Abstract
Two-thirds of adults in the United States are overweight or obese, and another 26 million have type 2 diabetes. Decreased insulin sensitivity in cardiovascular tissue is an underlying abnormality in these individuals. Insulin metabolic signaling increases endothelial cell nitric oxide production. Impaired vascular insulin sensitivity is an early defect leading to impaired vascular relaxation. In overweight and obese persons, as well as in those with hypertension, systemic and vascular insulin resistance often occurs in conjunction with activation of the cardiovascular tissue renin–angiotensin–aldosterone system (RAAS). Activated angiotensin II type 1 receptor and mineralocorticoid receptor signaling promote the development of vascular insulin resistance and impaired endothelial nitric oxide–mediated relaxation. Research in this area has implicated excessive serine phosphorylation and proteasomal degradation of the docking protein insulin receptor substrate and enhanced signaling through hybrid insulin/insulin-like growth factor (IGF-1) receptor as important mechanisms underlying RAAS impediment of downstream vascular insulin metabolic signaling. This review will present recent evidence supporting the notion that RAAS signaling represents a potential pathway for the development of vascular insulin resistance and impaired endothelial-mediated vasodilation.
Keywords: insulin resistance, endothelium, cardiovascular disease, obesity, nitric oxide
Introduction
Type 2 diabetes mellitus (DM2) affects 26 million people in the United States and 347 million worldwide.1 The prevalence of DM2 is closely associated with the alarming rates of physical inactivity and over-nutrition,2 leading contributors to current pandemic rates of obesity. Resistance to the metabolic actions of insulin, termed insulin resistance for the purposes of this article, and consisting mainly of suppression of hepatic gluconeogenesis and skeletal muscle glucose disposal, is a key event in both DM2 and obesity.3 Resistance to the vascular effects of insulin contributes to the pathogenesis of cardiovascular disease (CVD),3 which accounts for the majority of the deaths in diabetic patients.4 Thus, understanding the role of insulin in the vasculature in health and disease should help to elucidate new therapeutic strategies aimed at curbing the pandemic of CVD in diabetes. In this review, we will discuss the available evidence of normal and pathological actions of insulin in the vasculature, as well as its impact on glucose homeostasis and on the pathogenesis of CVD.
Distinct molecular effects of insulin in the vasculature
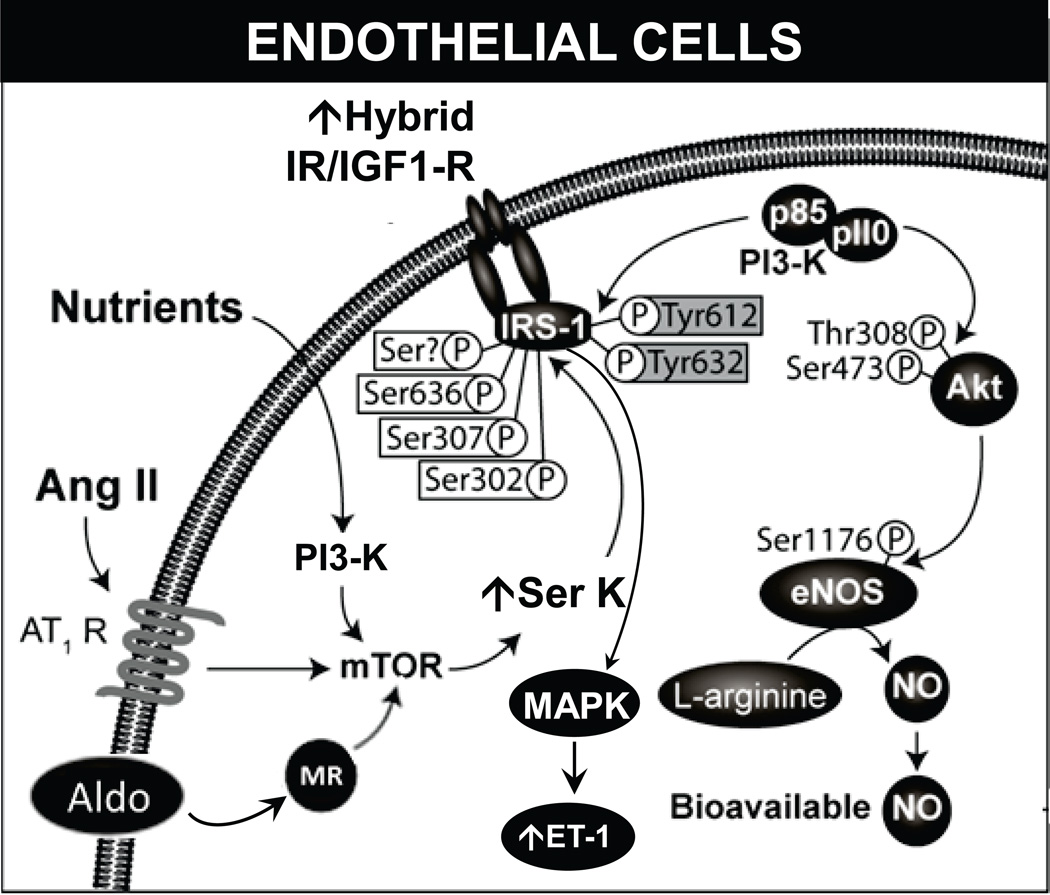
The net effects of insulin on the vasculature are determined by different cellular signaling pathways that are activated by stimulation of the insulin receptor (IR) (Fig. 1).5 Classically, insulin metabolic signaling results in vasodilation via increased nitric oxide (NO) production and increases in bioavailable NO. However, in conditions of insulin resistance, it promotes vasoconstriction and vascular proliferation.5
Figure 1.
Insulin effects on endothelial cells. Under normal conditions, stimulation of IR results in activation of the PI3K–Akt pathway, eNOS phosphorylation, and vasodilation. Insulin resistance induced by RAAS activation and excess nutrients causes increased serine phosphorylation of insulin receptor substrate and metabolic signaling with uninhibited activation of mitogenic and growth pathways. Aldo, aldosterone; Ang II, angiotensin II; AT1R, angiotensin II type 1 receptor; eNOS, endothelial NO synthase; ET-1,endothelin-1; IGF1-R, insulin-like growth factor-1 receptor; IR, insulin receptor; mTOR, mammalian target of rapamycin; MR, mineralocorticoid receptor; MAPK, mitogen activated protein kinase; NO, nitric oxide; PI3K, phosphatidylinositol 3-kinase; p, phosphorylation; Akt, protein kinase B; Ser, serine; Ser K, serine kinase; thr, threonine; Tyr, tyrosine.
Binding of insulin to IR triggers its phosphorylation and activation via an intrinsic kinase activity, leading to tyrosine phosphorylation of the insulin receptor substrate (IRS) proteins.6 Phosphorylation of the IRS molecules creates Src homology 2 (SH2) domain binding motifs that serve as docking points for SH2-containing proteins like phosphatidylinositol 3-kinase (PI3K) and Grb-2.6 The docking of PI3K to IRS-1 activates, via p85, the p110 catalytic subunit of PI3K, resulting in production of phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 promotes phosphorylation and activation of 3-phosphoinositide-dependent protein kinase-1 (PDK-1), which then activates different serine/threonine kinases such as Akt. (Fig.1).6 In turn, Akt activates the endothelial NO synthase (eNOS) by phosphorylation in serine residue 1177.7,8 eNOS catalyzes the conversion of L-arginine and O2 to L-citrulline and NO.6 eNOS is expressed in caveola, where it is inhibited by caveolin-1, and its activity is modulated in a Ca+2/calmodulin–sensitive manner.9 Nevertheless, in endothelial cells, eNOS activation by insulin stimulation is only partially blunted by calmodulin inhibitors, suggesting a calcium-independent mechanism of insulin-mediated eNOS activation.7
Insulin also promotes eNOS phosphorylation of threonine 495 in human endothelial cells (threonine 497 bovine).10 Dephosphorylation of this residue is involved in uncoupling eNOS and increasing production of reactive oxygen species (ROS).10 Importantly, the PI3K–Akt cascade is not the only determinant of eNOS activity; heat-shock protein 90 modulates eNOS activity11 and an inadequate supply of tetrahydrobiopterin (eNOS cofactor) limits the enzyme activity and results in eNOS uncoupling.12 The NO produced by eNOS decreases vascular tone and vascular smooth muscle cell (VSMC) proliferation and diminishes adhesion of inflammatory cells and platelet aggregation to the endothelium.5 Furthermore, insulin modulates production of prostaglandins and endothelium-derived hyperpolarizing factors.5
In addition to vasodilatation, insulin can promote vasoconstriction. Under some circumstances, insulin activates the mitogen-activated protein kinase (MAPK) cascade that coordinates insulin vasoconstriction and growth-promoting effects.5 These effects of insulin are mediated, in part, by the increased production of endothelin-1 (ET-1) and the activation of signaling through the vascular tissue RAAS.5,6 ET-1 is produced in the endothelium and acts via the ET-1 receptor A to cause vasoconstriction, increase oxidative stress, and promote cell growth and mitogenesis in VSMC.13 Supraphysiological levels of insulin have been shown to increase the production of ET-1 in cultured endothelial cells and in rats.14 Similar deleterious effects are seen with the activation of vascular tissue RAAS.5
It has been recently demonstrated that insulin activates the insulin-like growth factor-1 (IGF-1) receptor (IGF1-R)15 and stimulates NO production through this alternative mechanism. However, production of NO can be limited by the fact that insulin binds less avidly to IGF1-R than to its native receptor, and in conditions of insulin resistance, IRs are outnumbered by IGF1-Rs.15
In diabetic CVD, insulin resistance is generated by chronic low-grade inflammation, increased oxidative stress, lipotoxicity, and activation of the renin angiotensin aldosterone system (RAAS) (16). These conditions promote serine phosphorylation of different insulin signaling molecules such as IRS-1 and the impairment of the normal tyrosine phosphorylation cascade (17), thus impairing insulin metabolic signaling.
Actions of insulin in the skeletal muscle vasculature
Skeletal muscle is one of the main metabolic targets of insulin and promotes glucose uptake into skeletal muscle fibers.18 Insulin plays a modulatory role on skeletal muscle vasculature by increasing blood flow and regulating its own delivery.19 Skeletal muscle vasculature is extremely flexible.
Under basal, non-exercising conditions, the skeletal muscle receives 0.03–004 ml/min of blood flow per gram of tissue,19 but upon initiation of exercise, blood flow increases up to 100-fold.20 Capillary recruitment in the skeletal muscle is initially determined by vasomotor changes in the terminal arterioles.19 Increased metabolic demands (such as exercise) result in a drop in tissue oxygen tension below the threshold necessary to maintain oxidative phosphorylation.21,22 Once oxygen extraction is maximized, an ascending vasodilation response extends from the contracting skeletal muscle arterioles to the proximal feed arteries, resulting in increased blood flow to the contracting muscle.22
The mechanism behind localized ascending vasodilation is dependent on an intact endothelium and involves activation of the α adrenergic receptors to restrict the vasodilation to actively contracting areas of the muscle.22 Once the terminal arterioles are maximally dilated, capillary perfusion is determined by tissue metabolic demands and the anatomic arrangement of the vessels.19 Adequate skeletal muscle capillary recruitment is crucial for the normal metabolic effects of insulin,23 and clinical conditions characterized by insulin resistance such as obesity and DM2 manifest with impaired capillary recruitment.24
Although insulin is critical for vasodilation in the vasculature, there are several additional factors involved in vascular reactivity that are independent of insulin, including catecholamine release, glucagon-like protein 1 (GLP-1) secretion, and RAAS activation, as will be discussed in this review.
The effects of insulin in the vasculature have been examined using different invasive and non-invasive approaches like the 1-methylxantnine (1-MX),25,26 thermodilution method, laser-Doppler perfusion,27 contrast-enhanced ultrasound (CEU), and positron-emission tomography.28,29 The CEU is a non-invasive technique adapted from heart muscle imaging.25 It uses lipid-coated microbubbles filled with perfluorocarbon that behave similarly to red blood cells and remain in the intravascular compartment.25,28 The microbubbles are destroyed using harmonic ultrasound imaging and their rate of reappearance in the microcirculation parallels the microvascular blood volume and the rate of flow of the area studied.19 This technique requires anesthesia when used in rodents and immobilization in humans, which may interfere with the behavior of the microvasculature.25,28 CEU has been widely used by several authors to describe the response of the skeletal muscle vasculature to different stimuli.19,25,28
Whole-limb blood flow
Even though effects of insulin on the vasculature have been described for several decades,18 controversy exists regarding the significance of insulin-mediated vasodilation in glucose uptake.30 Earlier reports in humans studied the effect of insulin on whole-limb blood flow using the thermodilution method and the hyperinsulinemic–euglycemic clamp.31–35 Thermodilution allows for uninterrupted monitoring of limb blood flow, which potentially gives a more accurate description of the hemodynamic changes produced by insulin.19 Insulin increases whole-limb blood flow in a dose-dependent manner in lean humans, and it correlates with increased skeletal muscle glucose uptake.36 The effect of insulin on leg blood flow is largely dependent on increased NO production and is blunted by eNOS inhibition.37 Furthermore, eNOS inhibition results in blunting of insulin-mediated skeletal muscle glucose uptake via decreased skeletal muscle blood flow.35
Nevertheless, some investigators have challenged these concepts. Yki– Järvinen and colleagues showed that, in lean subjects, augmentation of limb blood flow through bradykinin infusion did not translate into increased insulin-mediated glucose uptake by skeletal muscle (measured by [18F]-fluoro-deoxy-glucose and positron emission tomography).30 Furthermore, the same group did not find any evidence that increased blood flow to skeletal muscle in response to insulin relates to the muscle areas where insulin stimulated glucose uptake is increased.38 Even though the opposing findings can be partially explained by the different techniques used by the authors to assess blood flow and glucose uptake, the current available knowledge does not completely clarify the role of insulin-mediated vasodilation on whole-limb blood flow and its impact on insulin-stimulated glucose uptake.
Microvascular recruitment
Recruitment of under-perfused capillaries by ascending vasodilation is necessary at times of increased metabolic demand such as during exercise.28,29. Capillary recruitment increases the endothelial surface available for nutrient exchange, as well as for the delivery of insulin to the skeletal muscle.28 Insulin has been proposed to have an exercise-like effect on the vasculature of skeletal muscle.28 The evidence of capillary recruitment secondary to insulin is derived from using the indirect methods described above. Insulin infusion in healthy non-obese adults (systemically and through the brachial artery) results in increased forearm blood volume without significant changes in whole-limb blood flow.39 In addition, insulin-mediated increases in microvascular blood flow, which parallel microvascular recruitment, precede increases in total limb blood flow caused by insulin40 and are dependent on NO production.23 These increases are seen at lower insulin doses compared to those required to increase whole-limb blood flow.23
Finally, transport of insulin across the endothelial barrier has gained relevance as it has been postulated to be the rate-limiting factor for the action of insulin in skeletal muscle. One investigative group has proposed that the delivery of insulin to skeletal muscle depends on binding of insulin to IR in the caveloae, resulting in internalization of the vesicles and transendothelial transport of the hormone.19
RAAS modulation of skeletal muscle microvasculature
Overweight/obesity and DM2 are characterized by inappropriate activation of the RAAS, which plays an important role in the modulation of the skeletal muscle vasculature.3,5,16 Both beneficial and deleterious effects have been ascribed to the activation of the system. The vascular effects of angiotensin II (Ang II) have been extensively characterized.41 Ang II signals through G-coupled membrane-bound type 1 and type 2 receptors (AT1R and AT2R, respectively). In the vasculature, AT1R activation increases oxidative stress and promotes vasoconstriction and remodeling.27 In endothelium, chronic AT1R activation leads to increased activity of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymatic complex, enhanced production of ROS, and uncoupling of eNOS.41 Conversely, eNOS uncoupling decreases bioavailable NO and results in increased production of ROS.42 Furthermore, in endothelial cells, Ang II induces impairment of insulin-stimulated eNOS activation (via decreased Ser1177 phosphorylation) and diminishes NO production via the mammalian target of rapamycin/p70S6 kinase 1 pathway (mTOR/S6K1).43
On the other hand, AT2R activation counteracts the deleterious effects of Ang II via AT1R signaling.44 Vasodilation mediated by AT2R results from activation of the bradykinin/NO system.45 AT2R increases the expression of prolylcarboxipeptidase,46 which converts prekallikrein to kallikrein and cleaves high–molecular weight kininogen (HMWK), resulting in release of bradykinin.47 Likewise, in a rodent model of diabetes and insulin resistance, increased ROS resulted in reduced AT2R-mediated dilatation.48 In this regard, it has been reported that treatment for one year with an AT1R blocker in hypertensive diabetic persons resulted in increased AT2R expression and enhanced vasodilatory response in resistance arteries.49
Angiotensin II regulates skeletal muscle perfusion through AT2R and AT1R
The differential effects of Ang II receptor activation and its impact on glucose homeostasis have been extensively investigated. Acute infusion of pressor and non-pressor doses of Ang II in control rats resulted in a twofold increase in microvascular blood volume. Moreover, AT1R blockade with losartan results in a further increment in blood flow and glucose extraction, which was abolished by the NO inhibitor NG-nitro-L-arginine methyl ester (L-NAME). Not surprisingly, AT2R blockade decreased microvascular blood flow by 80%, with a parallel drop in glucose extraction.50 Additionally, this study examined the interaction between Ang II receptors and skeletal muscle insulin-stimulated vasodilation and glucose uptake. During continuous insulin infusion, AT1R blockade did not have an additive effect on these parameters. On the other hand, AT2R antagonism resulted in whole-body insulin resistance and attenuation of microvasculature recruitment. These changes paralleled a decrease in plasma NO and skeletal muscle eNOS activation (as evaluated by eNOS phosphorylation).51
Lastly, RAAS exerts direct effects on the skeletal muscle vasculature to impair endothelial-mediated vasorelaxation.5,18 For example, Ang II, acting via AT1R signaling, has been shown to impair insulin metabolic signaling and glucose uptake in cultured myotubules through activation of the NAPDH oxidase and increased oxidative stress.52 The resultant increase in oxidative stress promotes the activation of redox-sensitive serine kinases that, in turn, promote the serine phosphorylation of IRS and result in its proteasomal degradation and reduced downstream insulin metabolic degradation. This reduced insulin metabolic signaling also results in attenuated activation of eNOS, increased destruction of NO, and enhanced vascular smooth muscle intracellular calcium and calcium sensitization (Fig. 1).3,5,16
Insulin resistance in the vasculature
As previously noted, obesity and DM2 are associated with impaired endothelial-dependent vasodilation.53 After a mixed meal, lean humans exhibit increased brachial blood flow and forearm microvascular recruitment, and obese subjects have a blunted response in the post-prandial state despite hyperinsulinemic conditions.34,54 This blunted response is related to insulin resistance and associated endothelial dysfunction, which contributes to increased CVD.55 Endothelial dysfunction manifests as decreased NO bioavailability, abnormal vasoreactivity, increased oxidative stress, and increased expression of inflammatory, immune and pro-thrombotic mediators.55 The impaired endothelial-dependent vasodilation of skeletal muscle in insulin-resistant models correlates with arteriolar remodeling and increased stiffness of the vessel wall.56 Resistance to the vascular effects of insulin has been shown to selectively involve the PI3K–Akt–NO pathway with intact activation of the MAPK pro-mitotic pathway.55 Induction of insulin resistance in cultured endothelial cells, via blockade of the PI3K pathway, results in blunted production of NO with increased expression of pro-atherosclerotic molecules.8 In the vasculature of obese fa/fa rats, tyrosine phosphorylation of IR and IRS-1/IRS-2, as well as activation of PI3K and Akt, are markedly decreased, while basal phosphorylation of MAPK is augmented.57 The spontaneously hypertensive rats exhibit selective insulin resistance in the vasculature with decreased insulin-stimulated NO production but enhanced ET-1 secretion.58 Finally, although they are not the focus of the present paper, factors other than impaired vasodilatation are important in the pathogenesis of obesity and insulin resistance–related vascular dysfunction, such as impaired nutrient and insulin delivery due to abnormalities in extracellular matrix.
Lipotoxicity
Lipotoxicity is a common finding in DM2 and obesity, and its effects have been extensively characterized in the vasculature. Rodents subjected to intravenous infusion of lipids and heparin demonstrated decreased muscle glucose uptake and blunted insulin-mediated microvascular recruitment in skeletal muscle.31 Similarly, in healthy humans, lipid infusion causes systemic insulin resistance and decreased forearm microvascular recruitment.59 Both lipotoxicity and glucotoxicity converge in augmented production of diacylglycerol (DAG) and ceramides.60 In the vasculature, DAG activates protein kinase C (PKC) isoforms β1 and β2.61 Protein kinase C (PKC) isoform β is known to inhibit insulin effects in the vasculature.62 In insulin-resistant Zucker fatty rats, activation of PKC isoform β relates to decreased Akt-dependent eNOS activation.62 Conversely, treatment with ruboxistaurin, a PKCβ inhibitor, restored the insulin-induced eNOS activation in fatty rats.62 Overexpression of PKC-β2 and concomitant ApoE knockout (KO) in mice fed a high-fat diet resulted in a significant impairment in insulin-stimulated Akt/eNOS activation, with augmented leukocyte–endothelial binding and increased ET-1 production.63 Aortic atherosclerosis was 70% greater in the KO rodents when compared to control mice.63 Despite those findings, whole-body insulin sensitivity and blood pressure were not different.63 Recently, Tabit and colleagues demonstrated that PKCβ expression is markedly increased in endothelial cells isolated from DM2 patients. Insulin-induced eNOS activation was severely depressed in cells from diabetic patients, with a parallel increased in oxidative stress and expression of inflammatory markers.64 Ex vivo use of a PKCβ inhibitor restores eNOS activation by insulin.64
The deleterious effects of lipotoxicity also involve the activation of inflammatory pathways.65 Jang and colleagues examined the effect of Toll-like receptor 2 (TLR2) activation by saturated fatty acids in vascular insulin resistance.65 In vascular cultured endothelial cells, exposure to palmitate results in activation of pro-inflammatory molecules with impaired insulin-driven production of NO. This effect was ameliorated by the TLR2 knockdown. Furthermore, mice that lack TLR2 were protected from whole-body and endothelium insulin resistance upon high-fat feeding.65
Vascular insulin resistance: evidence from transgenic models
The molecular defects that lead to impairment of insulin signaling in the vasculature have been explored with different KO and overexpression models. Mice with vascular endothelial IR knockout have impaired ET-1 and eNOS expression.66 Interestingly, using a low salt–diet feeding, mice lacking endothelial IR became insulin resistant relative to controls,66 and the high salt diet–feeding impaired insulin sensitivity in the control group but not in the KO group.66 Systemic insulin sensitivity, assessed via a hyperinsulinemic–euglycemic clamp, was not different under basal condition in KO mice.66
Investigators utilizing a transgenic mouse with endothelium overexpression of a dominant-negative mutant human IR containing a mutation in the tyrosine kinase domain (ESMIRO) demonstrated normal whole-body insulin sensitivity but decreased vasodilation in response to acetylcholine (Ach).67 These changes occurred in concert with blunted vascular responses to insulin stimulation and augmented production of superoxide derived from the Nox2 isoform of NAPDH oxidase. Interestingly, ex vivo and in vivo pharmacological inhibition of Nox2 under insulin-resistant conditions resulted in decreased production of ROS and improvement in Ach-mediated vasodilation.68 In this study, genetic deletion of Nox2 in the setting of the ESMIRO model resulted in improved aortic response to Ach and decreased oxidative stress.
In the ApoE KO mice fed a high-fat diet, the classical model of atherosclerosis, additional KO of IR in endothelial cells resulted in accelerated atherosclerosis in areas of turbulent flow with a parallel increase in endothelial oxidative stress.69 Intriguingly, this model did not exhibit whole-body insulin resistance. As noted earlier, the IGF1-R is also involved in the actions of insulin in the vasculature.15 IGF1-Rs heterodimerize with the IR and form a hybrid with a 50-fold weaker affinity for insulin than for IGF-1.70 The presence of this hybrid receptor has been correlated in humans with hyperinsulinemia and obesity.71,72 Others used a rodent model of whole-body haploinsufficiency of IGF1-R and endothelium-specific deletion of IGF1-R to demonstrate that IGF1-R negatively regulates insulin sensitivity (evaluated by insulin stimulated eNOS phosphorylation and NO production).73 On the other hand, targeted endothelial models of human IGF1-R overexpression resulted in an increased vasoconstrictor response to phenylephrine, endothelial insulin resistance, reduced NO bioavailability, and enhanced endothelium regeneration after denuding wire injury (Fig. 1).74
Insulin-resistant conditions coexist with hyperinsulinemia for as long as pancreatic β cells are able to increase production of insulin.2 Compensatory hyperinsulinemia is not a benign process, and increasing data support its role in the pathogenesis of vascular disease.75 To this point, investigators have explored the role of forkhead box, sub-group O (FoxO) transcription factors in the pathogenesis of vascular disease.76 FoxO proteins are classically considered to impair insulin signaling, and are known to decrease eNOS transcription and promote oxidative stress. In physiologic conditions, FoxO proteins are excluded from the nuclei upon phosphorylation by Akt.75 Earlier studies showed that mice lacking low-density lipoprotein (LDL) receptors, fed a high fat diet, exhibited insulin resistance and atherosclerosis with reduced aortic IRS-1, IRS-2, Akt, eNOS, FoxO1, and FoxO3a phosphorylation.76 Mice lacking LDL receptors in addition to triple knockout of Foxo1, Foxo3a, and Foxo4 had restored vascular endothelial-dependent vasodilation and decreased atherosclerotic lesions in the coronary artery by 80% without an effect on glucose homeostasis.76 Endothelial cells derived from these mice exhibited increased NO production, decreased expression of adhesion molecules, and reduced oxidative stress.76 Further work from this group described the metabolic and vascular phenotype of triple Foxo knockout mice under conditions of regular chow and LDL receptor expression. Surprisingly, the of triple Foxo knockout mice exhibited insulin resistance and impaired glucose homeostasis, with decreased insulin signaling in the liver but not in muscle or adipose tissue. Further examination demonstrated that insulin resistance in this model is secondary to hyperinsulinemia-driven increased NO production by the endothelium and correlates with nitration of insulin-signaling cascade proteins in the liver.77
RAAS-system activation and insulin resistance in the vasculature
RAAS activation mediated by signaling through AT1R, as well as through the mineralocorticoid receptor (MR) promote fibrosis, remodeling, proliferation, migration, and hypertrophy in vascular tissues.42,78 Again, ROS production plays a critical role in this process, through Rac-mediated activation of NADPH oxidase,42 and triggers several pro-inflammatory pathways, including Janus activated kinase (JAK), Rho/RhoK, and MAPK.79,80 Mineralocorticoids are typically elevated in the setting of insulin resistance and obesity78 and result in impaired insulin sensitivity in the vasculature in healthy humans.81 Furthermore, mild increases in aldosterone levels, even within currently accepted normal ranges, may result in increased cardiovascular mortality in individuals with coronary artery disease.16
Aldosterone promotes insulin resistance by increasing the proteasomal degradation of IRS-1 in a c-Src- and ROS-mediated mechanism in vascular tissue.82 Ang II also reduces IRS-1 levels in VSMC via a similar mechanism involving c-Src and ROS.83 As a result, insulin signaling through the PI3K–Akt pathway is impaired, leading to insulin resistance. Importantly, aldosterone-induced vascular dysfunction is prevented by blockade of the MR as well as use of antioxidants and Src inhibition, thus underscoring the participation of signaling through MR. In turn, serine phosphorylation of IRS molecules can be the result of several pathways involved in insulin resistance, including the mTOR/S6K1, JNK, IκB kinase (IKK), tumor necrosis factor α (TNF-α) and PKCθ.84 As previously mentioned, aldosterone also induces vascular insulin resistance and dysfunction through upregulation and activation of IGF1-R and its hybrid insulin/IGF1-R in VSMC and in rats, which was prevented by treatment via MR blockade and antioxidants.85
Therapeutic strategies
Diet and exercise
Increased physical activity on a regular basis has been shown to improve insulin sensitivity in the vasculature. In a spontaneously hypertensive insulin-resistant rat model, vascular insulin sensitivity, assessed as insulin-induced vasodilation of the mesenteric vasculature, improved significantly after 10 weeks of exercise and was correlated with a reduction in blood pressure.86 Furthermore, exercise decreased the expression and activity of G protein–coupled kinase-2 (GRK-2).86 GRK-2 is known to inhibit insulin signaling in classical insulin-sensitive tissues like liver and fat,87,88 as well as in vascular endothelium.89 Similarly, in the Otsuka Long–Evans Tokushima fatty (OLEFT) rat, a model of hyperphagia-induced obesity and insulin resistance, voluntary running resulted in improved insulin response in the microvasculature of skeletal muscle.90
Caloric restriction leading to weight loss, through decreased visceral fat, improves endothelial function in conduit and resistance arteries of overweight and obese adults. Changes are related to an increase in NO bioavailability.91 The effects of lifestyle modifications are also seen once obese patients become diabetic.92 Six months of caloric restriction (500 calorie negative balance) and 150 minutes of weekly exercise augmented brachial artery flow–mediated dilation along with an improvement in systemic insulin sensitivity and decreased inflammatory markers.92 Weight loss also decreases circulating levels of ET-1 in obese males.93
Insulin sensitizers: 5′-adenosine monophosphate–activated kinase activation/metformin
Metformin, a commonly used antidiabetic medication and insulin sensitizer, has been shown to improve endothelial-dependent vasodilation independently of NO production in a rodent model of insulin resistance.94 In contrast, in cultured aortic endothelial cells, metformin activated eNOS and increased NO production in a PI3K-dependent manner. The vascular effects of metformin requires 5′-AMP–activated kinase (AMPK), as mice lacking AMPK do not obtain these beneficial effects.95 In women with polycystic ovary syndrome, another condition characterized by insulin resistance, metformin therapy improves flow-mediated dilation and decreases levels of ET-1.96 In DM2 patients with coronary artery disease, metformin, which improves insulin sensitivity and reduces glucose liver output, has been shown to significantly decrease cardiovascular events in comparison with glipizide, which enhances insulin secretion by β cells.97
RAAS blockade
Four weeks of treatment with irbesartan, an AT1R blocker, was associated with improved endothelial function assessed by flow-mediated vasodilation of the brachial artery and decreased systemic markers of oxidative stress and inflammation in subjects with metabolic syndrome.52 The vascular effects were potentiated by the use of an α-lipoic acid (an antioxidant). A similar study with quinapril, an angiotensin-converting enzyme, and an α-lipoic acid showed improvement in endothelial function and albuminuria with 8 weeks of treatment in hypertensive patients.98
Antioxidants
As previously discussed, the use of antioxidants in experimental conditions improves markers of insulin resistance in the vasculature and positively influences its function. From a clinical standpoint, however, not all interventions aimed at improving endothelial function with antioxidants in insulin-resistant conditions have yielded positive results.99 Chen and colleagues examined the effects of high-dose vitamin C in vascular and systemic insulin resistance. Sadly, no beneficial effect was attained with this intervention, which according to the authors might have been linked to failure to reach therapeutic levels of vitamin C in DM2 patients relative to control individuals.99
Another intervention in DM2 patients comparing lipoic acid and vitamin C (low and high dose) showed that high doses of vitamin C and lipoic acid improved NO-mediated dilation.100 Use of vitamin C or vitamin E for six months did not have a beneficial effect in the endothelial mediated vasodilation in DM2 patients, whereas it did improve endothelial function in type 1 diabetics.101
Polyphenols
Epigallocatechin gallate (EGCG), a green tea polyphenol, has been explored as a potential therapeutic agent against vascular insulin resistance.102 In human aortic endothelial cells, EGCG decreases expression and secretion of ET-1.103 Similarly, in a rodent model of insulin resistance induced by high fat–diet feeding, EGCG treatment resulted in decreased insulin resistance, improved insulin-mediated vasodilation of mesenteric arteries, and decreased macrophage vascular infiltration.104 Furthermore, in humans, green tea consumption has been associated with decreased risk of cardiovascular events and improvement in blood glucose control.105,106
Another polyphenol, hesperidin, also increases NO production in endothelial cells and decreases the expression of inflammatory markers.107 Oral administration of hesperidin to patients with metabolic syndrome improves endothelial function assessed by flow-mediated dilation and decreases circulating levels of inflammatory markers.107 The mechanisms underlying the effects of polyphenols on insulin sensitivity are largely unknown, and it has even been suggested that green tea can actually impair the absorption of some medications. Thus, additional studies in this area are warranted before polyphenols can be added to currently accepted medications.108
Gut-derived peptides: incretin hormones
Incretin hormones are produced in the gut in response to nutrients and act as insulin secretagogues.109 However, their effects seem to extend beyond being antihyperglycemic agents.109 Incretins currently used clinically are the glucagon like peptide-1 (GLP-1) analogs and the dipeptidyl peptidase-4 (DDP-4) inhibitors (DDP-4 metabolizes GLP-1). The vascular effects of incretins have been explored in different models. Initially, the cardiovascular vascular effects of GLP-1 were described in models of cardiomyopathy and ischemic reconditioning.110–112 More recently, the effect of GLP-1 and GLP-1 receptor activation of skeletal muscle vasculature and endothelial cells have been explored. Lie and colleagues demonstrated that GLP-1 treatment on bovine aortic endothelial cells results in increased Akt and eNOS phosphorylation, as well as cyclic AMP (cAMP)-dependent protein kinase (PKA) activity. In vivo, using adult Sprague–Dawley rats, GLP-1 infusion for 2 hours resulted in marked increments of skeletal muscle blood flow and glucose extraction. These effects were abolished by co-administration of L-NAME.113 In hypertensive rodents, sitagliptin, a DDP-4 inhibitor, reduced blood pressure, improved renal endothelial vasodilation, and increased eNOS activation in an AMPK-dependent fashion. These effects were mediated via the GLP-1 receptor, as they were abolished by the use of an antagonist.114 In contrast, treatment with alogliptin (another DDP-4 inhibitor), resulted in aortic relaxation in an endothelium-dependent manner, but a GLP-1 receptor antagonist did not blunt the effect.115 As recently reviewed,115 DPP-4 inhibitors may also exert beneficial cardiovascular and renal effects by reducing inflammation and maladaptive immune modulation.
A recent randomized, placebo-controlled study examined the role of saxagliptin, a DDP-4 inhibitor, in 16,492 patients with DM2 and increased cardiovascular risk. There was no cardiovascular protective effect (primary outcome), but the use of the DDP-4 inhibitor improved glycemic control and microalbuminuria while increasing the risk of heart failure hospitalization and hypoglycemia.116 Another recent double-blinded trial explored the effect of alogliptin after an acute coronary event in 5380 patients with DM2. The DDP-4 inhibitor had no effect in the rate of cardiovascular events in this population.117
Summary
Insulin resistance and hyperinsulinemia play a major role in the pathophysiology of obesity and DM2, which are leading risk factors for hypertension and CVD. It has also become increasingly clear that insulin resistance not only affects tissues considered classic targets for insulin action, but also significantly affects cardiovascular tissue, leading to vascular dysfunction and contributing to atherosclerosis. Expanding knowledge about the mechanisms of vascular injury mediated by resistance to the metabolic actions of insulin has uncovered novel roles for inflammation, excessive oxidative stress, incretins, and inappropriate RAAS activation in the pathophysiology of CVD as it relates to obesity and DM2, which can to some extent be manipulated with therapeutic objectives. Among non-pharmacologic interventions, weight-loss strategies are of paramount importance. Moderate caloric restriction, as well as regular physical activity, has been demonstrated to improve parameters of insulin resistance and vascular function, and add to our already abundant pharmacologic alternatives. RAAS modulation focused on MR and AT1R blockade are established forms of treatment, whereas anti-inflammation, antioxidation, and insulin-sensitizing agents have an encouraging therapeutic potential. Further research is needed, however, in order to provide more effective and comprehensive management of CVD, which is beyond doubt the most important burden for public health systems worldwide.
Acknowledgements
This research was supported by the National Institutes of Health (R01-HL73101 and R01-HL107910) and Veterans Affairs Merit System 0018. The authors wish to thank Brenda Hunter for her assistance in preparing this manuscript.
References
- 1.Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, et al. Prevalence of Diabetes and Prediabetes according to Fasting Plasma Glucose and HbA1c. Diabetes & metabolism journal. 2013 Oct;37(5):349–357. doi: 10.4093/dmj.2013.37.5.349. PubMed PMID: 24199164. Pubmed Central PMCID:3816136. Epub 2013/11/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta D, Krueger C, Lastra G. Over-nutrition, obesity and insulin resistance in the development of β-cell dysfunction. Curr Diabetes Rev. 2012 Mar 1;8(2):76–83. doi: 10.2174/157339912799424564. 2012. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ferrannini E. Insulin Resistance: A Multifaceted Syndrome Responsible for NIDDM, Obesity, Hypertension, Dyslipidemia, and Atherosclerotic Cardiovascular Disease. Diabetes Care. 1991 Mar 1;14(3):173–194. doi: 10.2337/diacare.14.3.173. 1991. [DOI] [PubMed] [Google Scholar]
- 4.Morrish N, Wang S, Stevens L, Fuller J, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001 Sep 1;44(Suppl 2):S14–S21. doi: 10.1007/pl00002934. 2001. [DOI] [PubMed] [Google Scholar]
- 5.Muniyappa R, Yavuz S. Metabolic Actions of Angiotensin II and Insulin: A Microvascular Endothelial Balancing Act. Mol Cell Endocrinol. 2012;378(1–2):59–69. doi: 10.1016/j.mce.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular Actions of Insulin. Endocr Rev. 2007 Aug 1;28(5):463–491. doi: 10.1210/er.2007-0006. 2007. [DOI] [PubMed] [Google Scholar]
- 7.Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin Receptor Substrate-1 and Phosphoinositide-Dependent Kinase-1 Are Required for Insulin-Stimulated Production of Nitric Oxide in Endothelial Cells. Mol Endocrinol. 2002 Aug 1;16(8):1931–42. doi: 10.1210/me.2002-0074. 2002. [DOI] [PubMed] [Google Scholar]
- 8.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, et al. Inhibition of Phosphatidylinositol 3-Kinase Enhances Mitogenic Actions of Insulin in Endothelial Cells. J Biol Chem. 2002 Jan 11;277(3):1794–1799. doi: 10.1074/jbc.M103728200. 2002. [DOI] [PubMed] [Google Scholar]
- 9.Michel JB, Feron O, Sacks D, Michel T. Reciprocal Regulation of Endothelial Nitric-oxide Synthase by Ca2+-Calmodulin and Caveolin. J Biol Chem. 1997 Jun 20;272(25):15583–15586. doi: 10.1074/jbc.272.25.15583. 1997. [DOI] [PubMed] [Google Scholar]
- 10.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, et al. Phosphorylation of Threonine 497 in Endothelial Nitric-oxide Synthase Coordinates the Coupling of L-Arginine Metabolism to Efficient Nitric Oxide Production. J Biol Chem. 2003 Nov 7;278(45):44719–44726. doi: 10.1074/jbc.M302836200. 2003. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard KA, Jr, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, et al. Heat Shock Protein 90 Mediates the Balance of Nitric Oxide and Superoxide Anion from Endothelial Nitric-oxide Synthase. J Biol Chem. 2001 May 18;276(21):17621–17624. doi: 10.1074/jbc.C100084200. 2001. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree M, Channon K. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011 Aug 1;25(2):81–88. doi: 10.1016/j.niox.2011.04.004. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khimji A, Rockey D. Endothelin--biology and disease. Cell Signal. 2010 Nov 1;22(11):1615–1625. doi: 10.1016/j.cellsig.2010.05.002. 2010. [DOI] [PubMed] [Google Scholar]
- 14.Ferri C, Pittoni V, Piccoli A, Laurenti O, Cassone MR, Bellini C, et al. Insulin stimulates endothelin-1 secretion from human endothelial cells and modulates its circulating levels in vivo. Journal of Clinical Endocrinology & Metabolism. 1995 Mar 1;80(3):829–835. doi: 10.1210/jcem.80.3.7883838. 1995. [DOI] [PubMed] [Google Scholar]
- 15.Zeng G, Quon M. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996 Aug 15;98(4):894–898. doi: 10.1172/JCI118871. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid Receptor-Mediated Vascular Insulin Resistance: An Early Contributor to Diabetes-Related Vascular Disease? Diabetes. 2013 Feb 1;62(2):313–319. doi: 10.2337/db12-0905. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo R. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010 Jul 1;53(7):1270–1287. doi: 10.1007/s00125-010-1684-1. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiebaud D, Jacot E, Defronzo RA, Maeder E, Jequier E, Felber J-P. The Effect of Graded Doses of Insulin on Total Glucose Uptake, Glucose Oxidation, and Glucose Storage in Man. Diabetes. 1982 Nov 1;31(11):957–963. doi: 10.2337/diacare.31.11.957. 1982. [DOI] [PubMed] [Google Scholar]
- 19.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab. 2011 Aug 1;301(2):E252–E263. doi: 10.1152/ajpendo.00186.2011. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saltin B, Radegran G, Koskolou M, Roach R. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998 Mar 1;162(3):421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. 1998. [DOI] [PubMed] [Google Scholar]
- 21.Klitzman B, Damon D, Gorczynski R, Duling B. Augmented tissue oxygen supply during striated muscle contraction in the hamster. Relative contributions of capillary recruitment, functional dilation, and reduced tissue PO2. Circ Res. 1982 Dec 1;51(6):711–721. doi: 10.1161/01.res.51.6.711. 1982. [DOI] [PubMed] [Google Scholar]
- 22.Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol. 2001 Nov 1;536(3):937–946. doi: 10.1111/j.1469-7793.2001.00937.x. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003 Jul 1;285(1):E123–E129. doi: 10.1152/ajpendo.00021.2003. 2003. [DOI] [PubMed] [Google Scholar]
- 24.Womack L, Peters D, Barrett E, Kaul S, Price W, Lindner J. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009 Jun 9;53(23):2175–2183. doi: 10.1016/j.jacc.2009.02.042. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab. 2008 Oct 1;295(4):E732–E750. doi: 10.1152/ajpendo.90477.2008. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley EA, Clark MG, Rattigan S. Acute effects of wortmannin on insulin's hemodynamic and metabolic actions in vivo. Am J Physiol Endocrinol Metab. 2007 Mar 1;292(3):E779–E787. doi: 10.1152/ajpendo.00407.2006. 2007. [DOI] [PubMed] [Google Scholar]
- 27.Ketel IJG, Stehouwer CDA, Serne EH, Korsen TJM, Hompes PGA, Smulders YM, et al. Obese But Not Normal-Weight Women with Polycystic Ovary Syndrome Are Characterized by Metabolic and Microvascular Insulin Resistance. J Clin Endocrinol Metab. 2008 Sep 1;93(9):3365–3372. doi: 10.1210/jc.2008-0626. 2008. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Ko S, Chai W, Cao W. Regulation of muscle microcirculation in health and diabetes. Diabetes & metabolism journal. 2012 Apr 1;36(2):83–89. doi: 10.4093/dmj.2012.36.2.83. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruotsalainen U, Raitakari M, Nuutila P, Oikonen V, Sipila H, Teras M, et al. Quantitative Blood Flow Measurement of Skeletal Muscle Using Oxygen-15-Water and PET. J Nucl Med. 1997 Feb 1;38(2):314–319. 1997. [PubMed] [Google Scholar]
- 30.Nuutila P, Raitakari M, Laine H, Kirvela O, Takala T, Utriainen T, et al. Role of blood flow in regulating insulin-stimulated glucose uptake in humans. Studies using bradykinin, [15O]water, and [18F]fluoro-deoxy-glucose and positron emission tomography. J Clin Invest. 1996 Apr 1;97(7):1741–1747. doi: 10.1172/JCI118601. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clerk LH, Rattigan S, Clark MG. Lipid Infusion Impairs Physiologic Insulin-Mediated Capillary Recruitment and Muscle Glucose Uptake In Vivo. Diabetes. 2002 Apr 1;51(4):1138–1145. doi: 10.2337/diabetes.51.4.1138. 2002. [DOI] [PubMed] [Google Scholar]
- 32.Baron AD. Hemodynamic actions of insulin. Am J Physiol Endocrinol Metab. 1994 Aug 1;267(2):E187–E202. doi: 10.1152/ajpendo.1994.267.2.E187. 1994. [DOI] [PubMed] [Google Scholar]
- 33.Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of Insulin Resistance in Insulin-Dependent Diabetes Mellitus: A Major Role for Reduced Skeletal Muscle Blood Flow. J Clin Endocrinol Metab. 1991 Sep 1;73(3):637–643. doi: 10.1210/jcem-73-3-637. 1991. [DOI] [PubMed] [Google Scholar]
- 34.Baron AD, Laakso M, Brechtel G, Hoit B, Watt C, Edelman SV. Reduced Postprandial Skeletal Muscle Blood Flow Contributes to Glucose Intolerance in Human Obesity. J Clin Endocrinol Metab. 1990 Jun 1;70(6):1525–1533. doi: 10.1210/jcem-70-6-1525. 1990. [DOI] [PubMed] [Google Scholar]
- 35.Baron A, Steinberg H, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest. 1995 Aug 1;96(2):786–792. doi: 10.1172/JCI118124. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laakso M, Edelman S, Brechtel G, Baron A. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990 Jun 1;85(6):1844–1852. doi: 10.1172/JCI114644. 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg H, Brechtel G, Johnson A, Fineberg N, Baron A. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994 Sep 1;94(3):1172–1179. doi: 10.1172/JCI117433. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raitakari M, Nuutila P, Ruotsalainen U, Laine H, Teras M, Iida H, et al. Evidence for Dissociation of Insulin Stimulation of Blood Flow and Glucose Uptake in Human Skeletal Muscle: Studies Using [15O]H2O, [18F]fluoro-2-deoxy-D-glucose, and Positron Emission Tomography. Diabetes. 1996 Nov 1;45(11):1471–1477. doi: 10.2337/diab.45.11.1471. 1996. [DOI] [PubMed] [Google Scholar]
- 39.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, et al. Physiologic Hyperinsulinemia Enhances Human Skeletal Muscle Perfusion by Capillary Recruitment. Diabetes. 2001 Dec 1;50(12):2682–2690. doi: 10.2337/diabetes.50.12.2682. 2001. [DOI] [PubMed] [Google Scholar]
- 40.Vincent MA, Dawson D, Clark ADH, Lindner JR, Rattigan S, Clark MG, et al. Skeletal Muscle Microvascular Recruitment by Physiological Hyperinsulinemia Precedes Increases in Total Blood Flow. Diabetes. 2002 Jan 1;51(1):42–48. doi: 10.2337/diabetes.51.1.42. 2002. [DOI] [PubMed] [Google Scholar]
- 41.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007 Jan 1;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. 2007. [DOI] [PubMed] [Google Scholar]
- 42.Lassegue B, Griendling KK. NADPH Oxidases: Functions and Pathologies in the Vasculature. Arterioscler Thromb Vasc Biol. 2010 Apr 1;30(4):653–661. doi: 10.1161/ATVBAHA.108.181610. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J-a, Jang H-J, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012 Jan 15;302(2):E201–E208. doi: 10.1152/ajpendo.00497.2011. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funke-Kaiser H, Reinemund J, Steckelings UM, Unger T. Adapter proteins and promoter regulation of the angiotensin AT2 receptor -- implications for cardiac pathophysiology. Journal of Renin-Angiotensin-Aldosterone System. 2010 Mar 1;11(1):7–17. doi: 10.1177/1470320309343652. 2010. [DOI] [PubMed] [Google Scholar]
- 45.Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999 Oct 1;104(7):925–935. doi: 10.1172/JCI7886. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu L, Carretero OA, Liao T-D, Harding P, Li H, Sumners C, et al. Role of Prolylcarboxypeptidase in Angiotensin II Type 2 Receptor-Mediated Bradykinin Release in Mouse Coronary Artery Endothelial Cells. Hypertension. 2010 Sep 1;56(3):384–390. doi: 10.1161/HYPERTENSIONAHA.110.155051. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motta G, Rojkjaer R, Hasan AAK, Cines DB, Schmaier AH. High Molecular Weight Kininogen Regulates Prekallikrein Assembly and Activation on Endothelial Cells: A Novel Mechanism for Contact Activation. Blood. 1998 Jan 15;91(2):516–528. 1998. [PubMed] [Google Scholar]
- 48.Retailleau K, Belin de Chantemele EJ, Chanoine S, Guihot A-L, Vessieres E, Toutain B, et al. Reactive Oxygen Species and Cyclooxygenase 2-Derived Thromboxane A2 Reduce Angiotensin II Type 2 Receptor Vasorelaxation in Diabetic Rat Resistance Arteries. Hypertension. 2010 Feb 1;55(2):339–344. doi: 10.1161/HYPERTENSIONAHA.109.140236. 2010. [DOI] [PubMed] [Google Scholar]
- 49.Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin Type 2 Receptor in Resistance Arteries of Type 2 Diabetic Hypertensive Patients. Hypertension. 2007 Feb 1;49(2):341–346. doi: 10.1161/01.HYP.0000253968.95136.b8. 2007. [DOI] [PubMed] [Google Scholar]
- 50.Chai W, Wang W, Liu J, Barrett EJ, Carey RM, Cao W, et al. Angiotensin II Type 1 and Type 2 Receptors Regulate Basal Skeletal Muscle Microvascular Volume and Glucose Use. Hypertension. 2010 Feb 1;55(2):523–530. doi: 10.1161/HYPERTENSIONAHA.109.145409. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II Receptors Modulate Muscle Microvascular and Metabolic Responses to Insulin In Vivo. Diabetes. 2011 Nov 1;60(11):2939–2946. doi: 10.2337/db10-1691. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM-E, Clark SE, et al. Angiotensin II-induced NADPH Oxidase Activation Impairs Insulin Signaling in Skeletal Muscle Cells. J Biol Chem. 2006 Nov 17;281(46):35137–35146. doi: 10.1074/jbc.M601320200. 2006. [DOI] [PubMed] [Google Scholar]
- 53.Steinberg H, Chaker H, Leaming R, Johnson A, Brechtel G, Baron A. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996 Jun 1;97(11):2601–2610. doi: 10.1172/JCI118709. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity Blunts Microvascular Recruitment in Human Forearm Muscle After a Mixed Meal. Diabetes Care. 2009 Sep 1;32(9):1672–1677. doi: 10.2337/dc09-0206. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14(1):5–12. doi: 10.1007/s11154-012-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frisbee JC. Remodeling of the skeletal muscle microcirculation increases resistance to perfusion in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2003 Jun 5;285(1):H104–H111. doi: 10.1152/ajpheart.00118.2003. 2003. [DOI] [PubMed] [Google Scholar]
- 57.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104(4):447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005;289(2):25. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free Fatty Acids Induce Insulin Resistance in Both Cardiac and Skeletal Muscle Microvasculature in Humans. J Clin Endocrinol Metab. 2011 Feb 1;96(2):438–446. doi: 10.1210/jc.2010-1174. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samuel V, Petersen K, Shulman G. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010 Jun 26;375(9733):2267–2277. doi: 10.1016/S0140-6736(10)60408-4. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25(3):487–496. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 62.Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55(3):691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 63.Li Q, Park K, Li C, Rask-Madsen C, Mima A, Qi W, et al. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-beta isoform in the endothelium. Circ Res. 2013;113(4):418–427. doi: 10.1161/CIRCRESAHA.113.301074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, et al. Protein kinase C-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127(1):86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jang HJ, Kim HS, Hwang DH, Quon MJ, Kim JA. Toll-like receptor 2 mediates high-fat diet-induced impairment of vasodilator actions of insulin. Am J Physiol Endocrinol Metab. 2013;304(10):26. doi: 10.1152/ajpendo.00578.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111(9):1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duncan ER, Crossey PA, Walker S, Anilkumar N, Poston L, Douglas G, et al. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57(12):3307–3314. doi: 10.2337/db07-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sukumar P, Viswambharan H, Imrie H, Cubbon RM, Yuldasheva N, Gage M, et al. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes. 2013;62(6):2130–2134. doi: 10.2337/db12-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gage MC, Yuldasheva NY, Viswambharan H, Sukumar P, Cubbon RM, Galloway S, et al. Endothelium-specific insulin resistance leads to accelerated atherosclerosis in areas with disturbed flow patterns: a role for reactive oxygen species. Atherosclerosis. 2013;230(1):131–139. doi: 10.1016/j.atherosclerosis.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 70.Slaaby R, Schaffer L, Lautrup-Larsen I, Andersen AS, Shaw AC, Mathiasen IS, et al. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J Biol Chem. 2006;281(36):25869–25874. doi: 10.1074/jbc.M605189200. [DOI] [PubMed] [Google Scholar]
- 71.Federici M, Porzio O, Lauro D, Borboni P, Giovannone B, Zucaro L, et al. Increased abundance of insulin/insulin-like growth factor-I hybrid receptors in skeletal muscle of obese subjects is correlated with in vivo insulin sensitivity. J Clin Endocrinol Metab. 1998;83(8):2911–2915. doi: 10.1210/jcem.83.8.4935. [DOI] [PubMed] [Google Scholar]
- 72.Federici M, Porzio O, Zucaro L, Giovannone B, Borboni P, Marini MA, et al. Increased abundance of insulin/IGF-I hybrid receptors in adipose tissue from NIDDM patients. Mol Cell Endocrinol. 1997;135(1):41–47. doi: 10.1016/s0303-7207(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 73.Abbas A, Imrie H, Viswambharan H, Sukumar P, Rajwani A, Cubbon RM, et al. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes. 2011;60(8):2169–2178. doi: 10.2337/db11-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imrie H, Viswambharan H, Sukumar P, Abbas A, Cubbon RM, Yuldasheva N, et al. Novel role of the IGF-1 receptor in endothelial function and repair: studies in endothelium-targeted IGF-1 receptor transgenic mice. Diabetes. 2012;61(9):2359–2368. doi: 10.2337/db11-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kearney MT. Changing the way we think about endothelial cell insulin sensitivity, nitric oxide, and the pathophysiology of type 2 diabetes: the FoxO is loose. Diabetes. 2013;62(5):1386–1388. doi: 10.2337/db13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, et al. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15(3):372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuchiya K, Accili D. Liver sinusoidal endothelial cells link hyperinsulinemia to hepatic insulin resistance. Diabetes. 2013;62(5):1478–1489. doi: 10.2337/db12-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lastra G, Dhuper S, Johnson MS, Sowers JR. Salt, aldosterone, and insulin resistance: impact on the cardiovascular system. Nat Rev Cardiol. 2010;7(10):577–584. doi: 10.1038/nrcardio.2010.123. [DOI] [PubMed] [Google Scholar]
- 79.Lastra-Lastra G, Sowers JR, Restrepo-Erazo K, Manrique-Acevedo C, Lastra-González G. Role of aldosterone and angiotensin II in insulin resistance: an update. Clinical Endocrinology. 2009;71(1):1–6. doi: 10.1111/j.1365-2265.2008.03498.x. [DOI] [PubMed] [Google Scholar]
- 80.Rossi G, Boscaro M, Ronconi V, Funder JW. Aldosterone as a cardiovascular risk factor. Trends Endocrinol Metab. 2005;16(3):104–107. doi: 10.1016/j.tem.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 81.Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautiere K, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33(2):191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- 82.Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, et al. Aldosterone Suppresses Insulin Signaling Via the Downregulation of Insulin Receptor Substrate-1 in Vascular Smooth Muscle Cells. Hypertension. 2007 Oct 1;50(4):750–755. doi: 10.1161/HYPERTENSIONAHA.107.093955. 2007. [DOI] [PubMed] [Google Scholar]
- 83.Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of Reactive Oxygen Species-Dependent Downregulation of Insulin Receptor Substrate-1 by Angiotensin II. Arterioscler Thromb Vasc Biol. 2005 Jun 1;25(6):1142–1147. doi: 10.1161/01.ATV.0000164313.17167.df. 2005. [DOI] [PubMed] [Google Scholar]
- 84.Draznin B. Molecular Mechanisms of Insulin Resistance: Serine Phosphorylation of Insulin Receptor Substrate-1 and Increased Expression of p85{alpha}: The Two Sides of a Coin. Diabetes. 2006 Aug 1;55(8):2392–2397. doi: 10.2337/db06-0391. 2006. [DOI] [PubMed] [Google Scholar]
- 85.Sherajee SJ, Fujita Y, Rafiq K, Nakano D, Mori H, Masaki T, et al. Aldosterone induces vascular insulin resistance by increasing insulin-like growth factor-1 receptor and hybrid receptor. Arterioscler Thromb Vasc Biol. 2012;32(2):257–263. doi: 10.1161/ATVBAHA.111.240697. [DOI] [PubMed] [Google Scholar]
- 86.Xing W, Li Y, Zhang H, Mi C, Hou Z, Quon MJ, et al. Improvement of vascular insulin sensitivity by downregulation of GRK2 mediates exercise-induced alleviation of hypertension in spontaneously hypertensive rats. American Journal of Physiology - Heart and Circulatory Physiology. 2013 doi: 10.1152/ajpheart.00290.2013. 2013-10-15 16:00:50;305(8):H1111–H9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shahid G, Hussain T. GRK2 negatively regulates glycogen synthesis in mouse liver FL83B cells. J Biol Chem. 2007;282(28):20612–20620. doi: 10.1074/jbc.M700744200. [DOI] [PubMed] [Google Scholar]
- 88.Usui I, Imamura T, Babendure JL, Satoh H, Lu JC, Hupfeld CJ, et al. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol Endocrinol. 2005;19(11):2760–2768. doi: 10.1210/me.2004-0429. [DOI] [PubMed] [Google Scholar]
- 89.Taguchi K, Matsumoto T, Kamata K, Kobayashi T. G protein-coupled receptor kinase 2, with beta-arrestin 2, impairs insulin-induced Akt/endothelial nitric oxide synthase signaling in ob/ob mouse aorta. Diabetes. 2012;61(8):1978–1985. doi: 10.2337/db11-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mikus CR, Roseguini BT, Uptergrove GM, Morris EM, Rector RS, Libla JL, et al. Voluntary wheel running selectively augments insulin-stimulated vasodilation in arterioles from white skeletal muscle of insulin-resistant rats. Microcirculation. 2012;19(8):729–738. doi: 10.1111/j.1549-8719.2012.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, et al. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52(1):72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26(7):2119–2125. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- 93.Maeda S, Jesmin S, Iemitsu M, Otsuki T, Matsuo T, Ohkawara K, et al. Weight loss reduces plasma endothelin-1 concentration in obese men. Exp Biol Med. 2006;231(6):1044–1047. [PubMed] [Google Scholar]
- 94.Katakam PV, Ujhelyi MR, Hoenig M, Miller AW. Metformin improves vascular function in insulin-resistant rats. Hypertension. 2000;35(1 Pt 1):108–112. doi: 10.1161/01.hyp.35.1.108. [DOI] [PubMed] [Google Scholar]
- 95.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55(2):496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 96.Diamanti-Kandarakis E, Alexandraki K, Protogerou A, Piperi C, Papamichael C, Aessopos A, et al. Metformin administration improves endothelial function in women with polycystic ovary syndrome. Eur J Endocrinol. 2005;152(5):749–756. doi: 10.1530/eje.1.01910. [DOI] [PubMed] [Google Scholar]
- 97.Hong J, Zhang Y, Lai S, Lv A, Su Q, Dong Y, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36(5):1304–1311. doi: 10.2337/dc12-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rahman ST, Merchant N, Haque T, Wahi J, Bhaheetharan S, Ferdinand KC, et al. The impact of lipoic acid on endothelial function and proteinuria in quinapril-treated diabetic patients with stage I hypertension: results from the QUALITY study. J Cardiovasc Pharmacol Ther. 2012;17(2):139–145. doi: 10.1177/1074248411413282. [DOI] [PubMed] [Google Scholar]
- 99.Chen H, Karne RJ, Hall G, Campia U, Panza JA, Cannon RO, 3rd, et al. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol. 2006;290(1):26. doi: 10.1152/ajpheart.00768.2005. [DOI] [PubMed] [Google Scholar]
- 100.Heitzer T, Finckh B, Albers S, Krohn K, Kohlschutter A, Meinertz T. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radic Biol Med. 2001;31(1):53–61. doi: 10.1016/s0891-5849(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 101.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Keaney JF, Jr, Creager MA. Oral antioxidant therapy improves endothelial function in Type 1 but not Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285(6):24. doi: 10.1152/ajpheart.00403.2003. [DOI] [PubMed] [Google Scholar]
- 102.Kim JA. Mechanisms underlying beneficial health effects of tea catechins to improve insulin resistance and endothelial dysfunction. Endocr Metab Immune Disord Drug Targets. 2008;8(2):82–88. doi: 10.2174/187153008784534349. [DOI] [PubMed] [Google Scholar]
- 103.Reiter CE, Kim JA, Quon MJ. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology. 2010;151(1):103–114. doi: 10.1210/en.2009-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jang HJ, Ridgeway SD, Kim JA. Effects of the Green Tea Polyphenol, Epigallocatechin-3-Gallate (EGCG), on High Fat Diet-Induced Insulin Resistance and Endothelial Dysfunction. Am J Physiol Endocrinol Metab. 2013;22:22. doi: 10.1152/ajpendo.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296(10):1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 106.Polychronopoulos E, Zeimbekis A, Kastorini CM, Papairakleous N, Vlachou I, Bountziouka V, et al. Effects of black and green tea consumption on blood glucose levels in non-obese elderly men and women from Mediterranean Islands (MEDIS epidemiological study) Eur J Nutr. 2008;47(1):10–16. doi: 10.1007/s00394-007-0690-7. [DOI] [PubMed] [Google Scholar]
- 107.Rizza S, Muniyappa R, Iantorno M, Kim JA, Chen H, Pullikotil P, et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(5):2010–2879. doi: 10.1210/jc.2010-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Misaka S, Yatabe J, Muller F, Takano K, Kawabe K, Glaeser H, et al. Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clinical pharmacology and therapeutics. 2014 Jan 13; doi: 10.1038/clpt.2013.241. PubMed PMID: 24419562. Epub 2014/01/15. Eng. [DOI] [PubMed] [Google Scholar]
- 109.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 110.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110(8):955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 111.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289(6):15. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 112.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317(3):1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 113.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61(4):888–896. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60(3):833–841. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]
- 115.Shah Z, Pineda C, Kampfrath T, Maiseyeu A, Ying Z, Racoma I, et al. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vascul Pharmacol. 2011;55(1–3):2–9. doi: 10.1016/j.vph.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 117.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]