Abstract
Benign prostatic hyperplasia (BPH) is a histological diagnosis associated with unregulated proliferation of connective tissue, smooth muscle and glandular epithelium. BPH may compress the urethra and result in anatomic bladder outlet obstruction (BOO); BOO may present as lower urinary tract symptoms (LUTS), infections, retention and other adverse events. BPH and BOO have a significant impact on the health of older men and health-care costs. As the world population ages, the incidence and prevalence of BPH and LUTS have increased rapidly. Although non-modifiable risk factors – including age, genetics and geography – play significant roles in the etiology of BPH and BOO, recent data have revealed modifiable risk factors that present new opportunities for treatment and prevention, including sex steroid hormones, the metabolic syndrome and cardiovascular disease, obesity, diabetes, diet, physical activity and inflammation. We review the natural history, definitions and key risk factors of BPH and BOO in epidemiological studies.
Keywords: Etiology, benign prostatic hyperplasia, bladder outflow obstruction, epidemiology, genetics, public health
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a histological diagnosis associated with unregulated proliferation of connective tissue, smooth muscle and glandular epithelium within the prostatic transition zone.[1] Prostate tissue is composed of two basic elements: A glandular element composed of secretory ducts and acini; and a stromal element composed primarily of collagen and smooth muscle. In BPH, cellular proliferation leads to increased prostate volume and increased stromal smooth muscle tone. McNeal describes two phases of BPH progression. The first phase consists of an increase in BPH nodules in the periurethral zone and the second a significant increase in size of glandular nodules.[2]
BPH may cause physical compression of the urethra and result in anatomic bladder outlet obstruction (BOO) through two distinct mechanisms: First, an increase in prostate volume, termed the static component; second, an increase in stromal smooth muscle tone, termed the dynamic component.[3] BOO, in turn, may present clinically as lower urinary tract symptoms (LUTS), urinary tract infections, acute urinary retention (AUR), renal failure hematuria, and bladder calculi.[4]
Notably, two factors complicate the natural history and clinical presentation of BPH, BOO and LUTS; first, prostate volume does not linearly correlate with the severity of BOO or LUTS; and second, progressive BPH and BOO can lead to primary bladder dysfunction, which in turn can exacerbate the severity of LUTS independently of BOO.[3] Collectively, BPH, BOO and LUTS are associated with increased risks of mortality, depression, falls and diminished health-related quality-of-life as well as with billions of US dollars in annual health expenditures.[5,6]
In the last decade, epidemiological models of BPH and BOO have evolved substantially. Although age and genetics play important roles in the etiology of BPH and BOO, recent data have revealed novel, modifiable risk factors that present new opportunities for treatment and prevention. These risk factors appear to potentially influence the natural history of BPH and BOO throughout the different stages of clinical progression [Figure 1]. Herein, we review current concepts in the epidemiology and etiology of BPH and BOO.
Figure 1.
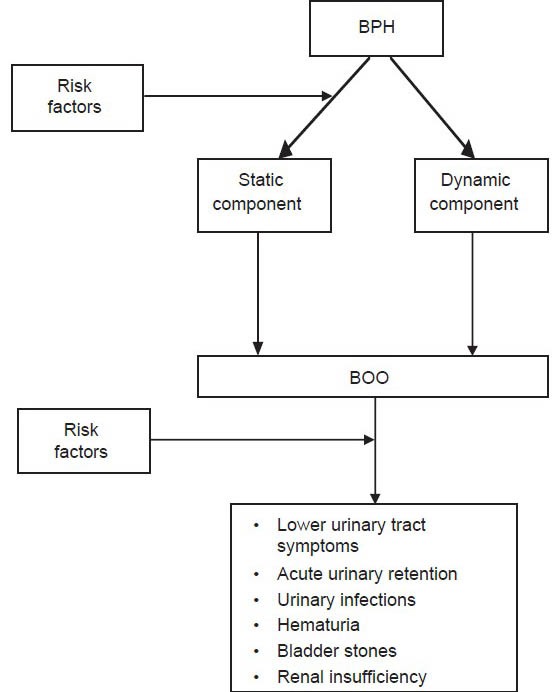
Natural history of benign prostatic hyperplasia and bladder outlet obstruction
DEFINITIONS OF BPH AND BOO IN EPIDEMIOLOGICAL STUDIES
A persistent challenge in the interpretation of data from population based studies of BPH and BOO is the heterogeneity of the case definitions in the literature. Investigators have utilized many different definitions for BPH, including histological analysis of prostate tissue, radiologic benign prostate enlargement (BPE), decreased urinary flow rates, urodynamic studies consistent with BOO, need for BPH surgery, AUR, and physician-diagnosed BPH and LUTS.
LUTS describes a distinct phenotype of a group of disorders affecting the prostate and bladder that share a common clinical manifestation. In recent years, LUTS has become the preferred term for studying urinary symptoms in male populations[5,7] because it allows for a broad, epidemiological description of urinary symptoms without identification of organ- or disease- specific etiologies. The most commonly used measures of LUTS in epidemiologic studies are the American Urological Association Symptom Index (the AUA-SI) and its internationally validated counterpart, the International Prostate Symptom Score (I-PSS). The AUA-SI and I-PSS are robust and reliable metrics for measuring male LUTS. The AUA, European Association of Urology and the World Health Organization International Consultation on Urologic Disease recommend the routine use of the I-PSS in the clinical evaluation of patients with suspected BPH and BOO.[3,8]
The terms BPH, BOO and LUTS remain interconnected in the contemporary treatment and study of urinary disorders in older men. However, prior epidemiological studies have not consistently utilized the term “BOO.” Instead, the two terms routinely used in the literature to describe the clinical manifestations of BPH – i.e., the adverse clinical effects of BOO – are “BPH” and “LUTS.” Therefore, the remainder of this review will focus primarily on epidemiological risk factors associated with the etiologies of BPH and male LUTS.
PUBLIC HEALTH EFFECTS OF BPH AND BOO: ADVERSE EVENTS AND COSTS
BPH and BOO have substantial adverse effects on the public health. Despite widespread use of medical therapy, BPH remains, on a population level, associated with a substantial incidence of BOO-associated adverse events, including LUTS, urinary infections, bladder calculi, urinary retention and acute renal failure. In a study of 3.7 million US men presenting to emergency rooms in the state of California, for example, the incidence of urinary retention increased 25% from 2007 to 2010.[4,9] Another important public health issue is the costs associated with diagnosis and treatment. In 2000, BPH accounted for $1.1 billion dollars in direct health-care expenditures, 4.4 million office visits, 117,000 emergency room visits, 105,000 hospitalizations and 21-38 million h in lost productivity in the US estimated annual costs of BPH treatment in the US total at least $3.9 billion dollars.[10,11]
RISK FACTORS FOR BPH AND LUTS
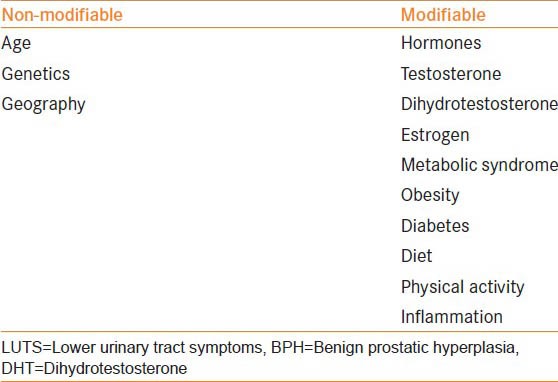
On a population level, there are 2 broad categories of risk factors associated with BPH and LUTS: Non-modifiable (age, geography and genetics) and modifiable (sex steroid hormones, the metabolic syndrome, obesity, diabetes, physical activity, diet, and inflammation) [Table 1].
Table 1.
Risk factors for BPH and male LUTS
Age
The prevalence of BPH rises markedly with age. Autopsy studies have observed a histological prevalence of 8%, 50% and 80% in the 4th, 6th and 9th decades of life, respectively.[12] Multiple observational studies from Europe, the US and Asia have demonstrated older age to be a risk factor for BPH onset and clinical progression by several different metrics.[5,10,13,14,15,16,17,18]
Prostate volume also increases with age, with data from the Krimpen and Baltimore Longitudinal Study of Aging (BLSA) cohorts suggesting a prostate growth rate of 2.0% to 2.5% per year in older men.[19,20,21] Although prostate volume does not directly correlate with symptom severity, prostate growth is a risk factor for LUTS progression and larger volume prostates are associated with increased risks of BPH clinical progression, urinary retention and need for prostate surgery.[22,23]
LUTS incidence also increased among older men. In the osteoporotic fractures in men study cohort, a prospective study of 6000 community dwelling men over the age of 65 years in the US, 29% of those without LUTS at baseline developed clinically significant LUTS within 2 years of follow-up; among those ≥ 80 years, this proportion increased to 34%.[24] In the US Olmsted County cohort, 14% of men without LUTS at baseline subsequently reported moderate or severe symptoms within 18 months of follow-up and 22% reported moderate or severe symptoms within 42 months of follow-up.[25] Similarly, 21% of Japanese, 26% of black American and 20% of Austrian men with no or mild LUTS at baseline reported worsened symptoms after 3, 4 and 5 years of follow-up, respectively.[26,27,28] A recent study by Platz et al., followed 9628 men for progression of LUTS over 18 years based on I-PSS and observed that the incidence and progression rates of LUTS increased steeply as the men aged, with progression rates being higher than incidence rates.[29]
Both prevalence and incidence of BPH and LUTS in the US increased steadily between 1994 and 2000.[30] Between 1998 and 2007, the age-adjusted prevalence of BPH among hospitalized patients in the US nearly doubled.[4] Notably, increases in BPH and LUTS prevalence and incidence are occurring within the context of an aging global population. By 2030, for example, 20% of the US population will be 65 years or older, including over 20 million men. Significantly, the fastest growing segment of the elderly population is the oldest age group: Those over age 85 years. Current estimates are that the number of individuals 80 years and older in the US will rise from 9.3 million in 2000 to 19.5 million in 2030, an increase of over 100%.[31]
Geography
International studies have also demonstrated geographic heterogeneity in prostate volume and LUTS prevalence. Significantly lower prostate volumes have been observed in men from Southeast Asia compared to western populations.[32] However, smaller volume did not always correlate with a decreased prevalence of LUTS: Ganpule, et al., demonstrated lower prostate volume, but higher mean IPSS values in a population of 2406 Indian men compared to men in western regions.[33]
Genetics
Evidence suggests that there are genetic components to both BPH and LUTS. One case control analysis, in which cases were men less than 64 years of age who underwent surgery for BPH, noted 4-fold and 6-fold increase in the age-specific risks of BPH surgery among all male relatives and brothers, respectively, of cases. These investigators further estimated that 50% of men undergoing surgery for BPH less than 60 years of age had an inheritable form of the disease.[34] These findings and those of others have suggested an autosomal dominant pattern of inheritance.[35] Men with inherited forms of BPH tend to have a larger volume prostates and earlier age of onset of clinical symptoms then men with sporadic BPH.[36]
Monozygotic twin concordance rates of 63% and 26% have been observed for LUTS and BPH, respectively, with one study estimating that genetic factors may contribute as much as 72% to the risk of high-moderate or severe LUTS among older men.[37,38]
Gene polymorphisms have also been implicated in the development of BPH. In a study of 160 North Indian men with LUTS, deletions of Glutathone S-transferase enzyme genes, thought to confer cellular resistance to oxidative stress, were significantly associated with an increased risk of symptomatic BPH.[39] Another North Indian study demonstrated a 2-fold risk of histologic BPH in men with CAG repeats in the androgen receptor gene and a 16-fold risk in the presence of the prostate specific antigen (PSA) G-158A single nucleotide polymorphism.[40]
Sex steroid hormones: Testosterone, dihydrotestosterone and estrogen
In prostatic secretory cells, the hormone 5-alpha reductase converts testosterone to DHT, a potent stimulator of prostate growth that, in addition to being necessary for prostate development, appears to play a central role in BPH pathogenesis. Multiple studies have explored associations of endogenous sex steroid hormones – namely testosterone, DHT and estrogen – with BPH and LUTS.
At least 7 observational studies have reported no associations and 5 inverse associations of serum testosterone (total, bioavailable, or free) with BPH or LUTS.[41,42,43] No studies to date have reported an increased risk of BPH or LUTS with higher serum testosterone levels. Furthermore, data from a subset of men in the Proscar long-term efficacy and Safety trial demonstrate low testosterone (<300 ng/dl) in 21.7% of aging men with BPH.[44] A salient, but theoretical, concern of testosterone replacement therapy is the potential for it to exacerbate BPH, BOO and LUTS.[45] These observations, however, imply that higher serum testosterone concentrations do not promote BPH and even are potentially protective.
Several studies have noted an increased risk of BPH with increased serum concentrations of DHT and its metabolites. In one prospective study of community men, those with the highest midlife levels of DHT had nearly 3 times the risk of subsequent BPH compared with those with the lowest levels.[42] These results are consistent with 3 prior studies of serum concentrations of two DHT metabolites: 17b-diol-glucuronide and androstanediol glucuronide. These metabolites are surrogate markers for DHT activity, with higher concentrations indicating increased and lower concentrations decreased levels of DHT. Two cross-sectional and one prospective study have shown direct associations of these DHT metabolites with BPH or LUTS.[42,43]
Five-alpha reductase inhibitors (finasteride and dutasteride) decrease serum concentrations of DHT[46] and prevent clinical progression of BPH and LUTS.[47] In addition, a recent analysis of the Prostate Cancer Prevention Trial observed that finasteride reduced the risk of incident symptomatic BPH and LUTS – the first study to suggest that BOO onset may be prevented in asymptomatic men.[48]
In contrast to DHT, no clear patterns of estrogen, BPH and LUTS have as yet emerged. Prior studies have reported positive, negative and null associations of endogenous estrogens with BPH and LUTS.[42] However, one study has observed increased efficacy for reducing stromal cell proliferation in human BPH through the use of selective estrogen receptor modulators in combination with five-alpha reductase inhibitors.[49]
The metabolic syndrome and cardiovascular disease
A notable and relatively recent development in the epidemiology of BPH and BOO is the recognition that modifiable life-style factors influence the natural history of these conditions. Accumulating data suggest that many of the same metabolic disturbances associated with cardiovascular disease – and the life-style factors that alter these disturbances – influence the risk of BPH and LUTS. These observations are important because they suggest novel targets for prevention and treatment.
The metabolic syndrome is a collection of metabolic abnormalities – obesity, glucose intolerance, dyslipidemia and hypertension – that increases the risk of cardiovascular disease and results primarily from dietary and other life-style practices endemic to Westernized societies.[50]
Despite heterogeneity in definitions and diagnosis, accumulating evidence suggests associations of metabolic syndrome with increased risks of BPH and LUTS.[51] In one cohort, men diagnosed with at least three components of the metabolic syndrome had an 80% increased prevalence of LUTS compared with those with no components. Other studies have shown that men with heart disease are at significantly increased risk of both BPH and LUTS.[52,53]
Obesity
Prior studies have consistently observed that increased adiposity is positively associated with prostate volume: The greater the amount of adiposity, the greater the prostate volume. Body weight, body mass index (BMI) and waist circumference have all been positively associated with prostate volume in multiple different study populations.[54] In the BLSA cohort, for example, each 1 kg/m2 increase in BMI corresponded to a 0.41 cc increase in prostate volume. Moreover, obese (BMI ≥ 35 kg/m2) participants had a 3.5-fold increased risk of prostate enlargement compared with non-obese (BMI <25 kg/m2) participants.[55]
A preponderance of evidence also demonstrates that obesity increases the risks of BPH surgery, initiation of BPH medical therapy and LUTS[54,55,56] and decreases the efficacy of finasteride[48] and dutasteride[57] for the treatment of BOO.
Diabetes and disruptions in glucose homeostasis
Disruptions in glucose homeostasis at multiple different levels – from alterations in serum insulin growth factor (IGF) concentrations to diagnosis of clinical diabetes – are associated with higher likelihoods of BPH, BPE and LUTS. Higher serum concentrations of IGF-1 and insulin-like growth factor binding protein 3 have been associated with increased risk of clinical BPH and BPH surgery.[58] Physician-diagnosed diabetes, increased serum insulin and elevated fasting plasma glucose have been associated with increased prostate size and increased risks of prostate enlargement, clinical BPH, BPH surgery and LUTS in multiple different cohorts cumulatively incorporating tens of thousands of men.[54,58,59,60] Recent findings from Olmsted County demonstrate that diabetic men on medical therapy had decreased odds of moderate/severe LUTS compared with those men not on medications.[61]
Physical activity
Increased physical activity and exercise have been robustly and consistently linked with decreased risks of BPH surgery, clinical BPH, histological BPH and LUTS.[54,56] A meta-analysis of 11 published studies (n = 43,083 men) indicated that moderate to vigorous physical activity reduced the risk of BPH or LUTS by as much as 25% relative to a sedentary life-style, with the magnitude of the protective effect increasing with higher levels of activity.[62]
Diet
There are some indications that both macronutrients and micronutrients may affect the risk of BPH and LUTS, although the patterns are inconsistent. For macronutrients, increased total energy intake, energy-adjusted total protein intake, red meat, fat, milk and dairy products, cereals, bread, poultry and starch potentially increase the risks of symptomatic BPH and BPH surgery; vegetables (particularly carotenoids), fruits, polyunsaturated fatty acids, linoleic acid, Vitamin A and Vitamin D potentially decrease the risks of symptomatic BPH and LUTS.[55,63,64] For micronutrients, higher circulating concentrations of vitamin E, lycopene, selenium and carotene have been inversely associated with BPH and LUTS;[55,63,65] zinc and vitamin C have been associated with both increased and decreased risk.[63,64,65,66]
Finally, with respect to alcohol consumption, a meta-analysis of 19 studies observed that alcohol intake was associated with decreased and increased risks, respectively, of BPH and LUTS.[67]
Inflammation
A majority of observational studies suggests that inflammation is linked to the development of BPH and LUTS. The mechanisms underlying this relationship are unclear. One potential explanation is that the metabolic syndrome, which promotes systemic inflammation and oxidative stress, mediates the connection. Inflammation has been implicated as a primary stimulus for prostate carcinogenesis and it is possible that BPH represents a non-malignant proliferative pathway promoted by oxidative stress and inflammatory mediators.[51,68]
There are strong links between BPH and histological inflammation in surgical specimens, with the extent and severity of the inflammation corresponding to the magnitude of prostate enlargement and BPH area.[69,70] Data from the REDUCE trial suggest that more severe inflammation detected in prostate biopsy core specimens is correlated with higher IPSS scores.[71] Men with LUTS are more likely to have higher serum C-reactive protein, a marker of systemic inflammation[72] while prior gonorrheal infection or prostatitis increase the likelihoods of BPH surgery and LUTS.[73] A history of infection with gonorrhea, chlamydia or trichomonosis increases the risk of elevated PSA;[74] high serum IgG antibody titers to cytomegalovirus, herpes virus, human papilloma virus and hepatitis are associated with LUTS.[75]
Conversely, inhibition of inflammatory pathways potentially attenuates BPH risk. In one community cohort, men who reported daily non-steroidal anti-inflammatory (NSAID) use experienced significantly decreased risks of LUTS, low urinary flow rate, increased prostate volume and elevated PSA.[76]
Other risk factors
Other modifiable risk factors for which clear patterns of risk have not yet emerged include hypertension, serum lipids and lipoproteins and smoking.[77]
CONCLUSION
In summary, BPH and BOO are of significant importance to public health, affecting tens of millions of older men globally. Current disease trends in the US, Europe and other regions suggest that the incidence and prevalence of these conditions will increase in the near future due to aging of the world population and the increased prevalence of the metabolic syndrome and its components, thereby placing even greater burdens on finite resources. While age and genetic factors play a role in the development of BPH and BOO, many modifiable variables contribute as well - factors that potentially may be altered in order to delay onset, prevent progression or diminish symptoms. Potential strategies include inhibition of DHT synthesis with five-alpha reductase inhibitors, modulation of metabolic risk factors with comprehensive life-style interventions incorporating diet change and physical activity and suppression of inflammatory pathways with NSAIDs.
Footnotes
Source of Support: Nil
Conflict of Interest: Watson Pharmaceuticals, consultant (JKP); AMS, lecturer (JKP).
REFERENCES
- 1.Auffenberg GB, Helfand BT, McVary KT. Established medical therapy for benign prostatic hyperplasia. Urol Clin North Am. 2009;36:443–59. doi: 10.1016/j.ucl.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990;17:477–86. [PubMed] [Google Scholar]
- 3.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 4.Stroup SP, Palazzi-Churas K, Kopp RP, Parsons JK. Trends in adverse events of benign prostatic hyperplasia (BPH) in the USA, 1998 to 2008. BJU Int. 2012;109:84–7. doi: 10.1111/j.1464-410X.2011.10250.x. [DOI] [PubMed] [Google Scholar]
- 5.Taylor BC, Wilt TJ, Fink HA, Lambert LC, Marshall LM, Hoffman AR, et al. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: The MrOS study. Urology. 2006;68:804–9. doi: 10.1016/j.urology.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Parsons JK, Mougey J, Lambert L, Wilt TJ, Fink HA, Garzotto M, et al. Lower urinary tract symptoms increase the risk of falls in older men. BJU Int. 2009;104:63–8. doi: 10.1111/j.1464-410X.2008.08317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons JK, Bergstrom J, Silberstein J, Barrett-Connor E. Prevalence and characteristics of lower urinary tract symptoms in men aged>;or=80 years. Urology. 2008;72:318–21. doi: 10.1016/j.urology.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Rosette JJ, Alivizatos G, Madersbacher S, Perachino M, Thomas D, Desgrandchamps F, et al. EAU Guidelines on benign prostatic hyperplasia (BPH) Eur Urol. 2001;40:256–63. doi: 10.1159/000049784. [DOI] [PubMed] [Google Scholar]
- 9.Groves HK, Chang D, Palazzi K, Cohen S, Parsons JK. The incidence of acute urinary retention secondary to BPH is increasing among California men. Prostate Cancer Prostatic Dis. 2013;16:260–5. doi: 10.1038/pcan.2013.11. [DOI] [PubMed] [Google Scholar]
- 10.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: Benign prostatic hyperplasia. J Urol. 2005;173:1256–61. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 11.Hu TW, Wagner TH, Bentkover JD, LeBlanc K, Piancentini A, Stewart WF, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123–8. doi: 10.1016/s0090-4295(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 12.Barry MJ, Fowler FJ, Jr, Bin L, Pitts JC, 3rd, Harris CJ, Mulley AG., Jr The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. J Urol. 1997;157:10–4. [PubMed] [Google Scholar]
- 13.Kok ET, Schouten BW, Bohnen AM, Groeneveld FP, Thomas S, Bosch JL. Risk factors for lower urinary tract symptoms suggestive of benign prostatic hyperplasia in a community based population of healthy aging men: The Krimpen study. J Urol. 2009;181:710–6. doi: 10.1016/j.juro.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, et al. Natural history of prostatism: Risk factors for acute urinary retention. J Urol. 1997;158:481–7. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 15.Bosch JL, Hop WC, Kirkels WJ, Schröder FH. Natural history of benign prostatic hyperplasia: Appropriate case definition and estimation of its prevalence in the community. Urology. 1995;46:34–40. doi: 10.1016/s0090-4295(99)80248-9. [DOI] [PubMed] [Google Scholar]
- 16.Fong YK, Milani S, Djavan B. Natural history and clinical predictors of clinical progression in benign prostatic hyperplasia. Curr Opin Urol. 2005;15:35–8. doi: 10.1097/00042307-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Guess HA, Arrighi HM, Metter EJ, Fozard JL. Cumulative prevalence of prostatism matches the autopsy prevalence of benign prostatic hyperplasia. Prostate. 1990;17:241–6. doi: 10.1002/pros.2990170308. [DOI] [PubMed] [Google Scholar]
- 18.Tantiwong A, Nuanyong C, Vanprapar N, Swasdipala P, Chittapraphai S. Benign prostatic hyperplasia in elderly Thai men in an urban community: The prevalence, natural history and health related behavior. J Med Assoc Thai. 2002;85:356–60. [PubMed] [Google Scholar]
- 19.Bosch JL, Tilling K, Bohnen AM, Bangma CH, Donovan JL. Establishing normal reference ranges for prostate volume change with age in the population-based Krimpen-study: Prediction of future prostate volume in individual men. Prostate. 2007;67:1816–24. doi: 10.1002/pros.20663. [DOI] [PubMed] [Google Scholar]
- 20.Loeb S, Kettermann A, Carter HB, Ferrucci L, Metter EJ, Walsh PC. Prostate volume changes over time: Results from the Baltimore Longitudinal Study of Aging. J Urol. 2009;182:1458–62. doi: 10.1016/j.juro.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams AM, Simon I, Landis PK, Moser C, Christens-Barry W, Carter HB, et al. Prostatic growth rate determined from MRI data: Age-related longitudinal changes. J Androl. 1999;20:474–80. [PubMed] [Google Scholar]
- 22.Bosch JL, Bangma CH, Groeneveld FP, Bohnen AM. The long-term relationship between a real change in prostate volume and a significant change in lower urinary tract symptom severity in population-based men: The Krimpen study. Eur Urol. 2008;53:819–25. doi: 10.1016/j.eururo.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 23.Roehrborn CG. BPH progression: Concept and key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int. 2008;101(Suppl 3):17–21. doi: 10.1111/j.1464-410X.2008.07497.x. [DOI] [PubMed] [Google Scholar]
- 24.Parsons JK, Wilt TJ, Wang PY, Barrett-Connor E, Bauer DC, Marshall LM, et al. Progression of lower urinary tract symptoms in older men: A community based study. J Urol. 2010;183:1915–20. doi: 10.1016/j.juro.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: Longitudinal changes in voiding symptoms in community dwelling men. J Urol. 1996;155:595–600. doi: 10.1016/s0022-5347(01)66461-9. [DOI] [PubMed] [Google Scholar]
- 26.Masumori N, Tsukamoto T, Rhodes T, Girman CJ. Natural history of lower urinary tract symptoms in men – Result of a longitudinal community-based study in Japan. Urology. 2003;61:956–60. doi: 10.1016/s0090-4295(02)02594-3. [DOI] [PubMed] [Google Scholar]
- 27.Sarma AV, McLaughlin JC, Jacobsen SJ, Logie J, Dolin P, Dunn RL, et al. Longitudinal changes in lower urinary tract symptoms among a cohort of black American men: The Flint Men's Health Study. Urology. 2004;64:959–65. doi: 10.1016/j.urology.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Temml C, Brössner C, Schatzl G, Ponholzer A, Knoepp L, Madersbacher S, et al. The natural history of lower urinary tract symptoms over five years. Eur Urol. 2003;43:374–80. doi: 10.1016/s0302-2838(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 29.Platz EA, Joshu CE, Mondul AM, Peskoe SB, Willett WC, Giovannucci E. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. J Urol. 2012;188:496–501. doi: 10.1016/j.juro.2012.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: Benign prostatic hyperplasia. J Urol. 2008;179:S75–80. doi: 10.1016/j.juro.2008.03.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC). Trends in aging-United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52(6):101–4. 106. [PubMed] [Google Scholar]
- 32.Jin B, Turner L, Zhou Z, Zhou EL, Handelsman DJ. Ethnicity and migration as determinants of human prostate size. J Clin Endocrinol Metab. 1999;84:3613–9. doi: 10.1210/jcem.84.10.6041. [DOI] [PubMed] [Google Scholar]
- 33.Ganpule AP, Desai MR, Desai MM, Wani KD, Bapat SD. Natural history of lower urinary tract symptoms: Preliminary report from a community-based Indian study. BJU Int. 2004;94:332–4. doi: 10.1111/j.1464-410X.2004.04931.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanda MG, Beaty TH, Stutzman RE, Childs B, Walsh PC. Genetic susceptibility of benign prostatic hyperplasia. J Urol. 1994;152:115–9. doi: 10.1016/s0022-5347(17)32831-8. [DOI] [PubMed] [Google Scholar]
- 35.Pearson JD, Lei HH, Beaty TH, Wiley KE, Isaacs SD, Isaacs WB, et al. Familial aggregation of bothersome benign prostatic hyperplasia symptoms. Urology. 2003;61:781–5. doi: 10.1016/s0090-4295(02)02509-8. [DOI] [PubMed] [Google Scholar]
- 36.Sanda MG, Doehring CB, Binkowitz B, Beaty TH, Partin AW, Hale E, et al. Clinical and biological characteristics of familial benign prostatic hyperplasia. J Urol. 1997;157:876–9. [PubMed] [Google Scholar]
- 37.Rohrmann S, Fallin MD, Page WF, Reed T, Partin AW, Walsh PC, et al. Concordance rates and modifiable risk factors for lower urinary tract symptoms in twins. Epidemiology. 2006;17:419–27. doi: 10.1097/01.ede.0000219723.14476.28. [DOI] [PubMed] [Google Scholar]
- 38.Partin AW, Page WF, Lee BR, Sanda MG, Miller RN, Walsh PC. Concordance rates for benign prostatic disease among twins suggest hereditary influence. Urology. 1994;44:646–50. doi: 10.1016/s0090-4295(94)80197-5. [DOI] [PubMed] [Google Scholar]
- 39.Konwar R, Manchanda PK, Chaudhary P, Nayak VL, Singh V, Bid HK. Glutathione S-transferase (GST) gene variants and risk of benign prostatic hyperplasia: A report in a North Indian population. Asian Pac J Cancer Prev. 2010;11:1067–72. [PubMed] [Google Scholar]
- 40.Soni A, Bansal A, Mishra AK, Batra J, Singh LC, Chakraborty A, et al. Association of androgen receptor, prostate-specific antigen, and CYP19 gene polymorphisms with prostate carcinoma and benign prostatic hyperplasia in a north Indian population. Genet Test Mol Biomarkers. 2012;16:835–40. doi: 10.1089/gtmb.2011.0322. [DOI] [PubMed] [Google Scholar]
- 41.Trifiro MD, Parsons JK, Palazzi-Churas K, Bergstrom J, Lakin C, Barrett-Connor E. Serum sex hormones and the 20-year risk of lower urinary tract symptoms in community-dwelling older men. BJU Int. 2010;105:1554–9. doi: 10.1111/j.1464-410X.2009.09090.x. [DOI] [PubMed] [Google Scholar]
- 42.Parsons JK, Palazzi-Churas K, Bergstrom J, Barrett-Connor E. Prospective study of serum dihydrotestosterone and subsequent risk of benign prostatic hyperplasia in community dwelling men: The Rancho Bernardo Study. J Urol. 2010;184:1040–4. doi: 10.1016/j.juro.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 43.Kristal AR, Schenk JM, Song Y, Arnold KB, Neuhouser ML, Goodman PJ, et al. Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: Results from the prostate cancer prevention trial. Am J Epidemiol. 2008;168:1416–24. doi: 10.1093/aje/kwn272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan SA, O’Neill E, Lowe R, Hanson M, Meehan AG. Prevalence of low testosterone in aging men with benign prostatic hyperplasia: Data from the Proscar Long-term Efficacy and Safety Study (PLESS) Aging Male. 2013;16:48–51. doi: 10.3109/13685538.2013.773421. [DOI] [PubMed] [Google Scholar]
- 45.Bhasin S, Singh AB, Mac RP, Carter B, Lee MI, Cunningham GR. Managing the risks of prostate disease during testosterone replacement therapy in older men: Recommendations for a standardized monitoring plan. J Androl. 2003;24:299–311. doi: 10.1002/j.1939-4640.2003.tb02676.x. [DOI] [PubMed] [Google Scholar]
- 46.Amory JK, Wang C, Swerdloff RS, Anawalt BD, Matsumoto AM, Bremner WJ, et al. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab. 2007;92:1659–65. doi: 10.1210/jc.2006-2203. [DOI] [PubMed] [Google Scholar]
- 47.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 48.Parsons JK, Schenk JM, Arnold KB, Messer K, Till C, Thompson IM, et al. Finasteride reduces the risk of incident clinical benign prostatic hyperplasia. Eur Urol. 2012;62:234–41. doi: 10.1016/j.eururo.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar R, Verma V, Sarswat A, Maikhuri JP, Jain A, Jain RK, et al. Selective estrogen receptor modulators regulate stromal proliferation in human benign prostatic hyperplasia by multiple beneficial mechanisms - Action of two new agents. Invest New Drugs. 2012;30:582–93. doi: 10.1007/s10637-010-9620-2. [DOI] [PubMed] [Google Scholar]
- 50.Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003;108:1541–5. doi: 10.1161/01.CIR.0000088845.17586.EC. [DOI] [PubMed] [Google Scholar]
- 51.De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. Eur Urol. 2012;61:560–70. doi: 10.1016/j.eururo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Meigs JB, Mohr B, Barry MJ, Collins MM, McKinlay JB. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol. 2001;54:935–44. doi: 10.1016/s0895-4356(01)00351-1. [DOI] [PubMed] [Google Scholar]
- 53.Joseph MA, Harlow SD, Wei JT, Sarma AV, Dunn RL, Taylor JM, et al. Risk factors for lower urinary tract symptoms in a population-based sample of African-American men. Am J Epidemiol. 2003;157:906–14. doi: 10.1093/aje/kwg051. [DOI] [PubMed] [Google Scholar]
- 54.Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: Clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189:S102–6. doi: 10.1016/j.juro.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 55.Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: New approaches to old problems. J Urol. 2007;178:395–401. doi: 10.1016/j.juro.2007.03.103. [DOI] [PubMed] [Google Scholar]
- 56.Parsons JK, Messer K, White M, Barrett-Connor E, Bauer DC, Marshall LM, et al. Obesity increases and physical activity decreases lower urinary tract symptom risk in older men: The Osteoporotic Fractures in Men study. Eur Urol. 2011;60:1173–80. doi: 10.1016/j.eururo.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller RL, Gerber L, Moreira DM, Andriole G, Jr, Hamilton RJ, Fleshner N, et al. Obesity is associated with increased prostate growth and attenuated prostate volume reduction by dutasteride. Eur Urol. 2013;63:1115–21. doi: 10.1016/j.eururo.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 58.Sarma AV, Parsons JK, McVary K, Wei JT. Diabetes and benign prostatic hyperplasia/lower urinary tract symptoms – What do we know? J Urol. 2009;182:S32–7. doi: 10.1016/j.juro.2009.07.088. [DOI] [PubMed] [Google Scholar]
- 59.Parsons JK, Bergstrom J, Barrett-Connor E. Lipids, lipoproteins and the risk of benign prostatic hyperplasia in community-dwelling men. BJU Int. 2008;101:313–8. doi: 10.1111/j.1464-410X.2007.07332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta A, Gupta S, Pavuk M, Roehrborn CG. Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: A prospective cohort study of Air Force veterans. Urology. 2006;68:1198–205. doi: 10.1016/j.urology.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 61.Sarma AV, St Sauver JL, Hollingsworth JM, Jacobson DJ, McGree ME, Dunn RL, et al. Diabetes treatment and progression of benign prostatic hyperplasia in community-dwelling black and white men. Urology. 2012;79:102–8. doi: 10.1016/j.urology.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parsons JK, Kashefi C. Physical activity, benign prostatic hyperplasia, and lower urinary tract symptoms. Eur Urol. 2008;53:1228–35. doi: 10.1016/j.eururo.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 63.Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Goodman P, Penson DF, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: Results from the prostate cancer prevention trial. Am J Epidemiol. 2008;167:925–34. doi: 10.1093/aje/kwm389. [DOI] [PubMed] [Google Scholar]
- 64.Maserejian NN, Giovannucci EL, McVary KT, McKinlay JB. Dietary, but not supplemental, intakes of carotenoids and vitamin C are associated with decreased odds of lower urinary tract symptoms in men. J Nutr. 2011;141:267–73. doi: 10.3945/jn.110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tavani A, Longoni E, Bosetti C, Maso LD, Polesel J, Montella M, et al. Intake of selected micronutrients and the risk of surgically treated benign prostatic hyperplasia: A case-control study from Italy. Eur Urol. 2006;50:549–54. doi: 10.1016/j.eururo.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 66.Holton K, Parsons JK, Shannon J, Lapidus J, Shikany J, Bauer D, Marshall L, et al. San Diego, CA: Presented at the Annual Meeting of the American Urological Association; 2013. Higher dietary intakes of vitamin C and some carotenoids are associated with reduced progression of lower urinary tract symptoms in elderly men: The MrOS study. [Google Scholar]
- 67.Parsons JK, Im R. Alcohol consumption is associated with a decreased risk of benign prostatic hyperplasia. J Urol. 2009;182:1463–8. doi: 10.1016/j.juro.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 68.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976–81. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 70.Anim JT, Udo C, John B. Characterisation of inflammatory cells in benign prostatic hyperplasia. Acta Histochem. 1998;100:439–49. doi: 10.1016/S0065-1281(98)80040-8. [DOI] [PubMed] [Google Scholar]
- 71.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: Examination of baseline data from the REDUCE trial. Eur Urol. 2008;54:1379–84. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rohrmann S, De Marzo AM, Smit E, Giovannucci E, Platz EA. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey (NHANES III) Prostate. 2005;62:27–33. doi: 10.1002/pros.20110. [DOI] [PubMed] [Google Scholar]
- 73.Sutcliffe S, Giovannucci E, De Marzo AM, Willett WC, Platz EA. Sexually transmitted infections, prostatitis, ejaculation frequency, and the odds of lower urinary tract symptoms. Am J Epidemiol. 2005;162:898–906. doi: 10.1093/aje/kwi299. [DOI] [PubMed] [Google Scholar]
- 74.Sutcliffe S, Zenilman JM, Ghanem KG, Jadack RA, Sokoll LJ, Elliott DJ, et al. Sexually transmitted infections and prostatic inflammation/cell damage as measured by serum prostate specific antigen concentration. J Urol. 2006;175:1937–42. doi: 10.1016/S0022-5347(05)00892-X. [DOI] [PubMed] [Google Scholar]
- 75.Sutcliffe S, Rohrmann S, Giovannucci E, Nelson KE, De Marzo AM, Isaacs WB, et al. Viral infections and lower urinary tract symptoms in the third national health and nutrition examination survey. J Urol. 2007;178:2181–5. doi: 10.1016/j.juro.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 76.St Sauver JL, Jacobson DJ, McGree ME, Lieber MM, Jacobsen SJ. Protective association between nonsteroidal antiinflammatory drug use and measures of benign prostatic hyperplasia. Am J Epidemiol. 2006;164:760–8. doi: 10.1093/aje/kwj258. [DOI] [PubMed] [Google Scholar]
- 77.Parsons JK. Lifestyle factors, benign prostatic hyperplasia, and lower urinary tract symptoms. Curr Opin Urol. 2011;21:1–4. doi: 10.1097/MOU.0b013e32834100c9. [DOI] [PubMed] [Google Scholar]


