Abstract
Introduction:
The finding of only sperm heads and/or short tails (SHST) during vasectomy reversal (VR) creates a difficult decision for the best method of vasal reconstruction, i.e. vasovasostomy (VV) or epididymovasostomy (EV). Using outcome analyses, we report the impact of SHST alone and combined with qualitative analysis of gross fluid quality in predicting successful VR.
Materials and Methods:
The records of 356 men who underwent VR by a single surgeon from 2005 to 2012 were retrospectively reviewed. Intravasal fluid was assessed for gross quality (i.e., clear, opaque, pasty or creamy) as well as microscopic composition (i.e., motile or non-motile whole sperm, SHST or no sperm). The post-operative patency rates and semen analysis parameters were assessed.
Results:
Fourteen men (3.9%) demonstrated SHST bilaterally in the vasal fluid. The median duration from vasectomy was 6.0 years (interquartile range 4.0-9.8). Bilateral VVs were performed on 12 men (86%), while two men (14%) had a unilateral VV and a contralateral EV. Of the 26 vasa undergoing VR, the majority of the fluid quality was classified as creamy (n = 20 vasa, 76.9%). The remaining fluid was classified as pasty (n = 3 vasa, 11.5%), opaque (n = 2 vasa, 7.7%) and clear (n = 1 vasa, 3.8%). In cases undergoing bilateral VV with only SHST, patency rates were 90.9%, and both cases of unilateral EV were patent (100%). Conclusions: VV was successful in 90.9% of patients undergoing VR in the setting of SHST alone. Even when creamy or pasty fluid was present, the results surpassed the expected patency rate for an EV. Therefore, the presence of only SHST, regardless of fluid quality, should not dissuade the surgeon from performing a VV.
Conclusions:
VV was successful in 90.9% of patients undergoing VR in the setting of SHST alone. Even when creamy or pasty fluid was present, the results surpassed the expected patency rate for an EV. Therefore, the presence of only SHST, regardless of fluid quality, should not dissuade the surgeon from performing a VV.
Keywords: Infertility, male, spermatozoa, vasectomy reversal, vasovasostomy
INTRODUCTION
The modern era of vasectomy reversal (VR) arose following the increased utilization of vasectomy as a means for sterilization after World War I.[1,2] Current estimates indicate that over 50 million men worldwide undergo vasectomy as a means of contraception, and up to 6% of men will request a reversal.[3,4] Contemporary microsurgical techniques of vasal reconstruction include a modified one-layer and multi-layered vasovasostomy (VV) as well as end-to-end, end-to-side and intussuscepted end-to-side epididymovasostomy (EV).[5]
During surgery, the physician's decision to proceed with VV or EV depends upon the gross fluid quality expressed from the testicular end of the vas deferens and the microscopic examination of the fluid for sperm. Findings may include motile or non-motile whole sperm, sperm heads alone, sperm heads with short tails or no sperm. Fluid characteristics may be classified on a diminishing quality continuum of clear, opaque, creamy or pasty. VV is routinely performed when whole sperms are identified in the vasal fluid or the nature of the fluid is clear and copious, even in the absence of sperm. In contrast, when the fluid quality is poor and sperms are absent, EV is generally required.[5] Modern series indicate a VV patency rate of 99.5% in the presence of whole sperm.[6] Even in cases of bilateral intravasal azoospermia, patency rates in some series approach 80% and pregnancy rates are 38% when the obstructive interval is less than 11 years.[7]
On occasion, sperm heads and/or short tails (SHST) are identified. Few studies have commented on VR success rates when sperm fragments (i.e., SHST) alone are present. Similarly, prior studies have been limited in the number of patient outcomes reviewed and a lack of reported correlation with gross fluid quality.
In the Vasovasostomy Study Group, Belker et al. found patency rates exceeding 90% in the presence of whole sperm compared with 75% when only sperm heads were present.[8,9] Similarly, pregnancy rates were lower when only SHST were identified intraoperatively.[8] Given the lower patency and pregnancy rates in the setting of sperm fragments and the advances in successful EV, microsurgeons have historically had to consider EV when incomplete sperm are seen in the vasal fluid, particularly in the setting of poor intravasal fluid quality.[8,10,11,12,13]
Some series have utilized a modified, intraoperative Silber score as a means of classifying seminal consistency and quality while attempting to predict post-operative vas patency and fertility. Using a Silber score, semen quality is assessed as follows: Grade 1 — mainly normal, motile spermatozoa; Grade 2 — mainly normal, non-motile spermatozoa; Grade 3 — mainly sperm heads; Grade 4 — only sperm heads; and Grade 5 — no sperm.[8,11,14,15] While some series report successful VV in the setting of Silber Grade 4 cases,[1,8] others prefer to perform EV.[9,11] The gross characteristics of the fluid also have an influence on return of sperm to the semen and the likelihood of pregnancy. These rates are highest when the fluid is clear, lower when fluid is opaque and lowest when it is thick and creamy.[8,11]
The goal of the current study was to evaluate the outcomes of microsurgical VR in the setting of SHST alone in a variety of vasal fluid conditions. It is hoped that by examining the outcomes in a contemporary patient cohort, the historical paradigm that an EV needs to be considered in the presence of SHST could be reconsidered.
MATERIALS AND METHODS
This retrospective review was approved by the Baylor College of Medicine Institutional Review Board. The intraoperative findings and subsequent outcomes for a total of 356 men who underwent VR by a single, experienced microsurgeon in an academic medical center from 2005 through 2012 were analyzed. Patients were included for analysis if they demonstrated SHST within the vasal fluid bilaterally and had at least one post-operative semen analysis or documented pregnancy. Intraoperative exclusion criteria included patients with motile sperm, non-motile whole sperm or no sperm. Furthermore, patients who lacked a post-operative semen analysis and in whom a documented pregnancy (to indirectly assess patency) could not be assessed were also excluded.
Microscopic examination of the vasal fluid was performed intraoperatively by placing a drop of vasal fluid, minimally diluted in human tubual fluid, on a slide. The decision to proceed with VV versus EV was based on the gross quality of the intravasal fluid (clear, opaque, creamy or pasty) as well as findings on microscopic examination (motile or non-motile whole sperm, SHST or no sperm). Poor fluid quality was defined as either creamy or pasty in gross appearance. Vasal character and fluid volume were also assessed intraoperatively. Each vasal anastomosis was described as either within the straight or convoluted vas. Intravasal fluid volume was characterized as copious, medium, low or none, and this assessment was included as a potential adjunctive indicator of proximal epididymal obstruction. Microsurgical VV was performed under general anesthesia using a previously described two-layer technique, and EV was completed using an end-to-side intussuscepted technique.[5]
Post-operative patency was defined by the presence of motile sperm on follow-up semen analysis, typically obtained at a minimum of 6 weeks post-operatively, or by pregnancy. Semen analysis was also used to assess sperm density among patent men. For men with more than one follow-up semen analysis, the highest reported sperm concentration was used for statistical analysis. Baseline demographic and clinical variables were described using counts and percentages or mean and standard deviation for categorical and continuous variables, respectively. Patency rates across various subgroups were analyzed using the Fisher exact test, while sperm density was assessed using the Kruskal — Wallis test. Statistical significance was defined as P < 0.05.
RESULTS
A total of 14 men (3.9%) with bilateral SHST on intraoperative vasal fluid examination were identified. Thirteen men were included in the final analysis as one man was lost to follow-up in the post-operative period and was therefore excluded. These men had a median age of 38.5 years [interquartile range (IQR) 36.0-41.0 years], were predominantly Caucasian and were all married at the time of VR [Table 1]. The median occlusive interval was 6.0 years (IQR 4.0-9.8 years). In terms of gross vasal fluid quality, 76.9% of the time the fluid was described intraoperatively by the primary surgeon as creamy, 11.5% of the time as pasty, 7.7% of the time as opaque and 3.8% of the time as clear. This highights the fact that the majority of anastomoses were performed in the setting of poor-quality fluid [Table 2]. Occlusive intervals were nearly evenly distributed among short (<5 years; 30.8%), medium (5-9 years; 38.5%) and long (>9 years; 30.8%) durations. VV was the primary method of vasal reconstruction (92.3%), and most anastamoses occurred within the straight portion of the vas deferens (53.8%). Two-thirds of the time, vasal fluid volume was characterized as copious.
Table 1.
Demographic and clinical characteristics of the study participants, n = 14 patients
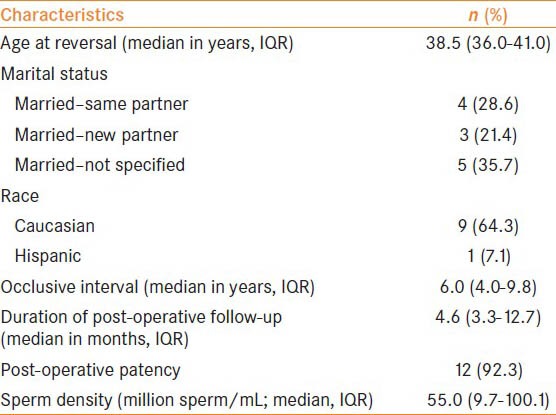
Table 2.
Patency rates by intravasal fluid quality and occlusion interval
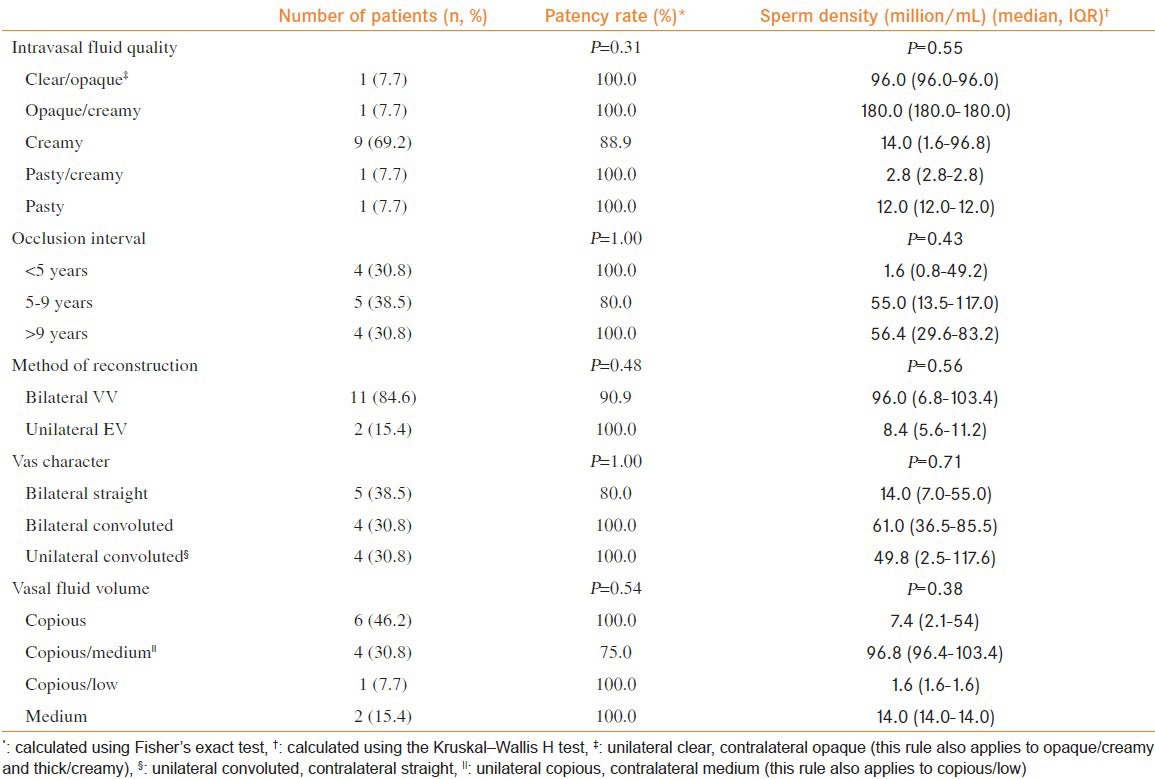
Thirteen patients followed-up after a median duration of 4.6 months (IQR 3.3-12.7), with post-operative semen analysis or documented pregnancy, with 12 (92.3%) achieving patency. Patients undergoing bilateral VV demonstrated patency rates of 90.9%, while 100% of the cases of unilateral VV and contralateral EV were patent (n = 2). One man was azoospermic on post-operative semen analysis and underwent a successful microsurgical epididymal sperm aspiration. The median sperm density for the nine patients demonstrating post-operative patency was 55.0 million sperm/mL (IQR 9.7-100.1). Three additional patients were patent post-operatively, as demonstrated by pregnancy, but had unavailable follow-up sperm density data at the time of analysis. Patency rates remained high in the setting of poor fluid quality (90.9%), including patients who had documented creamy or pasty fluid. Post-operative sperm density, among all the groups, was lowest when VR was performed in the setting of pasty fluid bilaterally or pasty/creamy fluid.
Of those patients with at least one vas deferens demonstrating copious vasal fluid volume, the patency rates were 90.9%. Patients undergoing VR after a longer occlusive interval (>9 years) retained high patency rates (100%) and median post-operative sperm density (56.4 million/mL, IQR 29.6-83.2). Overall, gross intravasal fluid quality, occlusion interval, method of vasal reconstruction, vasal character and vasal fluid volume were not significantly associated with patency rates nor post-operative sperm density (P > 0.05; Table 2).
DISCUSSION
The finding of only SHST during VR creates a difficult decision for the surgeon regarding the best choice of vasal reconstruction, i.e. VV or EV. Patency rates in the Vasovasostomy Study Group decreased from greater than 90% to 75% when only sperm heads were noted.[8] Similarly, Sigman examined the relationship between intravasal sperm quality and patency rates after VV, finding an overall patency rate of 98%.[1] Patency rates were 95% for the subgroups with sperm heads alone and 100% for sperm with short tails. The post-operative sperm density for these groups was 40 million/mL and 44 million/mL, respectively. Gross fluid quality was similar between each of the groups examined, but, because it was not specified in the reported data, it was unavailable for comparison in the context of this study.[1]
The Sigman series was limited in the number of patients with sperm fragments; however, the presence of only SHST did not appear to adversely affect patency rates after VV.[1] In comparison with our series of 14 men, we found an overall patency rate of 90.9% in patients undergoing VV with intravasal fluid demonstrating SHST. Post-operative sperm density was higher in the current study, demonstrating a median sperm concentration of 55.0 million sperm/mL. Pregnancy was noted in three men; however, follow-up data regarding conception was not available for all patients and follow-up was of a limited duration (median 4.6 months, IQR 3.3-12.7).
In 2006, Kolettis examined the outcomes for VV in the presence of sperm fragments within the vasal fluid.[9] Patients with SHST in the vasal fluid bilaterally or SHST unilaterally and intravasal azoospermia contralaterally were included in the analysis. The patency rate for patients with SHST bilaterally was 77% and the pregnancy rate was 35%.[9] These rates are less than that typically seen with VV and closer to that of EV.[8,9] The authors concluded that VV should be performed when sperm fragments are seen in the intravasal fluid, in line with the Vasovasostomy Study Group; however, Kolettis suggested that EV should be considered when only an occasional sperm head is identified. Of note, the authors were unable to find an obstructive interval threshold in which EV would be indicated in patients found to have SHST within the vasal fluid.[8,9] This is in agreement with our series and that of Sigman, which demonstrated high patency rates when SHST are present bilaterally.[1] In the current study, our patency rates were higher than those seen within the Kolettis series and the Vasovasostomy Study Group; however, we caution that this may be a reflection of the smaller sample size in the current series.
Our study addresses some limitations of the Kolettis and Sigman studies by capturing gross fluid quality and the location of the vasal anastomoses. These data were recorded in our cohort, and the patency rates remained high even in the presence of poor fluid quality (creamy and pasty) and longer occlusive intervals. It is noteworthy that post-operative sperm density was maintained in patients with bilateral creamy fluid (median 14.0 million/mL, IQR 1.6-96.8), which is generally accepted to be a poor prognostic indicator. We did note a trend toward diminishing post-operative sperm concentrations as one progresses toward poorer fluid quality, with the lowest results in those with unilateral or bilateral pasty fluid; however, conclusions are limited by the number of patients demonstrating pasty fluid. Our study contrasts with those previously reporting an association of creamy and pasty fluid with poorer outcomes as those patients with creamy fluid in our cohort demonstrated high patency and post-operative sperm concentrations.[8] Despite these trends, gross fluid quality did not correlate with patency (P = 0.31) or post-operative sperm density (P = 0.55) in our cohort.
This study does confirm the previous finding by Sandlow et al. that location of the anastomosis within the convoluted vas deferens does not appear to negatively impact outcomes.[16] In contrast, vasal fluid volume was not a significant predictor of patency (P = 0.54) or post-operative sperm density (P = 0.38).
Patency and pregnancy rates for VV may depend on multiple factors beyond intravasal fluid quality and microscopic assessment for sperm. These may include surgeon experience, age of the female partner, whether the female partner is the same before and after VR and obstructive interval.[7,8,17,18,19] As aforementioned, in the current study, patency rates and post-operative semen concentration remained high despite an occlusive interval of greater than 9 years. The significance of this finding, however, is limited by the size of this subgroup of men in our cohort. Similarly, pregnancy was noted in three men in the current series; however, data regarding post-operative pregnancy was not available for all patients and duration of follow-up was limited (median 4.6 months). All patients in this series were married at the time of VR; however, information regarding female partner age and whether the same partner was maintained before and after VR was not captured.
The macroscopic assessment of vasal fluid quality relies heavily upon subjective interpretation, which may limit its utility as a predictive intraoperative criterion. In addition, as a small, single-surgeon, retrospective case series, we are limited in applying these findings to a larger group of patients. Furthermore, in this data series, we do not delineate between sperm heads alone and sperm heads with short tails. Similarly, we do not routinely distinguish quantitatively between occasional SHST and more abundant specimens. This could potentially impact the generalizability of these results should there be unforeseen discrepant outcomes between these subcategories of sperm fragments. Although this represents a small series, which lacks a control group, our study suggests that VV is indicated when SHST are seen, regardless of the occlusive interval and fluid quality.
CONCLUSIONS
This study suggests that VV is the preferred method of reconstruction during VR when SHST are present within the intravasal fluid. The high patency rates in this cohort exceed the expected patency of EV, despite poor fluid quality and longer occlusive intervals. Our study adds further credence to the growing body of literature suggesting that VV is preferred in this subpopulation of men undergoing VR. Urologic microsurgeons may be reassured about performing VV in the setting of SHST irrespective of fluid quality and occlusive interval.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sigman M. The relationship between intravasal sperm quality and patency rates after vasovasostomy. J Urol. 2004;171:307–9. doi: 10.1097/01.ju.0000102322.90257.8b. [DOI] [PubMed] [Google Scholar]
- 2.O’Conor VJ. Anastomosis of vas deferens after purposeful division for sterility. J Am Med Assoc. 1948;136:162. doi: 10.1001/jama.1948.02890200016004. [DOI] [PubMed] [Google Scholar]
- 3.Potts JM, Pasqualotto FF, Nelson D, Thomas AJ, Jr, Agarwal A. Patient characteristics associated with vasectomy reversal. J Urol. 1999;161:1835–9. [PubMed] [Google Scholar]
- 4.Weiske WH. Vasectomy. Andrologia. 2001;33:125–34. doi: 10.1046/j.1439-0272.2001.00445.x. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz LI, Rumohr JA, Bennett RC. Techniques for vasectomy reversal. Urol Clin North Am. 2009;36:375–82. doi: 10.1016/j.ucl.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: The microdot technique of precision suture placement. J Urol. 1998;159:188–90. doi: 10.1016/s0022-5347(01)64053-9. [DOI] [PubMed] [Google Scholar]
- 7.Kolettis PN, D’Amico AM, Box L, Burns JR. Outcomes for vasovasostomy with bilateral intravasal azoospermia. J Androl. 2003;24:22–4. [PubMed] [Google Scholar]
- 8.Belker AM, Thomas AJ, Jr, Fuchs EF, Konnak JW, Sharlip ID. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol. 1991;145:505–11. doi: 10.1016/s0022-5347(17)38381-7. [DOI] [PubMed] [Google Scholar]
- 9.Kolettis PN, Burns JR, Nangia AK, Sandlow JI. Outcomes for vasovasostomy performed when only sperm parts are present in the vasal fluid. J Androl. 2006;27:565–7. doi: 10.2164/jandrol.05190. [DOI] [PubMed] [Google Scholar]
- 10.Fenig DM, Kattan MW, Mills JN, Gisbert M, Yu C, Lipshultz LI. Nomogram to preoperatively predict the probability of requiring epididymovasostomy during vasectomy reversal. J Urol. 2012;187:215–8. doi: 10.1016/j.juro.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Practice Committee of American Society for Reproductive M: Vasectomy reversal. Fertil Steril. 2008;90:S78–82. doi: 10.1016/j.fertnstert.2008.08.097. [DOI] [PubMed] [Google Scholar]
- 12.Crain DS, Roberts JL, Amling CL. Practice patterns in vasectomy reversal surgery: Results of a questionnaire study among practicing urologists. J Urol. 2004;171:311–5. doi: 10.1097/01.ju.0000100801.40282.b0. [DOI] [PubMed] [Google Scholar]
- 13.Chawla A, O’Brien J, Lisi M, Zini A, Jarvi K. Should all urologists performing vasectomy reversals be able to perform vasoepididymostomies if required? J Urol. 2004;172:1048–50. doi: 10.1097/01.ju.0000135118.43383.b1. [DOI] [PubMed] [Google Scholar]
- 14.Silber SJ. Pregnancy after vasovasostomy for vasectomy reversal: A study of factors affecting long-term return of fertility in 282 patients followed for 10 years. Hum Reprod. 1989;4:318–22. doi: 10.1093/oxfordjournals.humrep.a136896. [DOI] [PubMed] [Google Scholar]
- 15.Belker AM, Konnak JW, Sharlip ID, Thomas AJ., Jr Intraoperative observations during vasovasostomy in 334 patients. J Urol. 1983;129:524–7. doi: 10.1016/s0022-5347(17)52215-6. [DOI] [PubMed] [Google Scholar]
- 16.Sandlow JI, Kolettis PN. Vasovasostomy in the convoluted vas deferens: Indications and outcomes. J Urol. 2005;173:540–2. doi: 10.1097/01.ju.0000149981.89230.50. [DOI] [PubMed] [Google Scholar]
- 17.Kolettis PN, Woo L, Sandlow JI. Outcomes of vasectomy reversal performed for men with the same female partners. Urology. 2003;61:1221–3. doi: 10.1016/s0090-4295(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 18.Kolettis PN, Sabanegh ES, Nalesnik JG, D’Amico AM, Box LC, Burns JR. Pregnancy outcomes after vasectomy reversal for female partners 35 years old or older. J Urol. 2003;169:2250–2. doi: 10.1097/01.ju.0000063780.74931.d6. [DOI] [PubMed] [Google Scholar]
- 19.Kolettis PN, Sabanegh ES, D’Amico AM, Box L, Sebesta M, Burns JR. Outcomes for vasectomy reversal performed after obstructive intervals of at least 10 years. Urol. 2002;60:885–8. doi: 10.1016/s0090-4295(02)01888-5. [DOI] [PubMed] [Google Scholar]


