Abstract
Most men will develop histological BPH if they live long enough. Approximately, half will develop benign prostatic enlargement (BPE) and about half of these will get BOO with high bladder pressures and low flow, this in turn leads to detrusor wall hypertrophy. Many of these men will only have lower urinary tract symptoms (LUTS) but a significant number will also suffer the other complications of BPH. These include urinary retention (acute and chronic), haematuria, urinary tract infection, bladder stones, bladder wall damage, renal dysfunction, incontinence and erectile dysfunction. Recognition of the complications of BPH/BOO early allows more effective management of these complications. This is particularly important for the more serious urinary infections and also for high-pressure chronic retention (HPCR). Complications of LUTS/BPH are very rare in clinical trials because of their strict inclusion and exclusion criteria but are more common in real life practice.
Keywords: Bladder calculi, renal dysfunction, urinary retention, urinary tract infection
INTRODUCTION
LUTS/BPH is a condition that affects an increasing number of men within the aging population. In 1995, a population based cross-sectional study found roughly 5.6 million white men aged between 50 and 79 living in the United States were suitable for treatment for BPH based on guidelines written by Agency for Health Care Policy and Research. It is thought this figure will be doubled by 2020. The causes of LUTS however involve more than just the prostate. It can also be caused by diseases of the central nervous, endocrine, cardiac and renal systems as well as the bladder[1] [Figure 1].
Figure 1.
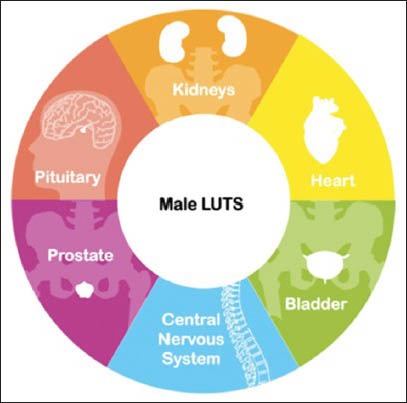
The causes of LUTS and their potential complications are multifactorial
The most common presenting complication of BPH that requires hospitalization is acute urinary retention, which greatly affects patients’ quality of life and is an important health issue. Many of the other complications of BPH/BOO are in part due to complications of chronic urinary retention; these include recurrent urinary tract infections (UTIs), formation of bladder calculi, hematuria, and damage to bladder wall and kidneys. Finally, there is an important association between BPH/BOO and male erectile dysfunction. The relationships between LUTS/BPH/BOO and their complications are complex and some of these associations are shown in Figure 2.
Figure 2.
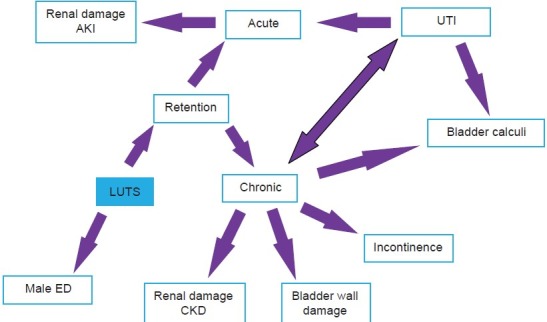
Some of the associations and links between the complications of LUTS AKI-Acute kidney Injury, CKD- Chronic kidney disease, ED- Erectile dysfunction, UTI-Urinary tract infection
Figure 3.
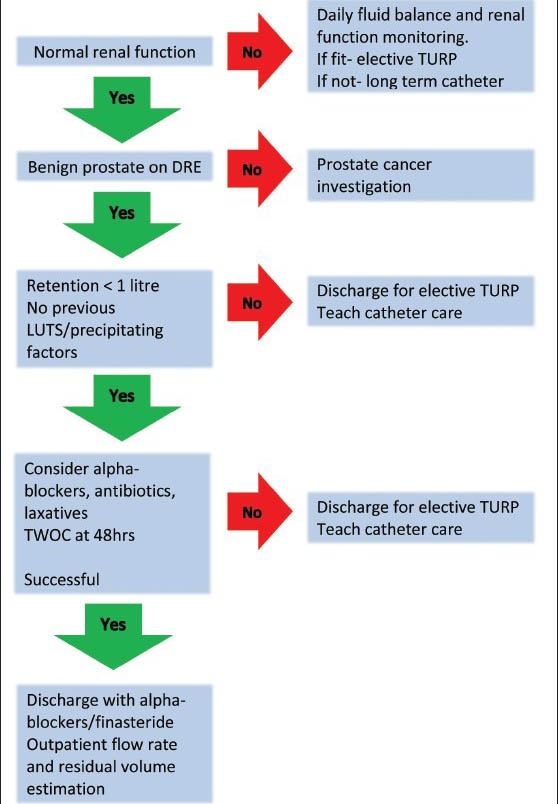
Management of AUR adapted from O Kalejaiye and MJ Speakman[16], TURP Transurethral resection of the Prostate
Figure 4.
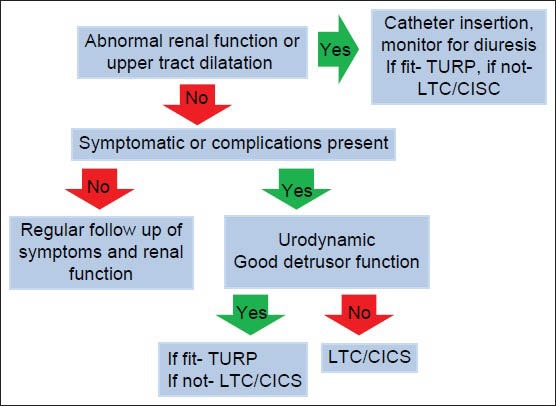
Management of CUR adapted from O Kalejaiye and MJ Speakman[16], LTC Long term catheter, CISC clean intermittent self catheterisation, TURP Transurethral resection of prostate
Complications are rare in clinical trials; for example in the famous MTOPS trial, of the 737 patients on placebo; only one patient got recurrent UTIs and no patient developed renal insufficiency.[2] In real life practice admissions with retention, frank hematuria, recurrent infections, and acute kidney injury are a regular event.
Urinary retention
Urinary retention is the inability to completely empty the bladder and can be defined as acute, chronic or acute on chronic. Acute urinary retention (AUR) is sudden onset and painful due to stretching of the bladder caused by overfilling; the pain can be so severe that it is likened to that of renal colic or childbirth.[3,4] Typically, patients present as a urological emergency with bladder volumes between 500 mls and 1 L. In chronic urinary retention (CUR) patients tend to suffer from incomplete voiding with significant post-void residual urine volumes (PVR) ranging between 300 ml and 1000 mls and present with acute on chronic retention. CUR is usually painless due to its slow onset and presents with higher retained volumes, between 450 mls and 4.5 L or more. The International Continence Society (ICS) published the definition of CUR as “a non-painful bladder, which remains palpable or percussable after the patient has passed urine, such patients may be incontinent.”[5]
Acute retention
AUR can be divided into precipitated where there is a triggering event or spontaneous, thought to be due to the natural course of BPH, which accounts for the vast majority.[6,7] The triggering event can be surgery (effects of general anaesthetic, pain, prolonged immobilisation, opiate analgesia or periods of anaesthesia, most typically more than 60 min),[8,9] excessive fluid intake (especially alcohol which is a sympathetic stimulator), urinary tract infections (UTI) or medications with sympathomimetic or anticholinergic effects.[3] Table 1 summarizes the causes of AUR and the percentage each cause accounts for in men with lower urinary tract symptoms (LUTS) in Europe, Latin America, Middle-East, Asia, and Canada.[10]
Table 1.
Causes of AUR in 170 men with LUTS in Europe, Latin America, Middle-East, Asian and Canada
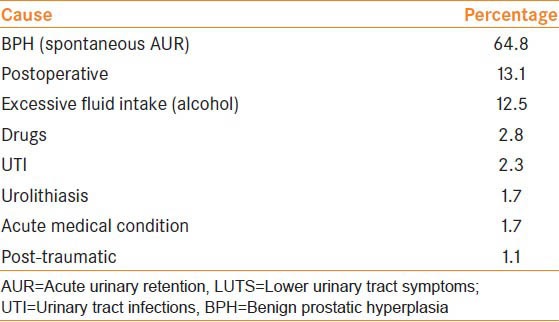
It is important to differentiate between spontaneous and precipitated AUR due to its clinical implications. It has been found in spontaneous AUR that 15% of patients go on to experience another episode of retention and 75% underwent surgery. In precipitated AUR, 9% of patients experienced another episode and only 26% required surgery.[11]
The assessment of AUR involves examination of the patient's abdomen for a palpable suprapubic mass that is dull to percussion. A bladder scanner is helpful to confirm the diagnosis and a digital rectal examination should be carried out to assess the size and texture of the prostate and the presence or absence of constipation. The initial treatment for AUR is urgent catheterization (urethral or suprapubic) and the volume drained in the first 10-15 min (residual volume) should be accurately recorded to aid the diagnosis of AUR or acute on chronic retention. The National Prostatectomy Audit reported greater mortality and morbidity in patients who underwent prostatectomy immediately after AUR in comparison to those who underwent elective surgery for symptomatic relief.[12]
Chronic retention
CUR can be divided into high-pressure chronic retention (HPCR) and low-pressure chronic retention (LPCR).[13,14,15] The term high or low pressure refers to the subtracted detrusor pressure at the end of micturition.[14,15] In HPCR, there is usually bladder outflow obstruction (BOO) and therefore high voiding detrusor pressure with poor flow rate leading to persistently high pressure within the bladder causing retrograde pressure and bilateral hydronephrosis. The latter can lead to varying degrees of renal dysfunction. In LPCR, the bladder tends to be floppy, more compliant and therefore there is no increased pressure on the upper tracts. Both types of CUR can lead to nocturnal enuresis where bladder pressure overcomes urethral resistance.[16]
Patients with CUR are unable to empty their bladder to completion; they can be asymptomatic or complain of increased frequency, poor flow, hesitancy, and nocturnal incontinence. The initial assessment of CUR is similar to that of AUR. Urinalysis should be performed to rule out infection and measurements of renal function, such as creatinine and eGFR. In patients with high volume retention and deranged renal function a renal ultrasound should be carried out. Unless there are signs of renal dysfunction — catheterization is usually less urgent. Patients with HPCR must be monitored closely for signs of post-obstructive diuresis where excessive urine output occurs within the first few days. It should be noted that post-catheterization, hematuria can be expected due to decompression of the upper tracts and usually settles within 48-72 h without need for slow decompression or other intervention.[17] Provided the patient is fit, long term management for CUR is to perform a prostatectomy, however, if they are not, then a long term catheter (LTC) or learning intermittent self-catheterization (ISC) is suitable.
Trial without catheter
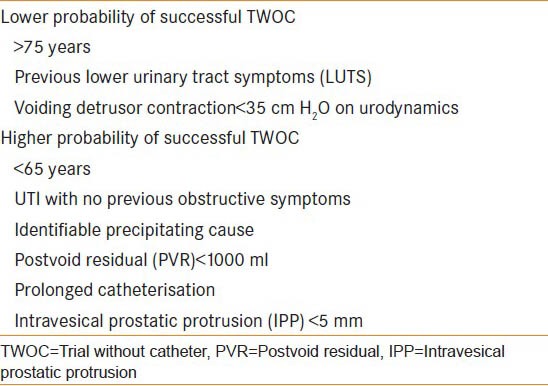
Trial without catheter is usually carried out 1-3 days post-catheterization and should be considered for patients with AUR and LPCR.[7,18] It is increasingly common as it allows 23-40% of patients to void and therefore decrease the complications associated with a catheter in situ.[3,19] Factors leading to less and more successful TWOC are summarized in Table 2. There is a commonly held belief that the use of a flip-flow catheter valve, by allowing the bladder to fill and empty, may also promote a better chance of a successful TWOC and patients certainly prefer this method.
Table 2.
Factors contributing to low and high probability of successful TWOC
Despite an initial successful TWOC, 50% of men will have another episode of AUR over the next year and a third of men will require surgery within 6 months. Therefore, it is important to provide adequate follow up for patients experiencing recurrent AUR despite successful TWOC.[3,19,20]
HPCR and TWOC
In patients with HPCR and evidence of renal dysfunction, TWOC should not be carried out even if their renal function settles with catheterization. This is due to the high risk of progressive renal failure.
Alpha-blockers and TWOC
Alpha-blockers such as tamsulosin and alfuzosin (2 or more doses) have been used before removing catheters to increase the likelihood of successful TWOC in both AUR and CUR. A study looking at the effects of alfuzosin in 360 men with first presentation of spontaneous AUR, where 236 were randomly assigned 10 mg alfuzosin and 121 received placebo, found alfuzosin almost doubled the chances of successful TWOC (OR 1.98, 95% CI 1226 to 3127).[21] A cochrane systematic review was carried out in 2009 where five randomized clinical trials using alpha-blockers pre-TWOC were reviewed (four trials used alfuzosin and one tamsulosin). The overall findings favored the use of alpha-blockers in comparison to placebo regardless of alpha-blocker used (alfuzosin: RR 1.31, 95% CI 1.10 to 1.56; tamsulosin: RR 1.86, 95% CI 1.17 to 2.97).[22] The use of alpha-blockers before and during TWOC means more patients are able to be discharged without the need of a catheter reducing the complications and discomfort associated with catheters.
Urinary tract infection
Urinary tract infection (UTI) can be classified into simple or complicated. Complicated UTI is secondary to structural or functional abnormality of the genitourinary system. Examples are prostate hyperplasia, urolithiasis, and instrumentation secondary to catheter insertion. Clinically, these patients tend to experience recurrent episodes of infection. These are not present in simple infections.[23]
Urinary infections associated with benign prostatic hyperplasia (BPH) occur as patients are unable to completely empty their bladder and the stagnant urine acts as a growth medium for bacteria. Although infections can be asymptomatic, symptoms can range from mild dysuria, frequency and urgency to severe systemic infection and frank hematuria causing acute retention.
Many organisms are responsible for complicated urinary infection and tend to be more resistant to antimicrobials.[24] Most commonly isolated organism is Escherichia Coli, which is more commonly found in women than in men.[25,26,27] In patients with indwelling catheters urease producing organisms such as Proteus mirabilis, Providencia Stuartii, and Morganella morganii are most prevalent. Other causative organisms for UTIs are Pseudomonas aeruginosa, Klebsiella pneumonia and Gram positive organisms as well as yeasts.
The management of complicated UTI includes assessing the patient clinically, performing a urine dipstick, collecting mid-stream urine (MSU) sample for culturing and commencing empirical antimicrobial treatment (as per hospital guideline) until culture results are available. There is no proven benefit for treating asymptomatic patients with indwelling catheters.[27] Patients with large residual volumes however can benefit considerably from learning clean intermittent self-catheterization. Patients with LUTS/BPH who experience frequent, recurrent and symptomatic UTI should be considered for long-term suppressive antimicrobial treatment. There is no determined regimen (dose and duration) or preferred antibiotic, therefore individual hospital guidelines should be consulted.
Hematuria
Hematuria is a recognised complication of BPH/BOO, and it is also an indication for secondary care referral from general practitioner LUTS management. It is usually caused by the friable hypervascularity of the enlarged prostate, the superficial vessels being disrupted by physical activity. It can occasionally result in clot retention, but generally it presents as initial hematuria with the subsequent urinary stream being clearer. Finasteride has been suggested as a treatment for BPH related hematuria as it may lower the microvessel density (MVD) and vascular endothelial growth factor (VEGF)[28]
Bladder calculi
In the Western world, bladder calculi account for 5% of all urinary tract calculi and BOO is a known risk factor along with chronic urinary infections due to urea-splitting organisms such as Proteus mirabilis.[29,30] Bladder calculi are usually preceded by recurrent UTI, residual volume in the bladder[31,32] and are a strong indication for outflow tract surgery. Multiple stones are more common in cases of large residual volume; incidences ranging between 25% and 30%.[32] The clinical presentation of bladder calculi include visible or non-visible hematuria, abdominal pain, urinary retention secondary to obstruction, recurrent UTI, and signs of sepsis in the most severe cases.
Investigations include urine dipstick, X-ray and ultrasound of kidneys, ureters and bladder. Small bladder stones can pass spontaneously, whereas larger bladder stones will need to be fragmented using endoscopic litholopaxy or by open cystolithotomy when large, this is usually associated with outflow tract surgery such as TURP. If prostate volume is greater than 70-100 cm3 and large stones are present then open surgery with open prostatectomy or possibly HoLEP laser enucleation and laser fragmentation would be indicated.[33]
Bladder wall damage
BPE causes increased intra-vesical pressure due to BOO which leads to a number of changes in the bladder wall. One of the main and well known changes is detrusor muscle hypertrophy. Using ultrasound to assess the morphology of hypertrophic bladders, it was found that in severe hypertrophic bladders (weight estimated to be 60 gm or more); there is an increase in the ratio of connective tissue to smooth muscle to greater than 30%.[34] It has also been suggested that initially in BPH/BPE, there is an increase in smooth muscle and a later increase in connective tissue. Overall, the reason for this is the need to overcome the resistance to emptying the bladder and therefore in part, it can be regarded as a positive compensatory mechanism. A positive correlation has been found between the ultrasound-estimated-bladder weight (UEBW) and bladder wall thickness and the severity of obstruction. The effects of BPH/BOO on the bladder wall can also be confirmed by the post-surgical relief of obstruction, where there is a decrease in bladder wall thickness and UEBW.[35]
Bladder wall fibrosis has been reported as another complication of BPH/BOO due to deposition of collagenous protein and other matrix constituents in response to recurrent ischemia/reperfusion injury caused by partial outlet obstruction. The process by which fibrosis occurs in the bladder is thought to be similar to the rest of the body where initially there is enhanced production of type III collagen followed by predominance of type I collagen in later stages. This leads to decreased bladder wall compliance and results in trabeculation and abnormal infiltrations of connective tissue. As a result, there is a low-volume, high pressure, and non-compliant bladder.[36] This is not a universal finding however.
Renal damage
Although AUR seen in BPH is sometimes associated with acute renal failure presumed secondary to obstructive uropathy, it is felt these patients most likely have underlying chronic renal failure prior to presentation. Chronic renal failure, also known as chronic kidney disease (CKD) is defined as glomerular filtration rate (GFR) less than 60 ml per minute per 1.73 m2 for 3 months or more,[34] has been linked with BPH. The main pathophysiological finding of patients with BPH presenting with CKD is chronic interstitial nephritis.[37]
CUR is the main mechanism for development of CKD in patients with LUTS/BPH. Upper tract dilatation or increased serum creatinine have been found in half of all patients with CUR secondary to BPH.[38,39] There is also an association between CUR and decreased GFR. Recurrent UTI seen in CUR is another contributing factor for progress to CKD and has been shown to be a predictor of renal failure in men with BPH.
Diagnosis of acute or chronic renal failure secondary to BPH traditionally involves imaging of the urinary tracts to check for hydronephrosis and also measures of eGFR and serum creatinine. Renal ultrasound is the favored method of imaging despite poorer images as it avoids the risk of contrast nephropathy seen in contrast CT scanning. The management of renal dysfunction involves treating the underlying condition — in this case BPH/BPE. This is most successfully done by performing TURP, which is proven to improve renal function post-surgery. If renal function does not respond to TURP, some patients may require ureteric stents to relieve the obstruction at the ureterovesical junction secondary to detrusor hypertrophy.[39]
Incontinence
BPH is not always recognized as a cause of incontinence in men, but BOO can result in the overactive bladder syndrome with urgency, with or without urgency incontinence as well as “overflow” incontinence in men with chronic retention, most commonly seen in men during the night.
Male erectile dysfunction
Several epidemiological studies have indicated that the association between LUTS and ED is more than a co-incidence of age, with a possible cause and effect relationship.[40] LUTS is more common in men with ED and there is a strong relationship between the severity of LUTS and the degree of erectile difficulty. Four pathophysiologies have been suggested to explain the relationship between LUTS and ED. There is a complex interaction between these mechanisms and there may be additional processes involved. These are: (1) alteration in nitric oxide levels; (2) autonomic hyperactivity; (3) changes in the Rho-kinase/endothelin pathway; and (4) pelvic vasculature atherosclerosis. It is therefore recommend that patients seeking consultation for one condition should always be screened for the other condition. Therefore, combination therapy with an alpha-blocker and a PDE-5 inhibitor appears appropriate for patients with both LUTS and ED. More recently tadalafil has been approved in many countries for the treatment of either LUTS or ED or the combination of conditions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Speakman M. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH): More than treating symptoms? Eur Urol Suppl. 2008;7:680–9. [Google Scholar]
- 2.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick J, Kirby R. Management of acute urinary retention. BJU Int. 2006;97(Suppl 2):16–20. doi: 10.1111/j.1464-410X.2006.06100.x. [DOI] [PubMed] [Google Scholar]
- 4.Thomas K, Oades G, Taylor-Hay C, Kirby RS. Acute urinary retention: What is the impact on patients’ quality of life? BJU Int. 2005;95:72–6. doi: 10.1111/j.1464-410X.2004.05254.x. [DOI] [PubMed] [Google Scholar]
- 5.The Standardisation of lower urinary tract function. [Last accessed on 2013 Nov 1]. Available from: http://www.ics.org/Documents/DocumentsDownload.aspx?DocumentID=22 .
- 6.Murray K, Massey A, Feneley RC. Acute urinary retention: A urodynamic assessment. Br J Urol. 1984;56:468–73. [PubMed] [Google Scholar]
- 7.Desgrandchamps F, De la Taille A, Doublet J. Management of acute urinary retention in France: A cross-sectional survey in 2618 men with benign prostatic hyperplasia. BJU Int. 2006;97:727–33. doi: 10.1111/j.1464-410X.2006.06109.x. [DOI] [PubMed] [Google Scholar]
- 8.Petros JG, Rimm EB, Robillard RJ, Argy O. Factors influencing postoperative urinary retention in patients undergoing elective inguinal herniorrhaphy. Am J Surg. 1991;161:431–3. doi: 10.1016/0002-9610(91)91105-r. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi-Fallah M, Hamedanchi S, Tayyebi-Azar A. Preventive effect of tamsulosin on postoperative urinary retention. Korean J Urol. 2012;53:419–23. doi: 10.4111/kju.2012.53.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elhilali M, Vallancien G, Emberton M, Harving N, van Moorselaar J, Matzkin H, Alcaraz A, et al. Management of acute urinary retention (AUR) in patients with BPH. a worldwide comparison. J Urol. 2004;171(Suppl):407. A1544, 407. [Google Scholar]
- 11.Roehrborn CG, Bruskewitz R, Nickel GC, Glickman S, Cox C, Anderson R, et al. Urinary retention in patients with BPH treated with finasteride or placebo over 4 years. Characterization of patients and ultimate outcomes. The PLESS study group. Eur Urol. 2000;37:528–36. doi: 10.1159/000020189. [DOI] [PubMed] [Google Scholar]
- 12.Pickard R, Emberton M, Neal DE. The management of men with acute urinary retention. National Prostatectomy Audit Steering Group. Br J Urol. 1998;81:712–20. doi: 10.1046/j.1464-410x.1998.00632.x. [DOI] [PubMed] [Google Scholar]
- 13.Ghalayini IF, Al-Ghazo MA, Pickard RS. A prospective randomized trial comparing transurethral prostatic resection and clean intermittent self-catheterization in men with chronic urinary retention. BJU Int. 2005;96:93–7. doi: 10.1111/j.1464-410X.2005.05574.x. [DOI] [PubMed] [Google Scholar]
- 14.Abrams P, Dunn M, George N. Urodynamic findings in chronic retention of urine and their relevance to results of surgery. BMJ. 1978;2:1258–60. doi: 10.1136/bmj.2.6147.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George NJ, O’Reilly PH, Barnard RJ, Blacklock NJ. High pressure chronic retention. Br Med J (Clin Res Ed) 1983;286:1780–3. doi: 10.1136/bmj.286.6380.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalejaiye O, Speakman MJ. Management of acute and chronic retention in men. Eur Urol Suppl. 2009;8:523–9. [Google Scholar]
- 17.Boettcher S, Lazica DA, Roth S. First randomized controlled trial to compare rapid vs. gradual decompression of urinary retention - the mythical danger of rapid emptying of the extended bladder. Eur Urol Suppl. 2012;11:1. 635. [Google Scholar]
- 18.Fitzpatrick JM, Desgrandchamps F, Adjali K, Gomez Guerra L, Hong SJ, El Khalid S, et al. Management of acute urinary retention: A worldwide survey of 6074 men with benign prostatic hyperplasia. BJU Int. 2012;109:88–95. doi: 10.1111/j.1464-410X.2011.10430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emberton M, Fitzpatrick J. The Reten-World survey of the management of acute urinary retention: Preliminary results. BJU Int. 2008;101(Suppl 3):2732. doi: 10.1111/j.1464-410X.2008.07491.x. [DOI] [PubMed] [Google Scholar]
- 20.Holtgrewe HL, Mebust WK, Dowd JB, Cockett AT, Peters PC, Proctor C. Transurethral prostatectomy: Practice aspects of the dominant operation in American urology. J Urol. 1989;141:248–53. doi: 10.1016/s0022-5347(17)40732-4. [DOI] [PubMed] [Google Scholar]
- 21.McNeill SA, Hargreave TB Members of the ALFAUR study group. Alfuzosin once daily facilitates return to voiding in patients in acute urinary retention. J Urol. 2004;171:2316–20. doi: 10.1097/01.ju.0000127743.80759.7a. [DOI] [PubMed] [Google Scholar]
- 22.Zeif HJ, Subramonian K. Alpha blockers prior to removal of a catheter for acute urinary retention in adult men. Cochrane Database Syst Rev. 2009;4:CD006744. doi: 10.1002/14651858.CD006744.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Nicolle LE AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16:349–60. doi: 10.1155/2005/385768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raz R, Schiller D, Nicolle LE. Chronic indwelling catheter replacement before antimicrobial therapy for symptomatic urinary tract infection. J Urol. 2000;164:1254–8. [PubMed] [Google Scholar]
- 25.Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am. 1997;11:647–67. doi: 10.1016/s0891-5520(05)70378-0. [DOI] [PubMed] [Google Scholar]
- 26.Bakke A, Digranes A. Bacteriuria in patients treated with clean intermittent catheterization. Scand J Infect Dis. 1991;23:577–82. doi: 10.3109/00365549109105181. [DOI] [PubMed] [Google Scholar]
- 27.Bennett CJ, Young MN, Darrington H. Differences in urinary tract infections in male and female spinal cord injury patients on intermittent catheterization. Paraplegia. 1995;33:69–72. doi: 10.1038/sc.1995.17. [DOI] [PubMed] [Google Scholar]
- 28.Kashif KM, Foley SJ, Basketter V, Holmes SA. Haematuria associated with BPH-Natural history and a new treatment option. Prostate Cancer Prostatic Dis. 1998;1:154–6. doi: 10.1038/sj.pcan.4500224. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz BF, Stoller ML. The vesical calculus. Urol Clin North Am. 2000;27:333–46. doi: 10.1016/s0094-0143(05)70262-7. [DOI] [PubMed] [Google Scholar]
- 30.Lingeman JE, Lifshitz DA, Evan AP. Surgical management of urinary lithiasis. In: Walsh PC, Vaughan D, Retik AB, editors. Campbell's Urology. 8th ed. Philadelphia: WB Saunders; 2002. pp. 3384–7. [Google Scholar]
- 31.De la Rossette J, Alivizatos G, Madersbacher S, Rioja Sanz C, Nordling J, Emberton M, Gravas S, Michel MC, Oelke M. Guidelines on Benign Prostatic Hyperplasia. EAUguideline. 2002. [Last accessed date on 2013 Sep 30]. http://www.uroweb.org/fileadmin/user_upload/Guidelines/11%20BPH.pdf .
- 32.Larsen EH, Bruskewitz RC. Urodynamic evaluation of male outflow obstruction. In: Krane RJ, Siroky B, editors. Clinical Neurourology. New York: Lippincott Williams and Wilkins; 1991. pp. 427–43. [Google Scholar]
- 33.Sofer M, Kaver I, Greenstein A, Bar Yosef Y, Mabjeesh NJ, Chen J, et al. Refinements in treatment of large bladder calculi: Simultaneous percutaneous suprapubic and transurethral cystolithotripsy. Urology. 2004;64:651–4. doi: 10.1016/j.urology.2004.04.067. [DOI] [PubMed] [Google Scholar]
- 34.Inui E, Ochiai A, Naya Y, Ukimura O, Kojima M. Comparative morphometric study of bladder detrusor between patients with benign prostatic hyperplasia and controls. J Urol. 1999;161:827–30. [PubMed] [Google Scholar]
- 35.Mirone V, Imbimbo C, Longo N, Fuscoo F. The detrusor muscle: An innocent victim of bladder outlet obstruction. Eur Urol. 2007;51:57–66. doi: 10.1016/j.eururo.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 36.Deveaud CM, Macarak EJ, Kucich U, Ewalt DH, Abrams WR, Howard PS. Molecular analysis of collagens in bladder fibrosis. J Urol. 1998;160:1518–27. [PubMed] [Google Scholar]
- 37.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 38.Styles RA, Ramsden PD, Neal DE. Chronic retention of urine. The relationship between upper tract dilatation and bladder pressure. Br J Urol. 1986;58:647–51. doi: 10.1111/j.1464-410x.1986.tb05904.x. [DOI] [PubMed] [Google Scholar]
- 39.Rule AD, Lieber MM, Jacobsen SJ. Is benign prostatic hyperplasia a risk factor for chronic renal failure? J Urol. 2005;173:691–6. doi: 10.1097/01.ju.0000153518.11501.d2. [DOI] [PubMed] [Google Scholar]
- 40.Speakman MJ. PDE5 inhibitors in the treatment of LUTS. Curr Pharm Des. 2009;15:3502–5. doi: 10.2174/138161209789207051. [DOI] [PubMed] [Google Scholar]


