SUMMARY
Phototherapy is a powerful noninvasive treatment approach for cancer treatment, with several agents currently being in clinical use. Despite the progress and promise, most current phototherapy agents have serious side effects as they can lead to damage to healthy tissue, even when the photosensitizers is fused to targeting molecules, due to non-specific light activation of the unbound photosensitizer. To overcome these limitations, we develop a phototherapy agent that combines a functional ligand and a near infrared phthalocyanine dye. The target we focus on here is type 2 cannabinoid receptor (CB2R) which has been considered an attractive therapeutic target for phototherapy given it is over-expressed by many types of cancers which are located at a surface or can be reached by an endoscope. We show that our CB2R-targeted phototherapy agent, IR700DX-mbc94, is specific for CB2R and effective only when bound to the target receptor, suggesting a receptor-selective phototherapy mechanism and eliminating key side effects. Overall, this opens up opportunity for development of an alternative treatment option for CB2R positive cancers.
Keywords: receptor-targeted, phototherapy, CB2, cancer, photosensitizer
INTRODUCTION
The therapeutic value of light has been known for more than three thousand years while a systematic understanding of phototherapy has only been established over the past century (Dolmans, et al., 2003; Tong and Kohane, 2012). Two types of phototherapy have been particularly well studied: photodynamic therapy (PDT) and photothermal therapy (PTT). As the conventional phototherapy, PDT destroys neoplastic lesions with reactive oxygen species (ROS), which are produced by using light of specific wavelengths to irradiate the PDT agents (Oleinick, et al., 2002). The main classes of PDT agents include porphyrin derivatives, chlorins, porphycenes and phthalocyaines (De Rosa and Bentley, 2000). PDT has now been clinically used as a noninvasive technique for cancer treatment, mainly in dermatology, ophthalmology and gastroenterology. To date, several PDT agents have been approved by the FDA to treat cancer, such as Photofrin, Levulan, Metvix and Foscan (Triesscheijn, et al., 2006). PTT ablates tumors by converting photonic energy to heat instead of ROS (Jori and Spikes, 1990). By causing temperatures to rise, PTT leads to irreversible damage to cancer cells (Tong and Kohane, 2012).
Despite the advances in phototherapy techniques, PDT and PTT have significant limitations. Most existing PDT and PTT agents lack tumor selectivity; therefore, normal tissues can also be damaged, leading to considerable side effects (Mitsunaga, et al., 2011; Tong and Kohane, 2012). Although targeting molecules, such as antibodies (Copland, et al., 2004; Lukianova-Hleb, et al., 2011), antibody fragments (Qian, et al., 2008), receptor ligands (Chen, et al., 2013), and peptides (Kim, et al., 2011; Kumar, et al., 2012) can be attached to photosensitizers for targeted delivery, unbound photosensitizers can still damage normal tissues upon light irradiation. As such, it is critical to develop highly target-selective phototherapy approaches.
Recently, Kobayashi and co-workers described a highly target-selective phototherapy approach, photoimmunotherapy (PIT), which uses target-specific photosensitizers based on monoclonal antibodies coupled with a near infrared (NIR) phthalocyanine dye, IR700DX (Mitsunaga, et al., 2011). Unlike conventional PIT technique (Goff, et al., 1991; Mew, et al., 1983), the new PIT agents reported by Kobayashi’s group caused effective therapeutic effect only when they bound to target cancer cells while unbound sensitizers did not produce phototoxicity. Considering the high cost and long circulation time of antibodies (Olafsen and Wu, 2010), we set out to investigate whether target-selective phototherapy can be achieved using small photosensitizers based on receptor ligands.
Here we report the first type 2 cannabinoid receptor (CB2R)-targeted phototherapy study using a novel phototherapy agent, IR700DX-mbc94. CB2R is a transmembrane G protein-coupled receptor (GPCR) that was first cloned in 1993 (Munro, et al., 1993). Under basal conditions, CB2R is expressed mainly in immune cells while the expression in other types of cells is low to undetectable (Munro, et al., 1993; Sexton, et al., 2011). However, CB2R is over-expressed by many types of cancers, such as prostate, skin, liver, and breast cancer, and moreover, the expression levels of CB2R appear to be associated with tumor aggressiveness (Qamri, et al., 2009; Velasco, et al., 2012; Xu, et al., 2006). Such up-regulation of the receptor in cancer cells provides an opportunity to specifically target CB2R, and thus lower the side effects. Importantly, because many types of the CB2R positive cancers are superficial or can be reached by endoscope, CB2R has great potential as a phototherapy target. We previously reported a NIR CB2R-targeted imaging probe, NIR-mbc94, and validated its specific binding to CB2R in vitro (Bai, et al., 2008; Sexton, et al., 2011). More recently, we reported the first CB2R-targeted in vivo optical imaging using another NIR fluorescent probe, NIR760-mbc94 (Zhang, et al., 2013). In this study, we developed IR700DX-mbc94 as the first CB2R receptor-targeted photosensitizer. We found that IR700DX-mbc94 caused significant cancer cell death only when bound to the target receptor. This novel photosensitizer appears to have great potential in cancer phototherapy with high target specificity.
RESULTS AND DISCUSSION
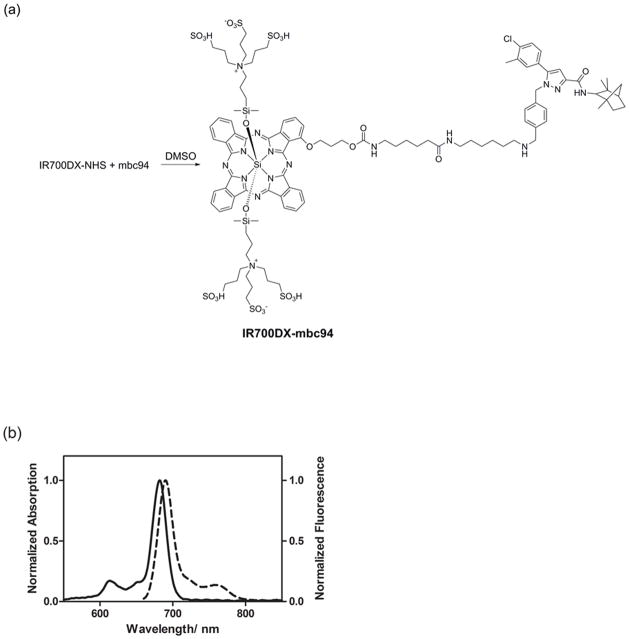
IR700DX-mbc94 was synthesized by coupling IR700DX with mbc94, a conjugable CB2R ligand previously reported by us (Figure 1a) (Bai, et al., 2008; Sexton, et al., 2011). As displayed in Figure 1b, IR700DX-mbc94 exhibited an intense NIR absorption centered at 682 nm and maximum emission at 690 nm in methanol. The relatively small Stokes shift is typical for phthalocyanine dyes (Li, et al., 2008).
Figure 1. Synthesis and photophysical properties of IR700DX-mbc94.
(a) Synthesis of IR700DX-mbc94. (b) Absorption (solid) and emission (dotted) spectra of IR700DX-mbc94 in MeOH at a concentration of 1 μM (λex=640 nm).
To study the phototherapeutic effect of IR700DX-mbc94, we used CB2-mid DBT cells, a transfected mouse malignant astrocytoma cell line that expresses CB2R at the endogenous levels. Wild type (WT) DBT cells that has no CB2R expression was used as negative control (Cudaback, et al., 2010).
To measure the cytotoxicity of IR700DX-mbc94 in the absence of light irradiation in CB2-mid DBT cells, we used the CellTiter-Glo Luminescent Cell Viability Assay kit. As shown in Figure S1a, cells incubated with as high as 10 μM of IR700DX-mbc94 exhibited comparable low cytotoxicity to those treated with indocyanine green (ICG), the only FDA approved NIR fluorescent dye. We also found that the cytotoxic effect of IR700DX-mbc94 was significantly lower than that of mbc94 alone. The relatively high cytotoxicity of mbc94 was expected as it had been reported that many CB2R ligands exhibited anti-cancer properties (Velasco, et al., 2012). It is possible that attaching IR700DX to mbc94 changed the binding profile of mbc94, leading to decreased CB2R activation and therapeutic effect. In addition, to show the toxicity of IR700DX-mbc94 in non-cancerous cells that express the CB2R, we used CB2R-transfected Chinese hamster ovarian (CHO-K1/CB2) cells (Gertsch, et al., 2008). The cytotoxicity of IR700DX-mbc94 in CHO-K1/CB2 cells was as low as that in CB2-mid DBT cells (Figure S1b). These combined data suggest that IR700DX-mbc94 is a safe agent in the absence of light irradiation.
To determine IR700DX-mbc94’s binding affinity to CB2R in intact cells, we used an in vitro saturation binding assay to measure the equilibrium dissociation constant (Kd) and the maximum specific binding (Bmax). A representative saturation binding curve is shown in Figure 2. From this data we estimated that IR700DX-mbc94 bound to CB2R with a Kd of 42.0 nM (± 19.6) and Bmax of 650.5 pmol/mg (± 93.1). Bmax over Kd value was roughly 15, indicating favorable binding for in vitro phototherapy experiments and future in vivo imaging studies.
Figure 2. In vitro CB2R saturation binding assay of IR700DX-mbc94.
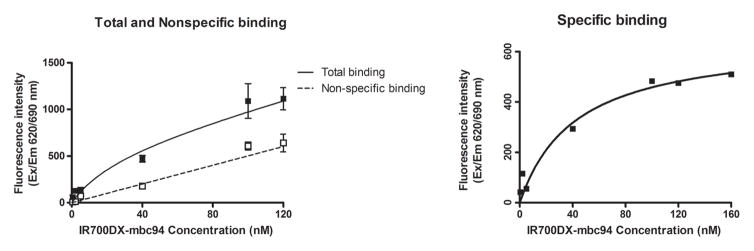
Cells were preincubated with (non-specific binding) or without (total binding) 1 μM of the blocking agent SR144528 for 1 h, and then incubated overnight with an increasing concentration of IR700DX-mbc94 at 37 °C. Cells were then rinsed and the fluorescence intensity was recorded. The specific binding was obtained by the subtraction of non-specific binding from the total binding. The dissociation constant (Kd) and receptor density (Bmax) were estimated from the non-linear fitting of specific binding versus IR700DX-mbc94 concentration using Prism software. Each data point represents the mean ± SD based on triplicate samples.
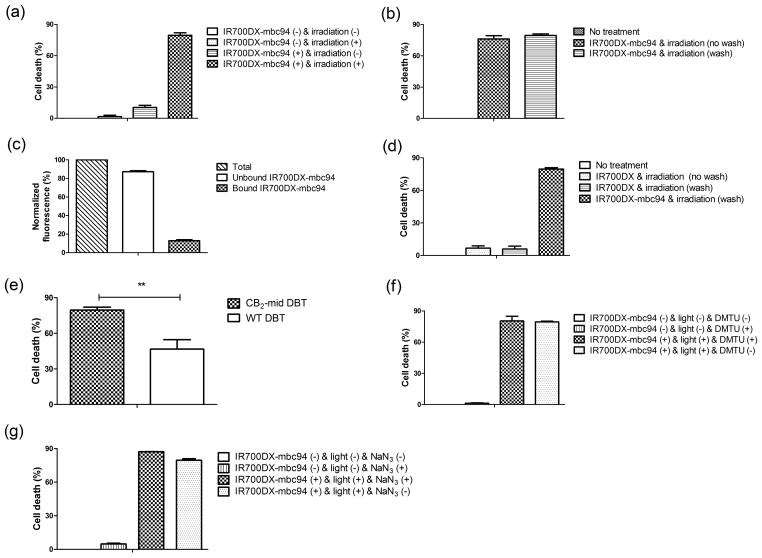
We then carried out in vitro phototherapy experiments using IR700DX-mbc94 and light irradiation. CB2-mid DBT cells were incubated with 5 μM of IR700DX-mbc94 at 37 °C overnight. Cells were then washed twice and irradiated with light from an LED light source at wavelengths of 670–710 nm (peak at 690 nm) and a power density of 30 mW/cm2. As shown in Figure 3a, CB2-mid DBT cells incubated with IR700DX-mbc94 did not show significant cell death (1.6% ± 1.4%) without light irradiation; however, irradiation of light for 20 minutes eradicated 79.5% ± 2.4% of the living cells. Similarly, there was little cell death associated with light irradiation in the absence of IR700DX-mbc94 (10.4% ± 2.0%). In addition, unbound IR700DX-mbc94 did not appear to contribute to the therapeutic effect, as we observed a similar level of cell death whether unbound IR700DX-mbc94 was washed (79.5% ± 2.4% cell death) or not (76.2 ± 5.3% cell death) (Figure 3b). We found that as much as 87.2% ± 2.0% of IR700DX-mbc94 was washed out (Figure 3c). To further investigate the target-specific phototherapy effect of IR700DX-mbc94, we compared phototoxicity between free IR700DX dye and IR700DX-mbc94. As expected, CB2-mid DBT cells incubated with the same concentration of free IR700DX (5 μM) showed minimal cell death under light irradiation, whether the cells were washed (6.0% ± 4.5% cell death) or not (6.7% ± 3.7% cell death) (Figure 3d). We also studied the role of IR700DX-mbc94 binding in phototherapy by comparing the therapeutic effect on CB2-mid and WT DBT cells. As shown in Figure 3e, WT-DBT cells treated with IR700DX-mbc94 followed by light irradiation showed significantly less cell death than CB2-mid DBT cells (46.7% ± 7.9% vs 79.5% ± 2.4%, p=0.002). This provides additional evidence that the ability of IR700DX-mbc94 to kill cancer cells required receptor binding. These combined data indicate that IR700DX-mbc94 causes cell death in a target-specific manner. Additionally, increased cell death was observed with an increased dose of light irradiation, higher concentration of IR700DX-mbc94 or longer incubation time (Figure S2). Furthermore, the potential of IR700DX-mbc94 for treating tumors in vivo was preliminarily assessed using a mouse tumor model. CB2-mid DBT cells were subcutaneously injected into mice to grow tumor. We found that tumor growth was significantly inhibited in phototherapy-treated group using IR700DX-mbc94 compared with non-treatment group (average tumor volume: 134.98 mm3 in phototherapy-treated mice vs 780.16 mm3 in untreated mice at day 7, p = 0.03) (Figure S3). This indicates that IR700DX-mbc94 has great potential as a new phototherapy agent.
Figure 3. In vitro phototherapy using IR700DX-mbc94.
(a) Comparison of phototherapy effect on CB2-mid DBT cells among four groups: IR700DX-mbc94 (−) & irradiation (−); IR700DX-mbc94 (−) & irradiation (+); IR700DX-mbc94 (+) & irradiation (−); and IR700DX-mbc94 (+) & irradiation (+). (b) Phototherapy effect on CB2-mid DBT cells incubated with 5 μM of IR700DX-mbc94 with or without wash. (c) Quantification of unbound (photosensitizer that was washed out) and bound (remaining photosensitizer after washing) IR700DX-mbc94 using fluorescence. (d) Phototherapy effect compared between CB2-mid DBT cells treated with IR700DX-mbc94 (5 μM) and free IR700DX dye (5 μM). (e) Comparison of cell death caused by IR700DX-mbc94 exposed to light irradiation between CB2-mid DBT and WT-DBT cells (**p<0.01). (f) Effect of DMTU (1 mM) inhibition on cell death caused by IR700DX-mbc94 phototherapy. (g) Effect of sodium azide (50 mM) inhibition on cell death caused by IR700DX-mbc94 phototherapy. Each data point represents the mean ± SD based on triplicate samples.
To study the mechanism underlying phototherapy of IR700DX-mbc94, we first investigated whether the therapeutic effect correlated with the generation of reactive oxygen species (ROS), which trigger cell death during PDT. There are two types of PDT mechanisms: type I produces free radicals such as hydroxyl radicals (HO·), which can be inhibited by 1,3-Dimethyl-2-thiourea (DMTU) (Sagone, et al., 1989), and type II produces singlet oxygen (1O2) (Huang, et al., 2012), which can be scavenged by sodium azide (NaN3) (Mitsunaga, et al., 2011). Studies have shown that DMTU could pass through cell membrane (Lai, et al., 1999) and NaN3 is an effective scavenger of intracellularly generated singlet oxygen (Sparrow, et al., 2002). Therefore, although IR700DX-mbc94 is primarily located inside the cells as discussed below, DMTU and NaN3 are effective ROS inhibitors. We found that treatment of CB2-mid DBT cells with DMTU (1 mM) did not significantly affect the therapeutic effect of IR700DX-mbc94 under light irradiation (cell death 80.5% ± 7.6% with DMTU vs 79.4% ± 1.2% without DMTU, Figure 3f). In addition, the use of NaN3 (50 mM) did not reverse the therapeutic effect either (cell death 87.1% ± 0.1% with NaN3 vs 79.5% ± 2.4% without NaN3, Figure 3g). Based on the above data, it appears that the phototherapeutic mechanism of IR700DX-mbc94 is distinct from the traditional PDT effect. However, further studies are needed to elucidate the mechanism of IR700DX-mbc94’s therapeutic effect, which are beyond the scope of this study.
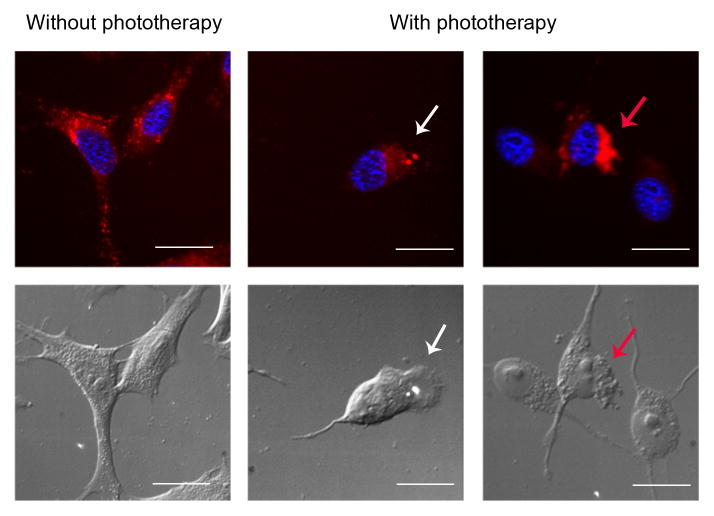
To visualize cell death caused by phototherapy of IR700DX-mbc94, CB2-mid DBT cells were imaged under fluorescence microscopy with or without phototherapy (Figure 4). Cells treated with IR700DX-mbc94 in the absence of irradiation exhibited a normal morphology, and IR700DX-mbc94 was mainly located in the cytoplasm. In contrast, cells treated with IR700DX-mbc94 with light irradiation showed morphological changes typically observed in necrotic cell death, such as small blebs formation, cytoplasmic swelling, cell membrane rupture and cell contents’ release (Golstein and Kroemer, 2007). These morphological changes are similar to those previously reported for PIT, in which an IR700DX-conjugated monoclonal antibody was used as the PIT probe (Mitsunaga, et al., 2011). To investigate whether internalization of IR700DX-mbc94 played an important role in the phototherapy effects, we used LysoTracker to find out if some IR700DX-mbc94 molecules were internalized and ended in lysosomal degradation. We observed certain co-localization (orange color) of LysoTracker and IR700DX-mbc94 fluorescence after 2 h of incubation, indicating that part of IR700DX-mbc94 was internalized. In addition, IR700DX-mbc94 was shown to be primarily localized in the cytoplasm (Figure 4 and S4), which is consistent with recent studies reporting that CB2R is primarily located at intracellular sites in certain cell lines (Castaneda, et al., 2013). Based on these observations, it is likely that some IR700DX-mbc94 molecules bound to the surface CB2R and internalized, whereas the majority of IR700DX-mbc94 molecules diffused into the cells and bound to intracellular CB2R. We also noted that compared with IR700DX-based PIT (Mitsunaga, et al., 2011), 10-fold of light was needed in CB2R-targeted phototherapy to achieve similar therapeutic effects. This is likely due to the relatively low binding affinity of IR700DX-mbc94 to CB2R compared with that of the antibody-antigen binding.
Figure 4. Fluorescence microscopy of CB2-mid DBT cells with or without phototherapy.
CB2-mid DBT cells were treated with 5 μM of IR700DX-mbc94 at 37 °C overnight, with or without light irradiation (30 mW/cm2, 20 min). Upper panel: IR700DX-mbc94 (red fluorescence) and DAPI (blue fluorescence) images. Lower panel: Differential Interference Contrast (DIC) images. With phototherapy treatment, cells experienced typical necrotic cell morphological changes, such as bleb formation (white arrowhead), cell membrane rupture and releasing of cells’ contents (red arrowhead). Scale: 20 μm.
SIGNIFICANCE
We report the first type 2 cannabinoid receptor-targeted phototherapy study using a novel photosensitizer (IR700DX-mbc94) based on a NIR phthalocyanine dye and a functional CB2R-targeted molecule. IR700DX-mbc94 was only effective when bound to the target receptor and produced no phototoxicity when not bound, and the extent of the phototherapeutic effect of IR700DX-mbc94 was much less in CB2R− than in CB2R+ cells, suggesting a highly specific receptor-targeted phototherapy mechanism. Overall, receptor-targeted phototherapy provides a facile, target-specific approach based on small molecule photosensitizers with the advantages of low cost, easy delivery and fast clearance. In addition, the discovery of receptor-targeted phototherapy may open new opportunities to develop receptor targeted phototherapy techniques in general because various receptor ligands or peptides can be conjugated to IR700DX as potential phototherapy agents. Therefore, IR700DX-mbc94 appears to be an attractive phototherapy and therapeutic monitoring agent for the treatment of cancers.
EXPERIMENTAL PROCEDURES
Synthesis of IR700DX-mbc94
A mixture of IR700DX-NHS ester (2.5 mg, 1.28 μmol) and mbc94 (3 mg, 5.08 μmol) in DMSO (1 mL) was stirred at room temperature under argon for 24 hours in the absence of light. DMSO was removed by lyophilization, and the residue was washed by ethyl acetate (10 mL ×3) and dried by lyophilization.
In vitro saturation binding assay of IR700DX-mbc94
We carried out intact cell saturation binding assay to determine CB2R binding affinity to IR700DX-mbc94 as previously reported (Zhang, et al., 2013).
Cytotoxicity
CB2-mid DBT cells were treated with indicated concentration of IR700DX-mbc94, mbc94, without light irradiation for 24 h in a water jacketed incubator. ICG (Sigma-Aldrich) was used as the negative control and Doxorubicin hydrochloride (Fisher Scientific) was used as the positive control. To test the toxicity of the IR700DX-mbc94 in cells that express the CB2 receptor but are not tumoral, CHO-K1/CB2 cells were treated with indicated concentration of IR700DX-mbc94 for 24 h. Cell viability was determined by CellTiter-Glo Luminescent Cell Viability Assay kit (Promega) according to the manufacturer’s instructions.
In Vitro Phototherapy
CB2-mid (CB2R+) and WT (CB2R−) DBT cells were grown to confluence in T75 flasks, harvested, seeded into 96-well optical plates and incubated in a water-jacketed incubator for 24 h prior to treatment. Each type of DBT cells was incubated with 5 μM of IR700DX-mbc94 at 37 °C overnight. Cells were then irradiated with light from an LED light source (L690-66-60, Marubeni America Co.) at wavelengths of 670–710 nm (peak at 690 nm) and a power density of 30 mW/cm2, as measured with an optical power meter (PM100, Thorlabs). We compared the number of dead cells after the following treatments: IR700DX-mbc94 (−) & irradiation (−); IR700DX-mbc94 (−) & irradiation (+); IR700DX-mbc94 (+) & irradiation (−); and IR700DX-mbc94 (+) & irradiation (+). In addition, to investigate whether a higher amount of IR700DX-mbc94 in the surrounding medium will induce more cell death, we compared the phototherapy effect in cells that were washed and not washed. To quantify the unbound and bound IR700DX-mbc94, we measured the fluorescence intensities of the combined serum free washing medium and the washed cells. Furthermore, we compared CB2-mid DBT cells treated with free IR700DX dye (5 μM) with those treated with IR700DX-mbc94 (5 μM). The CB2R-targeted phototherapy effect was then evaluated between CB2-mid and WT DBT cells. To study whether the mechanism of phototherapy differs from that of PDT, a free radical scavenger DMTU (1 mM) or a 1O2 quencher NaN3 (50 mM) was added with IR700DX-mbc94 to CB2-mid DBT cells. We also tested whether the phototherapeutic effect is dependent on the dose of light irradiation, incubation time and concentration of IR700DX-mbc94.
Fluorescent Microscopy
CB2-mid DBT cells were incubated with 5 μM of IR700DX-mbc94 at 37 °C overnight, and then washed with FBS free medium once. The cells were divided into two groups, with or without light irradiation (30 mW/cm2, 20 min). Cell fluorescent staining was carried out as previously reported (Zhang, et al., 2013). IR700DX-mbc94 fluorescent images were captured with a NIR camera equipped with a Cy5 filter set (Excitation/emission: 625–655 nm/665–715 nm). Nuclear images were obtained with a DAPI filter set (Excitation/emission: 335–383 nm/420–470 nm). Differential interference contrast (DIC) images were obtained through Trans light DIC.
Supplementary Material
Development of the first CB2 receptor-targeted phototherapy agent, IR700DX-mbc94
IR700DX-mbc94 was only effective when bound to the target receptor
Phototherapeutic effect of IR700DX-mbc94 was much less in CB2R− than in CB2R+ cells
CB2R-targeted phototherapy provides a promising target-specific approach
Acknowledgments
We thank Dr. Nephi Stella at the University of Washington for providing DBT cells and technical advice. This work was supported by the startup fund provided by the Department of Radiology, University of Pittsburgh. This project used the UPCI imaging facilities supported, in part, by award P30CA047904. We also thank the Grant of Shanghai Science and Technology (12DZ1940606,12ZR1439900) and Grant of Shanghai Municipal Health Bureau (20124195) for supporting Ningyang Jia’s work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai M, Sexton M, Stella N, Bornhop DJ. MBC94, a conjugable ligand for cannabinoid CB 2 receptor imaging. Bioconjugate chemistry. 2008;19:988–992. doi: 10.1021/bc700419e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda JT, Harui A, Kiertscher SM, Roth JD, Roth MD. Differential expression of intracellular and extracellular CB(2) cannabinoid receptor protein by human peripheral blood leukocytes. J Neuroimmune Pharmacol. 2013;8:323–332. doi: 10.1007/s11481-012-9430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Li K, Wen S, Liu H, Peng C, Cai H, Shen M, Zhang G, Shi X. Targeted CT/MR dual mode imaging of tumors using multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials. 2013;34:5200–5209. doi: 10.1016/j.biomaterials.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Copland JA, Eghtedari M, Popov VL, Kotov N, Mamedova N, Motamedi M, Oraevsky AA. Bioconjugated gold nanoparticles as a molecular based contrast agent: implications for imaging of deep tumors using optoacoustic tomography. Mol Imaging Biol. 2004;6:341–349. doi: 10.1016/j.mibio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Cudaback E, Marrs W, Moeller T, Stella N. The expression level of CB1 and CB2 receptors determines their efficacy at inducing apoptosis in astrocytomas. Plos One. 2010;5:e8702. doi: 10.1371/journal.pone.0008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa FS, Bentley MV. Photodynamic therapy of skin cancers: sensitizers, clinical studies and future directives. Pharm Res. 2000;17:1447–1455. doi: 10.1023/a:1007612905378. [DOI] [PubMed] [Google Scholar]
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A. 2008;105:9099–9104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff BA, Bamberg M, Hasan T. Photoimmunotherapy of human ovarian carcinoma cells ex vivo. Cancer Res. 1991;51:4762–4767. [PubMed] [Google Scholar]
- Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends in biochemical sciences. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Huang L, Xuan Y, Koide Y, Zhiyentayev T, Tanaka M, Hamblin MR. Type I and Type II mechanisms of antimicrobial photodynamic therapy: an in vitro study on gram-negative and gram-positive bacteria. Lasers Surg Med. 2012;44:490–499. doi: 10.1002/lsm.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori G, Spikes JD. Photothermal Sensitizers - Possible Use in Tumor-Therapy. J Photoch Photobio B. 1990;6:93–101. doi: 10.1016/1011-1344(90)85078-b. [DOI] [PubMed] [Google Scholar]
- Kim YH, Jeon J, Hong SH, Rhim WK, Lee YS, Youn H, Chung JK, Lee MC, Lee DS, Kang KW, et al. Tumor targeting and imaging using cyclic RGD-PEGylated gold nanoparticle probes with directly conjugated iodine-125. Small. 2011;7:2052–2060. doi: 10.1002/smll.201100927. [DOI] [PubMed] [Google Scholar]
- Kumar A, Ma H, Zhang X, Huang K, Jin S, Liu J, Wei T, Cao W, Zou G, Liang XJ. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials. 2012;33:1180–1189. doi: 10.1016/j.biomaterials.2011.10.058. [DOI] [PubMed] [Google Scholar]
- Lai YL, Chiou WY, Lu FJ, Chiang LY. Roles of oxygen radicals and elastase in citric acid-induced airway constriction of guinea-pigs. Br J Pharmacol. 1999;126:778–784. doi: 10.1038/sj.bjp.0702352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Pritchett TM, Huang JD, Ke MR, Shao P, Sun WF. Photophysics and nonlinear absorption of peripheral-substituted zinc phthalocyanines. J Phys Chem A. 2008;112:7200–7207. doi: 10.1021/jp7108835. [DOI] [PubMed] [Google Scholar]
- Lukianova-Hleb EY, Oginsky AO, Samaniego AP, Shenefelt DL, Wagner DS, Hafner JH, Farach-Carson MC, Lapotko DO. Tunable plasmonic nanoprobes for theranostics of prostate cancer. Theranostics. 2011;1:3–17. doi: 10.7150/thno/v01p0003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mew D, Wat CK, Towers GH, Levy JG. Photoimmunotherapy: treatment of animal tumors with tumor-specific monoclonal antibody-hematoporphyrin conjugates. J Immunol. 1983;130:1473–1477. [PubMed] [Google Scholar]
- Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40:167–181. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- Qamri Z, Preet A, Nasser MW, Bass CE, Leone G, Barsky SH, Ganju RK. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther. 2009;8:3117–3129. doi: 10.1158/1535-7163.MCT-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- Sagone AL, Jr, Husney RM, Wewers MD, Herzyk DJ, Davis WB. Effect of dimethylthiourea on the neutrophil myeloperoxidase pathway. J Appl Physiol (1985) 1989;67:1056–1062. doi: 10.1152/jappl.1989.67.3.1056. [DOI] [PubMed] [Google Scholar]
- Sexton M, Woodruff G, Horne EA, Lin YH, Muccioli GG, Bai M, Stern E, Bornhop DJ, Stella N. NIR-mbc94, a fluorescent ligand that binds to endogenous CB(2) receptors and is amenable to high-throughput screening. Chem Biol. 2011;18:563–568. doi: 10.1016/j.chembiol.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- Tong R, Kohane DS. Shedding light on nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:638–662. doi: 10.1002/wnan.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triesscheijn M, Baas P, Schellens JH, Stewart FA. Photodynamic therapy in oncology. Oncologist. 2006;11:1034–1044. doi: 10.1634/theoncologist.11-9-1034. [DOI] [PubMed] [Google Scholar]
- Velasco G, Sanchez C, Guzman M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer. 2012;12:436–444. doi: 10.1038/nrc3247. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu Y, Huang S, Liu G, Xie C, Zhou J, Fan W, Li Q, Wang Q, Zhong D, et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. 2006;171:31–38. doi: 10.1016/j.cancergencyto.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Zhang S, Shao P, Bai M. In vivo type 2 cannabinoid receptor-targeted tumor optical imaging using a near infrared fluorescent probe. Bioconjug Chem. 2013;24:1907–1916. doi: 10.1021/bc400328m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.