Abstract
Trovafloxacin (TVX) is a fluoroquinolone antibiotic known to cause idiosyncratic, drug-induced liver injury (IDILI) in humans. The mechanism underlying this toxicity remains unknown. Previously, an animal model of IDILI in mice revealed that TVX synergizes with inflammatory stress from bacterial lipopolysaccharide (LPS) to produce a hepatotoxic interaction. The liver injury required prolongation of the appearance of tumor necrosis factor-α (TNF) in the plasma. The results presented here describe a model of TVX/LPS coexposure in RAW 264.7 cells acting as a surrogate for TNF-releasing cells in vivo. Pretreating cells with TVX for 2 hours before LPS addition led to increased TNF protein release into culture medium in a concentration- and time-dependent manner relative to cells treated with LPS or TVX alone. During the pretreatment period, TVX increased TNF mRNA, but this was less apparent when cells were exposed to TVX after LPS addition, suggesting that the pivotal signaling events that increase TNF expression occurred during the TVX pretreatment period. Indeed, TVX exposure increased activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase. Inhibition of either ERK or JNK decreased the TVX-mediated increase in TNF mRNA and LPS-induced TNF protein release, but p38 inhibition did not. These results demonstrated that the increased TNF appearance from TVX-LPS interaction in vivo can be reproduced in vitro and occurs in an ERK- and JNK-dependent manner.
Introduction
Drug-induced liver injury (DILI) is responsible for more than half of acute liver failure cases in the United States (Ostapowicz et al., 2002). DILI is associated with significant morbidity and mortality. It is the most common adverse effect that prevents market approval for new drug entities, and it prompts removal of efficacious drugs from the market (Watkins, 2005). An important subset of DILI is idiosyncratic, drug-induced liver injury (IDILI), which accounts for 13% of all cases of acute liver failure (Ostapowicz et al., 2002). Although this represents a fraction of all instances of DILI, the bulk of Food and Drug Administration–imposed restrictions on the use of drugs is due to idiosyncratic adverse drug reactions (Lasser et al., 2002).
Causes of IDILI are not well understood. Among several hypotheses put forth to explain IDILI is the inflammatory stress hypothesis, which states that a mild inflammatory episode interacts with a drug, resulting in hepatotoxicity (Shaw et al., 2010). Animal models based on this hypothesis have been developed for several drugs that have caused IDILI in humans, including chlorpromazine, ranitidine, amiodarone, doxorubicin, sulindac, and trovafloxacin (TVX) (Buchweitz et al., 2002; Luyendyk et al., 2003; Shaw et al., 2007; Hassan et al., 2008; Zou et al., 2009; Lu et al., 2012). In each of these models, bacterial lipopolysaccharide (LPS) was used to cause a modest, nontoxic, acute inflammatory episode.
Binding of LPS to Toll-like receptor 4 on inflammatory cells leads to activation of proximal intracellular signaling factors in the MyD88 (myeloid differentiation primary response 88)-dependent pathway (Chow et al., 1999). The result is intracellular signaling that activates canonical mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 (Reimann et al., 1994; Sanghera et al., 1996; Swantek et al., 1997). Activated MAPKs induce the transactivation of genes that encode tumor necrosis factor-α (TNF) and other mediators of acute inflammation, as well as increase the stability of TNF mRNA (Wang et al., 2001; DeLeault et al., 2008). TNF has been implicated as a critical mediator of liver injury in drug-LPS cotreatment models using several of the aforementioned IDILI-associated drugs.
TVX is a broad-spectrum antibiotic in the fluoroquinolone class that acts by inhibiting bacterial DNA gyrase and topoisomerase IV (Brighty and Gootz, 1997). Soon after its introduction into the market, TVX use was severely restricted due to 14 cases of acute liver failure, of which six were fatal (Ball et al., 1999). An animal model of TVX IDILI was established in which mice coexposed to nontoxic doses of TVX and LPS developed pronounced hepatocellular injury (Shaw et al., 2007). A defining characteristic of this model was that the liver injury depended upon TNF. Pharmacological intervention with pentoxifylline to inhibit TNF transcription or with etanercept to neutralize released TNF prevented the injury (Shaw et al., 2007). Studies in TNF receptor knockout mice supported a key role for TNF in TVX/LPS hepatotoxicity (Shaw et al., 2009b). Importantly, the LPS-induced increase in plasma concentration of TNF was significantly prolonged by TVX, and this prolongation proved to be critical in the pathogenesis of liver injury. In animals treated with TVX and TNF rather than LPS, liver injury also occurred, and TVX caused the appearance of TNF in plasma to be prolonged due in part to enhanced TNF synthesis (Shaw et al., 2009a). Whether this resulted from a direct effect on TNF-producing cells was unknown. Accordingly, the purpose of the present study was to test the hypothesis that TVX pretreatment directly increases LPS-induced TNF synthesis and release by cells in vitro and to explore the underlying intracellular signaling involved in the response.
Materials and Methods
Chemicals and Inhibitors.
All chemicals and reagents in this study were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. Antibiotic/antimycotic and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Life Technologies (Grand Island, NY).
Cell Culture.
The RAW 264.7 macrophage cell line (American Type Culture Collection, Manassas, VA) was maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% antibiotic/antimycotic at 37°C in 5% CO2. Cells were harvested by detachment with a sterile spatula and plated at a density of 4 × 104 cells per well in 24-well plates (Costar, Lowell, MA) for cytokine release and RNA isolation or 1 × 106 cells per well in 10-cm plates (Costar) for protein isolation. Medium was replaced after 24 hours with DMEM (0.5% FBS) for cell synchronization prior to drug exposure 16 hours later.
TNF Release.
Synchronized RAW 264.7 cells, at 80–90% confluence, were pretreated for 2 hours with various concentrations of TVX (0–100 μM) in 0.5% fetal bovine serum–containing medium. TVX was dissolved in 0.1 N potassium hydroxide [vehicle (VEH)] at a stock concentration of 50 mM and diluted to the final concentration in 0.5% fetal bovine serum–containing medium. This 2-hour incubation was followed by a change to medium containing LPS from Escherichia coli serotype O55:B5 (lot 075K4038) at various concentrations (0.1–100 ng/ml) or saline vehicle (SAL) as control. The LPS had an activity of 3.3 × 106 endotoxin units/mg as determined using a colorimetric, Limulus amebocyte lysate assay (Kit 50-650U; Cambrex Corp., East Rutherford, NJ). For determination of TNF release, an enzyme-linked immunosorbent assay (ELISA) was performed (BD Biosciences, San Jose, CA). Cell culture medium was withdrawn at various times and stored at −20°C until the time of analysis. Ninety-six–well plates were coated with an anti-TNF capture antibody in a coating buffer overnight at 4°C. Medium was diluted to remain within standard curve concentrations.
Protein Isolation.
RAW 264.7 cells seeded in 10-cm plates as previously stated were exposed to TVX or an equivalent volume of VEH. At the indicated times, plates were washed twice with ice-cold phosphate-buffered saline and scraped with 1 ml of ice-cold phosphate-buffered saline. The cells were pelleted (400g for 2 minutes) and then resuspended in ice-cold radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Rockford, IL) supplemented with serine protease inhibitor phenylmethylsulfonyl fluoride and HALT protease and phosphatase inhibitor cocktails (Thermo Fisher Scientific). After a 10-minute incubation on ice, suspended pellets were sonicated twice with 5-second pulses. Suspensions were centrifuged at 22,000g for 30 minutes, and supernatants were withdrawn and stored at −80°C until analysis occurred.
Nuclear Factor κB Activation.
Activation of nuclear factor κB (NF-κB) was assessed from the binding of the p65 subunit to DNA using a TransAm NF-κB Transcription Factor ELISA Kit (ActiveMotif, Carlsbad, CA). Nuclear extracts of RAW cells were prepared per the manufacturer's instructions. Extracts were then incubated in a 96-well plate coated with an oligonucleotide sequence that corresponds to the consensus binding element NF-κB. After incubation with the extract, an antibody directed against an epitope of the p65 subunit of NF-κB was added. A secondary antibody was added that detects the primary antibody-bound p65. Finally, a developing solution was added, and p65 binding was assessed colorimetrically by measuring the optical density at 450 nm; background optical density was subtracted from all groups.
Western Analysis.
Protein concentration in extracts was determined by bicinchoninic acid assay (Thermo Fisher Scientific). All western analyses were performed by loading 20 μg of protein on precast NuPAGE SDS-PAGE gels (Life Technologies) using all NuPAGE reagents. For phospho-MAPK, samples were separated on precast 12% gels. After separation, proteins were transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA) for 1 hour at 4°C. Membranes were blocked in 5% bovine serum albumin dissolved in Tris-buffered saline plus 0.1% Tween 20 for 1 hour prior to incubation with primary antibody. They were probed with phospho-ERK1/2 (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), phospho-MAPKAPK-2 (Thr334), or phospho–activating transcription factor 2 (ATF2) (Thr71) rabbit polyclonal antibodies (Cell Signaling Technology, Danvers, MA). For subsequent probes, membranes were stripped with Restore Western Blot Stripping Agent (Thermo Fisher Scientific), washed for 30 minutes in Tris-buffered saline plus 0.1% Tween 20, and blocked prior to incubation with antibodies to total ERK, ATF, p38, or lamin B1.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction.
Total RNA was isolated using TRIzol reagent (Life Technologies) per the manufacturer's instructions. RNA quantity and quality were assessed using Nanodrop 2000 (Thermo Fisher Scientific). cDNA was prepared using 1 μg of RNA with the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). The expression of specific genes was analyzed using SYBR Green (Applied Biosystems, Foster City, CA). Expression level was normalized to β-actin. Polymerase chain reaction (PCR) primers used were as follows: mouse TNF [5′-TCTCATGCACCACCATCAAGGACT-3′ (forward) and 5′-ACCACTCTCCCTTTGCAGAACTCA-3′ (reverse)] and mouse β-actin [5′-TGTGATGGTGGGAATGGGTCAGAA-3′ (forward) and 5′-TGTGGTGCCAGATCTTCTCCATGT-3′ (reverse)].
Statistical Analysis.
A one- or two-way analysis of variance was performed on data sets with Tukey’s post-hoc test applied for multiple comparisons between groups. The criterion for significance was P < 0.05.
Results
Trovafloxacin Pretreatment Potentiates LPS-Induced TNF Release in a Dose- and Time-Dependent Manner.
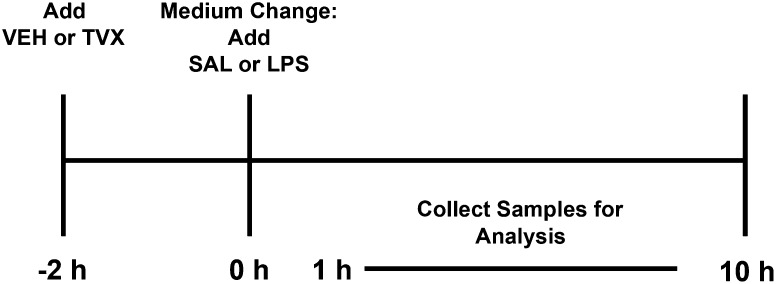
In mice, the maximal hepatotoxic response to TVX occurred when the animals were treated with TVX 3 hours before LPS exposure (Shaw et al., 2007). To approximate this exposure in vitro, RAW cells were exposed to TVX prior to LPS using the regimen depicted in Fig. 1. It is important to note that the TVX-LPS interaction described below required pretreatment with TVX followed by removing the TVX-containing medium and replacing it with medium containing LPS.
Fig. 1.
Protocol for treatment of RAW cells with TVX/LPS. After an overnight synchronization in 0.5% FBS-containing medium, VEH or TVX was added to RAW cells for a 2-hour pretreatment. The medium was then withdrawn and replaced with SAL- or LPS-containing medium and samples were collected at various times between 1 and 10 hours thereafter. For some studies, cultures were examined immediately after the TVX treatment (i.e., 0 hour).
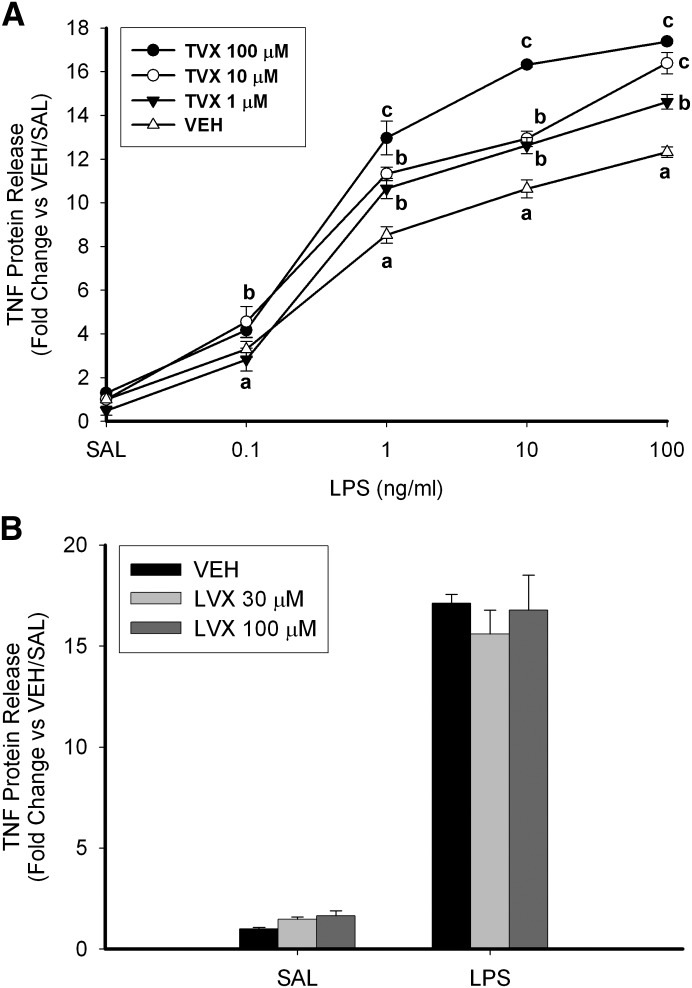
Exposure of cells for 6 hours to LPS (0.1–100 ng/ml) by itself resulted in a concentration-dependent increase in TNF concentration in the culture medium (Fig. 2A). Pretreatment with TVX (1, 10, or 100 μM) significantly increased LPS-induced TNF release in a concentration-dependent manner. The maximal difference between VEH- and TVX-pretreated cells was detected with a combination of 100 μM TVX and 10 ng/ml LPS, so further studies were conducted using this combination. Levofloxacin (LVX), a quinolone antibiotic used as a negative comparator because of its lesser association with IDILI, did not significantly increase TNF release 6 hours after LPS exposure (Fig. 2B). This is consistent with previous results in mice treated with LVX/LPS (Shaw et al., 2007).
Fig. 2.
TNF production in TVX/LPS-treated RAW cells: concentration-response. (A) RAW cells were pretreated with VEH or TVX (1, 10, or 100 μM) for 2 hours before exposure to SAL or various concentrations of LPS (0.1–100 ng/ml), and TNF concentration was assessed by ELISA. Values are the mean ± S.E.M. for fold change of TNF release relative to VEH/SAL-treated cells (n = 3). (B) RAW cells were pretreated with VEH or LVX (30 or 100 μM) for 2 hours before LPS addition (10 ng/ml). Values are the mean ± S.E.M. fold change of TNF release relative to VEH/SAL-treated cells (n = 3). Values with different letters differ significantly within LPS concentration (P < 0.05).
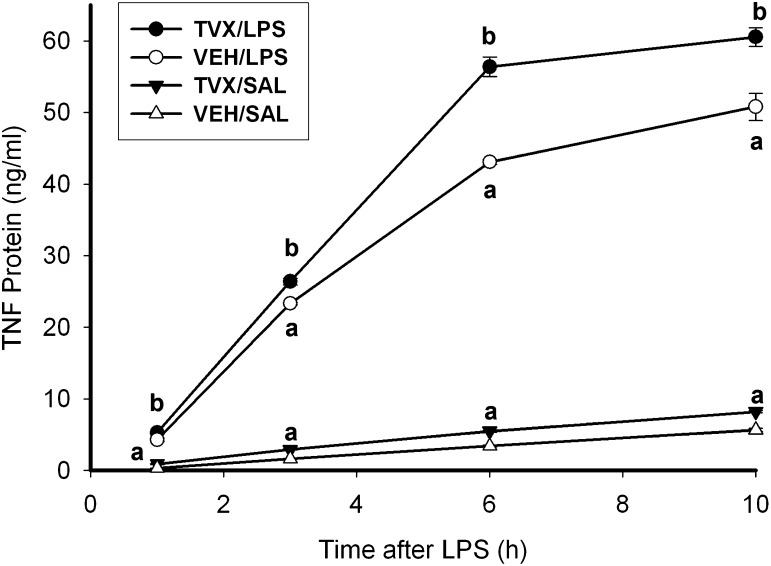
TVX pretreatment increased LPS-induced TNF release into cell culture medium at all times (1–10 hours) after LPS exposure (Fig. 3). TVX pretreatment also increased TNF release into the medium in SAL-treated cells at all times observed, although the increase was small. In cells treated only with LPS, the rate of TNF release (i.e., slope of lines in Fig. 3) was greatest from 1 to 3 hours and decreased thereafter. In comparison, the release rate in LPS/TVX-cotreated cells was greater from 1 to 3 hours and remained constant through 6 hours (Table 1). TVX pretreatment also increased the rate of release in the absence of LPS.
Fig. 3.
Time course of TNF release in TVX/LPS-treated RAW cells. RAW cells were pretreated with VEH or TVX (100 μM) then with SAL or LPS (10 ng/ml) as shown in Fig. 1, and TNF concentration was assessed by ELISA. Values are the mean concentration ± S.E.M. (n = 3). a, P < 0.05 versus VEH/SAL within a time point; b, P < 0.05 versus VEH/LPS within a time point.
TABLE 1.
Calculated rate of change in TNF release from RAW cells
Rate of TNF release from RAW cells was calculated from data in Fig. 3 as the Δ[TNF]/Δtime ± S.E.M.
| Treatment | Rate of Change in Medium TNF Concentration |
||
|---|---|---|---|
| 1–3 h | 3–6 h | 6–10 h | |
| ng/ml/h | |||
| VEH/SAL | 0.66 ± 0.01 | 0.59 ± 0.02 | 0.57 ± 0.07 |
| TVX/SAL | 1.01 ± 0.02a | 0.85 ± 0.04a | 0.69 ± 0.07a |
| VEH/LPS | 9.51 ± 0.14 | 6.59 ± 0.18 | 1.93 ± 0.55 |
| TVX/LPS | 10.55 ± 0.22b | 10.00 ± 0.32b | 1.04 ± 0.07b |
P < 0.05 versus VEH/SAL within a time point.
P < 0.05 versus VEH/LPS within a time point.
TVX, but Not LVX, Significantly Increases TNF mRNA Prior to LPS Addition.
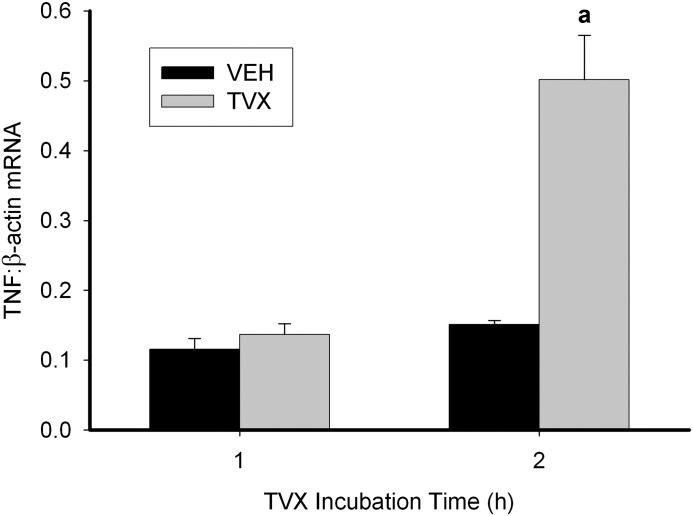
TVX pretreatment of RAW cells did not increase TNF mRNA from 1 to 3 hours after SAL or LPS exposure despite the consistent increases in TNF protein release, although a trend was observed (Supplemental Fig. 1). Accordingly, the effect of TVX on TNF mRNA prior to LPS addition was assessed. Exposure of RAW cells to TVX for 2 hours increased TNF mRNA (Fig. 4). In contrast, exposure to LVX for 2 hours failed to affect TNF mRNA (Supplemental Fig. 2).
Fig. 4.
Effect of TVX treatment on TNF mRNA in RAW cells. RNA was isolated from RAW cells after TVX treatment, converted to cDNA, and TNF mRNA was quantified by quantitative PCR and normalized to β-actin mRNA. Values are the mean ± S.E.M. (n = 3). a, P < 0.05 versus VEH within a time point.
TVX Treatment Increases MAPK Phosphorylation Prior to LPS Addition, and ERK and JNK Signaling Are Required for TVX-Mediated Increases in LPS-Induced TNF Release.
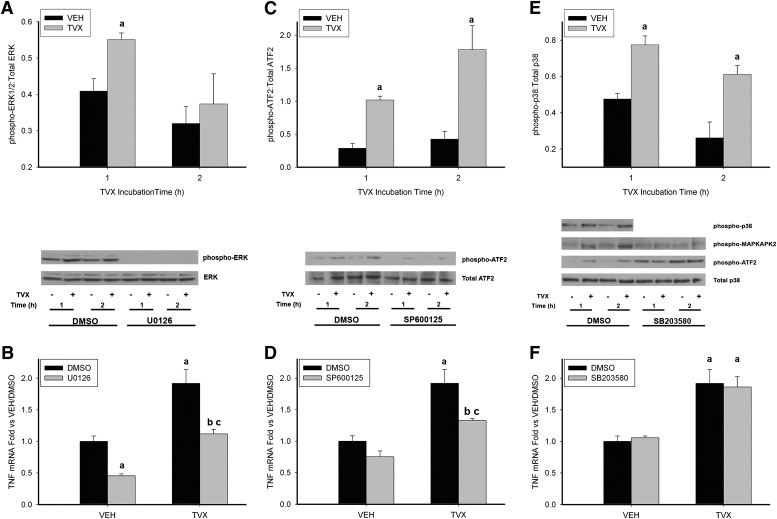
These results indicated that pivotal changes in signaling that led to increased TNF mRNA occurred during the TVX pretreatment, so the next studies investigated MAPK activation during this period. MAPK activation can increase TNF mRNA in inflammatory cells (reviewed in Guha and Mackman, 2001). Accordingly, the role of ERK, JNK, and p38 in TNF mRNA induction was assessed in TVX-treated RAW cells. ERK phosphorylation was increased after 1 hour, but not after 2 hours, of TVX exposure (Fig. 5A). The selective MEK1 (MAPK or Erk kinase 1) inhibitor and ERK-activation inhibitor, U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene) (Favata et al., 1998), abolished all phospho-ERK signal, indicating a complete inhibition of ERK1/2-mediated signaling. As expected, TVX caused an increase in TNF mRNA, and U0126 prevented this increase (Fig. 5B). TNF mRNA was also decreased in VEH-pretreated cells treated with U0126. The TVX-mediated increase in LPS-induced TNF release at 3 and 6 hours was completely prevented by U0126 (Fig. 6).
Fig. 5.
MAPK activation during TVX exposure and involvement in TVX-induced TNF mRNA expression. RAW cells were treated with VEH or TVX and with MAPK inhibitors or their dimethylsulfoxide (DMSO) vehicle. Cells were lysed at the indicated times, and MAPK phosphorylation was evaluated in protein extracts. RNA was isolated after a 2-hour incubation with VEH or TVX and with DMSO or MAPK inhibitors, and TNF mRNA was assessed by quantitative PCR. (A) RAW cells were treated with U0126 (500 nM), and extracts were probed for phosphorylated ERK and total ERK. Values for phosphorylated ERK were normalized to values for total ERK. (B) TNF mRNA expression from RAW cells exposed to TVX and U0126 (500 nM). (C) RAW cells were treated with SP600126 (10 μM), and extracts were probed for phospho-ATF2 and total ATF2. Values for phospho-ATF2 were normalized to values for total ATF2. (D) TNF mRNA expression in RAW cells exposed to SP600125 (10 μM). (E) RAW cells were treated with SB203580 (10 μM), and extracts were probed for phosphorylated p38, MAPKAPK-2, ATF2, and total p38. Values for phosphorylated p38 were normalized to values for total p38. (F) TNF mRNA expression in RAW cells exposed to SB203580 (10 μM). All data are represented as means ± S.E.M. of normalized densitometry (n = 3–6). a, P < 0.05 versus VEH within a time point or VEH/DMSO; b, P < 0.05 versus TVX/DMSO; c, P < 0.05 versus VEH/MAPK inhibitor.
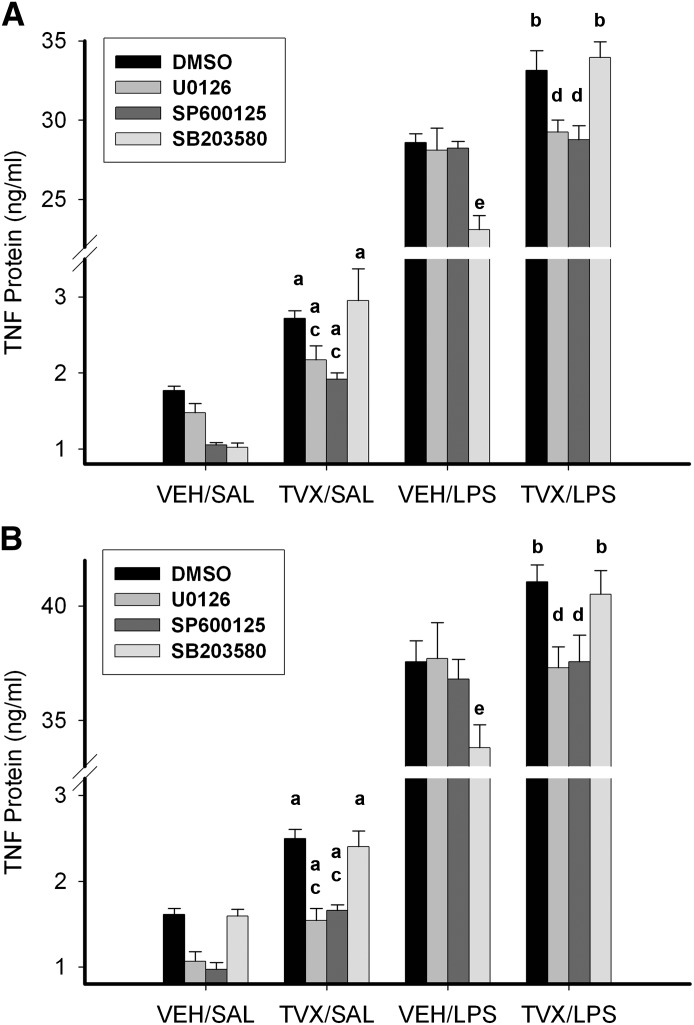
Fig. 6.
Effect of MAPK inhibition on TVX-mediated changes in LPS-induced TNF protein release. RAW cells were treated with MAPK inhibitors (U0126, SP600125, or SB203580) or 0.05% dimethylsulfoxide (DMSO) during a 2-hour TVX incubation, after which time the medium was replaced with one containing SAL or LPS (without inhibitors). TNF protein release was measured 3 hours (A) or 6 hours (B) after LPS addition. Values are the mean ± S.E.M. (n = 6). a, P < 0.05 versus respective VEH/SAL group; b, P < 0.05 versus respective VEH/LPS group; c, P < 0.05 versus TVX/SAL/DMSO; d, P < 0.05 versus TVX/LPS/DMSO; e, P < 0.05 versus VEH/LPS/DMSO.
Attempts were made to detect phosphorylated JNK, but despite using several different antibodies, the results were inconsistent. Instead, TVX-dependent activation of JNK was determined by phosphorylation of the known downstream target of JNK signaling, ATF2 (Fig. 5C). TVX treatment increased phospho-ATF2 at 1 and 2 hours. The selective inhibitor of JNK activity, SP600125 (1,9-pyrazoloanthrone) (Bennett et al., 2001), markedly reduced phosphorylation of ATF2 (Fig. 5C), which suggested that the phosphorylation of ATF2 was JNK-selective. SP600125 also decreased the TVX-mediated increase in TNF mRNA (Fig. 5D). SP600125 completely prevented TVX-mediated enhancement of LPS-induced increase in TNF protein release 3 and 6 hours after LPS exposure (Fig. 6).
p38 Phosphorylation was increased 1 and 2 hours after TVX exposure (Fig. 5E). SB203580 [4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole)] is a selective inhibitor of p38 activity (Cuenda et al., 1995), but it does not prevent its phosphorylation. Accordingly, the phosphorylation of MAPKAPK-2, a direct downstream target of activated p38 (Beyaert et al., 1996), was evaluated, and SB203580 markedly reduced MAPKAPK-2 phosphorylation. Despite the TVX-mediated increase in p38 phosphorylation (Fig. 5E), p38 inhibition did not reduce the TVX-induced increase in TNF mRNA (Fig. 5F). SB203580 also did not affect the TVX-induced increase in TNF protein release in the absence or presence of LPS (Fig. 6). Interestingly, SB203580 exposure during the pretreatment period decreased LPS-induced TNF release in VEH-pretreated controls at 3 and 6 hours after LPS (Fig. 6).
Discussion
The relative infrequency with which cases of IDILI occur in humans makes elucidation of the molecular pathogenesis of IDILI difficult. That being said, several animal models of IDILI have been generated that require increased or prolonged TNF in the plasma of animals to precipitate hepatotoxicity (Shaw et al., 2007; Tukov et al., 2007; Zou et al., 2009; Lu et al., 2012). Developing a model in vitro that reproduces increased TNF production is therefore important to be able to conduct studies to understand these drug-LPS interactions in animal models of IDILI. Such a model could be used to discover previously unidentified critical molecular targets of IDILI-associated drugs in cells.
In TVX/LPS-treated mice, the drug-induced increase in LPS-stimulated TNF appearance is relatively small in magnitude, and the increased duration of plasma TNF is relatively short (Shaw et al., 2007). However, this relatively small prolongation in plasma TNF proved to be essential to the hepatotoxic TVX-LPS interaction. Etanercept given at the time of peak TNF appearance in LPS-treated mice eliminated the prolongation of TNF appearance in TVX/LPS-cotreated mice and protected them from liver injury. In contrast, etanercept administered after the TVX-mediated prolongation of plasma TNF concentration had ended failed to protect against the hepatocellular necrosis. Thus, the brief prolongation of TNF appearance was required for the pathogenesis of liver injury in TVX/LPS-cotreated mice (Shaw et al., 2009b). Accordingly, we sought to determine whether a similar interaction between TVX and LPS could be reproduced in TNF-producing cells in vitro.
Kupffer cells (KCs) are the largest population of fixed macrophages in the body and a likely source of TNF in response to LPS (Su et al., 2000). RAW cells were chosen as a KC surrogate because of their well characterized LPS-induced TNF production and suitability for high-throughput screening (Beutler et al., 1985). As a transformed murine macrophage, RAW cells maintain many functions ascribed to KCs and other macrophages, including cytokine release, respiratory burst and phagocytosis, etc. TVX pretreatment increased LPS-induced TNF release in RAW cells (Figs. 2A and 3). In contrast, LVX did not, consistent with the failure of LVX to enhance LPS-mediated TNF appearance in mice (Shaw et al., 2007). Accordingly, the drug-induced increase in LPS-induced plasma TNF observed in TVX/LPS-cotreated mice was recapitulated in RAW cells in vitro.
In the time course study (Fig. 3), analysis of the rate of TNF release (Table 1) indicated that TVX-pretreated cells released TNF at a greater rate than VEH-pretreated controls through 6 hours after the addition of LPS, but not thereafter. The results suggested that the capacity to release TNF in RAW cells was nearly exhausted within 6 hours of exposure to LPS; this has been observed previously in LPS-exposed RAW cells (Rouzer et al., 2005; Wang et al., 2012). TVX pretreatment increased TNF release after removal of TVX-containing medium and replacement with LPS- or SAL-containing medium, even though TNF mRNA did not change after medium replacement (Supplemental Fig. 1). The results strongly suggest that the TVX-induced signaling that contributed to increased LPS-induced TNF mRNA occurred before the cells were exposed to LPS.
TVX treatment did increase TNF mRNA during the 2-hour drug exposure (Fig. 4). The increase in TNF mRNA occurred in a relatively short time, between 1 and 2 hours. LVX did not significantly increase TNF mRNA at this time (Supplemental Fig. 2), providing an explanation for the lack of effect of LVX on LPS-stimulated TNF appearance in vitro and in vivo (Fig. 2B; Shaw et al., 2007). LPS-induced TNF expression involves activation of NF-κB and/or MAPKs (Geppert et al., 1994; Swantek et al., 1997; Fischer et al., 1999), so these signaling proteins became a focus for investigation. Although NF-κB is a well characterized inducer of TNF expression, TVX did not induce p65 binding to DNA before or after LPS exposure (Supplemental Fig. 3), suggesting that MAPKs are likely responsible for the TVX-mediated increase in TNF mRNA.
All three MAPKs assessed were activated during TVX pretreatment (Fig. 5, A, C, and E). ERK activation occurred early (1 hour), whereas JNK and p38 activation were increased at both times assayed (1 and 2 hours). In this initial exploration, we used selective inhibitors of each MAPK to identify which one(s) contributed to increased TNF release. TNF biosynthesis can involve p38 at several levels: activation of trans-acting factors, stabilization of TNF mRNA, and shedding of membrane-bound TNF (Deleault et al., 2008; Rowlett et al., 2008; Scott et al., 2011). Surprisingly, p38 inhibition failed to alter the TVX-mediated increase in TNF mRNA (Fig. 5F). Inhibition of ERK, however, prevented the TVX-mediated increase in TNF mRNA and depressed basal levels of TNF mRNA (Fig. 5B). ERK phosphorylation was detected in VEH-pretreated cells (Fig. 5A), suggesting that basal TNF mRNA expression is ERK-mediated in RAW cells. The TVX-mediated increase in TNF mRNA was reduced but not totally eliminated when JNK signaling was inhibited (Fig. 5D). The marker of JNK activation, ATF2, is also a target of p38 signaling in inflammatory cells (Brown et al., 2008; Hirose et al., 2009), but as shown in Fig. 5E, SB203580 did not reduce phosphorylation of ATF2, arguing for ATF2 as a selective target for JNK in this model. Together, these results suggest that both ERK and JNK are involved causally in the increase in TNF mRNA caused by TVX.
U0126 and SP600125 are reversible inhibitors of ERK and JNK, respectively. They were present only during the TVX pretreatment period, not during exposure to LPS. If the increase in TNF mRNA during the TVX pretreatment period was linked to TNF protein release prompted by the later exposure to LPS, then these MAPK inhibitors should reduce the TVX-mediated increase in TNF release, as observed in Fig. 6. Despite an incomplete reduction in the TVX-mediated increase in TNF mRNA prior to LPS exposure, SP600125 completely eliminated the TVX interaction with LPS. It is possible that JNK contributes to this interaction not only through increased TNF mRNA, but also through a post-transcriptional mechanism, such as increased TNF translation (Swantek et al., 1997). It is also possible that ERK and JNK both contribute to the TVX-mediated increase in TNF expression through a similar mechanism, such as increased AP-1 (activator protein 1)–dependent transactivation of the TNF gene (Chan and Riches, 1998; Cuschieri et al., 2004), but this remains a topic for future investigation.
ERK has been shown to mediate increases in TNF mRNA and protein in other models. For example, long-term ethanol treatment increases LPS-induced TNF mRNA expression and TNF protein release by macrophages in an ERK-dependent manner (Kishore et al., 2002; Pritchard and Nagy, 2005). In addition, both pro- and anti-inflammatory effects of adiponectin on LPS-induced TNF release in RAW cells are mediated through ERK-dependent signaling (Park et al., 2007; Huang et al., 2008). ERK signaling plays a significant role in experimental models of inflammatory liver injury from alcohol or bile acid exposure (Mandrekar and Szabo, 2009; Allen et al., 2010). These results attest to the dynamic and important role ERK signaling plays in TNF biosynthesis in macrophages in models of hepatotoxicity involving inflammatory stress.
JNK has been implicated in cell-death signaling, and examples of JNK dependence in drug-inflammation interaction models of hepatocellular injury have been described (Gandhi et al., 2013; Beggs et al., 2014). Chlorpromazine (CPZ) is a phenothiazine antipsychotic drug associated with IDILI in humans, and coexposure of rats to CPZ and LPS precipitates liver injury (Buchweitz et al., 2002). Exposure of mice or primary murine hepatocytes to CPZ combined with LPS or the Toll-like receptor 2 agonist lipoteichoic acid resulted in hepatocellular injury that was associated with prolonged JNK activation (Gandhi et al., 2010). Toxicity was preceded by an increase and prolongation of TNF in the plasma of CPZ-LPS or CPZ-lipoteichoic acid coexposed animals, raising the possibility of a link between prolonged plasma TNF and hepatotoxicity, similar to what is observed during TVX-LPS coexposure. Another recent study described JNK-dependent cytotoxicity resulting from TVX/TNF coexposure in a hepatocyte cell line (Beggs et al., 2014). Therefore, it is possible that a common upstream stimulus in hepatocytes and macrophages activates JNK in response to TVX, and that this results in cell death in hepatocytes and increased cytokine release in macrophages. Both of these effects could contribute to liver injury from TVX and, more generally, from drugs associated with IDILI.
The knowledge generated in this model of TVX/LPS-treated RAW cells enhances our understanding of TVX-LPS interaction in the murine model of IDILI. Results from this study identified critical roles for TVX-mediated ERK and JNK signaling in macrophages. The findings in RAW cells are strongly supported by substantial evidence previously generated in vivo (Shaw et al., 2007, 2009a,b), which demonstrated a critical role for enhancement of LPS-induced TNF release by TVX in TVX-dependent hepatotoxicity. Furthermore, the current study provides significant rationale for studying the role of ERK and JNK signaling in models of IDILI. Since several drug-inflammation interaction models in animals are also associated with increases in TNF and require TNF for hepatotoxicity, the results of this study raise the possibility that common MAPK signaling mechanisms are at play in IDILI reactions.
Supplementary Material
Abbreviations
- ATF2
activating transcription factor 2
- CPZ
chlorpromazine
- DMEM
Dulbecco’s modified Eagle’s medium
- DILI
drug-induced liver injury
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- IDILI
idiosyncratic drug-induced liver injury
- JNK
c-Jun N-terminal kinase
- KC
Kupffer cell
- LPS
lipopolysaccharide
- LVX
levofloxacin
- MAPK
mitogen-activated protein kinase
- MAPKAPK-2
MAPK-activated protein kinase 2
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- PCR
polymerase chain reaction
- SAL
saline
- SB203580
4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole
- SP600125
1,9-pyrazoloanthrone
- TNF
tumor necrosis factor-α
- TVX
trovafloxacin
- U0126
1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene
- VEH
vehicle
Authorship Contributions
Participated in research design: Poulsen, Albee, Ganey, Roth.
Conducted experiments: Poulsen, Albee.
Performed data analysis: Poulsen.
Wrote or contributed to the writing of the manuscript: Poulsen, Ganey, Roth.
Footnotes
This study was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK061315] and National Institutes of Health National Institute of Environmental Health Sciences [Training Grant T32ES007255 (to K.L.P.)].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Allen K, Kim ND, Moon JO, Copple BL. (2010) Upregulation of early growth response factor-1 by bile acids requires mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol 243:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P, Mandell L, Niki Y, Tillotson G. (1999) Comparative tolerability of the newer fluoroquinolone antibacterials. Drug Saf 21:407–421 [DOI] [PubMed] [Google Scholar]
- Beggs KM, Fullerton AM, Miyakawa K, Ganey PE, Roth RA. (2014) Molecular mechanisms of hepatocellular apoptosis induced by trovafloxacin-tumor necrosis factor-alpha interaction. Toxicol Sci 137:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et al. (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98:13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Mahoney J, Le Trang N, Pekala P, Cerami A. (1985) Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med 161:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. (1996) The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J 15:1914–1923 [PMC free article] [PubMed] [Google Scholar]
- Brighty KE, Gootz TD. (1997) The chemistry and biological profile of trovafloxacin. J Antimicrob Chemother 39 (Suppl B):1–14 [DOI] [PubMed] [Google Scholar]
- Brown KK, Heitmeyer SA, Hookfin EB, Hsieh L, Buchalova M, Taiwo YO, Janusz MJ. (2008) p38 MAP kinase inhibitors as potential therapeutics for the treatment of joint degeneration and pain associated with osteoarthritis. J Inflamm (Lond) 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchweitz JP, Ganey PE, Bursian SJ, Roth RA. (2002) Underlying endotoxemia augments toxic responses to chlorpromazine: is there a relationship to drug idiosyncrasy? J Pharmacol Exp Ther 300:460–467 [DOI] [PubMed] [Google Scholar]
- Chan ED, Riches DW. (1998) Potential role of the JNK/SAPK signal transduction pathway in the induction of iNOS by TNF-alpha. Biochem Biophys Res Commun 253:790–796 [DOI] [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. (1999) Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 274:10689–10692 [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. (1995) SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett 364:229–233 [DOI] [PubMed] [Google Scholar]
- Cuschieri J, Gourlay D, Garcia I, Jelacic S, Maier RV. (2004) Implications of proteasome inhibition: an enhanced macrophage phenotype. Cell Immunol 227:140–147 [DOI] [PubMed] [Google Scholar]
- Deleault KM, Skinner SJ, Brooks SA. (2008) Tristetraprolin regulates TNF TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways. Mol Immunol 45:13–24 [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al. (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273:18623–18632 [DOI] [PubMed] [Google Scholar]
- Fischer C, Page S, Weber M, Eisele T, Neumeier D, Brand K. (1999) Differential effects of lipopolysaccharide and tumor necrosis factor on monocytic IkappaB kinase signalsome activation and IkappaB proteolysis. J Biol Chem 274:24625–24632 [DOI] [PubMed] [Google Scholar]
- Gandhi A, Guo T, Ghose R. (2010) Role of c-Jun N-terminal kinase (JNK) in regulating tumor necrosis factor-alpha (TNF-alpha) mediated increase of acetaminophen (APAP) and chlorpromazine (CPZ) toxicity in murine hepatocytes. J Toxicol Sci 35:163–173 [DOI] [PubMed] [Google Scholar]
- Gandhi A, Guo T, Shah P, Moorthy B, Ghose R. (2013) Chlorpromazine-induced hepatotoxicity during inflammation is mediated by TIRAP-dependent signaling pathway in mice. Toxicol Appl Pharmacol 266:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert TD, Whitehurst CE, Thompson P, Beutler B. (1994) Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med 1:93–103 [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. (2001) LPS induction of gene expression in human monocytes. Cell Signal 13:85–94 [DOI] [PubMed] [Google Scholar]
- Hassan F, Morikawa A, Islam S, Tumurkhuu G, Dagvadorj J, Koide N, Naiki Y, Mori I, Yoshida T, Yokochi T. (2008) Lipopolysaccharide augments the in vivo lethal action of doxorubicin against mice via hepatic damage. Clin Exp Immunol 151:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose N, Maekawa T, Shinagawa T, Ishii S. (2009) ATF-2 regulates lipopolysaccharide-induced transcription in macrophage cells. Biochem Biophys Res Commun 385:72–77 [DOI] [PubMed] [Google Scholar]
- Huang H, Park PH, McMullen MR, Nagy LE. (2008) Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol 23 (Suppl 1):S50–S53 [DOI] [PubMed] [Google Scholar]
- Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. (2002) ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol 282:G6–G15 [DOI] [PubMed] [Google Scholar]
- Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. (2002) Timing of new black box warnings and withdrawals for prescription medications. JAMA 287:2215–2220 [DOI] [PubMed] [Google Scholar]
- Lu J, Jones AD, Harkema JR, Roth RA, Ganey PE. (2012) Amiodarone exposure during modest inflammation induces idiosyncrasy-like liver injury in rats: role of tumor necrosis factor-alpha. Toxicol Sci 125:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyendyk JP, Maddox JF, Cosma GN, Ganey PE, Cockerell GL, Roth RA. (2003) Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J Pharmacol Exp Ther 307:9–16 [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Szabo G. (2009) Signalling pathways in alcohol-induced liver inflammation. J Hepatol 50:1258–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, et al. U.S. Acute Liver Failure Study Group (2002) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 137:947–954 [DOI] [PubMed] [Google Scholar]
- Park PH, McMullen MR, Huang H, Thakur V, Nagy LE. (2007) Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem 282:21695–21703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard MT, Nagy LE. (2005) Ethanol-induced liver injury: potential roles for egr-1. Alcohol Clin Exp Res 29(Suppl. 11):146S–150S [DOI] [PubMed] [Google Scholar]
- Reimann T, Büscher D, Hipskind RA, Krautwald S, Lohmann-Matthes ML, Baccarini M. (1994) Lipopolysaccharide induces activation of the Raf-1/MAP kinase pathway. A putative role for Raf-1 in the induction of the IL-1 beta and the TNF-alpha genes. J Immunol 153:5740–5749 [PubMed] [Google Scholar]
- Rouzer CA, Jacobs AT, Nirodi CS, Kingsley PJ, Morrow JD, Marnett LJ. (2005) RAW264.7 cells lack prostaglandin-dependent autoregulation of tumor necrosis factor-alpha secretion. J Lipid Res 46:1027–1037 [DOI] [PubMed] [Google Scholar]
- Rowlett RM, Chrestensen CA, Nyce M, Harp MG, Pelo JW, Cominelli F, Ernst PB, Pizarro TT, Sturgill TW, Worthington MT. (2008) MNK kinases regulate multiple TLR pathways and innate proinflammatory cytokines in macrophages. Am J Physiol Gastrointest Liver Physiol 294:G452–G459 [DOI] [PubMed] [Google Scholar]
- Sanghera JS, Weinstein SL, Aluwalia M, Girn J, Pelech SL. (1996) Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol 156:4457–4465 [PubMed] [Google Scholar]
- Scott AJ, O’Dea KP, O’Callaghan D, Williams L, Dokpesi JO, Tatton L, Handy JM, Hogg PJ, Takata M. (2011) Reactive oxygen species and p38 mitogen-activated protein kinase mediate tumor necrosis factor α-converting enzyme (TACE/ADAM-17) activation in primary human monocytes. J Biol Chem 286:35466–35476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Beggs KM, Sparkenbaugh EM, Dugan CM, Ganey PE, Roth RA. (2009a) Trovafloxacin enhances TNF-induced inflammatory stress and cell death signaling and reduces TNF clearance in a murine model of idiosyncratic hepatotoxicity. Toxicol Sci 111:288–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Ganey PE, Roth RA. (2009b) Tumor necrosis factor alpha is a proximal mediator of synergistic hepatotoxicity from trovafloxacin/lipopolysaccharide coexposure. J Pharmacol Exp Ther 328:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Ganey PE, Roth RA. (2010) Idiosyncratic drug-induced liver injury and the role of inflammatory stress with an emphasis on an animal model of trovafloxacin hepatotoxicity. Toxicol Sci 118:7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Hopfensperger MJ, Ganey PE, Roth RA. (2007) Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol Sci 100:259–266 [DOI] [PubMed] [Google Scholar]
- Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, Wang SC. (2000) Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and Toll-like receptor 4. Hepatology 31:932–936 [DOI] [PubMed] [Google Scholar]
- Swantek JL, Cobb MH, Geppert TD. (1997) Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol 17:6274–6282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukov FF, Luyendyk JP, Ganey PE, Roth RA. (2007) The role of tumor necrosis factor alpha in lipopolysaccharide/ranitidine-induced inflammatory liver injury. Toxicol Sci 100:267–280 [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346–351 [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang Y, Liu X, Wang N, Cao H, Lu Y, Zhou H, Zheng J. (2012) Inhibition of clathrin/dynamin-dependent internalization interferes with LPS-mediated TRAM-TRIF-dependent signaling pathway. Cell Immunol 274:121–129 [DOI] [PubMed] [Google Scholar]
- Watkins PB. (2005) Idiosyncratic liver injury: challenges and approaches. Toxicol Pathol 33:1–5 [DOI] [PubMed] [Google Scholar]
- Zou W, Beggs KM, Sparkenbaugh EM, Jones AD, Younis HS, Roth RA, Ganey PE. (2009) Sulindac metabolism and synergy with tumor necrosis factor-alpha in a drug-inflammation interaction model of idiosyncratic liver injury. J Pharmacol Exp Ther 331:114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.