Abstract
Pharmacology of the lower urinary tract provides the basis for medical treatment of lower urinary tract symptoms (LUTS). Therapy of LUTS addresses obstructive symptoms (frequently explained by increased prostate smooth muscle tone and prostate enlargement) in patients with benign prostate hyperplasia (BPH) and storage symptoms in patients with overactive bladder (OAB). Targets for medical treatment include G protein-coupled receptors (α1-adrenoceptors, muscarinic acetylcholine receptors, β3-adrenoceptors) or intracellular enzymes (5α-reductase; phosphodiesterase-5, PDE5). Established therapies of obstructive symptoms aim to induce prostate smooth muscle relaxation by α1-blockers or PDE5 inhibitors, or to reduce prostate growth and volume with 5α-reductase inhibitors. Available options for treatment of OAB comprise anitmuscarinics, β3-adrenoceptor agonists, and botulinum toxin A, which improve storage symptoms by inhibition of bladder smooth muscle contraction. With the recent approval of β3-antagonists, PDE inhibitors, and silodosin for therapy of LUTS, progress from basic research of lower urinary tract pharmacology was translated into new clinical applications. Further targets are in preclinical stages of examination, including modulators of the endocannabinoid system and transient receptor potential (TRP) channels.
Keywords: Alpha1 adrenoreceptor, arginine vasopressors, endocannabinoids, 5 alpha reductase, muscarinic receptors, phosphodiesterase, vitamin D
INTRODUCTION
α1-adrenoceptors, muscarinic acetylcholine receptors, 5α-reductase, and phosphodiesterases are established targets for pharmacologic therapies of lower urinary tract symptoms (LUTS). Further strategies are in preclinical stages of examination, or are awaiting approval following clinical studies. Therapy of LUTS includes voiding symptoms (“obstructive”) in patients with benign prostatic obstruction (BPO), and storage symptoms (“irritative”) in patients with an overactive bladder (OAB).
The aim of any pharmacological therapy is an amelioration of symptoms by relaxation of prostate smooth muscle, reduction of prostate volume, or relaxation of bladder smooth muscle. What these strategies have in common is that their mechanisms are closely related to pathophysiology of LUTS. Understanding the principles of pathophysiology and pharmacology in the lower urinary tract provides the basis for current and future therapies. Here, we briefly summarize the pharmacological basis of available therapies and targets showing promising results either in preclinical studies or in clinical stages of examination.
Pathophysiology of LUTS
Urethral obstruction in patients with BPO is frequently explained by exaggerated α1-adrenergic prostate smooth muscle contraction and by prostate growth [Figure 1].[1,2] Both may cause bladder outlet obstruction (BOO), resulting in obstructive storage symptoms.[1,2] Accordingly, α1-adrenoceptors and prostate growth are important targets for therapy of LUTS in patients with BPO.[3] In patients with OAB, irritative symptoms are caused by spontaneous, uncontrolled phasic contractions of bladder smooth muscle (detrusor overactivity, DO) [Figure 1].[4] Therefore, prevention of detrusor contraction and decreasing smooth muscle tone in the bladder is an important strategy for medical treatment of these symptoms.[4] It is now well known that relationships between LUTS, their etiology, and organ-specific context are highly variable.[5] It has been proposed that the causal relationship between BOO, maximum flow rate (Qmax), and symptom scores may be lower as previously assumed.[6]
Figure 1.

Pathophysiology and medical therapy of LUTS. Obstructive symptoms are frequently explained by benign prostatic obstruction, due to enhanced prostate smooth muscle tone and prostate enlargement. Both may contribute to urethral obstruction. Application of α1-blockers or PDE5 inhibitors cause improvement of obstructive symptoms by relaxation of prostate smooth muscle, while beneficial effects of 5α-reductase inhibitors occur by reduction of prostate growth and volume. Storage symptoms (“irritative”) are often caused by an overactive bladder, due to overactivity of detrusor smooth muscle contraction. Consequently, available options for treatment of storage symptoms are based on relaxation and quiescing of bladder smooth muscle tone, by application of muscarinic receptor antagonists, β3-adrenoceptors agonists, or botulinum toxin A
α1-adrenoceptors
Three subtypes of α1-adrenoceptors are known from the lower urinary tract, designated as α1A, α1B, and α1D.[7,8] In the human prostate, the α1A subtype covers around 70% of the total α1-adrenoceptors population and is responsible for smooth muscle contraction.[7,8] Prostatic expression of α1B-adrenoceptors is most likely confined to the glandular epithelium, while α1D is expressed by intraprostatic blood vessels.[9] α1-adrenoceptors in the prostate and elsewhere may occur in two phenotypes, designated as α1A and α1L, both belonging to the α1A subtype.[10,11] Although both are products of the same gene (Adra1A), factors deciding whether Adra1A mRNA is translated as α1A or α1L are still unknown.[11] It has been proposed that interaction with the binding partner CRELD1α (cysteine-rich epidermal growth factor-like domain 1α) may confer the unique pharmacological profile of α1L to α1A-adrenoceptors.[11] Both phenotypes show distinct pharmacological properties. A key difference is their affinity to the non-selective α1-adrenoceptor antagonist prazosin, which is high for α1A, but low for α1L.[11] α1A-adrenoceptors may also occur in the bladder, where they mediate smooth muscle contraction in the human trigonum and bladder base.[7,10] In animal models, the subtype distribution of α1-adrenoceptors in the lower urinary tract may differ.[7]
It is widely accepted that beneficial effects of α-blockers in patients with obstructive LUTS are explained by smooth muscle relaxation in the prostate.[1,2,7,8] In addition, it is now clear that mechanisms besides prostate smooth muscle relaxation are involved in therapeutic effects of α1-blockers.[12] These may include actions on the bladder microcirculation, and α1-adrenoceptors in the urothelium, in afferent nerves, or in bladder smooth muscle.[12] In fact, α1-blockers may improve symptoms in women, in men without BPO, or in animal models, where a prostate-dependent contribution can be excluded.[8,13,14,15]
While α1-adrenoceptors in the lower urinary tract were intensively studied at expression level, their intracellular signaling or posttranslational regulation attracted less attention.[7] Following receptor stimulation, activation of intracellular signaling cascades via receptor-associated heterotrimeric G proteins leads to contraction of prostate smooth muscle [Figure 2].[1] Activation of phospholipase C (PLC) causes formation of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), leading to activation of myosin light chain (MLC) kinase by calcium-dependent mechanisms, and to deactivation of MLC phosphatase via protein kinase C (PKC) [Figure 2].[1] Result is an increased MLC phosphorylation, being the prerequisite for smooth muscle contraction.[1] In parallel to PLC, the monomeric GTPase RhoA is activated by G proteins.[16] RhoA activates Rho kinase, which subsequently leads to contraction by MLC phosphatase inhibition [Figure 2].[16]
Figure 2.
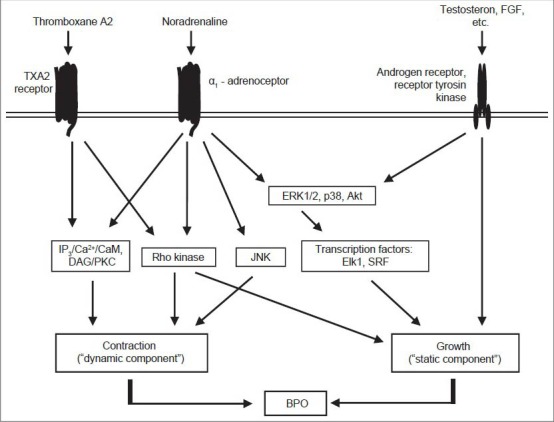
Mechanisms of prostate smooth muscle contraction and assumed connections to the regulation of prostate growth. In contrast to earlier concepts, α1-adrenoceptors in the prostate are no longer regarded as isolated receptors mediating exclusively contraction. In fact, α1-adrenoceptors in the prostate are part of a signaling network, where different receptors and non-adrenergic mediators cooperatively regulate prostate smooth muscle tone and growth, leading to benign prostate obstruction. Prostate α1-adrenoceptors lead to contraction by activation of the IP3/Ca2+/calmodulin pathway, of DAG/protein kinase C, of the RhoA/Rho kinase pathway, and by a JNK-dependent mechanism. At least the Ca2+- and Rho kinase-dependent mechanisms are shared by TXA2 receptors, which cause prostate smooth muscle contraction in parallel to α1-adrenoceptors. In addition, α1-adrenoceptors share intracellular effectors with hormone receptors and growth factors (e. g. fibroblast growth factor): Stimulation of prostate α1-adrenoceptors leads to activation of ERK1/2, Akt and transcription factors, which are well known to mediate growth and differentiation
Besides contraction, α1-adrenergic Rho kinase activation in the prostate has been linked to proliferation of prostate cells and therefore to prostate growth [Figure 2].[17] In fact, an involvement of α1-adrenoceptors in prostate growth and hyperplasia has been repeatedly suggested.[18,19,20] However, α1-blockers do not reduce prostate volume.[21,22] Recent evidence from experimental studies unequivocally proved the existence of signal transduction by prostate α1-adrenoceptors, which is not involved in contraction. This may be termed as “non-motoric” signaling, and comprises a panel of pathways including mitogen-activated protein kinases, Akt, and transcription factors, which are all activated by α1-adrenoceptors in the human prostate [Figure 2].[23,24,25,26]
Different α1-adrenoceptor antagonists (“α-blocker”) are routinely applied for treatment of obstructive symptoms.[27] Although their subtype selectivity may differ, their efficacy is similar in appropriate doses.[10,27] Application of α1-blockers still represents a gold standard for medical therapy of BPO.[27] The recent approval of silodosin in the USA and Europe reflects a high interest for α1-blockers with improved subtype selectivity and efficacy.[10,28,29] Before the introduction of silodosin, tamsulosin had the highest α1A-selectivity and was the most prescribed of all available α1-blockers.[10,30] Naftopidil, which is available for therapy of obstructive symptoms in India, blocks α1D-adrenoceptors in addition to α1A and has a comparable efficacy to tamsulosin.[31,32]
α1-blockers cause rapid amelioration of mild to moderate symptoms, which frequently persists for several years.[3,21,22,27] However, they do not prevent the progression of benign prostate hyperplasia (BPH), as the rate of acute urinary retention, the need for invasive therapy, or serum PSA levels are not reduced by α1-blockers.[21,22,27] Together, this leads to application of combination therapies (α1-blockers with 5α-reductase inhibitors) or non-medical, ablative therapies in many patients, if effects from α1-blockers are insufficient.[27] Despite the marked improvement of symptoms by α1-blockers, their efficacy is in fact limited. Symptom scores may be reduced 30-50% by α1-blockers, while placebos may cause an improvement of 10-34%.[3,27,33] Similarly, α1-blockers enhance maximum flow rate (Qmax) by 15-40%, while increases up to 27% were observed by treatment with placebos.[3,27,33] This points to non-adrenergic mediators of contraction, contributing to prostate smooth muscle tone in parallel to α1-adrenoceptors. Indeed, thromboxane A2 (TXA2) induces smooth muscle contraction in the human prostate, by activation of TXA2 receptors [Figure 2].[34] Finally, the contribution of further mediators cannot be excluded.
5α-reductase
Prostate growth in BPH depends on testosterone.[35,36] Testosterone is metabolized to dihydrotestosterone (DHT) by 5α-reductases (5-AR).[35,36] In the prostate, 5-AR-2 is the prevailing isoform, being located to stromal and basal cells.[35,36] DHT has a 4-5 fold higher affinity for androgen receptors as testosterone.[35,36] Consequently, inhibition of 5-AR by 5-AR inhibitors (5-ARI) abolishes prostate growth and reduces prostate size.[3,27] Therapy with 5-ARIs is applied to prevent the progression of BPH.[3,27] While finasteride selectively inhibits 5-AR-2, dutasteride inhibits both isoforms (5-AR-1, -2).[37] Beneficial effects of 5-ARIs become apparent 3-6 month after continuous application. Finasteride and dutasteride may reduce LUTS by 30% and prostate volume by 25%.[3,27,37]
In rats, reduction of prostate volume can be obtained by treatment with the luteinizing hormone-releasing hormone antagonist, cetrorelix.[38] Cetrorelix is available for anti-cancer treatment. However, approval for therapy of LUTS and BPH (as a benign disease) appears unlikely, due to the inappropriate balance of benefits and side effects.
Muscarinic receptors
In the lower urinary tract, muscarinic receptors are of outstanding importance for smooth muscle contraction in the bladder detrusor, while their relevance for smooth muscle tone in the prostate or urethra is minor.[39] Prevailing subtypes in the human detrusor are M2 and M3, which account for 70% and 20% of the total muscarinic receptor population.[39] Contraction of detrusor smooth muscle is primarily mediated by M3 receptors.[4,39] Muscarinic receptors are activated by acetylcholine, released from parasympathetic nerves.[4,39] In addition to smooth muscle cells, muscarinic receptors in the bladder are found in the urothelium and in presynaptic nerve terminals, the latter being involved in the regulation of neurotransmitter release.[4,39] Interestingly, the intracellular mechanisms leading to smooth muscle contraction by muscarinic receptors in the detrusor strongly resemble those used by α1-adrenoceptors in the prostate, as they involve IP3/Ca2+, DAG/PKC, and Rho kinase.[4]
Muscarinic antagonists are routinely applied for the treatment of storage symptoms in patients with OAB.[4,39] Several antagonists are available, despite different affinities and subtype selectivities. Nevertheless, side effects and efficacy are similar between all substances. Although application of antimuscarinics represents the gold standard of medical OAB therapy, the efficacy may not be fully satisfactory.[4] In fact, patients adherence to the therapy is quite low: Up to 45% or more patients discontinue the therapy, due to the perception that the medication is not working.[40]
Combinations with muscarinic antagonists may be effective in patients, where monotherapy with 5-AR or α1-blockers is insufficient. Despite initial concerns that such combinations may induce urinary retention, the combination of tolterodine with dutasteride may be effective and safe in patients with OAB and symptoms secondary to BPH.[41] Similarly, combinations of antimuscarinics with α1-blockers have been recently addressed by clinical studies.[27]
Phosphodiesterases
Phosphodiesterases hydrolyze the cyclic nucleotides, cGMP and cAMP, which both mediate smooth muscle relaxation in the lower urinary tract and other organs.[42] In the prostate, cGMP is synthesized by guanylyl cyclases, which are activated by nitric oxide (NO) released by neuronal NO synthase (nNOS) as a neurotransmitter, or by inducible NOS (iNOS) from macrophages.[43] Inhibitors for the cGMP-specific PDE5 were introduced in the 90's, for the treatment of erectile dysfunction (ED). PDE5 inhibition causes accumulation of cGMP in smooth muscle cells, promoting cGMP-mediated relaxation.[42] While PDE5 inhibitors are now available for treatment of LUTS in patients with BPH, cAMP-specific PDE4 is currently under preclinical investigation.[44]
The PDE5 inhibitor tadalafil has been approved very recently for treatment of obstructive symptoms in patients with BPH in the USA and Europe.[27,45] The advantage of tadalafil to other PDE5 inhibitors may be its extended half-life, allowing a once-daily application for treatment of LUTS.[45] The efficacy of tadalafil is comparable to that of α1-blockers.[46] In contrast to most other medical options for LUTS treatment, high attention has to be paid to possible contraindications, excluding the application of PDE5 inhibitors.[27] Patients receiving nitrates, potassium channel openers, nicroandil, or the α1-blockers doxazosin or terazosin cannot be treated with PDE5 inhibitors, due to high risks of dangerous interactions.[27] Further contraindications are unstable angina pectoris, recent myocardial infarction (<3 mo) or stroke (<6 mo), myocardial insufficiency, hypotension, poorly controlled blood pressure, hepatic or renal insufficiency, and anterior ischemic optic neuropathy with sudden loss of vision after previous use of PDE5 inhibitors.[27]
Arginine vasopressin
The antidiuretic hormone, arginine vasopressin (AVP), is a key regulator of body water homeostasis and in the control of urine production.[47] AVP promotes water reabsorption and decreases water as well as total urine volume.[47] It is released to compensate dehydrated conditions, resulting in water reasorption and formation of a concentrated, low volume urine.[47] In parallel, AVP induces moderate vasoconstriction and elevation of blood pressure by activation of AVP receptor 1 (V1), to counteract hypovolemic states.[47]
The vasopressin receptor 2 (V2)-selective agonist, desmopressin, is available for the treatment of nocturia secondary to nocturnal polyuria in adult patients.[27] Desmopressin reduces the overall number of nocturnal voids and prolongs the periods of undisturbed sleep.[27] Nevertheless, it is rarely used for treatment of nocturia in adults. According to the role of AVP for urine homeostasis, desmopressin has been considered for the treatment of OAB.[47] Urodynamic actions were addressed by two clinical studies, with promising results.[47] Nevertheless, this did not proceed to clinical application.
New targets
Preclinical studies revealed several targets, which were related to promising results in experimental models. Some of them were recently transferred into clinical stages of examination and may await approval for clinical application in LUTS therapy.
β3-adrenoceptor agonists
In the human lower urinary tract, β2- and β3-adrenoceptors induce smooth muscle relaxtion, while the function and expression of β1-adrenoceptors are of minor importance.[7,48] In addition to smooth muscle cells, β-adrenoceptos in the lower urinary tract may be present in the urothelium and in afferent nerves.[7,48] In the bladder, β-adrenoceptors enhance urine storage, while their function in the prostate or urethra is less understood.[7,48] β3-adrenoceptors cover > 95% of the total β-adrenoceptor mRNA pool in the human bladder and induce detrusor relaxation.[48] In the human prostate, β2 is the prevailing subtype at protein level; in fact, β-adrenergic activation inhibits α1-adrenergic prostate smooth muscle contraction via β2-adrenoceptors.[48]
With regard to clinical application, β3-adrenoceptors in the bladder attracted large attention. Following randomized clinical studies, mirabegron, a β3-adrenergic agonist, has now been approved for the treatment of OAB in Europe, Japan, and the USA.[49,50,51,52] Nevertheless, long-term experiences with mirabegron during clinical application are still missing.[53] Although proof-of-concept studies with two other agonists, solabegron and ritobegron, yielded promising results, these agonists did not proceed to clinical application to date.[53]
Endocannabinoids and TRP channels
The endocannabinoid system and transient receptor potential (TRP) channels have been recognized as important regulators of smooth muscle tone in the lower urinary tract.[54,55] Cannabinoid receptors, TRPA, and TRPV cooperatively mediate smooth muscle relaxation in the prostate, urethra, and bladder.[54,55] In this process, mechano-afferent signals cause activation of the cannabinoid receptor 2 (CB2) and TRP channels on sensory neurons, leading to the release of NO and cyclooxygenase activation by neurons, which finally results in postsynaptic smooth muscle relaxation [Figure 3].[55] In contrast to animal models, where CB1 strongly inhibits bladder smooth muscle contraction, endocannabinoid effects in the human lower urinary tract are prevailingly mediated by CB2.[54,56]
Figure 3.
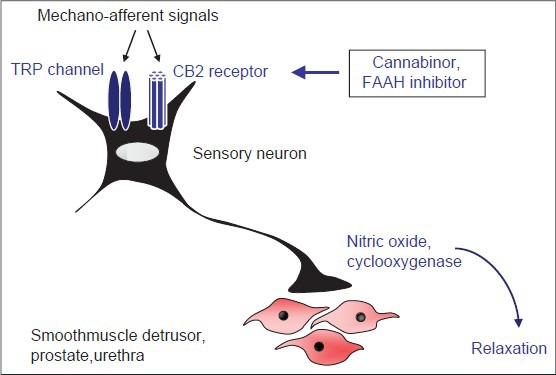
Role of endocannabinoids and TRP channels for regulation of smooth muscle tone in the lower urinary tract. Mechano-afferent signals lead to activation of CB2 receptors and TRP channels (TRPA, TRPV) in sensory neurons. This causes the release of nitric oxide and cyclooxygenase-dependent neurotransmission, finally resulting in smooth muscle relaxation in the detrusor, prostate, and urethra. Consequently, activation of CB2 receptors by Cannabinor or FAAH inhibitors improves LUTS in animal models.
While cannabinoid receptors and TRP channels have been intensively studied in vitro and in animal models, evidence for urodynamic effects in patients is still rare.[56,57,58,59,60] Accumulation of endocannabinoids by inhibition of their degradation has been proposed as a new strategy for improvement of LUTS.[61] Endocannabinoid degradation is promoted by fatty acid amide hydrolase (FAAH).[61] In rats, FAAH inhibition by oleoyl ethyl amide (OetA) caused urodynamic alterations, which may improve symptoms in OAB.[61] In proof of concept studies, intravesical application of the vanilloid TRPV agonist capsaicin or resiniferatoxin increased bladder capacity and decreased urge incontinence in patients with neurogenic and non-neurogenic DO.[62] Clinical studies focused on the application of cannabinoids for the treatment of bladder dysfunction in multiple sclerosis (MS).[54] However, results were divergent and are not easy to discriminate from placebo effects in MS.[54]
Botulinum toxin
Type A botulinum toxins (BTX-A), in particular onabotulinumtoxin A (BoNT-ONA, “botox”), has been investigated for use in the lower urinary tract. While intraprostatic injection is still controversially discussed and effects on obstructive symptoms may be limited,[63,64] its application in the bladder is now an established therapy of DO.[65] In the USA and Europe, BoNT-ONA is used for second-line treatment of neurogenic DO, as an alternative for anticholinergic therapy.[65,66] Approval for therapy of idiopathic DO may follow soon, as clinical trials provided encouraging results.[65,67,68]
The botulinum neurotoxins (type A to G) are proteins secreted by strains of Clostridium botulinum.[69] They disrupt neurotransmission at neuromuscular junctions by inhibition of presynaptic acetylcholine release.[69] In this process, BTX-A prevents complex formation of synaptic vesicles (containing acetylcholine) with synaptobrevin and syntaxin.[69] Under normal conditions, this is required for the transport of vesicles to the membrane, and subsequent neurotransmitter release.[69] Inhibition of this mechanism accounts for the beneficial effects of BoNT-ONA in DO, as detrusor contraction is explained by equipment of smooth muscle cells with muscarinic receptors, and parasympathetic cholinergic innervation.
Vitamin D
Preclinical studies with the vitamin D receptor agonist, BXL628 (elocalcitol), provided promising results and have been moved into the clinical testing stage. BXL628 prevents proliferation and contraction of smooth muscle cells in the bladder and the prostate, which is thought to be mediated by inhibition of the RhoA/Rho kinase pathway.[70,71,72] In a placebo-controlled phase II study in 119 patients with BPH (prostate volume >40 ml), application of BXL628 for 12 weeks caused a significant effect on prostate growth.[73] However, this was not paralleled by significant effects on Qmax, which may be related to the short treatment period.[73] The effects on storage symptoms were studied in another trial performed in 257 women with OAB due to idiopathic DO, who received BXL628 for 4 weeks.[74] In this study, treatment with elocalcitol significantly reduced the episodes of incontinence and significantly improved the Patient's Perception of Bladder Condition score (PPBC), while effects on other parameters were lacking.[74] The primary end point, a change in bladder volume at the first involuntary detrusor contraction, was not achieved.[74] Thus, a clinical progress in LUTS treatment by vitamin D-dependent therapies appears unlikely.
Peripheral mechanisms in uropharmacology
For most of the described pharmacologic agents with urodynamic effects in vivo, direct effects on smooth muscle cells are well established. These are exerted by receptors on the cell membrane (adrenoceptors, cholinergic receptors) or by intracellular enzymes (PDEs, 5-AR). Numerous studies demonstrated that peripheral mechanisms are of relevance for urodynamic effects as well. Evidence for a contribution of neuronal α1-adrenoceptors in the central and peripheral nervous system to urodynamic effects of α1-blockers was provided quite early by several investigators.[8] More recently, it has been demonstrated by intrathecal application that peripheral effects and actions in the spinal cord may contribute to urodynamic effects of muscarinic antagonists, PDE5 inhibitors, and β3-agonists.[75,76,77] Less surprising, but noteworthy was the finding that urodynamic alterations induced by FAAH inhibitors can be observed following an intrathecal application.[78]
CONCLUSIONS
Established therapies of obstructive symptoms aim to induce prostate smooth muscle relaxation by α1-blockers or PDE5 inhibitors, or to reduce prostate growth and volume with 5α-reductase inhibitors. Available options for treatment of OAB comprise antimuscarinics, β3-adrenoceptor agonists, and botulinum toxin A, which improve storage symptoms by inhibition of bladder smooth muscle contraction. With the recent approval of β3-adrenoceptor agonists, PDE inhibitors, and silodosin for therapy of LUTS, previous progress in basic research of lower urinary tract pharmacology was translated into new clinical applications. Further targets are in preclinical stages of examination, including modulators of the endocannabinoid system and transient receptor potential channels.
Footnotes
Source of Support: Nil
Conflict of Interest: C.G. is speaker/consultant/received honoraria for/from Astellas Pharma, Rottapharm Madaus, Lilly, Recordati Pharma, AMS, and Steba.
REFERENCES
- 1.Andersson KE, Lepor H, Wyllie MG. Prostatic alpha 1-adrenoceptors and uroselectivity. Prostate. 1997;30:202–15. doi: 10.1002/(sici)1097-0045(19970215)30:3<202::aid-pros9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol. 2004;171:1029–35. doi: 10.1097/01.ju.0000097026.43866.cc. [DOI] [PubMed] [Google Scholar]
- 3.Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The long-term outcome of medical therapy for BPH. Eur Urol. 2007;51:1522–33. doi: 10.1016/j.eururo.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Andersson KE. Antimuscarinic mechanisms and the overactive detrusor: An update. Eur Urol. 2011;59:377–86. doi: 10.1016/j.eururo.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Chapple CR, Wein AJ, Abrams P, Dmochowski RR, Giuliano F, Kaplan SA, et al. Lower urinary tract symptoms revisited: A broader clinical perspective. Eur Urol. 2008;54:563–9. doi: 10.1016/j.eururo.2008.03.109. [DOI] [PubMed] [Google Scholar]
- 6.Barendrecht MM, Abrams P, Schumacher H, de la Rosette JJ, Michel MC. Do alpha1-adrenoceptor antagonists improve lower urinary tract symptoms by reducing bladder outlet resistance? Neurourol Urodyn. 2008;27:226–30. doi: 10.1002/nau.20481. [DOI] [PubMed] [Google Scholar]
- 7.Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147(Suppl 2):S88–119. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson KE, Gratzke C. Pharmacology of alpha1-adrenoceptor antagonists in the lower urinary tract and central nervous system. Nat Clin Pract Urol. 2007;4:368–78. doi: 10.1038/ncpuro0836. [DOI] [PubMed] [Google Scholar]
- 9.Walden PD, Gerardi C, Lepor H. Localization and expression of the alpha1A-1, alpha1B and alpha1D-adrenoceptors in hyperplastic and non-hyperplastic human prostate. J Urol. 1999;161:635–40. [PubMed] [Google Scholar]
- 10.Yamada S, Ito Y. Alpha (1)-Adrenoceptors in the urinary tract. Handb Exp Pharmacol. 2011:283–306. doi: 10.1007/978-3-642-16499-6_14. [DOI] [PubMed] [Google Scholar]
- 11.Nishimune A, Yoshiki H, Uwada J, Anisuzzaman AS, Umada H, Muramatsu I. Phenotype pharmacology of lower urinary tract alpha (1)-adrenoceptors. Br J Pharmacol. 2012;165:1226–34. doi: 10.1111/j.1476-5381.2011.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel MC. The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: Alpha-blockers in the treatment of male voiding dysfunction-how do they work and why do they differ in tolerability? J Pharmacol Sci. 2010;112:151–7. doi: 10.1254/jphs.09r15fm. [DOI] [PubMed] [Google Scholar]
- 13.Low BY, Liong ML, Yuen KH, Chee C, Leong WS, Chong WL, et al. Terazosin therapy for patients with female lower urinary tract symptoms: A randomized, double-blind, placebo controlled trial. J Urol. 2008;179:1461–9. doi: 10.1016/j.juro.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 14.Thomas AW, Cannon A, Bartlett E, Ellis-Jones J, Abrams P. The natural history of lower urinary tract dysfunction in men: Minimum 10-year urodynamic followup of transurethral resection of prostate for bladder outlet obstruction. J Urol. 2005;174:1887–91. doi: 10.1097/01.ju.0000176740.76061.24. [DOI] [PubMed] [Google Scholar]
- 15.Gu B, Reiter JP, Schwinn DA, Smith MP, Korstanje C, Thor KB, et al. Effects of alpha 1-adrenergic receptor subtype selective antagonists on lower urinary tract function in rats with bladder outlet obstruction. J Urol. 2004;172:758–62. doi: 10.1097/01.ju.0000131609.96301.e6. [DOI] [PubMed] [Google Scholar]
- 16.Christ GJ, Andersson KE. Rho-kinase and effects of Rho-kinase inhibition on the lower urinary tract. Neurourol Urodyn. 2007;26(6 Suppl):948–54. doi: 10.1002/nau.20475. [DOI] [PubMed] [Google Scholar]
- 17.Rees RW, Foxwell NA, Ralph DJ, Kell PD, Moncada S, Cellek S. Y-27632, a Rho-kinase inhibitor, inhibits proliferation and adrenergic contraction of prostatic smooth muscle cells. J Urol. 2003;170:2517–22. doi: 10.1097/01.ju.0000085024.47406.6c. [DOI] [PubMed] [Google Scholar]
- 18.Kyprianou N. Doxazosin and terazosin suppress prostate growth by inducing apoptosis: Clinical significance. J Urol. 2003;169:1520–5. doi: 10.1097/01.ju.0000033280.29453.72. [DOI] [PubMed] [Google Scholar]
- 19.Golomb E, Kruglikova A, Dvir D, Parnes N, Abramovici A. Induction of atypical prostatic hyperplasia in rats by sympathomimetic stimulation. Prostate. 1998;34:214–21. doi: 10.1002/(sici)1097-0045(19980215)34:3<214::aid-pros9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Marinese D, Patel R, Walden PD. Mechanistic investigation of the adrenergic induction of ventral prostate hyperplasia in mice. Prostate. 2003;54:230–7. doi: 10.1002/pros.10170. [DOI] [PubMed] [Google Scholar]
- 21.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 22.Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57:123–31. doi: 10.1016/j.eururo.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 23.Bauer RM, Strittmatter F, Gratzke C, Göttinger J, Schlenker B, Reich O, et al. Coupling of alpha1-adrenoceptors to ERK1/2 in the human prostate. Urol Int. 2011;86:427–33. doi: 10.1159/000322639. [DOI] [PubMed] [Google Scholar]
- 24.Strittmatter F, Gratzke C, Walther S, Göttinger J, Beckmann C, Roosen A, et al. Alpha1-adrenoceptor signaling in the human prostate involves regulation of p38 mitogen-activated protein kinase. Urology. 2011;78:969.e7–13. doi: 10.1016/j.urology.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Strittmatter F, Walther S, Gratzke C, Göttinger J, Beckmann C, Roosen A, et al. Inhibition of adrenergic human prostate smooth muscle contraction by the inhibitors of c-Jun N-terminal kinase, SP600125 and BI-78D3. Br J Pharmacol. 2012;166:1926–35. doi: 10.1111/j.1476-5381.2012.01919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strittmatter F, Walther S, Roosen A, Rutz B, Schlenker B, Limmer S, et al. Activation of protein kinase B/Akt by alpha1-adrenoceptors in the human prostate. Life Sci. 2012;90:446–53. doi: 10.1016/j.lfs.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU Guidelines on the Treatment and Follow-up of Non-neurogenic Male Lower Urinary Tract Symptoms Including Benign Prostatic Obstruction. Eur Urol. 2013;64:118–40. doi: 10.1016/j.eururo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Chapple CR, Montorsi F, Tammela TL, Wirth M, Koldewijn E, Fernández Fernández E, et al. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: Results of an international, randomized, double-blind, placebo- and active-controlled clinical trial performed in Europe. Eur Urol. 2011;59:342–52. doi: 10.1016/j.eururo.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Osman NI, Chapple CR, Cruz F, Desgrandchamps F, Llorente C, Montorsi F. Silodosin: A new subtype selective alpha-1 antagonist for the treatment of lower urinary tract symptoms in patients with benign prostatic hyperplasia. Expert Opin Pharmacother. 2012;13:2085–96. doi: 10.1517/14656566.2012.714368. [DOI] [PubMed] [Google Scholar]
- 30.Ventura S, Oliver Vl, White CW, Xie JH, Haynes JM, Exintaris B. Novel drug targets for the pharmacotherapy of benign prostatic hyperplasia (BPH) Br J Pharmacol. 2011;163:891–907. doi: 10.1111/j.1476-5381.2011.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara N, Mizusawa T, Obara K, Takahashi K. The role of naftopidil in the management of benign prostatic hyperplasia. Ther Adv Urol. 2013;5:111–9. doi: 10.1177/1756287212461681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garimella PS, Fink HA, Macdonald R, Wilt TJ. Naftopidil for the treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;4:CD007360. doi: 10.1002/14651858.CD007360.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Kortmann BB, Floratos DL, Kiemeney LA, Wijkstra H, de la Rosette JJ. Urodynamic effects of alpha-adrenoceptor blockers: A review of clinical trials. Urology. 2003;62:1–9. doi: 10.1016/s0090-4295(02)02113-1. [DOI] [PubMed] [Google Scholar]
- 34.Strittmatter F, Gratzke C, Weinhold P, Steib CJ, Hartmann AC, Schlenker B, et al. Thromboxane A2 induces contraction of human prostate smooth muscle by Rho kinase- and calmodulin-dependent mechanisms. Eur J Pharmacol. 2011;650:650–5. doi: 10.1016/j.ejphar.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation. 2011;82:184–99. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8:29–41. doi: 10.1038/nrurol.2010.207. [DOI] [PubMed] [Google Scholar]
- 37.Rick FG, Saadat SH, Szalontay L, Block NL, Kazzazi A, Djavan B, et al. Hormonal manipulation of benign prostatic hyperplasia. Curr Opin Urol. 2013;23:17–24. doi: 10.1097/MOU.0b013e32835abd18. [DOI] [PubMed] [Google Scholar]
- 38.Rick FG, Schally AV, Block NL, Halmos G, Perez R, Fernandez JB, et al. LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate. 2011;71:736–47. doi: 10.1002/pros.21289. [DOI] [PubMed] [Google Scholar]
- 39.Andersson KE. Muscarinic acetylcholine receptors in the urinary tract. Handb Exp Pharmacol. 2011:319–44. doi: 10.1007/978-3-642-16499-6_16. [DOI] [PubMed] [Google Scholar]
- 40.Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Cardozo L, Subramanian D, et al. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: A systematic review of the literature. Int J Clin Pract. 2011;65:567–85. doi: 10.1111/j.1742-1241.2010.02626.x. [DOI] [PubMed] [Google Scholar]
- 41.Chung DE, Te AE, Staskin DR, Kaplan SA. Efficacy and safety of tolterodine extended release and dutasteride in male overactive bladder patients with prostates>;30 grams. Urology. 2010;75:1144–8. doi: 10.1016/j.urology.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Uckert S, Kuczyk MA. Cyclic nucleotide metabolism including nitric oxide and phosphodiesterase-related targets in the lower urinary tract. Handb Exp Pharmacol. 2011:527–42. doi: 10.1007/978-3-642-16499-6_23. [DOI] [PubMed] [Google Scholar]
- 43.Kedia GT, Uckert S, Jonas U, Kuczyk MA, Burchardt M. The nitric oxide pathway in the human prostate: Clinical implications in men with lower urinary tract symptoms. World J Urol. 2008;26:603–9. doi: 10.1007/s00345-008-0303-y. [DOI] [PubMed] [Google Scholar]
- 44.Waldkirch E, Uckert S, Sigl K, Langnaese K, Richter K, Stief CG, et al. Expression of cAMP-dependent protein kinase isoforms in the human prostate: Functional significance and relation to PDE4. Urology. 2010;76:515.e8–14. doi: 10.1016/j.urology.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 45.Cantrell MA, Baye J, Vouri SM. Tadalafil: A Phosphodiesterase-5 Inhibitor for Benign Prostatic Hyperplasia. Pharmacotherapy. 2013;33:639–49. doi: 10.1002/phar.1243. [DOI] [PubMed] [Google Scholar]
- 46.Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61:917–25. doi: 10.1016/j.eururo.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Pisipati S, Hashim H. Vasopressin receptors in voiding dysfunction. Handb Exp Pharmacol. 2011:453–83. doi: 10.1007/978-3-642-16499-6_21. [DOI] [PubMed] [Google Scholar]
- 48.Michel MC. Beta-Adrenergic Receptor Subtypes in the Urinary Tract. Handb Exp Pharmacol. 2011:307–18. doi: 10.1007/978-3-642-16499-6_15. [DOI] [PubMed] [Google Scholar]
- 49.Chapple CR, Amarenco G, López Aramburu MA, Everaert K, Liehne J, Lucas M, et al. A proof-of-concept study: Mirabegron, a new therapy for overactive bladder. Neurourol Urodyn. 2013 Feb 19; doi: 10.1002/nau.22373. doi: 10.1002/nau. 22373. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Chapple CR, Kaplan SA, Mitcheson D, Klecka J, Cummings J, Drogendijk T, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta (3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63:296–305. doi: 10.1016/j.eururo.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 51.Khullar V, Amarenco G, Angulo JC, Cambronero J, Høye K, Milsom I, et al. Efficacy and tolerability of mirabegron, a beta (3)-adrenoceptor agonist, in patients with overactive bladder: Results from a randomised European-Australian phase 3 trial. Eur Urol. 2013;63:283–95. doi: 10.1016/j.eururo.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a Randomized Phase III Trial of Mirabegron in Patients with Overactive Bladder. J Urol. 2013;189:1388–95. doi: 10.1016/j.juro.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Andersson KE, Martin N, Nitti V. Selective beta3-Adrenoceptor Agonists in the Treatment of the Overactive Bladder. J Urol. 2013;190:1173–80. doi: 10.1016/j.juro.2013.02.104. [DOI] [PubMed] [Google Scholar]
- 54.Ruggieri MR., Sr Cannabinoids: Potential targets for bladder dysfunction. Handb Exp Pharmacol. 2011:425–51. doi: 10.1007/978-3-642-16499-6_20. [DOI] [PubMed] [Google Scholar]
- 55.Andersson KE, Gratzke C, Hedlund P. The role of the transient receptor potential superfamily of cation-selective channels in the management of the overactive bladder. BJU Int. 2010;106:1114–27. doi: 10.1111/j.1464-410X.2010.09650.x. [DOI] [PubMed] [Google Scholar]
- 56.Gratzke C, Streng T, Park A, Christ G, Stief CG, Hedlund P, et al. Distribution and function of cannabinoid receptors 1 and 2 in the rat, monkey and human bladder. J Urol. 2009;181:1939–48. doi: 10.1016/j.juro.2008.11.079. [DOI] [PubMed] [Google Scholar]
- 57.Gratzke C, Streng T, Waldkirch E, Sigl K, Stief C, Andersson KE, et al. Transient receptor potential A1 activity in the human urethra-evidence for a functional role for TRPA1 in the outflow region. Eur Urol. 2009;55:696–704. doi: 10.1016/j.eururo.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 58.Gratzke C, Weinhold P, Reich O, Seitz M, Schlenker B, Stief CG, et al. Transient receptor potential A1 and cannabinoid receptor activity in human normal and hyperplastic prostate: Relation to nerves and interstitial cells. Eur Urol. 2010;57:902–10. doi: 10.1016/j.eururo.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Gratzke C, Streng T, Stief CG, Downs TR, Alroy I, Rosenbaum JS, et al. Effects of cannabinor, a novel selective cannabinoid 2 receptor agonist, on bladder function in normal rats. Eur Urol. 2010;57:1093–100. doi: 10.1016/j.eururo.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 60.Gratzke C, Streng T, Stief CG, Alroy I, Limberg BJ, Downs TR, et al. Cannabinor, a selective cannabinoid-2 receptor agonist, improves bladder emptying in rats with partial urethral obstruction. J Urol. 2011;185:731–6. doi: 10.1016/j.juro.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 61.Strittmatter F, Gandaglia G, Benigni F, Bettiga A, Rigatti P, Montorsi F, et al. Expression of fatty acid amide hydrolase (FAAH) in human, mouse, and rat urinary bladder and effects of FAAH inhibition on bladder function in awake rats. Eur Urol. 2012;61:98–106. doi: 10.1016/j.eururo.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Andersson KE. LUTS treatment: Future treatment options. Neurourol Urodyn. 2007;26(6 Suppl):934–47. doi: 10.1002/nau.20500. [DOI] [PubMed] [Google Scholar]
- 63.de Kort LM, Kok ET, Jonges TN, Rosier PF, Bosch JL. Urodynamic effects of transrectal intraprostatic Ona botulinum toxin A injections for symptomatic benign prostatic hyperplasia. Urology. 2012;80:889–93. doi: 10.1016/j.urology.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Sacco E, Bientinesi R, Marangi F, Totaro A, D’Addessi A, Racioppi M, et al. Patient-reported outcomes in men with lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia treated with intraprostatic OnabotulinumtoxinA: 3-month results of a prospective single-armed cohort study. BJU Int. 2012;110:E837–44. doi: 10.1111/j.1464-410X.2012.11288.x. [DOI] [PubMed] [Google Scholar]
- 65.Bauer RM, Gratzke C. Onabotulinumtoxin A for idiopathic overactive bladder: Raising the bar. Eur Urol. 2012;62:158–9. doi: 10.1016/j.eururo.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 66.Cruz F, Herschorn S, Aliotta P, Brin M, Thompson C, Lam W, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: A randomised, double-blind, placebo-controlled trial. Eur Urol. 2011;60:742–50. doi: 10.1016/j.eururo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 67.Fowler CJ, Auerbach S, Ginsberg D, Hale D, Radziszewski P, Rechberger T, et al. OnabotulinumtoxinA improves health-related quality of life in patients with urinary incontinence due to idiopathic overactive bladder: A 36-week, double-blind, placebo-controlled, randomized, dose-ranging trial. Eur Urol. 2012;62:148–57. doi: 10.1016/j.eururo.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Tincello DG, Kenyon S, Abrams KR, Mayne C, Toozs-Hobson P, Taylor D, et al. Botulinum toxin a versus placebo for refractory detrusor overactivity in women: A randomised blinded placebo-controlled trial of 240 women (the RELAX study) Eur Urol. 2012;62:507–14. doi: 10.1016/j.eururo.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 69.Yokoyama T, Chancellor MB, Oguma K, Yamamoto Y, Suzuki T, Kumon H, et al. Botulinum toxin type A for the treatment of lower urinary tract disorders. Int J Urol. 2012;19:202–15. doi: 10.1111/j.1442-2042.2011.02946.x. [DOI] [PubMed] [Google Scholar]
- 70.Crescioli C, Ferruzzi P, Caporali A, Scaltriti M, Bettuzzi S, Mancina R, et al. Inhibition of prostate cell growth by BXL-628, a calcitriol analogue selected for a phase II clinical trial in patients with benign prostate hyperplasia. Eur J Endocrinol. 2004;150:591–603. doi: 10.1530/eje.0.1500591. [DOI] [PubMed] [Google Scholar]
- 71.Morelli A, Vignozzi L, Filippi S, Vannelli GB, Ambrosini S, Mancina R, et al. BXL-628, a vitamin D receptor agonist effective in benign prostatic hyperplasia treatment, prevents RhoA activation and inhibits RhoA/Rho kinase signaling in rat and human bladder. Prostate. 2007;67:234–47. doi: 10.1002/pros.20463. [DOI] [PubMed] [Google Scholar]
- 72.Penna G, Fibbi B, Amuchastegui S, Corsiero E, Laverny G, Silvestrini E, et al. The vitamin D receptor agonist elocalcitol inhibits IL-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the RhoA/Rho kinase and NF-kappaB pathways. Prostate. 2009;69:480–93. doi: 10.1002/pros.20896. [DOI] [PubMed] [Google Scholar]
- 73.Colli E, Rigatti P, Montorsi F, Artibani W, Petta S, Mondaini N, et al. BXL628, a novel vitamin D3 analog arrests prostate growth in patients with benign prostatic hyperplasia: A randomized clinical trial. Eur Urol. 2006;49:82–6. doi: 10.1016/j.eururo.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 74.Digesu GA, Verdi E, Cardozo L, Olivieri L, Khullar V, Colli E. Phase IIb, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to determine effects of elocalcitol in women with overactive bladder and idiopathic detrusor overactivity. Urology. 2012;80:48–54. doi: 10.1016/j.urology.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 75.Füllhase C, Hennenberg M, Giese A, Schmidt M, Strittmatter F, Soler R, et al. Presence of phosphodiesterase type 5 in the spinal cord and its involvement in bladder outflow obstruction related bladder overactivity. J Urol. 2013;190:1430–5. doi: 10.1016/j.juro.2013.03.112. [DOI] [PubMed] [Google Scholar]
- 76.Füllhase C, Soler R, Westerling-Andersson K, Andersson KE. Beta3-adrenoceptors in the rat sacral spinal cord and their functional relevance in micturition under normal conditions and in a model of partial urethral obstruction. Neurourol Urodyn. 2011;30:1382–7. doi: 10.1002/nau.21071. [DOI] [PubMed] [Google Scholar]
- 77.Füllhase C, Soler R, Gratzke C, Brodsky M, Christ GJ, Andersson KE. Spinal effects of the fesoterodine metabolite 5-hydroxymethyl tolterodine and/or doxazosin in rats with or without partial urethral obstruction. J Urol. 2010;184:783–9. doi: 10.1016/j.juro.2010.03.104. [DOI] [PubMed] [Google Scholar]
- 78.Füllhase C, Russo A, Castiglione F, Benigni F, Campeau L, Montorsi F, et al. Spinal Cord FAAH in Normal Micturition Control and Bladder Overactivity in Awake Rats. J Urol. 2012;189:2364–70. doi: 10.1016/j.juro.2012.11.165. [DOI] [PubMed] [Google Scholar]


