Abstract
DETECHIP® is a novel molecular sensing array being developed for the detection and identification of a variety of compounds including controlled substances. This easy to use technology has the ability to produce a unique identifying binary code for each substance tested. Original analysis methodology relied on human vision to classify color and fluorescence changes within the array. New digital color image analysis techniques using red-green-blue (RGB) color values provided a higher degree of specificity and greater consistency. This image analysis technique was able to detect more subtle changes in color and was therefore able to properly discriminate between substances that previously produced identical codes. This technique was also expanded to analyze changes in RGB color values individually, increasing the length of the code to 48 digits and therefore potentially providing a further increase in specificity. To show the applicability of this new method, a blind study was performed, correctly identifying two unknown analytes.
Keywords: Image analysis, Detection, Red Green Blue, Abused narcotics, Drugs, Cocaine, Caffeine
Introduction
DETECHIP® Technology
There is a need for a simple, cost effective, time efficient and easy-to-operate method for identifying controlled substances [1, 2]. To meet this need, we developed DETECHIP® technology. DETECHIP® is a patent pending novel spot test device being developed for the detection and identification of specific compounds in both lab and field settings [3–6]. A chemical mix-and-measure assay that is capable of providing both colorimetric and fluorescent signals for the rapid detection of molecules of emerging interest, DETECHIP® identifies and discriminates multiple classes of compounds including narcotics, narcotics with cutting agents, over the counter medications, volatile organic compounds, explosives and the intermediates used to make them, microbial metabolites, and environmental contaminants like pesticides. The strength of DETECHIP® technology is that rather than producing a simple yes/no signal (i.e., color change indicates presence of substance of interest), it produces many simultaneous responses that can be combined to develop a unique identifying binary code for each substance. This allows users to quickly characterize and identify suspect materials based on multiple reactions. In addition to its advantages of speed, simplicity of operation, portability and affordability, DETECHIP® has a unique built-in quality control system, by running a control adjacent to each analyte.
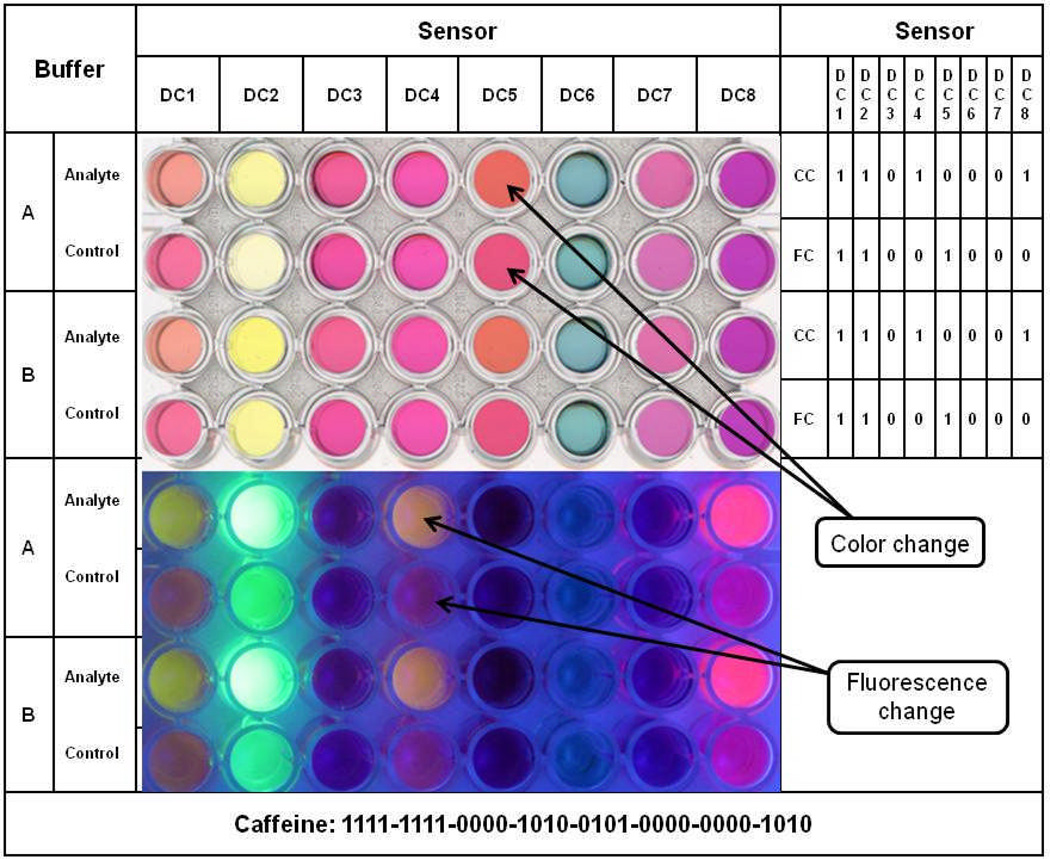
Figure 1 shows a single array excerpt (32-wells) of the original 8-sensor proof of concept version of DETECHIP®. In this test, the sample is mixed with the appropriate buffer (A or B), combined with each of the eight sensors, and analyzed for color and fluorescence changes. These immediate changes allow users to assemble a unique, substance specific binary code composed of ‘1’ and ‘0’. A ‘1’ represents a visual change in color or fluorescence when compared to the control, while a ‘0’ represents no visual change. Figure 1 shows how this combination of eight color signals and eight fluorescence signals for each of the two buffers is used to produce a 32-digit binary code describing the response of cocaine in macro- DETECHIP®.
Figure 1. An original DETECHIP® array.
A 32-well DETECHIP® array of the original proof of concept version is shown. Control wells are located adjacent to analyte wells for direct comparison. Color changes (CC) can be seen on the left while fluorescence changes (FC) are visualized under ultraviolet light at 254 nm. All values for DC1 are recorded beginning with CC in buffer A, then FC in buffer B, etc., resulting in the overall code 1111-1111-0000-1010-0101-0000-0000-1010. For each digit in the final combined code, the digit that prevailed was the one that the majority of the readers reported.
Image Analysis
In the original version of DETECHIP®, Figure 1, identifying codes were determined through visual interpretation of color and fluorescent changes. This method was both time-consuming and resulted in inconsistencies due to variation in human vision and subjective interpretation. This method of code determination also produced identical codes for caffeine and cocaine. To address these drawbacks, the system was replaced by digital colorimetric sensing image analysis techniques. Fluorescence changes were eliminated from this method to allow for stream-lined automated code determination using a single analysis system. We have recently published these new methods for analysis of DETECHIP® images. Analytes such as illegal and over the counter drugs were successfully detected and identified. Digital images of DETECHIP® arrays were obtained using a camera and a simple flat bed photo scanner. Several image analysis methods were evaluated it is was determined that, when compared to photographs, scanned images of DETECHIP® resulted in more consistent images through the elimination of parallax and shading caused by the wells themselves. Color information was obtained by measuring red-green-blue (RGB) values with software like GIMP, Adobe Photoshop, and ImageJ.
In the last several years, the use of colorimetric sensing using RGB values as an analysis method for digital images has increased in popularity [6–10]. Various analytes can be detected using RGB color space including pigments of green beans, nitrates, sugars, peroxide vapors, and biogenic amines [11–14]. However, only a few investigators, including the authors, use RGB analysis in conjunction with detection arrays. Therefore the extension of the RGB code adds a critical dimension to this technology.
Herein we compare visual interpretation and digital image analysis using ImageJ of caffeine and cocaine with DETECHIP® illustrating the ability of this new image analysis technique to detect more subtle changes in color when compared to human vision. We also introduce an expansion of this previously published digital image analysis technique with aims to further increase specificity and drastically reduce the likelihood of misidentification of drugs using DETECHIP®.
Materials and Methods
Standards and Reagents
All standards and reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO). DC1-DC8 were prepared in our laboratory and their chemical composition remains proprietary [4]. Digital images of DETECHIP® arrays were obtained using an Epson V700 Photo Scanner in transparency mode. Analysis of the resulting digital images was done using ImageJ, an in-house designed macro and Microsoft Excel.
DETECHIP® Design and Protocol
Each DETECHIP® array is composed of 32 wells (16 control and 16 exposed to analyte). The 96-well plate format allows for triplicate testing of a single analyte, or the ability to test three different analytes within the same plate. Stock solutions of the eight molecular sensors were dissolved in water at a concentration of 750µM, and 30µL of each sensor is added into a 96 well optical bottom plate (Thermo Fisher Scientific, Rochester, NY). Rows A-H each held one of the 8 sensors (i.e., DC1 in wells 1–12 of row A, DC2 in row B, etc.) Two separate buffers, Tris and phosphate, were prepared in deionized water at a concentration of 400 µM and a pH of 7.0. Each well in columns 1,2,5,6,9 and 10 contained 150µL of Tris buffer, while wells in columns 3,4,7,8,11 and 12 contained 150µL of phosphate buffer [4]. Aqueous solutions of caffeine and cocaine HCl were prepared at a concentration of 62.5mM. In each row, even numbered wells contained 120µL of the analyte of interest for a final analyte concentration of 25mM. Odd numbered wells served as control wells, where water was added in lieu of analyte. This final concentration of analyte was chosen because it rendered the most visually apparent color changes. This is not the lower limit of detection for DETECHIP®.
Visual Code Determination
As described in previously published work [5], control wells and analyte wells are compared to each other for color changes through visual inspection by five individuals, or readers. Changes are assigned a ‘1’ while instances where the control and analyte wells appear identical are assigned a ‘0’ for no change. The responses are then assembled into a binary code, which contains the digits reported by the majority of readers.
DETECHIP® Image Analysis and Digital Code Determination
The protocol for analysis of DETECHIP® was modified from previously published work [6]. Once prepared, each plate was scanned in triplicate using a V700 Epson photo scanner. Analysis of the resulting images was performed using ImageJ and a newly designed macro based upon previously published work [6, 7]. The macro in the initial publication [7], was written to use the color space data obtained for quantitative measurements. The macro was adapted for use with DETECHIP® to allow for direct comparison between two regions within the scanned image. In 16-bit color, the maximum value for each color channel (Red, Green or Blue) is 65,536. Each channel was measured using the macro designed for use with ImageJ. This macro was designed to select a circular area (47 × 50 pixels) in the center of each well. Within the selected region of the well, each pixel was analyzed for the individual color channels sequentially. These values were then assembled to calculate an average value representing the entire selected region of the well for each color channel. This sequence of measurements was performed on all 96 wells. The analysis of both analyte and control wells allowed for comparison of these color values. In order to determine if the change in a specific color channel was large enough to produce a ‘1’ in the binary code, an arbitrary value – referred to as the threshold – was set. For example, if the value for the Red channel in the control well was 60,000 and the value for the well containing analyte was 64,000 this would mean that a total value of 4,000 units of Red color space was changed by addition of the analyte. Our adapted macro compares this change to the set value for the threshold (2,000) and would therefore code this as a significant change – ‘1’ – because the size of the change is larger than the value of the threshold. If the change was smaller than 2,000 this change would not be significant enough and would be coded as a ‘0’. The value of 2,000 was chosen because it was a large enough change to produce consistent codes for our chosen analytes. This value can be lowered if smaller changes in color are to be monitored.
The initial version of this image analysis technique was designed to produce a ‘1’ in the code if there was a significant difference in the combined total value of Red, Green and Blue between the control and analyte wells – producing a 16 digit code (1 digit for each sensor/buffer combination). This technique was further optimized to analyze the color values of Red, Green and Blue separately. This tripled the length of the code to 48 digits and allowed for a higher degree of specificity when indicating a color change, allowing the experimenter to determine which of the three measured colors experienced a significant change.
Blind Study for Method Validation
For method validation, 30 DETECHIP® arrays were prepared according to the standard protocol and exposed to either cocaine or caffeine. These tests were scanned using a V700 Epson photo scanner and images were given to an investigator blind to the analyte identity in each array. The investigator used ImageJ and the macro to determine identifiable codes for each array.
Results and Discussion
Selectivity and Sensitivity
DETECHIP® has shown remarkable selectivity and sensitivity for a wide variety of substances ranging from drugs of abuse, to over the counter (OTC) products, to explosives to simple sugars [3–5]. When determined visually only two of the tested analytes resulted in identical codes – caffeine and cocaine (Table 1). For each digit in the final combined code, the digit that prevailed was the one that the majority of the readers reported.
Table 1. Codes determined for caffeine and cocaine through visual interpretation of color change and fluorescence change by 5 individuals.
The hyphenated code is assembled beginning with DC1 in buffer A for color then fluorescence – DC1 in buffer B for color then fluorescence – DC2 in buffer A for color then fluorescence – etc.
| Visual Code Determination (Color-Fluorescence) | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caffeine | 11 | - | 11 | - | 11 | - | 11 | - | 11 | - | 00 | - | 11 | - | 11 | - | 11 | - | 00 | - | 11 | - | 00 | - | 00 | - | 00 | - | 00 | - | 00 |
| Cocaine | 11 | - | 11 | - | 11 | - | 11 | - | 11 | - | 00 | - | 11 | - | 11 | - | 11 | - | 00 | - | 11 | - | 00 | - | 00 | - | 00 | - | 00 | - | 00 |
In an effort to not only address the issue of the overlapping codes in caffeine and cocaine, but also to streamline the process for DETECHIP® code determination, changes in fluorescence were eliminated, and a new digital color image analysis technique was developed.
For digital image analysis a V700 Epson photo scanner was used to scan the well-plates. After the image was saved as a Tiff file, it was opened in ImageJ. The resulting images were then analyzed using an in-house designed macro as a plugin for ImageJ and a code is determined within seconds. In its original form, the macro was designed to output a 16 digit code based on the significant color changes between the control and analyte wells. A ‘1’ was produced in the code if a significant change was present in either Red, Green or Blue color values.
Table 2 shows how incorporation of digital color image analysis was able to address the issue of overlapping codes between caffeine and cocaine. For direct comparison values for fluorescence changes were omitted from the visually determined codes. The percent error was determined by calculating the number of times a single digit deviated from the overall average code. The average codes were determined visually by five interpreters. Although the 10.00% error for cocaine was relatively low, the code for caffeine was noted to be different nearly 25% of the time. The digital image analysis method was able to produce unique 16 digit codes for caffeine and cocaine. These new codes were also achieved with a much lower error percentage than those determined using human vision. The changes in the caffeine code from that determined using human vision may be attributed to color changes that were subtle or hard to distinguish with human vision. It should also be noted that within the same time period the number of samples (N) can be increased greatly when using this method. The ability for the macro to determine codes quickly reduced the analysis time from minutes to seconds.
Table 2. Comparison of codes determined for caffeine and cocaine by both visual and digital color image analysis.
Visual codes were determined by 5 interpreters while digital codes were obtained using an in-house designed macro for use in ImageJ to measure changes in RGB values. The hyphenated code is assembled beginning with DC1 in buffer A – DC1 in buffer B – DC2 in buffer A – etc. N represents the number of total codes determined and the percentage error represents the total deviations from any of the 16 digits of this determined code.
| Visual Code Determination (Color Changes Only) | Error | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caffeine | N=15 | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 0 | - | 0 | - | 0 | - | 0 | 27.50% |
| Cocaine | N=10 | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 0 | - | 0 | - | 0 | - | 0 | 10.00% |
| Digital 16 Digit Code Determination (Threshold = 2000) | Error | ||||||||||||||||||||||||||||||||
| Caffeine | N=48 | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 0 | - | 0 | - | 1 | - | 1 | - | 0 | - | 0 | - | 1 | - | 1 | - | 0 | - | 0 | 7.16% |
| Cocaine | N=60 | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 1 | - | 0 | - | 0 | - | 0 | - | 0 | 4.06% |
While implementation of digital color image analysis solved the problem present by cocaine and caffeine, a simple modification to the method was made to increase specificity by expanding the code. This more unique code will prevent the occurrence of identical code for two substances. The original macro analyzed all three color values – Red, Green and Blue as a single value. If this value – representing a combined total of all three color channels – experienced a significant change, the code was determined to be a ‘1’. If this value did not show a significant change, the code was determined to be a ‘0’. This led to instances where one color channel, e.g. Green, increased, while another, e.g. Red, decreased by the same magnitude. These changes would not be reflected in the code because the overall value would not change. This could possibly lead to identical codes for different substances. Therefore, the macro was modified to output a value for each of the three color components. This resulted in a 48 digit code that was highly specific to the analyzed substance.
Table 3 shows the 48 digit codes for caffeine, cocaine, nicotine and oxycodone. The 3 digit hyphenated sections represent the Red-Green-Blue codes for each dye and buffer system. The threshold was set at 2000 for direct comparison of caffeine and cocaine with the 16 digits codes in Table 2. The expanded 48 digit code was unique for all substances tested with error percentages ranging from 4 to 12 percent. In addition to the four analytes presented in Table 3, suphedrine, aspirin, ketamine, hydrocodone, hydromorphone, and many more analytes have been tested – each producing a unique identifying binary code (data not shown).
Table 3. Codes determined for several different drugs using an in-house designed macro to measure individual changes in Red, Green, and Blue values.
The hyphenated code is assembled beginning with Red Green Blue values for DC1 in buffer A – Red Green Blue values for DC1 in buffer B – Red Green Blue values for DC2 in buffer A – etc. N represents the number of total codes determined and the percentage error represents the total deviations from any of the 48 digits of this determined code.
| Digital 48 Digit Code Determination (Threshold = 2000) | Error | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caffeine | N=42 | 011 | - | 011 | - | 001 | - | 001 | - | 011 | - | 011 | - | 000 | - | 000 | - | 011 | - | 011 | - | 000 | - | 000 | - | 001 | - | 101 | - | 000 | - | 000 | 9.47% |
| Cocaine | N=42 | 011 | - | 011 | - | 011 | - | 011 | - | 011 | - | 011 | - | 001 | - | 001 | - | 011 | - | 011 | - | 001 | - | 001 | - | 000 | - | 000 | - | 000 | - | 000 | 4.07% |
| Nicotine | N=30 | 011 | - | 011 | - | 011 | - | 011 | - | 111 | - | 011 | - | 101 | - | 011 | - | 010 | - | 010 | - | 001 | - | 001 | - | 101 | - | 001 | - | 001 | - | 001 | 12.01% |
| Oxycodone | N=20 | 111 | - | 111 | - | 011 | - | 111 | - | 111 | - | 111 | - | 111 | - | 111 | - | 011 | - | 011 | - | 001 | - | 001 | - | 111 | - | 111 | - | 001 | - | 001 | 9.20% |
To establish the validity of this method, a blind study was conducted to test the ability of this technique to identify an unknown substance. Thirty arrays were prepared containing either caffeine or cocaine and images were provided to an investigator blind to the identity of the analyte within each array. 48 digit codes were obtained using the macro within ImageJ. The investigator was able to identify the presence of two separate analytes and therefore determine an average code for each. These average codes were compared to an existing code library. Cocaine and caffeine were successfully discriminated and identified with error percentages of 5.20% (N=6) and 10.16% (N=24), respectively, when compared to the average codes within the existing code library.
Conclusions
DETECHIP® is a molecular sensor with the ability to produce an identifying code for a large variety of compounds. New image methodology was developed for the analysis of DETECHIP® arrays, resulting in a significant increase in reproducibility and specificity for the interpretation of colorimetric changes. Through the elimination of human vision as the primary means of code determination, high throughput analysis of RGB values in scanned images of DETECHIP® arrays was able to successfully produce unique 16 digit codes for caffeine and cocaine. The selectivity of DETECHIP® was further increased through the modification of this technique by analyzing individual Red, Green and Blue color values and ultimately producing a unique 48 digit code. This new image analysis technique allows for more accurate, more selective and more rapid code determination. The ability of this new methodology was put to the test when the expanded 48 digit code was used to successfully identify two unknown analytes.
Acknowledgements
Funding for this research was obtained from the NSF CHE-0747949 and NSF – EPSCoR-EPS-1004094. This publication was also made possible through funding by grants from the National Center for Research Resources (5P20RR016469) and the National Institute for General Medical Science (NIGMS) (8P20GM103427), a component of the National Institutes of Health (NIH) and its contents are the sole responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Abbreviations
- RGB
Red Green Blue
- DC1
DETECHIP® sensor 1, etc.
- CC
color change
- FC
fluorescence change
References
- 1.United Nations. Rapid Testing Methods of Drugs of Abuse. New York: 1994. http://www.unodc.org/pdf/publications/st-nar-13-rev1.pdf. [Google Scholar]
- 2.Montgomergy E. Trends in immunoassays for drugs-of-abuse testing. Med Lab Obs. 2011;43:8–17. http://www.mlo-online.com/features/201108/cover-story/trends-in-immunoassays-for-drugs-of-abuse-testing.aspx. [PubMed] [Google Scholar]
- 3.Burks R, Pacquette S, Guericke M, Wilson M, Symonsbergen D, et al. DETECHIP®: A sensor for drugs of abuse. J. Forensic Sci. 2010;55(3):723–727. doi: 10.1111/j.1556-4029.2010.01323.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1556-4029.2010.01323.x/abstract. [DOI] [PubMed] [Google Scholar]
- 4.Holmes AE, et al. DETECHIP: Molecular color and fluorescent sensory arrays for small molecules. United States Patent Office 20100197516. 2009 Jul 15; http://www.faqs.org/patents/app/20100197516.
- 5.Lyon M, Groathouse J, Beaber J, Turner LM, Rouhier KA, et al. DETECHIP®: An improved molecular sensing array. Journal of Forensic Research. 2011 http://www.omicsonline.org/2157-7145/2157-7145-2-126.php. [Google Scholar]
- 6.Lyon M, Wilson MV, Rouhier KA, Symonsbergen DJ, Bastola DK, et al. Image analysis of DETECHIP - A molecular sensing array. Advances in Intelligent and Soft Computing. 2012;166:145–158. [Google Scholar]
- 7.Soldat D, Barak P, Lepore B. Microscale Colorimetric Analysis Using a Desktop Scanner and Automated Digital Image Analysis. J. Chem. Ed. 2009;86(5):617–620. http://pubs.acs.org/doi/abs/10.1021/ed086p617. [Google Scholar]
- 8.Lin H, K S. A colorimetric sensor array for detection of triacetone triperoxide vapor. Journal of the American Chemical Society. 2010;132:15519–15521. doi: 10.1021/ja107419t. http://pubs.acs.org/doi/abs/10.1021/ja107419t. [DOI] [PubMed] [Google Scholar]
- 9.Rakow K, Suslick KS. A colorimetric sensor array for odour visualization. Nature. 2000;406:710–713. doi: 10.1038/35021028. http://www.scs.illinois.edu/suslick/documents/nature00710.pdf. [DOI] [PubMed] [Google Scholar]
- 10.Dugan DL, Wright DN. Quantitative infrared photoanalysis of selected bacteria. Applied and Environmental Microbiology. 1974;28(2):205–211. doi: 10.1128/am.28.2.205-211.1974. http://aem.asm.org/content/28/2/205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey E. Handbook of Digital Forensics and Investigation. Burlington, MA: Elsevier Academic Press; 2009. [Google Scholar]
- 12.Feng L, Musto CJ, Kemling JW, Lim SH, Suslick KS. A colorimetric sensor array for identification of toxic gases below permissible exposure limits. Chemical Communications. 2010;46:2037–2039. doi: 10.1039/b926848k. http://www.scs.illinois.edu/suslick/documents/chemcomm.20102037.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng L, Musto CJ, Suslick KS. A simple and highly sensitive colorimetric detection method for gaseous formaldehyde. Journal of the American Chemical Society. 2010;132(12):4046–4047. doi: 10.1021/ja910366p. http://pubs.acs.org/doi/abs/10.1021/ja910366p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon J, Chae SK, Kim J-M. Colorimetric sensors for volatile organic compounds (VOCs) based on conjugated polymer-embedded electrospun fibers. Journal of the American Chemical Society. 2007;129(11):3038–3039. doi: 10.1021/ja067856+. http://pubs.acs.org/doi/abs/10.1021/ja067856+ [DOI] [PubMed] [Google Scholar]