Abstract
Purpose
To investigate the relationship between human papillomavirus (HPV) and Epstein-Barr virus (EBV) in non-endemic nasopharyngeal carcinoma (NPC) and assess the prognostic implications of viral status.
Methods and Materials
Paraffin-embedded tumor specimens from 62 patients with primary NPC diagnosed between 1985 and 2011 were analyzed for EBV and high-risk HPV. EBV status was determined using in situ hybridization for EBV encoded RNA. HPV status was assessed with p16 immunohistochemistry and multiplex PCR-MassArray for determination of HPV type. Proportional hazards models were used to compare the risk of death among patients as stratified by viral status.
Results
Of 61 evaluable tumors, 26 (43%) were EBV-positive/HPV-negative, 18 (30%) were HPV-positive/EBV-negative, and 17 (28%) were EBV/HPV-negative. EBV and HPV infection was mutually exclusive. HPV-positivity was significantly correlated with WHO grade II tumors, older age, and smoking (all p <0.001). The racial distribution of the study population was 74% Caucasian, 15% African American, and 11% Asian/Middle Eastern. Among HPV-positive patients, 94% were Caucasian. At a median follow-up of 7 years, HPV-positive and EBV/HPV-negative tumors exhibited worse outcomes as compared to EBV-positive tumors, including decreased overall survival (hazard ratio [HR] 2.98, p=0.01 and HR 3.89, p=0.002], progression-free survival (HR 2.55, p=0.02 and HR 4.04, p <0.001], and locoregional control (HR 4.01, p=0.03 and HR 6.87, p=0.001).
Conclusion
In our Midwestern population high-risk HPV infection may play an etiologic role in the development of non-endemic, EBV-negative NPC. Compared with EBV-positive NPC, HPV positive and EBV/HPV-negative NPC are associated with worse outcomes. A larger confirmatory study is needed to validate these findings.
INTRODUCTION
While nasopharyngeal carcinoma (NPC) is relatively uncommon worldwide, it is endemic in certain populations including southern China, Southeast Asia, the Middle East, and North Africa. Interestingly, when persons of endemic regions migrate to non-endemic regions, their incidence of NPC remains higher than that of other races. Within the United States, rates of NPC are considerably higher among Asian Americans (2.5-3.8 cases per 100,000) as compared to White Americans (0.4), African Americans (0.6), and Hispanic Americans (0.4)(1). This unbalanced geographical and ethnic distribution of NPC suggests a multifactorial etiology involving viral, environmental, and genetic components.
Epstein-Barr virus (EBV), a ubiquitous human herpesvirus, is recognized as a primary etiologic agent in non-keratinizing NPC (WHO type II/III). In contrast, keratinizing carcinomas (WHO type I) lack a consistent association with EBV suggesting differences in the pathogenesis of NPC (2,3). Additional established risk factors for NPC in endemic areas include consumption of salt-preserved fish, tobacco exposure, and certain human leukocyte antigen class I genotypes (4). In non-endemic regions, tobacco exposure has been associated with type I NPC but not type II or III NPC (5).
High-risk human papillomavirus (HPV), particularly oncogenic HPV subtype 16, has recently been established as the primary etiologic agent in a subset of head and neck squamous cell carcinomas, the majority of which localize to the oropharynx and exhibit improved prognosis compared to patients with HPV-negative tumors (6,7). Given the similarities between the epithelium and lymphoid tissue of the oropharynx and nasopharynx, there has been recent interest in the potential role of HPV in NPC carcinogenesis. Reports, however, have been inconsistent likely due to limited patient numbers and ethnic and geographic differences in study populations. Several studies have detected HPV in NPC with some demonstrating a dichotomy between EBV and HPV infection (8-16) and others reporting cases of EBV and HPV co-infection predominantly in patients from endemic regions (17-20). Notably, the clinical significance of HPV infection in NPC remains unclear at this time. The present study aims to further investigate the relationship between HPV and EBV in non-endemic NPC and assess the prognostic implications of viral status.
PATIENTS AND METHODS
Study Population
As part of an institutional review board approved study, 155 patients with primary nasopharyngeal carcinoma evaluated at XXX from 1985 to 2011 were identified through the institutional tumor registry. Paraffin-embedded tumor specimens were available for 62 of these patients. Hematoxylin-and-eosin-stained slides of all specimens were re-evaluated by an experienced head and neck pathologist (J.B.M.) to confirm the diagnosis, assign a WHO grade, and select corresponding blocks of primary tumor tissue for further study. Clinical and radiographic records were reviewed to document patient and tumor characteristics as well as treatment details and clinical outcomes. All tumors were confirmed to originate within the nasopharynx.
HPV Detection and Identification
HPV status was assessed by both p16ink4a immunohistochemistry and multiplex polymerase chain reaction (PCR) MassArray(21). Immunohistochemical analysis for the CDKN2A protein, p16, was performed on 5-μm tissue sections. Following deparaffinization, rehydration, and antigen retrieval, tissue sections were incubated with a mouse monoclonal antibody against p16 (Roche mtm laboratories AG, Heidelberg, Germany) per manufacturer's protocol. Slides were then developed using chromagen DAB and counterstained with hematoxylin. The intensity and proportion of tumor cell staining was scored by a single pathologist (J.B.M.). Positive p16 expression was defined as strong and diffuse nuclear and cytoplasmic staining in 90% or more of tumor cells.
Multiplex PCR MassArray was performed on all samples following DNA extraction from core tissue as previously described (9,22). In brief, this method detects and identifies 15 high-risk HPV subtypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73) using type-specific, multiplex competitive PCR of the E6 region followed by probe specific single base extension. Matrix-assisted laser desorption/ ionization-time of flight mass spectroscopy allows separation of products on a matrix-loaded silicon chip array. All samples were assayed in quadruplicate. Normal human genomic DNA and water were used as negative controls. The positive controls were standard human HPV positive cell lines CaSki, HeLa and SiHa. The type specific competitors also served as internal positive controls. Samples that were p16 positive but negative for HPV DNA by Multiplex PCR-MassArray, were retested by PCR with PGMY L1 consensus primers to determine if an HPV type not represented in the multiplex PCR-MassArray assay was present (23).
Tumors were classified as HPV positive if they were positive for both p16 overexpression and HPV DNA.
EBV Detection
In situ hybridization for EBV encoded RNA (EBER) was performed on formalin-fixed, paraffin-embedded tissue sections using the automated Benchmark system (EBER 1 DNP probe, Ventana Medical Systems, Tucson, AZ) according to the manufacturer's protocol. A positive hybridization was defined as strong diffuse signals in the nucleus of nearly all of the tumor cells. Negative and positive (known EBV-positive NPC) controls were tested in tandem with the patient samples.
Treatment
Patients were treated with conventionally fractionated radiation therapy with or without concurrent and/or adjuvant chemotherapy. Radiation technique varied over time with the majority of patients receiving either three-dimensional conformal radiation therapy or intensity-modulated radiation therapy. Gross tumor volume included the primary tumor and any involved cervical or retropharyngeal lymph nodes. Uninvolved cervical lymph nodes were electively treated to lower dose levels. Concurrent chemotherapy typically consisted of cisplatin 100 mg/m2 every 21 days. Patients who developed toxicities to cisplatin were switched to weekly carboplatin (AUC 6). Adjuvant chemotherapy, if administered, consisted of a platinum agent and 5-flurouracil.
Statistics
The chi-square test was used to assess the association of viral grouping with categorical variables while ANOVA was used to assess the association with continuous variables. Overall survival, progression-free survival, locoregional control, and distant metastatic control were estimated with the Kaplan-Meier method and were compared between the viral groups using the log-rank test. Time was measured from the time of diagnosis until the event of interest. The impact of viral status and other covariates on clinical outcomes were assessed using univariate and multivariate Cox proportional hazard regression models. All tests were two-sided with a p-value of ≤0.05 considered significant. Analyses were performed using SAS/STAT for Windows, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics and Viral Status
Analyses were restricted to 61 of 62 patients for whom there was sufficient tumor present in the tissue specimen for comprehensive viral testing. The demographic, clinical, and tumor characteristics of the patients are shown in Table 1. The median age of the patients was 54 years and most were male (65.6%) and Caucasian (73.8%). Patients of Asian and Middle Eastern descent comprised only 11.5% of the study population. WHO type I, II, and III tumors assumed roughly equal distributions and were present in 27.9%, 36.1%, and 36.1% of patients, respectively. The majority of patients (75.4%) presented with locoregionally advanced (stage III/IV) disease. The median radiation dose was 70 Gy (range 64.8 to 74.4). Fifty-four patients (88.5%) received concurrent chemotherapy and 17 (27.9%) received adjuvant chemotherapy. The median follow-up for surviving patients was 7.0 years.
Table 1.
Baseline characteristics of study patients stratified according to viral status
| Characteristics | All Patients (n=61) | EBV positive (n=26) | HPV positive (n=18) | EBV/HPV negative (n=17) | P |
|---|---|---|---|---|---|
| Age – yr | <0.001 | ||||
| Median | 54.3 | 45.2 | 57.8 | 62.3 | |
| Range | 10-83 | 10-77 | 39-83 | 40-77 | |
| Sex – no. (%) | 0.30 | ||||
| Male | 40 (65.6) | 17 (65.4) | 14 (77.8) | 9 (52.9) | |
| Female | 21 (34.4) | 9 (34.6) | 4 (22.2) | 8 (47.1) | |
| Race – no. (%) | 0.29 | ||||
| Caucasian | 45 (73.8) | 16 (61.5) | 17 (94.4) | 12 (70.6) | |
| African American | 9 (14.8) | 5 (19.2) | 1 (5.6) | 3 (17.6) | |
| Asian | 5 (8.2) | 4 (15.4) | 0 | 1 (5.9) | |
| Middle Eastern | 2 (3.3) | 1 (3.8) | 0 | 1 (5.9) | |
| Tobacco exposure – no. (%) | |||||
| Never smoked | 24 (39.3) | 18 (69.2) | 4 (22.2) | 2 (11.8) | <0.001 |
| Current and former smokers | 36 (59.0) | 7 (26.9) | 14 (77.8) | 15 (88.2) | |
| Unknown | 1 (1.6) | 1 (3.8) | 0 | 0 | |
| WHO grade – no. (%) | <0.001 | ||||
| I | 17 (27.9) | 0 | 6 (33.3) | 11 (64.7) | |
| II | 22 (36.1) | 7 (26.9) | 11 (61.1) | 4 (23.5) | |
| III | 22 (36.1) | 19 (73.1) | 1 (5.6) | 2 (11.8) | |
| EBV encoded RNA – no. (%) | <0.001 | ||||
| Positive | 26 (42.6) | 26 (100) | 0 | 0 | |
| Negative | 35 (57.4) | 0 | 18 (100) | 17 (100) | |
| p16 overexpression – no. (%) | <0.001 | ||||
| Positive | 21 (34.4) | 0 | 18 (100) | 3 (17.6) | |
| Negative | 40 (65.6) | 26 (100) | 0 | 14 (82.4) | |
| HPV DNA – no. (%) | <0.001 | ||||
| Positive | 18 (29.5) | 0 | 18 (100) | 0 | |
| Negative | 43 (70.5) | 26 (100) | 0 | 17 (100) | |
| Tumor classification – no. (%) | 0.19 | ||||
| T1 | 8 (13.1) | 4 (15.4) | 3 (16.7) | 1 (5.9) | |
| T2 | 12 (19.7) | 6 (23.1) | 4 (22.2) | 2 (11.8) | |
| T3 | 7 (11.5) | 4 (15.4) | 2 (11.1) | 1 (5.9) | |
| T4 | 30 (49.2) | 10 (38.5) | 9 (50.0) | 11 (64.7) | |
| Unknown | 4 (6.6) | 2 (7.7) | 0 | 2 (11.8) | |
| Nodal classification – no. (%) | 0.07 | ||||
| N0 | 12 (19.7) | 2 (7.7) | 5 (27.8) | 5 (29.4) | |
| N1 | 19 (31.1) | 8 (30.8) | 6 (33.3) | 5 (29.4) | |
| N2 | 21 (34.4) | 11 (42.3) | 7 (38.9) | 3 (17.6) | |
| N3 | 6 (9.8) | 4 (15.4) | 0 | 2 (11.8) | |
| Unknown | 3 (4.9) | 1 (3.8) | 0 | 2 (11.8) | |
| AJCC stage – no. (%) | 0.24 | ||||
| I | 3 (4.9) | 1 (3.8) | 1 (5.6) | 1 (5.9) | |
| II | 8 (13.1) | 4 (15.4) | 3 (16.7) | 1 (5.9) | |
| III | 13 (21.3) | 7 (26.9) | 5 (27.8) | 1 (5.9) | |
| IVA | 27 (44.3) | 8 (30.8) | 9 (50.0) | 10 (58.8) | |
| IVB | 6 (9.8) | 4 (15.4) | 0 | 2 (11.8) | |
| Unknown | 4 (6.6) | 2 (7.7) | 0 | 2 (11.8) | |
| Year of diagnosis | 0.54 | ||||
| Mean | 2001.4 | 2000.3 | 2002.7 | 2001.7 | |
| Range | 1985-2011 | 1985-2011 | 1986-2011 | 1991-2011 | |
| Radiation dose – Gy | 0.44 | ||||
| Median | 70 | 70 | 70 | 70 | |
| Range | 64.8-74.4 | 64.8-74.4 | 69.0-72.4 | 69.0-74.0 | |
| Radiation technique | 0.34 | ||||
| 2D-RT / 3D-CRT | 30 (49.2) | 15 (57.7) | 6 (33.3) | 9 (52.9) | |
| IMRT | 30 (49.2) | 11 (42.3) | 11 (61.1) | 8 (47.1) | |
| Concurrent chemotherapy – no. (%) | 0.73 | ||||
| Yes | 54 (88.5) | 24 (92.3) | 16 (88.9) | 14 (82.3) | |
| No | 5 (8.2) | 2 (7.7) | 1 (5.6) | 2 (11.8) | |
| Unknown | 2 (3.3) | 0 | 1 (5.6) | 1 (5.9) | |
| Adjuvant chemotherapy – no. (%) | 0.10 | ||||
| Yes | 17 (27.9) | 6 (23.1) | 6 (33.3) | 5 (29.4) | |
| No | 38 (62.3) | 20 (76.9) | 8 (44.4) | 10 (58.8) | |
| Unknown | 6 (9.8) | 0 | 4 (22.2) | 2 (11.8) |
Abbreviations: EBV, Epstein-Barr virus; HPV, human papillomavirus;
*P values were calculated with the use of Pearson's chi-square test for all comparisons, except age and radiation dose for which ANOVA was used, and staging (tumor, nodal, and AJCC) for which Kruskal-Wallis test was used.
Overall, 26 of 61 (43%) carcinomas were positive for EBV and negative for HPV; 18 of 61 (30%) were positive for HPV and negative for EBV; and 17 of 61 (28%) were negative for both EBV and HPV (Table 1). EBV and HPV infection was mutually exclusive, thereby, allowing classification into three distinct viral groups: EBV-positive, HPV-positive, and EBV/HPV-negative. Among the HPV-positive patients, all 18 (100%) were strongly positive for p16 overexpression, consistent with transcriptionally active HPV serving as a driver of carcinogenesis. HPV types were more heterogeneous than is typically observed in oropharyngeal carcinoma with HPV-16 present in 9 of 18 tumors,HPV-18 in 5, HPV-59 in 2, HPV-39 in 1, and HPV-45 in 1. All carcinomas that were negative for p16 overexpression were also negative for HPV by multiplex PCR-MassArray. Three cases were p16 positive, but negative for HPV by multiplex PCR-MassArray and PCR with PGMY L1 consensus primers. As such, these three cases were classified as EBV/HPV-negative.
Viral status and WHO classification were significantly correlated (p <0.001). Non-keratinizing type II and III NPC predominated among EBV-positive tumors (100%) and HPV-positive tumors (66.7%), while keratinizing type I NPC was most prevalent among EBV/HPV-negative tumors (64.7%) (Table 1).
Patients with EBV-positive tumors were significantly younger than patients with HPV-positive and EBV/HPV-negative tumors with median ages of 45.2, 57.8, and 62.3 years, respectively (p <0.001). Patients with EBV-positive tumors were also more likely to be non-smokers (<10-pack-years), whereas HPV-positive and EBV/HPV-negative tumors were significantly associated with smoking (p <0.001). Among patients with HPV-positive tumors, 94.4% were Caucasian. HPV-positive and EBV/HPV-negative tumors were marginally correlated with node negative disease at presentation (p=0.07). There was no statistically significant association between viral status and patient sex, race, tumor or nodal classification, AJCC stage, radiation dose, or use of chemotherapy.
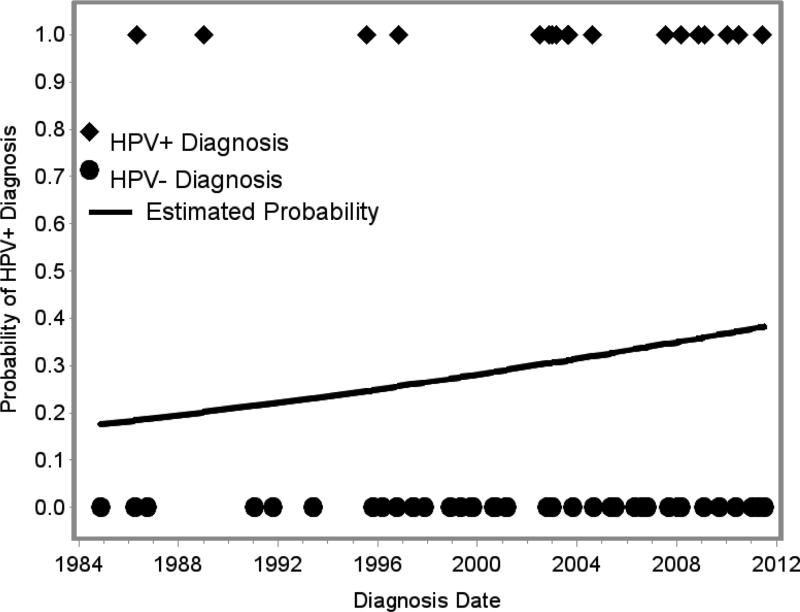
The type and frequency of oncogenic virus involvement did not vary significantly with year of diagnosis although there was a trend towards more frequent involvement by HPV in recent years (p=0.35, Figure 1).
Figure 1.
Increasing incidence of human papillomavirus (HPV)-positive nasopharyngeal carcinoma over time.
Viral Status and Clinical Outcomes
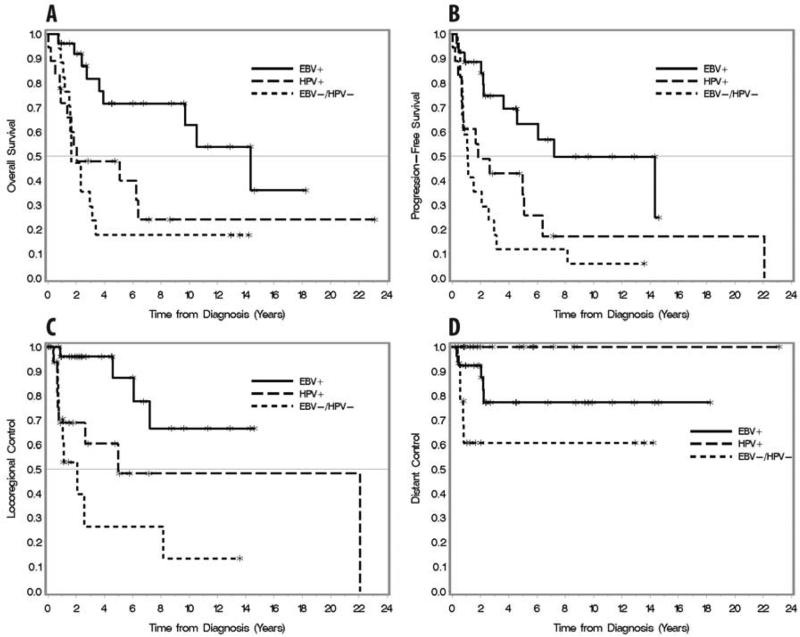
Patients with HPV-positive and EBV/HPV-negative tumors exhibited significantly worse overall survival, progression-free survival, and locoregional control than patients with EBV-positive tumors (p=0.003, p=0.001, p=0.002 respectively; Fig 2A-C). At 5-years, estimates for overall survival, progression-free survival, and locoregional control were 71.6%, 63.1%, and 87.3% for EBV-positive, 47.9%, 34.2%, and 48.1% for HPV-positive, and 17.6%, 11.8%, and 26.4% for EBV/HPV-negative, respectively (Table 2). Compared with EBV-positive tumors, HPV-positive and EBV/HPV-negative tumors were associated with increased risk of death [hazard ratio (HR) 2.98, p=0.01 and 3.89, p=0.002, respectively], increased risk of relapse or death [HR 2.55, p=0.02; HR 4.04, p<0.001], and increased risk of locoregional relapse [HR 4.01, p=0.03; HR 6.87, p=0.001] (Table 3).
Figure 2.
Kaplan-Meier plots stratified by viral status (EBV-positive, HPV-positive, and EBV/HPV-negative). Overall survival (A), progression-free survival (B), locoregional control (C), and distant control (D).
Table 2.
Survival estimates according to viral status
| Clinical Outcome | EBV Positive (n=26) | HPV Positive (n=18) | EBV/HPV negative (n=17) | P |
|---|---|---|---|---|
| Overall survival at 5 yr – % (95% CI) | 71.6% (47.1-86.2) | 47.9% (23.6-68.7) | 17.6% (4.3-38.3) | 0.003 |
| Progression-free survival at 5 yr – % (95% CI) | 63.1% (38.6-80.0) | 34.2% (12.7-57.3) | 11.8% (2.0 -31.2) | 0.001 |
| Locoregional control at 5 yr – % (95% CI) | 87.3% (54.6-97.0) | 48.1% (18.6-72.7) | 26.4% (4.6-56.1) | 0.002 |
| Distant metastatic control at 5 yr – % (95% CI) | 77.2% (53.1-89.9) | 100% | 60.5% (29.1-81.5) | 0.02 |
Abbreviations: EBV, Epstein-Barr virus; HPV, human papillomavirus
Table 3.
Associations of clinical outcomes with tumor and treatment characteristics.
| Covariate | Overall survival | Progression-free survival | Locoregional control | Distant metastatic control | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age† | 1.03 (1.01-1.05) | 0.02 | 1.02 (1.00-1.04) | 0.03 | 1.02 (0.99-1.05) | 0.13 | 0.99 (0.96-1.03) | 0.71 |
| Sex | 0.40 | 0.63 | 0.53 | 0.68 | ||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Male | 1.35 (0.67-2.73) | 1.18 (0.61-2.27) | 1.36 (0.52-3.60) | 0.76 (0.21-2.73) | ||||
| Race | 0.10 | 0.2 | 0.06 | 0.06 | ||||
| Caucasian | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| African American | 0.22 (0.05-0.91) | 0.37 (0.13-1.07) | 0.30 (0.07-1.32) | 0.81 (0.10-6.99) | ||||
| Asian / Middle Eastern | 0.68 (0.24-1.94) | 0.87 (0.33-2.25) | -* | 4.59 (1.23-17.16) | ||||
| Tobacco exposure | 0.03 | 0.03 | 0.04 | 0.81 | ||||
| Never smoked | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Current / former smokers | 2.23 (1.09-4.81) | 2.12 (1.07-4.18) | 2.93 (1.06-8.13) | 0.86 (0.25-2.98) | ||||
| WHO classification | <0.001 | <0.001 | 0.002 | 0.24 | ||||
| Non-keratinizing (II/III) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Keratinizing (I) | 4.04 (1.97-4.04) | 3.36 (1.72-6.56) | 4.36 (1.73-10.99) | 2.32 (0.58-9.30) | ||||
| Viral status | 0.006 | 0.002 | 0.006 | 0.02 | ||||
| EBV-positive | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| HPV-positive | 2.98 (1.25-7.11) | 2.55 (1.13-5.71) | 4.01 (1.16-13.84) | -* | ||||
| EBV/HPV-negative | 3.89 (1.66-9.13) | 4.04 (1.84-8.84) | 6.87 (2.09-22.57) | 2.85 (0.80-10.20) | ||||
| Primary tumor stage† | 1.33 (0.95-1.88) | 0.10 | 1.35 (0.98-1.87) | 0.07 | 1.41 (0.87-2.27) | 0.16 | 1.53 (0.82-2.86) | 0.18 |
| Nodal stage† | 0.94 (0.62-1.42) | 0.77 | 0.96 (0.65-1.41) | 0.82 | 0.66 (0.37-1.16) | 0.15 | 2.28 (0.99-5.25) | 0.05 |
| AJCC stage† | 1.53 (0.99-2.36) | 0.06 | 1.50 (0.99-2.27) | 0.06 | 1.29 (0.74-2.25) | 0.38 | 4.76 (1.34-16.9) | 0.02 |
| RT dose† | 1.14 (0.93-1.40) | 0.20 | 1.11 (0.93-1.34) | 0.24 | 1.06 (0.82-1.38) | 0.66 | 1.15 (0.80-1.66) | 0.45 |
| Radiation technique | 0.15 | 0.26 | 0.69 | 0.94 | ||||
| 2D-RT / 3D-CRT | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| IMRT | 1.72 (0.81-3.62) | 1.49 (0.75-2.97) | 1.23 (0.45-3.37) | 0.95 (0.27-3.32) | ||||
| Concurrent chemotherapy | 0.80 | 0.58 | 0.82 | 0.13 | ||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| No | 0.85 (0.25-2.89) | 1.35 (0.47-3.87) | 1.18 (0.27-5.14) | 3.38 (0.72-15.9) | ||||
| Adjuvant chemotherapy | 0.31 | 0.73 | 0.38 | 0.20 | ||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| No | 0.69 (0.34-1.41) | 0.88 (0.44-1.77) | 0.65 (0.26-1.67) | 3.85 (0.48-30.4) | ||||
Abbreviations: EBV, Epstein-Barr virus; HPV, human papillomavirus; HR, hazard ratio; CI, confidence interval.
Analyzed as continuous covariates.
There were no locoregional events among patients of Asian / Middle Eastern descent and no distant metastatic events among the HPV-positive cohort.
At 5-years, distant metastasis were most common among EBV/HPV-negative tumors followed by EBV-positive tumors (Fig 2D). There were no distant metastases among patients with HPV-positive tumors.
The influence of patient-, tumor-, and treatment-specific factors on clinical outcomes is presented in Table 3. In addition to viral status, which was associated with all clinical endpoints as outlined above, tobacco exposure and WHO type I keratinizing tumors were associated with poorer overall survival, progression-free survival, and locoregional control. Increasing age was also associated with shorter overall survival and progression-free survival. No treatment related variables significantly influenced any of the clinical outcomes. To adjust for competing risk factors, multivariate analyses were performed adjusting for age, tobacco exposure, WHO grade, and viral status (Table 4). In these models, there was a non-significant trend toward worse overall survival, progression-free survival, and locoregional control among patients with HPV-positive and EBV-HPV negative tumors compared to patients with EBV-positive tumors. Outcomes between viral groups were also compared by accounting for differing distributions of WHO grade across viral group using a stratified Cox model with strata equal to WHO grade. Sensitivity analyses were performed in which WHO grade was included as a covariate in this model and the results were qualitatively similar (data not presented). These results suggest that viral status independently impacts clinical outcomes, though its influence is attenuated due to the high correlation between viral status and WHO grade. However, given the correlation between tobacco exposure and the HPV-positive and EBV/HPV-negative subgroups, these differences in outcomes cannot be attributed to viral status alone.
Table 4.
Multivariate cox proportional hazards models for overall survival, progression-free survival, and locoregional control.
| Covariate | Overall survival | Progression-free survival | Locoregional control | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age† | 1.02 (0.99-1.04) | 0.23 | 1.01 (0.98-1.03) | 0.56 | - | - |
| Tobacco exposure | ||||||
| Never smoked | 1.00 | 1.00 | 1.00 | |||
| Current / former smokers | 1.50 (0.67-3.35) | 0.32 | 1.43 (0.67-3.05) | 0.35 | 1.47 (0.45-4.76) | 0.52 |
| WHO classification | ||||||
| Non-keratinizing (II/III) | 1.00 | 1.00 | 1.00 | |||
| Keratinizing (I) | 3.25 (1.24-8.50) | 0.02 | 2.12 (0.86-5.23) | 0.10 | 2.13 (0.69-6.58) | 0.19 |
| Viral status | ||||||
| EBV-positive | 1.00 | 1.00 | 1.00 | |||
| HPV-positive | 1.83 (0.71-4.72) | 0.39 | 1.86 (0.77-4.47) | 0.36 | 3.00 (0.78-11.50) | 0.24 |
| EBV/HPV-negative | 1.26 (0.37-4.20) | 0.39 | 1.87 (0.62-5.63) | 0.36 | 3.42 (0.69-16.97) | 0.24 |
Abbreviations: EBV, Epstein-Barr virus; HPV, human papillomavirus; HR, hazard ratio; CI, confidence interval.
Analyzed as continuous covariates.
Primary Nasopharyngeal Site Verification
Given the increasing incidence of HPV-associated oropharyngeal carcinomas, existing clinical, operative, and imaging records were reviewed for all patients to confirm the site of origin of the primary tumor. Diagnostic imaging was available for 39 of 43 patients from 1999-2011 with magnetic resonance imaging in 35 (90%) cases and computed tomography in 4 (10%). All scans were reviewed by a single head and neck radiologist (S.K.M.) blinded to the clinical and pathological findings. Of the 39 cases (15 EBV-positive, 15 HPV-positive, and 9 EBV/HPV-negative), 37 (95%) were scored as primary NPC on blinded imaging review including all 15 (100%) HPV-positive cases. One case was scored as a nasopharyngeal versus tonsillar primary (EBV-positive tumor), and one as a nasopharyngeal versus lateral pharyngeal wall primary (EBV/HPV-negative tumor). Thus, in the context of viral status, there is strong evidence supporting a diagnosis of primary NPC in 38 of 39 (97%) cases.
DISCUSSION
While EBV is recognized as the predominant etiologic agent in non-keratinizing NPC worldwide, there is a subset of EBV-negative NPCs, occurring primarily in Caucasians, for which the etiology remains largely undefined. This study identifies two unique subsets of EBV-negative NPC among North Americans both of which exhibit distinct clinical behavior from that of EBV-positive NPC with increased risks of locoregional relapse and death. Of these two subsets, one demonstrates a strong association with oncogenic HPV while the other lacks an association with both EBV and HPV. The HPV-positive cohort comprised 30% of our NPC cases with 94% occurring in Caucasians and 33%, 61%, and 6% in WHO type I, II, and III tumors, respectively. The EBV/HPV-negative group constituted only 28% of all cases but the majority (65%) of WHO type I tumors. The high correlation between viral status and WHO type suggests that viral status may well be the driving factor behind WHO type. Within our study cohort, EBV and HPV infection was mutually exclusive with no tumors harboring EBV/HPV co-infection, thereby, supporting an independent role for HPV-mediated carcinogenesis.
To the best of our knowledge, this is the largest study to date establishing the role of HPV in both keratinizing and non-keratinizing NPC and the first to demonstrate that tumor HPV status is an adverse prognostic factor for overall survival, progression-free survival, and locoregional relapse among patients with NPC. These findings are consistent with the hypothesis that non-endemic and endemic NPC are distinct entities biologically and clinically. The differences in clinical outcomes, however, cannot be attributed to viral status alone given the correlation between tobacco exposure and the HPV-positive and EBV/HPV-negative subgroups. Regardless, the divergent clinical behavior of NPC subtypes has important implications in the movement towards risk-stratified treatment paradigms. Current efforts are aimed at intensifying systemic therapy because distant metastasis is now the predominant pattern of failure in patients with endemic NPC treated with modern chemoradiotherapy. This pattern is replicated in our data among patients with EBV-related NPC. In contrast, patients with HPV-related NPC exhibited high rates of locoregional failure and death in the absence of distant metastasis. Thus, patients with HPV-related NPC, for whom the incidence approaches 40% among Caucasians in our study, may derive a benefit from further improvement of local therapy.
To help exclude benign HPV infection, all tumors were evaluated not only for HPV DNA by multiplex PCR-MassArray but also for the expression of the cyclin-dependent kinase inhibitor p16, a known biomarker of HPV oncoprotein function (24). We observed a strong agreement between p16 overexpression (as defined by strong and diffuse nuclear and cytoplasmic staining in 90% or more of the tumor cells) and high-risk HPV DNA detection with HPV DNA present in 18 of 21 (86%) p16 positive cases. There were no cases in which HPV DNA was detected in the absence of p16 overexpression. Interestingly, while HPV type 16 accounts for an estimated 90% of HPV-positive oropharyngeal carcinomas (25,26), it was only observed in 50% of our HPV-positive nasopharyngeal tumors. Additional oncogenic HPV types detected in our NPC cohort included HPV-18, HPV-59, HPV-39, and HPV-45.
Prior studies examining the relationship of HPV and NPC in North American patients have shown conflicting results and are limited by either small patient numbers or racial distributions that are typically associated with endemic EBV-positive NPC. In a study of 17 patients with NPC, Rassekh et al. found high-risk HPV to be present only in association with EBV, with co-detection in 4 of 10 keratinizing carcinomas and 5 of 7 non-keratinizing carcinomas (19). Conversely, Lo et al. detected high-risk HPV in 4 of 8 keratinizing cases and 1 of 22 non-keratinizing cases with no reported cases of HPV and EBV co-infection (8). Three more recent studies (14-16) demonstrated an association between HPV and EBV-negative NPC, primarily among non-keratinizing tumors in Caucasian patients. In these studies, HPV was detected in 5 of 88 (5.7%) (14), 6 of 63 (9.5%) (15), and 11 of 67 (16.4%) cases (16), respectively. These reports attempted to assess overall survival, but were notably limited by an absence of data on treatment receipt and clinical follow-up, small numbers within the HPV-positive groups, and incomplete demographic and smoking data.
Our study detected HPV in 18 of 61 cases with 67% occurring in non-keratinizing tumors and no cases of co-infection. The higher incidence of HPV-positive cases in our report is a reflection of our study population, which is representative of non-endemic NPC found within North America with Caucasians comprising the majority (74%) of patients. In contrast to the prior studies, our study includes comprehensive clinical data including patient characteristics, treatment details, and clinical outcomes, and is the first to fully assess the prognostic implications of viral status on overall survival, progression-free survival, locoregional control, and distant metastasis
It has been suggested that HPV-related carcinomas in sites adjacent to the oropharynx, such as the nasopharynx, may represent subepithelial extension from the oropharynx rather than primary involvement given the absence of anatomic constraints within Waldeyer's ring. Singhi et al. detected HPV in 4 of 45 non-keratinizing NPCs, but noted that all had synchronous involvement of the oropharynx (27). The authors cite that certain features of HPV-positive oropharyngeal carcinoma, such as their ability to propagate in the subepithelium and their histologic resemblance with EBV-positive NPC, may result in mistaken classification as primary NPC. However, our findings suggest that HPV-associated NPC exhibit distinct clinical and histologic characteristics from both EBV-associated NPC and HPV-associated oropharyngeal carcinoma. For instance, EBV-associated NPC and HPV-associated oropharyngeal carcinomas tend to occur more often in younger patients with no or brief tobacco exposure and are typically poorly differentiated non-keratinizing squamous cell carcinomas. In contrast, our findings indicate that HPV-associated NPC occur more often in older patients with a history of tobacco exposure and are either keratinizing or differentiated non-keratinizing squamous cell carcinomas. Furthermore, the high rates of locoregional relapse and death among the HPV-associated tumors in our study stand in stark contrast to the excellent response rates seen in HPV-associated oropharyngeal carcinoma and further support the notion that these tumors are a distinct entity and do not simply represent extension from the oropharynx. In this sense, HPV-associated NPC is similar to oral cavity, where an association with HPV does not confer any advantage.
The association between HPV and NPC among Caucasian patients raises the issue of whether or not the nasopharynx should be included in the electively irradiated mucosal volume for patients with head and neck carcinoma of unknown primary. In general, we do not advocate for routine inclusion of the nasopharynx, especially in non-smokers, given the low incidence of NPC as compared to oropharyngeal carcinoma in Caucasians as well as the strong correlation between HPV-positive NPC and smoking. Instances, in which inclusion of the nasopharynx should be considered, include patients with a heavy smoking history or those with nodal involvement consistent with NPC patterns of spread, such as retropharyngeal or level V lymph nodes.
In conclusion, our data in conjunction with other published studies (8,10,19) support the hypothesis that HPV is a possible etiologic agent in the development of NPC among Caucasians. Furthermore, our data suggest that non-endemic forms of NPC are clinically distinct from EBV-positive NPC with high rates of locoregional relapse and death. These patients may ultimately benefit from intensification of local therapy with either dose-escalated radiation therapy or additional radiation sensitizing agents. Due to the relative scarcity of non-endemic NPC at any one institution in the United States, it would be worthwhile to conduct a multi-center trial to determine the true incidence of HPV-positive and EBV/HPV-negative NPC, especially as these tumors appear to be less responsive to conventional treatment and rising in incidence.
SUMMARY.
This study identifies a unique subset of Epstein-Barr virus (EBV)-negative nasopharyngeal carcinoma among Caucasian patients that is strongly associated with oncogenic human papillomavirus (HPV). Patients with HPV-positive nasopharyngeal carcinoma exhibit distinct clinical behavior from those with EBV-positive nasopharyngeal carcinoma with increased risks of locoregional relapse and death. The differences in clinical outcomes, however, cannot be attributed to viral status alone given a correlation between tobacco exposure and HPV-positive tumors.
Acknowledgments
Research support: This project was supported by Federal Funds from the National Cancer Institute, National Institutes of Health under the University of Michigan Specialized Programs of Research Excellence (SPORE): P50CA097248. The Molecular Basis of Head and Neck Cancer Biology, Treatment and Prevention, Principal Investigator Gregory T. Wolf, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presented at ASTRO 55th Annual Meeting, Atlanta, GA, September 24, 2013
Conflicts of interest: none
REFERENCES
- 1.Howlander N, Noone AM, M. K, et al. Seer cancer statistics review, 1975-2009 (vintage 2009 populations) National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER website, April 2012. [Google Scholar]
- 2.Niedobitek G, Hansmann ML, Herbst H, et al. Epstein-barr virus and carcinomas: Undifferentiated carcinomas but not squamous cell carcinomas of the nasopharynx are regularly associated with the virus. J Pathol. 1991;165:17–24. doi: 10.1002/path.1711650105. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls JM, Agathanggelou A, Fung K, et al. The association of squamous cell carcinomas of the nasopharynx with epstein-barr virus shows geographical variation reminiscent of burkitt's lymphoma. J Pathol. 1997;183:164–168. doi: 10.1002/(SICI)1096-9896(199710)183:2<164::AID-PATH919>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 5.Vaughan TL, Shapiro JA, Burt RD, et al. Nasopharyngeal cancer in a low-risk population: Defining risk factors by histological type. Cancer Epidemiol Biomarkers Prev. 1996;5:587–593. [PubMed] [Google Scholar]
- 6.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo EJ, Bell D, Woo JS, et al. Human papillomavirus and who type i nasopharyngeal carcinoma. Laryngoscope. 2010;120:1990–1997. doi: 10.1002/lary.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxwell JH, Kumar B, Feng FY, et al. Hpv-positive/p16-positive/ebv-negative nasopharyngeal carcinoma in white north americans. Head Neck. 2010;32:562–567. doi: 10.1002/hed.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punwaney R, Brandwein MS, Zhang DY, et al. Human papillomavirus may be common within nasopharyngeal carcinoma of caucasian americans: Investigation of epstein-barr virus and human papillomavirus in eastern and western nasopharyngeal carcinoma using ligation-dependent polymerase chain reaction. Head Neck. 1999;21:21–29. doi: 10.1002/(sici)1097-0347(199901)21:1<21::aid-hed3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Tung YC, Lin KH, Chu PY, et al. Detection of human papilloma virus and epstein-barr virus DNA in nasopharyngeal carcinoma by polymerase chain reaction. Kaohsiung J Med Sci. 1999;15:256–262. [PubMed] [Google Scholar]
- 12.Giannoudis A, Ergazaki M, Segas J, et al. Detection of epstein-barr virus and human papillomavirus in nasopharyngeal carcinoma by the polymerase chain reaction technique. Cancer Lett. 1995;89:177–181. doi: 10.1016/0304-3835(94)03667-8. [DOI] [PubMed] [Google Scholar]
- 13.Hording U, Nielsen HW, Daugaard S, et al. Human papillomavirus types 11 and 16 detected in nasopharyngeal carcinomas by the polymerase chain reaction. Laryngoscope. 1994;104:99–102. doi: 10.1288/00005537-199401000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Lin Z, Khong B, Kwok S, et al. Human papillomavirus 16 detected in nasopharyngeal carcinomas in caucasian americans but not in endemic southern chinese patients. Head Neck. 2013 doi: 10.1002/hed.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogan S, Hedberg ML, Ferris RL, et al. Human papillomavirus and epstein-barr virus in nasopharyngeal carcinoma in a low-incidence population. Head Neck. 2013 doi: 10.1002/hed.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson M, Suh YE, Paleri V, et al. Oncogenic human papillomavirus-associated nasopharyngeal carcinoma: An observational study of correlation with ethnicity, histological subtype and outcome in a uk population. Infect Agent Cancer. 2013;8:30. doi: 10.1186/1750-9378-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laantri N, Attaleb M, Kandil M, et al. Human papillomavirus detection in moroccan patients with nasopharyngeal carcinoma. Infect Agent Cancer. 2011;6:3. doi: 10.1186/1750-9378-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirzamani N, Salehian P, Farhadi M, et al. Detection of ebv and hpv in nasopharyngeal carcinoma by in situ hybridization. Exp Mol Pathol. 2006;81:231–234. doi: 10.1016/j.yexmp.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Rassekh CH, Rady PL, Arany I, et al. Combined epstein-barr virus and human papillomavirus infection in nasopharyngeal carcinoma. Laryngoscope. 1998;108:362–367. doi: 10.1097/00005537-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Tyan YS, Liu ST, Ong WR, et al. Detection of epstein-barr virus and human papillomavirus in head and neck tumors. J Clin Microbiol. 1993;31:53–56. doi: 10.1128/jcm.31.1.53-56.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walline HM, Komarck C, McHugh JB, et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and, oral cavity cancers: Comparison of multiple methods. Otolaryngol Head Neck Surg. 2013 doi: 10.1001/jamaoto.2013.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Yang K, Khafagi A, et al. Sensitive detection of human papillomavirus in cervical, head/neck, and schistosomiasis-associated bladder malignancies. Proc Natl Acad Sci U S A. 2005;102:7683–7688. doi: 10.1073/pnas.0406904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coutlee F, Gravitt P, Kornegay J, et al. Use of pgmy primers in l1 consensus pcr improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol. 2002;40:902–907. doi: 10.1128/JCM.40.3.902-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed AL, Califano J, Cairns P, et al. High frequency of p16 (cdkn2/mts-1/ink4a) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56:3630–3633. [PubMed] [Google Scholar]
- 25.Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singhi AD, Califano J, Westra WH. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34:213–218. doi: 10.1002/hed.21714. [DOI] [PubMed] [Google Scholar]