Abstract
The assessment of men with bladder outflow obstruction relies on an adequate history and examination. Uroflowmetry and post-void residue estimation are very revealing and may be sufficient in the majority of men. The prostate-specific antigen test may be used to select men who are at a high risk of progression. In specific situations, cystometry may be required. We discuss the use of cystometry and the newer less-invasive methods of assessment that have emerged over the last few years, including ultrasound estimation of intravesical prostatic protrusion, prostatic urethra angle, detrusor wall thickness, ultrasound-estimated bladder weight, near-infrared spectroscopy and the condom catheter and penile cuff tests. Although these techniques show promise, they still require further modifications, standardization and testing in larger populations. In addition, they should be used in men where only specific questions need to be answered.
Keywords: Cystometry, detrusor wall thickness, intravesical prostatic protrusion, non-invasive cystometry, near-infrared spectroscopy, penile cuff test, uroflowmetry
HISTORY AND EXAMINATION
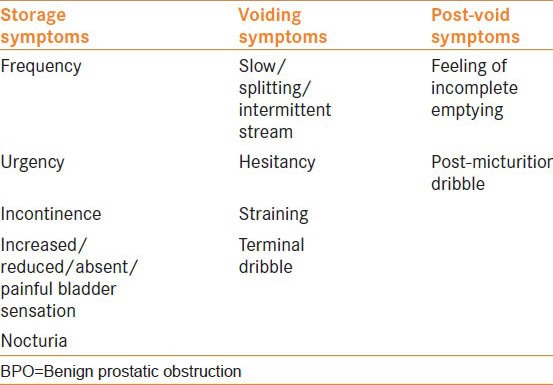
A detailed history and examination form the cornerstone of assessment in men with benign prostatic obstruction (BPO). The International Continence Society has made large strides in standardizing and categorizing lower urinary tract symptoms (LUTS).[1] The three categories that have been devised relate to phases of voiding and are described as storage, voiding and post-void [Table 1].
Table 1.
Lower urinary tract symptoms, common symptoms of BPO in red
Investigations
Numerous investigations may help in the diagnosis and management of BPO. They are not all necessary and a clinician needs to weigh the benefits of improved evidence against the cost, time and invasiveness of each test. All tests should have a formulated question of what needs answering and how the result will affect the management decision.
Blood tests
A serum creatinine test should be considered in men with suspected renal impairment, i.e., with a palpable bladder, nocturnal enuresis or history of stone disease. Prostate-specific antigen (PSA) has a useful role in the assessment of men with BPO by acting as a surrogate marker of benign enlargement. A PSA of 4-6 ng/mL is predictive of BOO in 65% of the cases, whereas a PSA of 6-10 ng/mL is 81% predictive.[2] The evidence suggests that men with a PSA > 1.4 ng/mL should be considered to be at an increased risk of symptom progression.[3,4]
Uroflowmetry
The Qmax in healthy males is 20.3 mL/s, which reduces with age.[5] In elderly men, a Qmax of 15 mL/s may be acceptable without noticeable symptoms. The uroflowmetry parameters have recently been studied for the first time in an Indian population in over 1000 men.[6] Nomograms according to age and voided volume are now available. The Qmax was shown to increase up to the age of 15 years, followed by a slow decline up to the age of 50 years. The mean Qmax in 16-50 year olds was found to be 22.5 + 9.2 mL/s.
The proportion of men with bladder outflow obstruction (BOO) with a Qmax < 10 mL/s has been reported at 90%, whereas 67% with a Qmax between 11 and 14 mL/s and 30% with a Qmax greater than 15 mL/s will have BOO.[7] This distinction is important as some men with low pressure low flow will not improve after prostatic surgery.
Cystometry
Cystometry is perhaps not essential in men with clear obstructive symptoms and a classical flow rate pattern. It is more likely to be useful in men with mixed lower urinary tract symptoms or chronic urinary retention where detrusor overactivity and underactivity are under question, respectively. Another use may be in men who have failed BOO surgery.
The assessment of detrusor contractility includes the PdetQmax, which allows the calculation of the bladder outlet obstruction index (BOOI). The BOOI = PdetQmax - (2 × Qmax).[8] If the BOOI is greater than 40 cmH2O, then the patient is considered obstructed. A BOOI value of 20-40 cmH2O is equivocal and a value less than 20 cmH2O suggests detrusor underactivity rather than obstruction.
Similarly, the voiding trace may be plotted on an International Continence Society ICS nomogram.[8] These are pre-marked graphs with the Qmax plotted on the x-axis and PdetQmax plotted on the y-axis. The patients’ void phase is plotted on the graph by the urodynamics machine, and the furthest point on the x-axis is equivalent to the BOOI. The graph contains a BOOI greater than 40 cmH2O cut-off for each Qmax value to signify obstruction, a BOOI less than 20 cmH2O for unobstructed and a BOOI of 20-40 cmH2O range for equivocal results.
Bladder contractility has also been defined by the ICS. The Bladder Contractility Index (BCI) is calculated as pdet Qmax + 5 Qmax. BCI greater than 150 cmH2O reveals good bladder contractility, 100-150 cmH2O is normal contractility and less than 100 cmH2O is reduced contractility.[8] Similarly, a bladder contractility nomogram may be used. The BOOI and BCI will be elevated in men with BPO and will be low in men with detrusor underactivity. Thus, these parameters will help differentiate between the two diagnoses.
Cystometry may also be combined with synchronous fluoroscopic imaging of the bladder outlet.[5] The urodynamicist looks at the bladder neck and urethra during a sustained detrusor contraction. This investigation may reveal the level of obstruction that guides the clinician to what may be causing the obstruction. This may be followed by cystoscopy or magnetic resonance imaging depending on the level and nature of the obstruction.
Non-invasive cystometry
Because of the invasiveness of cystometry, a number of non-invasive urodynamic investigations have been proposed. These provide either traditional cystometric measures in a non-invasive manner or they provide a surrogate marker of obstruction.
Intravesical prostatic protrusion (IPP) is measured via ultrasound.[9] If the length of the prostatic protrusion from its base is greater than 10 mm, then it has been shown that alpha blockers may be less effective at relieving symptoms;[10,11] conversely, surgery is likely to be more effective.[12] A recent study has reported an area under the receiver-operating characteristic (ROC) curve of 0.71 for predicting BOO symptoms.[13] Another study reported an area under the ROC curve of 0.84 compared with the BOOI.[14] Also, there is a higher probability of failing a trial without catheter in men with acute urinary retention if the IPP is >10 mm.[15] Progression, defined as worsening of the International Prostate Symptom Score (IPPS) by 4 points, or development of urinary retention, has been shown to occur with a higher frequency in men with an IPP > 10 mm.[16] From 259 men, followed for a mean of 32 months, 52 progressed; of these men who progressed, 44% had an IPP > 10 mm compared with 6% with an IPP < 5 mm.
The prostatic urethral angle occurs between the membranous and prostatic urethra. When correlated with the BOOI, the area under the ROC curve was 0.63 in one study.[17] An angle of > 35° was shown to correlate with BOO, but an increasing angle did not correlate with degree of BOO. These surrogate measures cannot replace cystometry but may be useful in helping clinicians decide which treatments to give to patients.
Bladder wall thickness is also measured by ultrasound. Detrusor wall thickness (DWT) has been shown to correlate better with BOO than bladder wall thickness.[18] Oelke et al. have prospectively compared anterior DWT > 2 mm with a 7.5-MHz ultrasound to standard urodynamics. The technique had a sensitivity of 83%, specificity of 95% and positive and negative predictive values of 94% and 86% respectively.[19] The area under the ROC curve for DWT was found to be 0.723 for successful Transurethral resection of prostate TURP in 239 men.[20] The limitations of this technique are that minimal changes of less than 2 mm need to be interpreted, the filling volume affects the readings and the region of measurement has not been standardized. Interobserver variability is reported in up to 12% and is affected by frequency of the ultrasound probe.[21] However, using a higher cut-off of > 2.9 mm has been reported to have a 100% sensitivity for obstruction and, therefore, this technique will allow for exclusion of men who do not then need to progress to cystometry.
More recently, ultrasound-estimated bladder weight (UEBW) has been proposed to increase accuracy over DWT as it also takes account of the bladder filling volume.[22] The area under the ROC curve for this technique is reported at 0.72 for success with TURP.[20] Men with an UEBW > 35 g were 13-times more likely to go into acute urinary retention.[23] These studies, however, have all been in Japanese men and, when performed in South Americans and Europeans, a significant correlation between UEBW and BOO has not been shown.[24,25] A limitation may be that UEBW varies according to height and weight and therefore these factors need to be considered.
Near-infrared spectroscopy (NIRS) measures the concentration of oxyhemoglobin and deoxyhemoglobin levels in the tissue. Increased work by the detrusor muscle (as occurs in BOO) leads to a reduction in the oxyhemoglobin levels (downward slope), whereas in an unobstructed system the oxyhemoglobin levels increase (upward slope). The sensitivity of NIRS has been reported to be around 86%.[26,27,28] However algorithms vary between studies and may also include the Qmax and post void residual PVR assessments in a combined score. Problems with this technique are, however, that motion artefact and chronic health conditions such as peripheral vascular disease/diabetes may the affect readings. Moreover, these patient groups have been excluded from these studies.[28,27]
The condom catheter method uses a sheath with a pressure transducer. This measures the detrusor pressure against a closed bladder outlet, the iso-volumetric pressure (Pves.iso). With the patient voiding, the outflow is interrupted several times during flow. This allows determination of maximum intravesical pressure and urethral resistance. Correct categorization of men into obstructed and non-obstructed categories was found in 42 of 46 patients with reference to urodynamics,[29] and also has good repeatability.[30,31] Problems occur if the flow rates are less than 5.4 mL/s or if the bladder volumes are less than 250 mL. Additionally, straining may lead to urethral closure or leaking.
The penile cuff test assumes a continuous flow of fluid from the urethra to the bladder. A pneumatic cuff is placed around the penis and is inflated to interrupt the urine flow and is thereafter rapidly deflated, resulting in a surge of urine (Qsurg), followed by a steady state flow (Qss). The maximum value of cuff interruption pressure plotted on a non-invasive pressure flow nomogram has been proposed to provide the best diagnostic accuracy.[32] A positive predictive value of 82% and a negative predictive value of 88% have been reported.[33] More interestingly, men shown to be obstructed had an 87% chance of a good outcome after TURP compared with only 56% who were shown to be unobstructed.[34] The limiting factors are the high trace exclusion rate, technical failures and a high proportion of equivocal outcomes.
Doppler assessment of urine flow through the prostatic urethra allows the plotting of flow velocity curves. In obstructed men, the Qmax/max velocity ratio is lower and has been suggested to correlate with obstruction.[35] In a small study of 22 men, a ratio > 1.6 correctly classified men according to urodynamic criteria. Limitations are the lack of large studies and the cost and expertise to perform this procedure.
Histological assessment of detrusor muscle has been shown to correlate with detrusor failure and obstruction. Urodynamically, obstructed men have detrusor myo-hypertrophy with wide spaces between the muscle cells.[36] Contrarily, unobstructed men show muscular and axonal degeneration followed by fibrosis.[36] As it is much more invasive than urodynamics and highly subjective in its interpretation, this methodology is unlikely to replace urodynamic assessment but may be useful in studying and identifying new methods for treating unobstructed men. Clearly, at present, an important area for standardization of terminology and assessment of the underlying pathophysiology is the subject of underactive detrusor function.
CONCLUSIONS
The majority of men with the correct history and examination findings probably do not need to undergo invasive and expensive urodynamic assessment. A simple flow rate should suffice to make a diagnosis and commence treatment. However, emerging evidence does suggest a role for PSA assessment and IPP measurement in deciding which men are likely to progress. Men with equivocal symptoms or after previous surgery may require more invasive assessments. Cystometry still remains the gold standard assessment tool in differentiating obstructed from unobstructed men. The use of prostatic measurements such as IPP and prostatic-urethral angle will not inform about bladder contractility. The use of DWT does reflect bladder contractility better, but is still a limited technique that requires further standardization. Similarly, the penile cuff test and condom catheter method require more evidence in larger populations but do carry the benefit of being less invasive, potentially providing more information than a flow rate, but being more labor intensive and costly to perform and not providing the same degree of accuracy as a pressure flow study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Laniado ME, Ockrim JL, Marronaro A, Tubaro A, Carter SS. Serum prostate-specific antigen to predict the presence of bladder outlet obstruction in men with urinary symptoms. BJU Int. 2004;94:1283–6. doi: 10.1111/j.1464-410X.2004.05158.x. [DOI] [PubMed] [Google Scholar]
- 3.Bartsch G, Fitzpatrick JM, Schalken JA, Isaacs J, Nordling J, Roehrborn CG. Consensus statement: The role of prostate-specific antigen in managing the patient with benign prostatic hyperplasia. BJU Int. 2004;93(Suppl 1):27–9. doi: 10.1111/j.1464-410x.2004.04646.x. [DOI] [PubMed] [Google Scholar]
- 4.Roehrborn CG, McConnell J, Bonilla J, Rosenblatt S, Hudson PB, Malek GH, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol. 2000;163:13–20. [PubMed] [Google Scholar]
- 5.Girman CJ, Panser LA, Chute CG, Oesterling JE, Barrett DM, Chen CC, et al. Natural history of prostatism: Urinary flow rates in a community-based study. J Urol. 1993;150:887–92. doi: 10.1016/s0022-5347(17)35640-9. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, Dhabalia JV, Nelivigi GG, Punia MS, Suryavanshi M. Age, gender, and voided volume dependency of peak urinary flow rate and uroflowmetry nomogram in the Indian population. Indian J Urol. 2009;25:461–6. doi: 10.4103/0970-1591.57912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrams P. 3rd ed. London: Springer; 2006. Urodynamics. [Google Scholar]
- 8.Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: Three simple indices to define bladder voiding function. BJU Int. 1999;84:14–5. doi: 10.1046/j.1464-410x.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- 9.Lieber MM, Jacobson DJ, McGree ME, St Sauver JL, Girman CJ, Jacobsen SJ. Intravesical prostatic protrusion in men in Olmsted County, Minnesota. J Urol. 2009;182:2819–24. doi: 10.1016/j.juro.2009.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumpanas AA, Botoca M, Minciu R, Bucuras V. Intravesical prostatic protrusion can be a predicting factor for the treatment outcome in patients with lower urinary tract symptoms due to benign prostatic obstruction treated with tamsulosin. Urology. 2013;81:859–63. doi: 10.1016/j.urology.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Seo YM, Kim HJ. Impact of intravesical protrusion of the prostate in the treatment of lower urinary tract symptoms/benign prostatic hyperplasia of moderate size by alpha receptor antagonist. Int Neurourol J. 2012;16:187–90. doi: 10.5213/inj.2012.16.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JW, Ryu JH, Yoo TK, Byun SS, Jeong YJ, Jung TY. Relationship between Intravesical Prostatic Protrusion and Postoperative Outcomes in Patients with Benign Prostatic Hyperplasia. Korean J Urol. 2012;53:478–82. doi: 10.4111/kju.2012.53.7.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aganovic D, Hasanbegovic M, Prcic A, Kulovac B, Hadziosmanovic O. Which is a better indicator of bladder outlet obstruction in patients with benign prostatic enlargement-intravesical protrusion of prostate or bladder wall thickness? Med Arh. 2012;66:324–8. doi: 10.5455/medarh.2012.66.324-328. [DOI] [PubMed] [Google Scholar]
- 14.Franco G, De NC, Leonardo C, Tubaro A, Ciccariello M, De DC, et al. Ultrasound assessment of intravesical prostatic protrusion and detrusor wall thickness--new standards for noninvasive bladder outlet obstruction diagnosis? J Urol. 2010;183:2270–4. doi: 10.1016/j.juro.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Syazarina SO, Zulkifli MZ, Hamzaini AH. Predicting outcome of trial of voiding without catheter in acute urinary retention with intravesical prostatic protrusion. Malays J Med Sci. 2013;20:56–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee LS, Sim HG, Lim KB, Wang D, Foo KT. Intravesical prostatic protrusion predicts clinical progression of benign prostatic enlargement in patients receiving medical treatment. Int J Urol. 2010;17:69–74. doi: 10.1111/j.1442-2042.2009.02409.x. [DOI] [PubMed] [Google Scholar]
- 17.Ku JH, Ko DW, Cho JY, Oh SJ. Correlation between prostatic urethral angle and bladder outlet obstruction index in patients with lower urinary tract symptoms. Urology. 2010;75:1467–71. doi: 10.1016/j.urology.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Bright E, Oelke M, Tubaro A, Abrams P. Ultrasound estimated bladder weight and measurement of bladder wall thickness-useful noninvasive methods for assessing the lower urinary tract? J Urol. 2010;184:1847–54. doi: 10.1016/j.juro.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Oelke M, Hofner K, Jonas U, de la Rosette JJ, Ubbink DT, Wijkstra H. Diagnostic accuracy of noninvasive tests to evaluate bladder outlet obstruction in men: Detrusor wall thickness, uroflowmetry, postvoid residual urine, and prostate volume. Eur Urol. 2007;52:827–34. doi: 10.1016/j.eururo.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Huang T, Qi J, Yu YJ, Xu D, Jiao Y, Kang J, et al. Predictive value of resistive index, detrusor wall thickness and ultrasound estimated bladder weight regarding the outcome after transurethral prostatectomy for patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Int J Urol. 2012;19:343–50. doi: 10.1111/j.1442-2042.2011.02942.x. [DOI] [PubMed] [Google Scholar]
- 21.Kessler TM, Gerber R, Burkhard FC, Studer UE, Danuser H. Ultrasound assessment of detrusor thickness in men-can it predict bladder outlet obstruction and replace pressure flow study? J Urol. 2006;175:2170–3. doi: 10.1016/S0022-5347(06)00316-8. [DOI] [PubMed] [Google Scholar]
- 22.Kojima M, Inui E, Ochiai A, Naya Y, Ukimura O, Watanabe H. Ultrasonic estimation of bladder weight as a measure of bladder hypertrophy in men with infravesical obstruction: A preliminary report. Urology. 1996;47:942–7. doi: 10.1016/S0090-4295(96)00059-3. [DOI] [PubMed] [Google Scholar]
- 23.Miyashita H, Kojima M, Miki T. Ultrasonic measurement of bladder weight as a possible predictor of acute urinary retention in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Ultrasound Med Biol. 2002;28:985–90. doi: 10.1016/s0301-5629(02)00545-8. [DOI] [PubMed] [Google Scholar]
- 24.Bright E, Pearcy R, Abrams P. Ultrasound estimated bladder weight in men attending the uroflowmetry clinic. Neurourol Urodyn. 2011;30:583–6. doi: 10.1002/nau.21049. [DOI] [PubMed] [Google Scholar]
- 25.Macnab AJ, Stothers L. Near-infrared spectroscopy: Validation of bladder-outlet obstruction assessment using non-invasive parameters. Can J Urol. 2008;15:4241–8. [PubMed] [Google Scholar]
- 26.Stothers L, Guevara R, Macnab A. Classification of male lower urinary tract symptoms using mathematical modelling and a regression tree algorithm of noninvasive near-infrared spectroscopy parameters. Eur Urol. 2010;57:327–32. doi: 10.1016/j.eururo.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Yurt M, Suer E, Gulpinar O, Telli O, Arikan N. Diagnosis of bladder outlet obstruction in men with lower urinary tract symptoms: Comparison of near infrared spectroscopy algorithm and pressure flow study in a prospective study. Urology. 2012;80:182–6. doi: 10.1016/j.urology.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Pel JJ, Bosch JL, Blom JH, Nijeholt AA, van MR. Development of a non-invasive strategy to classify bladder outlet obstruction in male patients with LUTS. Neurourol Urodyn. 2002;21:117–25. doi: 10.1002/nau.10046. [DOI] [PubMed] [Google Scholar]
- 29.Mastrigt R, Huang Foen Chung JW. Comparison of repeatability of non-invasive and invasive urodynamics. Neurourol Urodyn. 2004;23:317–21. doi: 10.1002/nau.20043. [DOI] [PubMed] [Google Scholar]
- 30.Huang Foen Chung JW, Bohnen AM, Pel JJ, Bosch JL, Niesing R, van MR. Applicability and reproducibility of condom catheter method for measuring isovolumetric bladder pressure. Urology. 2004;63:56–60. doi: 10.1016/j.urology.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Harding CK, Robson W, Drinnan MJ, Ramsden PD, Griffiths C, Pickard RS. Variation in invasive and noninvasive measurements of isovolumetric bladder pressure and categorization of obstruction according to bladder volume. J Urol. 2006;176:172–6. doi: 10.1016/S0022-5347(06)00497-6. [DOI] [PubMed] [Google Scholar]
- 32.Borrini L, Lukacs B, Ciofu C, Gaibisso B, Haab F, Amarenco G. [Predictive value of the penile cuff-test for the assessment of bladder outlet obstruction in men] Prog Urol. 2012;22:657–64. doi: 10.1016/j.purol.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Harding C, Robson W, Drinnan M, Sajeel M, Ramsden P, Griffiths C, Pickard R. Predicting the outcome of prostatectomy using noninvasive bladder pressure and urine flow measurements. Eur Urol. 2007;52:186–92. doi: 10.1016/j.eururo.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Ozawa H, Chancellor MB, Ding YY, Nasu Y, Yokoyama T, Kumon H. Noninvasive urodynamic evaluation of bladder outlet obstruction using Doppler ultrasonography. Urology. 2000;56:408–12. doi: 10.1016/s0090-4295(00)00684-1. [DOI] [PubMed] [Google Scholar]
- 35.Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. IV. Bladder outlet obstruction. J Urol. 1993;150:1681–95. doi: 10.1016/s0022-5347(17)35869-x. [DOI] [PubMed] [Google Scholar]
- 36.Nepomnyashchikh LM, Lushnikova EL, Neimark AI. Remodeling of the muscle layer (detrusor muscle) of hyperactive bladder disease in patients with benign prostatic hyperplasia. Bull Exp Biol Med. 2012;153:778–83. doi: 10.1007/s10517-012-1825-2. [DOI] [PubMed] [Google Scholar]


