH potency at the DAT is unchanged. Furthermore, IntA produces a sensitized effect for all psychostimulants tested to date, and one would expect differential effects of cocaine and MPH compared with amphetamine if inward versus outward selectivity explained the effects reported within the current study. Therefore, the changes are likely not global changes to the conformation of the transporter, but rather a specific site that only alters the potency of a select group of stimulants.
It is tempting to postulate that LgA does not change the potency of amphetamine-like compounds because shifts in MPH potency closely resemble the profile of the dopamine releaser amphetamine; however, it is more likely that the differences between blockers and releasers and MPH are attributed to not only structure per se but rather the way in which the compounds interact with the DAT directly, possibly at specific sites on the transporter protein. Giving further support to this idea is the fact that the structurally dissimilar releaser benzylpiperidine was also unaffected following extended-access cocaine self-administration (Ferris et al., 2012). Although structurally dissimilar from the other releasers tested, benzylpiperidine is transported via the DAT and thus interacts with the transporter in a way that is similar to other structurally dissimilar releasers. Thus, although structural components are integral to determining unique conformational interactions with the DAT, a number of different structures can interact with the same motifs on the DAT protein (Schmitt et al., 2013). Consistent with this hypothesis, recent studies comparing DAT inhibitors have demonstrated that the effects of DAT ligands are not based on class (blocker versus releaser) but rather are specific to how each compound interacts with the transporter (Schmitt et al., 2013). Here, we suggest that particular structural components of the DAT are altered following LgA cocaine self-administration, and that compounds that bind to the transporter at that site, regardless of structure, are affected by the alterations. This hypothesis could be tested by conducting a comprehensive structure-function analysis of a wide range of compounds that bind to the DAT and alter its function. One particularly interesting avenue would be the utilization of many structurally distinct DAT inhibitor compounds to determine whether a specific structural component of the molecule is predictive of the changes in psychostimulant potency directly at the DAT following each of these cocaine self-administration paradigms.
In contrast to LgA, IntA resulted in increased potency for MPH and amphetamine. Previous work has also shown that IntA results in increased cocaine potency (Calipari et al., 2013c), suggesting that the effects are not specific to class or interactions of each compound with the DAT, but rather affect all DAT-interacting compounds. The increased potency of MPH and amphetamine following IntA self-administration could be due to the fact that IntA results in increased DAT levels in these animals (Calipari et al., 2013c). Previous work has demonstrated that increased DAT levels lead to an increase in the potency of both amphetamine and MPH (Salahpour et al., 2008; Calipari et al., 2013b). If this is the case, then the increase in cocaine potency would have to occur via a different mechanism, as numerous previous studies have demonstrated that increases in DAT levels do not increase cocaine potency. If anything, increased DAT levels have been demonstrated to decrease cocaine potency (Chen and Reith, 2007; Rao et al., 2013). Thus, it is possible that the increase in amphetamine and MPH potency occurs via increased DAT levels, whereas increased cocaine potency manifests via an intrinsic change to the DAT, both of which are occurring simultaneously.
Although the mechanism driving the IntA-induced DAT changes is unclear, the fact that IntA results in increased potency for all of the psychostimulant drugs tested suggests that many drugs may have an elevated abuse liability/addiction potential following intermittent cocaine administration. Indeed, for cocaine, it has been demonstrated that the reinforcing efficacy of cocaine is increased following an IntA self-administration paradigm compared with LgA and ShA (Zimmer et al., 2012). Although this work has not been extended to other psychostimulants, it is likely that the reinforcing efficacy of the drugs tested here may be increased as well.
Here, we demonstrate that access conditions and the pattern of intake dictate not only the effects on cocaine potency as seen previously, but also whether these changes are conferred to other psychostimulants. We suggest that changes in psychostimulant potency following LgA are dictated by altered motifs on the DAT protein, which decrease the potency of a select group of stimulants, while intermittent patterns of administration induce nonspecific increases in the potency of psychostimulants. The current work supports the hypothesis that the binding of DAT ligands to the transporter is not based on psychostimulant class but rather is specific to interactions with the DAT. Additionally, the divergent effects of LgA and IntA highlight the importance of considering pattern and total intake when modeling the behavioral and neurochemical processes involved in addiction. The aim of future studies should be to identify the specific DAT motifs that are altered following LgA cocaine self-administration. The identification of the specific DAT changes that occur following clinically relevant models of cocaine abuse could lead to targeted therapies that may allow for the reduction in cocaine potency without altering other critical aspects of DAT protein function.
Acknowledgments
The authors thank Holly Buben for many of the surgeries that were performed for self-administration studies.
Abbreviations
- ANOVA
analysis of variance
- app. Km
apparent affinity
- DAT
dopamine transporter
- FR1
fixed-ratio 1
- GBR12909
1-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride
- IntA
intermittent access
- LgA
long access
- MPH
methylphenidate
- NAc
nucleus accumbens
- ShA
short access
Authorship Contributions
Participated in research design: Calipari, Ferris, Jones.
Conducted experiments: Calipari, Ferris, Siciliano, Zimmer.
Performed data analysis: Calipari, Ferris, Siciliano.
Wrote or contributed to the writing of the manuscript: Calipari, Ferris, Siciliano, Jones.
Footnotes
This work was funded by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA024095, R01-DA030161, R01-DA14030, P50-DA006634 (to S.R.J.), T32-DA007246, F31-DA031533 (to E.S.C.), K99-DA031791 (to M.J.F.), and T32-AA007565 (to C.A.S.)].
References
- Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM. (2010) Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. J Neurophysiol 104:922–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. (2005) Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 180:473–490 [DOI] [PubMed] [Google Scholar]
- Calipari ES, Beveridge TJ, Jones SR, Porrino LJ. (2013a) Withdrawal from extended-access cocaine self-administration results in dysregulated functional activity and altered locomotor activity in rats. Eur J Neurosci 38:3749–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR. (2014a) Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem 128:224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC, Jones SR. (2012) Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict Biol DOI: 10.1111/j.1369-1600.2012.00456.x [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Salahpour A, Caron MG, Jones SR. (2013b) Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nat Commun 4:2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR. (2013c) Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 38:2385–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Jones SR. (2014b) Sensitized nucleus accumbens dopamine terminal responses to methylphenidate and dopamine transporter releasers after intermittent-access self-administration. Neuropharmacology DOI: 10.1016/j.neuropharm.2014.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Reith ME. (2007) Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells. J Neurochem 101:377–388 [DOI] [PubMed] [Google Scholar]
- Dar DE, Mayo C, Uhl GR. (2005) The interaction of methylphenidate and benztropine with the dopamine transporter is different than other substrates and ligands. Biochem Pharmacol 70:461–469 [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR. (2012) Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology 37:1708–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Melchior JR, Roberts DC, España RA, Jones SR. (2013a) Paradoxical tolerance to cocaine after initial supersensitivity in drug-use-prone animals. Eur J Neurosci 38:2628–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Yorgason JT, Jones SR. (2013b) Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem Neurosci 4:693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DC, Jones SR. (2011) Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry 69:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froimowitz M, Patrick KS, Cody V. (1995) Conformational analysis of methylphenidate and its structural relationship to other dopamine reuptake blockers such as CFT. Pharm Res 12:1430–1434 [DOI] [PubMed] [Google Scholar]
- Hermans A, Keithley RB, Kita JM, Sombers LA, Wightman RM. (2008) Dopamine detection with fast-scan cyclic voltammetry used with analog background subtraction. Anal Chem 80:4040–4048 [DOI] [PubMed] [Google Scholar]
- Hirabayashi M, Okada S, Tadokoro S. (1991) Comparison of sensitization to ambulation-increasing effects of cocaine and methamphetamine after repeated administration in mice. J Pharm Pharmacol 43:827–830 [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U. (1989) Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain Res 498:199–203 [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. (1995) Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J Pharmacol Exp Ther 274:396–403 [PubMed] [Google Scholar]
- Kinsey BM, Kosten TR, Orson FM. (2010) Active immunotherapy for the Treatment of Cocaine Dependence. Drugs Future 35:301–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT. (1989) Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 98:357–362 [DOI] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. (2005) Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology 30:1455–1463 [DOI] [PubMed] [Google Scholar]
- Pan HT, Menacherry S, Justice JB., Jr (1991) Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem 56:1299–1306 [DOI] [PubMed] [Google Scholar]
- Peoples LL, Kravitz AV, Lynch KG, Cavanaugh DJ. (2007) Accumbal neurons that are activated during cocaine self-administration are spared from inhibitory effects of repeated cocaine self-administration. Neuropsychopharmacology 32:1141–1158 [DOI] [PubMed] [Google Scholar]
- Peoples LL, Lynch KG, Lesnock J, Gangadhar N. (2004) Accumbal neural responses during the initiation and maintenance of intravenous cocaine self-administration. J Neurophysiol 91:314–323 [DOI] [PubMed] [Google Scholar]
- Rao A, Sorkin A, Zahniser NR. (2013) Mice expressing markedly reduced striatal dopamine transporters exhibit increased locomotor activity, dopamine uptake turnover rate, and cocaine responsiveness. Synapse 67:668–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. (1989) Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther 248:1010–1017 [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. (1977) On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav 6:615–620 [DOI] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, et al. (2008) Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci USA 105:4405–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G. (2002) Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection. J Pharm Biomed Anal 30:247–255 [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Rothman RB, Reith ME. (2013) Nonclassical pharmacology of the dopamine transporter: atypical inhibitors, allosteric modulators, and partial substrates. J Pharmacol Exp Ther 346:2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, Reith ME. (2008) Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wild-type and mutant human dopamine transporters: molecular features that differentially determine antagonist-binding properties. J Neurochem 107:928–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. (2005a) Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology 30:853–863 [DOI] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. (2005b) Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron 46:661–669 [DOI] [PubMed] [Google Scholar]
- Tilley MR, O’Neill B, Han DD, Gu HH. (2009) Cocaine does not produce reward in absence of dopamine transporter inhibition. Neuroreport 20:9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayment HK, Deutsch H, Schweri MM, Schenk JO. (1999) Effects of methylphenidate analogues on phenethylamine substrates for the striatal dopamine transporter: potential as amphetamine antagonists? J Neurochem 72:1266–1274 [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr (1995) Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 120:10–20 [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. (2011) Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202:158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DCS. (2012) The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37:1901–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]