Abstract
Background.
Ethnicity is an important determinant of post-renal transplant outcomes. Limited data are available on cardiovascular risk differences in kidney transplant recipients (KTR) based on ethnicity.
Methods.
A group of 129 clinically stable age-matched KTR [43 South Asian (SA), 86 Caucasian]) were assessed for plasma total and high-molecular-weight (HMW) adiponectin, cystatin C, apolipoproteins A1 and B, C-reactive protein, uric acid, urine albumin-to-creatinine ratio, estimated glomerular filtration rate (eGFR) and transplant-specific plus traditional Framingham risk factors. SA and Caucasians were compared by t-tests, Wilcoxon rank-sum or chi-square testing. Accounting for the matched design, multivariable linear regression was performed to determine predictors of adiponectin concentrations.
Results.
SA did not differ from Caucasians in background cardiac disease or cardioprotective medication use or risk factors other than smoking (26 versus 56%, P = 0.001). Total adiponectin (9.5 ± 3.5 versus 12.9 ± 6.7 μmg/mL, P < 0.001) and HMW adiponectin (22 ± 9 versus 29 ± 11%, P < 0.001) were significantly lower in SA. Determinants of total adiponectin included SA ethnicity (P = 0.02), cystatin C-eGFR (P < 0.001), high-density lipoprotein (HDL) cholesterol (P < 0.0001) and waist-to-hip ratio (P < 0.001), while those of HMW adiponectin included SA ethnicity (P < 0.001), cystatin C-eGFR (P = 0.03) and HDL cholesterol (P = 0.001). There were no important differences in the other measured biomarkers.
Conclusion.
Total and HMW adiponectin concentrations are lower in SA KTR and may be promising exploratory biomarkers of post-transplant cardiovascular risk.
Keywords: cardiovascular disease, ethnicity, risk factors, renal transplantation
Introduction
Patients with end-stage renal disease remain at increased cardiovascular risk post-kidney transplantation [1] and South Asians (SA) are at particular risk for post-transplant cardiovascular disease (CVD) compared to other ethnic groups [2]. Although conventional risk factors explain a substantial portion of transplant-related cardiovascular risk, the most powerful models utilizing these such as the Framingham risk score (FRS) significantly underpredict post-transplant cardiac events [3]. The directed investigation of etiological factors especially relevant to large transplant population subgroups can therefore yield useful information that may help inform the design and implementation of measures to reduce overall post-transplant CVD burden.
Adiponectin is a protein secreted by adipose tissue that has both anti-inflammatory and anti-atherogenic properties [4]. Reduced adiponectin concentrations have been noted in SA compared to other ethnic groups in non-transplant populations [5]. Compared to Caucasians, SA also have a higher prevalence of glucose intolerance and lipid abnormalities [6], a higher C-reactive protein (CRP) level [7], waist-to-hip ratio [8] and apoB/apoA-I ratio [8]. Cystatin C, a marker of atherosclerosis and cardiovascular mortality [9], varies by ethnicity in patients with kidney impairment [10]. However, extrapolating such observations from other populations to post-transplant groups is inadvisable [2] since the relationship of risk factor profiles to outcomes may be atypical [11] due to peculiarities in the post-transplant milieu. The primary objective of this hypothesis-generating study therefore was to determine if ethnic-specific differences exist in adiponectin concentrations, with the secondary objective being to identify other candidate novel cardiovascular risk factors unique to South Asian kidney transplant recipients (SA KTR).
Materials and methods
St. Michael's Hospital is a university-affiliated tertiary care medical surgical centre that currently provides post-transplant care to ∼1200 adult single-organ KTR and performs 120 such transplants annually. The existing clinical database was queried to identify recipients of SA and Caucasian ancestry. A group of 129 clinically stable KTR matched 1:2 by ethnicity (43 SA and 86 Caucasian) and age (±10 years) who were 3–60 months post-transplant were recruited at the time of a routine clinic visit for the measurement of plasma adiponectin concentrations and other biomarkers. This time frame was chosen due to the greater number of SA recipients transplanted in recent years and also to minimize the effects of advancing age or graft dysfunction on post-transplant outcomes. Informed consent was obtained from all recipients with translation assistance if needed, and the study was approved by the St. Michael's Hospital Research Ethics Board (08-233, 26 September 2008).
SA status was defined as any self-reported ancestry from the Indian subcontinent (India, Pakistan, Bangladesh, Sri Lanka) whether born in those countries or elsewhere [2], and Caucasian status as any ancestry from Europe. Those with known combined ancestry were deemed ineligible. For study inclusion, stable renal function was defined as a fluctuation in the serum creatinine value of <20% from baseline [12] in the 2 months immediately preceding recruitment. Multiorgan transplant recipients and hospitalized patients were excluded. Traditional Framingham risk factor-related information (demographic data, automated blood pressure using the BP Tru® device, smoking and diabetes history) as well as body mass index (BMI) and waist-to-hip ratio as indices of obesity, in addition to estimated glomerular filtration rate (eGFR) as estimated by the modified Modification of Diet in Renal Diseases (MDRD) equation [13], glycosylated hemoglobin (HbA1c), serum uric acid, highly sensitive CRP, parathyroid hormone (PTH) and morning urine albumin-to-creatinine ratio (ACR) were all collected at the time of recruitment. Detailed information on immunosuppressive medication exposure, as well as cardiovascular risk-related medication, was obtained by chart review at the time of informed consent. Background CVD prevalence defined as major adverse cardiac events (MACE; myocardial infarction, coronary revascularization by angioplasty or bypass grafting or cardiac death) was assessed from charts blinded to individual patient identity by a cardiologist not associated with recruitment. FRS was calculated according to the method of Wilson et al. [14]. Delayed graft function was defined as the need for dialysis in the first post-operative week, acute rejection based on Banff 1997 criteria and new-onset diabetes based on the 2008 Canadian Diabetes Association guidelines [15].
Standardized lipoprotein measurements on non-fasting samples were determined by ultracentrifugation as described earlier using methodology that does not require fasting [16] since non-fasting determinations of apolipoprotein B (apoB), apoA-I and triglycerides have been shown to be predictive of ischemic heart disease [17] and exercise and fasting/feeding do not affect serum adiponectin concentrations.
Total adiponectin was measured by enzyme-linked immunosorbent assay (Kit#EZHADP-61-K; Millipore, St. Charles, MO) with a between-day coefficient of variation of 4.4 and 6.8% at values of 5.5 and 27.4 μg/mL, respectively. High-molecular-weight (HMW) adiponectin was separated by density gradient ultracentrifugation using an SW60 Beckman rotor and measured as previously described [17]. The method's between-day coefficient of variation was 14% for a serum pool with an HMW proportion of 32%. Cystatin C, apolipoprotein B and apolipoprotein AI were measured using the BN Prospec (Dade-Behring, Mississauga, ON). The eGFR estimated using cystatin C was derived using the Arnal-Dade formula (eGFR = 74.835/[cystatin_c × 1.333]) [18].
The required sample size for this study was determined on the basis of adiponectin measurements in a previous study of women with gestational diabetes [19] wherein attaining 80% power required 40 SA/70 Caucasians in order to detect a 5.7% difference in adiponectin concentrations. Due to known gender differences in adiponectin, with females having higher levels, analyses were performed separately by gender. Between-group comparisons were made by Student's t-test, Wilcoxon rank-sum or chi-square testing as appropriate. Normality of adiponectin or log10 adiponectin distribution was tested using the Kolmogorov–Smirnov test. Multivariable analysis of variance was performed using a mixed linear model for matched observations. A two-tailed P-value of <0.05 was considered significant for all analyses. SAS® (Cary, NC) 9.1 was the statistical software used.
Results
There were 43 matched triplet sets of patients (43 SA, 86 Caucasians) studied. Their demographic and anthropometric characteristics are provided in Table 1. There was no difference in any of the FRS contained risk factors between the two groups apart from smoking which was less in SA overall and non-existent among SA females. Among the non-FRS parameters, BMI but not waist-to-hip ratio was higher in Caucasian females. SA and Caucasian recipients differed in cyclosporine use (16 versus 5%, P = 0.03) but there were no other differences in the immunosuppressive medication profile, including tacrolimus (81 versus 91%, P = 0.13), mycophenolate mofetil (77 versus 76%, P = 0.88) or prednisone (79 versus 79%, P = 1.00). All patients received induction therapy with either basiliximab or anti-thymocyte globulin. SA and Caucasian recipients did not differ in use of cardioprotective medications such as angiotensin-converting enzyme (ACE) inhibitors (26 versus 17%, P = 0.27), 3-hydroxyl 3-methylglutaryl coenzyme A reductase inhibitors (67 versus 69%, P = 0.89), aspirin (35 versus 38%, P = 0.69) or insulin (26 versus 14%, P = 0.10). However, beta-blocker use was more prevalent in SA (42 versus 23%, P = 0.02), particularly in women (69 versus 15%, P = 0.001). SA women were taking on average 1.5 anti-hypertensive medications compared to 0.8 for Caucasian women (P = 0.03).
Table 1.
Demographic and cardiovascular risk factor comparison of South Asian (SA, N = 43) and Caucasian (C, N = 86) KTRa
| All patients |
Male |
Female |
|||||||
| Parameter | SA (N = 43) | C (N = 86) | P-value | SA (N = 30) | C (N = 60) | P-value | SA (N = 13) | C (N = 26) | P-value |
| Demographics | |||||||||
| Age at transplant (years) | 48 ± 11 | 50 ± 11 | NS | 49 ± 11 | 51 ± 12 | NS | 47 ± 11 | 48 ± 10 | NS |
| Age at study visit (years) | 50 ± 10 | 52 ± 11 | NS | 51 ± 11 | 53 ± 12 | NS | 49 ± 10 | 50 ± 10 | NS |
| Gender (male/female) | 30/13 | 60/26 | NS | ||||||
| Time post-transplant (months) | 65 ± 47 | 71 ± 48 | NS | 65 ± 40 | 73 ± 48 | NS | 63 ± 64 | 67 ± 50 | NS |
| Donor source (live/deceased) | 28/15 | 57/29 | NS | 24/6 | 40/20 | NS | 4/9 | 17/9 | <0.05 |
| No. of transplants (1/2) | 42/1 | 81/5 | NS | 29/1 | 55/5 | NS | 13/0 | 26/0 | NS |
| Cause of end-stage renal disease N (%) | |||||||||
| Diabetes | 9 (21) | 12 (14) | NS | 7 (23) | 9 (15) | NS | 2 (15) | 3 (11) | NS |
| Hypertension | 5 (12) | 4 (5) | 4 (13) | 3 (5) | 1 (8) | 1 (4) | |||
| Glomerulonephritis | 22 (51) | 29 (34) | 15 (50) | 21 (35) | 7 (54) | 8 (31) | |||
| Polycystic kidney disease | 3 (7) | 24 (28) | 2 (7) | 15 (25) | 1 (7) | 9 (35) | |||
| Others | 4 (9) | 17 (19) | 2 (7) | 12 (20) | 2 (16) | 5 (19) | |||
| Acute rejection N (%) | 7 (16) | 11 (13) | NS | 4 (13) | 9 (15) | NS | 3 (23) | 2 (8) | NS |
| Delayed graft function N (%) | 1 (2) | 1 (1) | NS | 0 (0) | 1 (2) | NS | 1 (8) | 0 (0) | NS |
| Cardiovascular risk factors | |||||||||
| BMI (kg/m2) | 26.9 ± 4.1 | 28.0 ± 6.0 | NS | 28 ± 4 | 27 ± 5 | NS | 25 ± 4 | 29 ± 8 | NS |
| Waist-to-hip ratio | 0.94 ± 0.04 | 0.93 ± 0.07 | NS | 0.96 ± 0.05 | 0.96 ± 0.06 | NS | 0.90 ± 0.05 | 0.88 ± 0.05 | NS |
| Diabetes N (%) | |||||||||
| Pre-existing | 13 (30) | 17 (20) | NS | 10 (33) | 13 (22) | NS | 3 (23) | 4 (15) | NS |
| New-onset | 4 (9) | 5 (6) | 2 (9) | 3 (5) | 2 (15) | 2 (8) | |||
| Total | 17 (40) | 22 (26) | 12 (40) | 16 (26) | 5 (38) | 6 (23) | |||
| Systolic blood pressure (mmHg) | 130 ± 16 | 128 ± 16 | NS | 132 ± 17 | 129 ± 14 | NS | 127 ± 16 | 126 ± 19 | NS |
| Diastolic blood pressure (mmHg) | 80 ± 11 | 80 ± 10 | NS | 80 ± 9 | 79 ± 10 | NS | 81 ± 14 | 83 ± 10 | NS |
| Total cholesterol (mmol/L) | 4.8 ± 1.6 | 4.4 ± 1.1 | NS | 4.5 ± 1.3 | 4.2 ± 0.9 | NS | 5.5 ± 2.0 | 4.8 ± 1.3 | NS |
| HDL cholesterol (mmol/L) | 1.1 ± 0.4 | 1.1 ± 0.3 | NS | 1.0 ± 0.3 | 1.0 ± 0.3 | NS | 1.4 ± 0.5 | 1.3 ± 0.4 | NS |
| LDL cholesterol (mmol/L) | 3.1 ± 1.2 | 2.7 ± 0.9 | NS | 3.0 ± 1.1 | 2.7 ± 0.8 | NS | 3.6 ± 1.5 | 2.9 ± 1.0 | NS |
| Triglycerides (mmol/L) | 1.8 ± 0.9 | 1.6 ± 0.7 | NS | 1.8 ± 0.9 | 1.6 ± 0.7 | NS | 1.8 ± 1.0 | 1.7 ± 0.7 | NS |
| VLDL cholesterol | 0.5 ± 0.4 | 0.5 ± 0.3 | NS | 0.5 ± 0.4 | 0.5 ± 0.3 | NS | 0.5 ± 0.5 | 0.5 ± 0.4 | NS |
| VLDL triglycerides | 1.3 ± 0.8 | 1.2 ± 0.6 | NS | 1.3 ± 0.8 | 1.2 ± 0.7 | NS | 1.2 ± 0.9 | 1.2 ± 0.6 | NS |
| Smoking N (%) | 11 (26) | 48 (56) | 0.001 | 11 (36) | 34 (56) | NS | 0 (0) | 14 (54) | 0.001 |
| Parathyroidectomy N (%) | 4 (9) | 6 (7) | NS | 0 (0) | 4 (7) | NS | 4 (31) | 2 (8) | NS |
| Major cardiac event (MACE) N (%) | 12 (28) | 14 (16) | NS | 10 (33) | 13 (22) | NS | 2 (15) | 1 (4) | NS |
| FRS | 9.5 ± 8.3 | 8.6 ± 6.7 | NS | 11.1 ± 9.0 | 9.7 ± 7.0 | NS | 5.7 ± 5.1 | 6.1 ± 5.2 | NS |
| Adiponectin (μg/mL) | 9.5 ± 3.5 | 12.9 ± 6.7 | <0.001 | 8.9 ± 3.6 | 11.5 ± 4.3 | <0.01 | 11.0 ± 3.1 | 16.3 ± 9.7 | 0.02 |
| HMW adiponectin (%) | 22 ± 9 | 29 ± 11 | <0.001 | 21 ± 9 | 28 ± 10 | <0.01 | 23 ± 8 | 33 ± 12 | 0.02 |
| ApoB/apoAI ratio | 0.65 ± 0.23 | 0.59 ± 0.16 | NS | 0.66 ± 0.21 | 0.61 ± 0.16 | NS | 0.63 ± 0.27 | 0.55 ± 0.18 | NS |
| Serum creatinine (μmol/L) | 110 ± 39 | 118 ± 34 | NS | 112 ± 27 | 124 ± 34 | NS | 107 ± 59 | 106 ± 32 | NS |
| eGFR by MDRD (mL/min/1.73m2) | 66 ± 21 | 60 ± 19 | NS | 68 ± 19 | 62 ± 20 | NS | 62 ± 25 | 56 ± 18 | NS |
| Cystatin C (mg/L) | 1.2 ± 0.4 | 1.2 ± 0.3 | NS | 1.2 ± 0.3 | 1.3 ± 0.3 | NS | 1.2 ± 0.6 | 1.2 ± 0.3 | NS |
| eGFR by cystatin C (mL/min/1.73m2) | 65 ± 22 | 62 ± 20 | NS | 64 ± 17 | 61 ± 20 | NS | 69 ± 30 | 65 ± 20 | NS |
| C-reactive protein (mg/L) | 4.5 ± 7.9 | 6.0 ± 13 | NS | 4.2 ± 8.5 | 6.0 ± 15.7 | NS | 5.3 ± 7.0 | 5.9 ± 7.1 | NS |
| Uric acid (μmol/L) | 359 ± 82 | 381 ± 95 | NS | 374 ± 81 | 399 ± 89 | NS | 325 ± 77 | 338 ± 98 | NS |
| Urine ACR (mg/mmol) | 6.8 ± 14.8 | 5.5 ± 10.6 | NS | 5.4 ± 5.2 | 6.5 ± 12.3 | NS | 10.3 ± 26.3 | 3.4 ± 4.3 | NS |
| Hemoglobin A1c (%) | 0.068 ± 0.01 | 0.060 ± 0.01 | <0.01 | 0.067 ± 0.01 | 0.060 ± 0.01 | <0.05 | 0.071 ± 0.02 | 0.060 ± 0.00 | NS |
| PTH (pmol/L) | 10 ± 6.3 | 10 ± 7.7 | NS | 11 ± 6 | 10 ± 6 | NS | 9 ± 7 | 12 ± 11 | NS |
Apo, apolipoprotein; VLDL, very low-density lipoprotein.
There was no difference between groups in prior MACE rates or the FRS (Table 1). The most striking difference between SA and Caucasian recipients was seen in total adiponectin concentrations and the percentage of HMW adiponectin. Both adiponectin and HMW adiponectin were significantly lower in SA of either gender. By contrast, there were no significant differences in the other novel risk factors (apoB/apoA-I ratio; cystatin C, CRP, uric acid or urine ACR). Interestingly, HbA1c values were higher in SA (Table 1). Common health indicators such as hemoglobin and albumin were not different (data not shown). Overall, there was no difference in renal function as determined by either MDRD eGFR or cystatin C (Table 1).
Adiponectin concentrations were significantly lower in males than females (P = 0.001) and in SA than Caucasians (P < 0.001). The mean concentration of adiponectin found in SA males (8.9 μg/mL) and females (11.0 μg/mL) was found to be similar to that observed in our previous study of incident diabetes [20]. There was an insignificant difference in total adiponectin concentrations between those that experienced or did not experience previous MACE (10.6 ± 3.8 versus 12.1 ± 6.5 μg/mL, P = 0.12), and similarly, no difference in HMW adiponectin (26.2 ± 10.6 versus 26.8 ± 10.8%, P = 0.79).
Normality of distribution was noted for log10 adiponectin but not untransformed adiponectin (Figure 1). Univariate analysis of the determinants for log10 adiponectin and HMW adiponectin is provided in Table 2. The eGFR (MDRD) correlated inversely with log10 adiponectin when all subjects were combined (r = −0.271, P = 0.002) and for females (r = −0.398, P = 0.01), but not males. By contrast, however, eGFR based on cystatin C was correlated inversely with log10 adiponectin for all subjects combined (r = −0.260, P = 0.003), females (r = −0.333, P = 0.03) and males (r = −0.283, P = 0.007). High-density lipoprotein (HDL) cholesterol was positively correlated with log10 adiponectin for both females (r = 0.471, P < 0.001) and males (r = 0.271, P = 0.01). HMW adiponectin was highly correlated with log10 adiponectin in females (r = 0.836, P < 0.001) and both log10 adiponectin (r = 0.809, P < 0.001) and HDL cholesterol (r = 0.25, P = 0.01) in males.
Fig. 1.
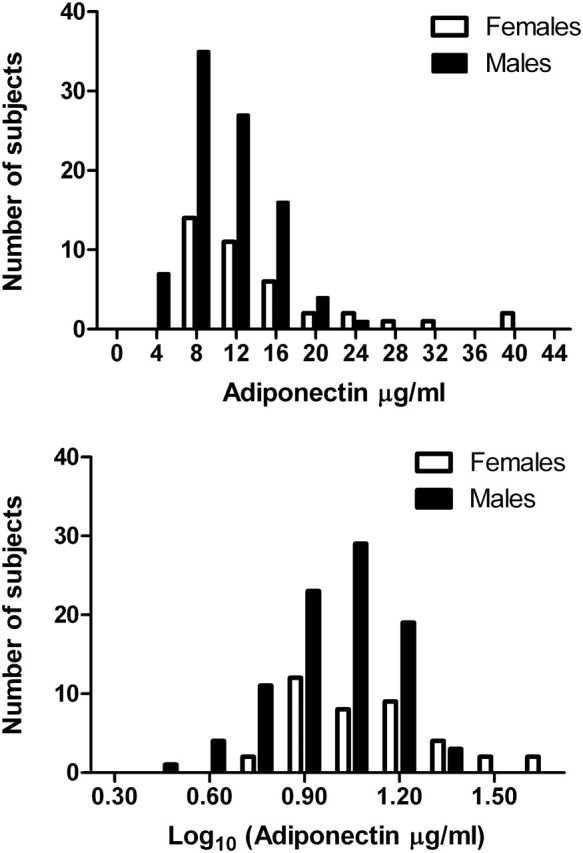
Distribution of total adiponectin and log10-transformed total adiponectin concentrations for the total population.
Table 2.
Univariate correlation between log-transformed adiponectin and selected risk factors
| Risk factor | All patients (N = 129) |
Male (N = 90) |
Female (N = 39) |
||||||
| Continuous | Pearson correlation coefficients | R 2 | P-value | Pearson correlation coefficients | R 2 | P-value | Pearson correlation coefficients | R 2 | P-value |
| Age at transplant | 0.040 | 0.002 | NS | 0.075 | 0.006 | NS | 0.044 | 0.002 | NS |
| Age at study visit | 0.046 | 0.002 | NS | 0.088 | 0.008 | NS | 0.047 | 0.002 | NS |
| BMI | −0.134 | 0.018 | NS | −0.076 | 0.006 | NS | −0.248 | 0.061 | NS |
| eGFR (MDRD) | −0.271 | 0.073 | 0.002 | −0.166 | 0.028 | NS | −0.398 | 0.158 | 0.01 |
| eGFR (cystatin C) | −0.260 | 0.068 | 0.003 | −0.283 | 0.080 | 0.01 | −0.333 | 0.111 | 0.04 |
| Categoricala | |||||||||
| Present mean ± SD (N) | Absent mean ± SD (N) | P-value | Present mean ± SD (N) | Absent mean ± SD (N) | P-value | Present mean ± SD (N) | Absent mean ± SD (N) | P-value | |
| Diabetes (pre-existing and new-onset) | 1.04 ± 0.21 (39) | 1.02 ± 0.19 (90) | NS | 0.98 ± 0.18 (28) | 1.0 ± 0.18 (62) | NS | 1.19 ± 0.22 (11) | 1.08 ± 0.20 (28) | NS |
| South Asian ethnicity | 0.95 ± 0.16 (43) | 1.06 ± 0.2 (86) | 0.002 | 0.92 ± 0.16 (30) | 1.03 ± 0.18 (60) | 0.005 | 1.02 ± 0.12 (13) | 1.15 ± 0.24 (26) | 0.04 |
| Smoking | 1.05 ± 0.22 (59) | 1.01 ± 0.17 (70) | NS | 1.02 ± 0.21 (45) | 0.96 ± 0.14 (45) | NS | 1.12 ± 0.25 (14) | 1.10 ± 0.19 (25) | NS |
Log10 adiponectin values are presented under categorical variables.
All significant variables from the univariate analysis (Table 2) were included in the initial multivariate models for total and HMW adiponectin, with waist-to-hip ratio substituted for BMI in separately and smoking and HDL cholesterol forced back in to the model. Results of the final multivariate model for total adiponectin demonstrated SA ethnicity (F = 10.3, P = 0.02), cystatin C-eGFR (F = 15.48, P < 0.001), HDL cholesterol (F = 41.3, P < 0.0001) and waist-to-hip ratio (F = 9.52, P < 0.001) as significant predictors, while those for HMW adiponectin included SA ethnicity (F = 16.66, P < 0.001), cystatin C-eGFR (F = 4.6, P = 0.03) and HDL cholesterol (F = 11.77, P = 0.001). The difference in total adiponectin was statistically significant independent of inclusion of variables for obesity, whether this was BMI or waist-to-hip ratio. Unlike for total adiponectin, waist-to-hip ratio (F = 1.3, P = 0.26) was not a significant predictor of HMW adiponectin. In gender-based subanalysis, SA ethnicity, cystatin C-eGFR and HDL cholesterol were significant independent predictors of total adiponectin in both males and females (Table 3). Total proportion of the variance in log10 adiponectin (model R 2) in males was 26.7%, with HDL cholesterol explaining 8.1%, ethnicity 9% and cystatin C-eGFR 9% (partial R 2). Total proportion of the variance in log10 adiponectin in females was 48%, with HDL cholesterol explaining 23.5%, ethnicity 11.8% and cystatin C-eGFR 9.6%. HMW adiponectin was also significantly associated with ethnicity in both males and females (Table 3). It was also significantly associated with HDL cholesterol in men and of borderline significance in women. Thus, the association of both adiponectin and HMW adiponectin with ethnicity appears to be independent of HDL cholesterol and weight.
Table 3.
Multivariable analysis of factors affecting log10 total and HMW adiponectin concentrations by gendera
| Males |
Females |
|||||
| Total adiponectin | ||||||
| Model | DF | F value | Pr > F | DF | F value | Pr > F |
| SA ethnicity | 1 | 9.35 | 0.003 | 1 | 8.67 | 0.006 |
| Cystatin C-eGFR | 1 | 10.45 | 0.001 | 1 | 4.86 | 0.03 |
| BMI | 1 | 0.75 | NS | 1 | 1.98 | NS |
| HDL-CHOL | 1 | 11.58 | 0.001 | 1 | 12.46 | 0.001 |
| HMW adiponectin | ||||||
| SA ethnicity | 1 | 10.42 | 0.001 | 1 | 11.34 | 0.002 |
| Cystatin C-eGFR | 1 | 2.97 | NS | 1 | 2.42 | NS |
| BMI | 1 | 0.01 | NS | 1 | 0.11 | NS |
| HDL-CHOL | 1 | 8.36 | 0.005 | 1 | 3.58 | 0.06 |
CHOL, cholesterol.
Discussion
SA KTR experience more early and late post-transplant major cardiac events than other ethnic groups despite similar pre-transplant screening, pre-transplant cardiac disease and FRS-related factors [2]. In this matched cross-sectional analysis, we have demonstrated that KTR of SA origin have significantly diminished total adiponectin and HMW adiponectin concentrations compared to Caucasians despite the presence of a uniquely complex post-transplant milieu including ongoing chronic kidney disease and immunosuppressive therapy that is common to all KTR. The effect of ethnicity is independent of several other important independent predictors of adiponectin including renal function, HDL cholesterol and weight. Several studies have previously demonstrated lower adiponectin in SA in healthy subjects [21, 22]. The current study extends the existing literature to show that lower adiponectin is maintained in SA KTR compared with Caucasian recipients, by extending this observation to HMW adiponectin and by using the gold-standard ultracentrifugation method of separation. Thus, it is reasonable to hypothesize for future prospective studies that adiponectin is an important independent ethnicity-specific cardiac risk factor in KTR.
Plasma adiponectin, an adipokine secreted almost exclusively by adipose tissue, represents almost 0.01% of total plasma protein. It is anti-atherogenic, anti-inflammatory and has insulin-sensitizing properties. Independent of the latter, it is associated with endothelium-dependent vasodilation [23]. Reconciling information about the significance of adiponectin concentrations in chronic kidney disease is difficult. Adiponectin concentrations are elevated in hemodialysis patients likely due in part to decreased clearance, but low adiponectin concentrations are associated with increased cardiovascular events [24]. Thus, the mechanisms of action of adiponectin and disease outcomes are consistent in dialysis patients with low adiponectin. It is unclear whether the mechanisms for increasing adiponectin, or adiponectin itself, are the determining factor for adverse outcomes for dialysis patients presenting with high adiponectin. Likewise, although adiponectin may be lowered after kidney transplantation [25, 26] from increased clearance, at least in male transplant recipients, low adiponectin is associated with increased CVD prevalence [27]. Opposing findings with higher adiponectin concentrations being associated with CVD and mortality have been reported in other populations [28, 29]. Low adiponectin also associates with more new-onset diabetes [30]. Moreover, although the HMW isoform (12mer or 18mer) that correlates best with insulin sensitivity is also elevated in end-stage renal disease, it may be lowered to a greater extent than total adiponectin after transplantation [26]. Therefore, if both low total and HMW adiponectin concentrations are indeed reflective of post-transplant CVD, then the particular role of ethnicity assumes considerable importance.
Understanding the mechanism by which SA ethnicity results in lower adiponectin will be beneficial in prioritizing the evaluation of pharmacologic and other therapies. Since adiponectin concentrations were found to be different in both men and women by ethnicity, it is unlikely that the known effects of androgens are responsible. Genetic, dietary and environmental causes need to be considered. A provocative hypothesis is that vitamin D is related to insulin resistance and adiponectin [31]. Deeper skin pigmentation along with cultural practices of reduced sun exposure may contribute to vitamin D deficiency in SA. In addition, KTR are often advised to avoid sun exposure to reduce the risk of cutaneous malignancy. Vitamin D receptor polymorphisms have also been associated with vitamin D concentrations in Canadians of different ethnicity [32]. Thus, genetic, dietary and environmental factors affecting vitamin D are candidates to explain the effect of ethnicity on adiponectin.
Measures, such as weight reduction and lifestyle modification, that are typically advocated for obese patients are known to increase plasma adiponectin concentration. HMW adiponectin in particular is increased after weight reduction [33]. Since the current study demonstrates that SA ethnicity is associated with both reduced total and HMW adiponectin independent of weight parameters, studies may be required of the specific effects of weight change in women of different ethnicities. Pharmacological interventions, such as thiazolidinediones, ACE inhibitors, angiotensin II receptor blockers and fenofibrate, may all increase plasma adiponectin concentrations [4]. An evaluation of some of these drugs in SA transplant recipients would therefore seem potentially rewarding. The lack of a difference in apolipoprotein A1 and B and cystatin C with ethnicity indicates that adiponectin holds the greatest promise as a biomarker of cardiovascular risk post-transplantation in select transplant subpopulations. Although smoking cessation increases adiponectin [34], this effect may depend on weight change and there were too few SA smokers in our cohort to examine its effect. Our study was limited by sample size in determining the relationship of adiponectin and prior MACE burden. It is also important to note that the current study was not designed to correlate specific risk factors to cardiovascular outcomes or the direction of the risk factor–cardiac event relationship but was powered sufficiently for its primary objective of detecting ethnic differences in adiponectin.
Canadian SA in the general population possess an increased risk for CVD [6]. North American SA have higher coronary heart disease mortality rates than individuals of either European or Chinese descent [35], have more severe disease and may also present at an earlier age [36]. Canada has a large SA transplant recipient population at higher cardiovascular risk. Thus, determining the relationship of adiponectin concentrations to cardiovascular events and evaluating therapies to affect adiponectin concentrations in SA to potentially reduce their cardiovascular risk seem worthwhile endeavors.
Acknowledgments
The authors would like to thank Rosane Nisenbaum, Li Ka Shing Knowledge Institute for guidance with the statistical analysis.
Funding. This study was funded by the Heart and Stroke Foundation of Ontario (PEA-6532, 2008).
Conflict of interest statement. None declared.
Footnotes
A version has been published to make this article Open Access.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.Prasad GV, Vangala SK, Silver SA, et al. South Asian ethnicity as a risk factor for major adverse cardiovascular events post-renal transplantation. Clin J Am Soc Nephrol. 2011;6:204–211. doi: 10.2215/CJN.03100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver SA, Huang M, Nash MM, et al. Framingham risk score and novel cardiovascular risk factors under-predict major adverse cardiovascular events in kidney transplant recipients. Transplantation. 2011;92:183–189. doi: 10.1097/TP.0b013e31821f303f. [DOI] [PubMed] [Google Scholar]
- 4.Cui J, Panse S, Falkner B. The role of adiponectin in metabolic and vascular disease: a review. Clin Nephrol. 2011;75:26–33. [PubMed] [Google Scholar]
- 5.Raji A, Gerhard-Herman MD, Warren M, et al. Insulin resistance and vascular dysfunction in nondiabetic Asian Indians. J Clin Endocrinol Metab. 2004;89:3965–3972. doi: 10.1210/jc.2004-0087. [DOI] [PubMed] [Google Scholar]
- 6.Anand SS, Yusuf S, Vuksan V, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and risk in Ethnic groups (SHARE) Lancet. 2000;356:279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 7.Anand SS, Razak F, Yi Q, et al. C-reactive protein as a screening test for cardiovascular risk in a multiethnic population. Arterioscler Thromb Vasc Biol. 2004;24:1509–1515. doi: 10.1161/01.ATV.0000135845.95890.4e. [DOI] [PubMed] [Google Scholar]
- 8.Smith J, Cianflone K, Al-Amri M, et al. Body composition and the apoB/apoA-I ratio in migrant Asian Indians and white Caucasians in Canada. Clin Sci (Lond) 2006;111:201–207. doi: 10.1042/CS20060045. [DOI] [PubMed] [Google Scholar]
- 9.Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 10.de Boer IH, Astor BC, Kramer H, et al. Lipoprotein abnormalities associated with mild impairment of kidney function in the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol. 2008;3:125–132. doi: 10.2215/CJN.03390807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jardine AG, Fellstrom B, Logan JO, et al. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis. 2005;46:529–536. doi: 10.1053/j.ajkd.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Wong BM, Huang M, Zaltzman JS, et al. Mycophenolate mofetil and C-reactive protein in renal transplant recipients. Transplantation. 2007;83:48–53. doi: 10.1097/01.tp.0000248864.21574.92. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Wilson PWF, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 15.Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32(Suppl 1):S10–S11. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Lee TC, Hanlon JG, Ben-David J, et al. Risk factors for cardiovascular disease in homeless adults. Circulation. 2005;111:2629–2635. doi: 10.1161/CIRCULATIONAHA.104.510826. [DOI] [PubMed] [Google Scholar]
- 17.Retnakaran R, Connelly PW, Maguire G, et al. Decreased high molecular weight adiponectin in gestational diabetes: implications for the pathophysiology of type 2 diabetes. Diabet Med. 2007;24:245–252. doi: 10.1111/j.1464-5491.2007.02077.x. [DOI] [PubMed] [Google Scholar]
- 18.Beauvieux MC, Le Moigne F, Lasseur C, et al. New predictive equations improve monitoring of kidney function in patients with diabetes. Diabetes Care. 2007;30:1988–1994. doi: 10.2337/dc06-2637. [DOI] [PubMed] [Google Scholar]
- 19.Retnakaran R, Hanley AJG, Raif N, et al. Hypoadiponectinemia in South Asian women during pregnancy: evidence of ethnic variation in adiponectin concentration. Diabet Med. 2004;21:388–392. doi: 10.1111/j.1464-5491.2004.1151.x. [DOI] [PubMed] [Google Scholar]
- 20.Ley SH, Harris SB, Connelly PW, et al. Adipokines and incident type 2 diabetes in an Aboriginal Canadian population: the Sandy Lake Health and Diabetes Project. Diabetes Care. 2008;31:1410–1415. doi: 10.2337/dc08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mente A, Razak F, Blankenberg S, et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care. 2010;33:1629–1634. doi: 10.2337/dc09-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal N, Anderson SG, Vyas A, et al. Adiponectin and lipid profiles compared with insulins in relation to early growth of British South Asian and European children: the Manchester children's growth and vascular health study. J Clin Endocrinol Metab. 2011;96:2567–2574. doi: 10.1210/jc.2011-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein BJ, Saclia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 24.Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 25.Chudek J, Adamczak M, Karkoszka H, et al. Plasma adiponectin concentration before and after successful kidney transplantation. Transplant Proc. 2003;35:2186–2199. doi: 10.1016/j.transproceed.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Shen YY, Charlesworth JA, Kelly JJ, et al. The effect of renal transplantation on adiponectin and its isoforms and receptors. Metabolism. 2007;56:1201–1208. doi: 10.1016/j.metabol.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Kaisar MO, Armstrong K, Hawley C, et al. Adiponectin is associated with cardiovascular disease in male renal transplant recipients: baseline results from the LANDMARK 2 study. BMC Nephrol. 2009;10:29. doi: 10.1186/1471-2369-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin concentrations and risk of acute myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 29.Laughlin GA, Barrett-Connor E, May S, et al. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007;165:164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayes B, Lauzurica R, Granada ML, et al. Adiponectin and risk of new-onset diabetes mellitus after kidney transplantation. Transplantation. 2004;78:26–30. doi: 10.1097/01.tp.0000132561.48217.b1. [DOI] [PubMed] [Google Scholar]
- 31.Vaidya A, Williams JS, Forman JP. The independent association between 25-hydroxy vitamin D and adiponectin and its relation with BMI in two large cohorts: the NHS and the HPFS. Obesity (Silver Spring) 2012;20:186–191. doi: 10.1038/oby.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gozdzik A, Zhu J, Wong BY, et al. Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. J Steroid Biochem Mol Biol. 2011;127:405–412. doi: 10.1016/j.jsbmb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Bobbert T, Rochlitz H, Wegewitz U, et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 34.Inoue K, Takeshima F, Kadota K, et al. Early effects of smoking cessation and weight gain on plasma adiponectin concentrations and insulin resistance. Intern Med. 2011;50:707–712. doi: 10.2169/internalmedicine.50.4600. [DOI] [PubMed] [Google Scholar]
- 35.Sheth T, Nair C, Nargundkar M, et al. Cardiovascular and cancer mortality among Canadians of European, South Asian and Chinese origin from 1979 to 1993: an analysis of 1.2 million deaths. CMAJ. 1999;161:132–138. [PMC free article] [PubMed] [Google Scholar]
- 36.Enas EA, Garg A, Davidson MA, et al. Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart J. 1996;48:343–353. [PubMed] [Google Scholar]


