Abstract
The transverse relaxation time constant, T2, is sensitive to brain tissue’s free water content and the presence of paramagnetic materials such as iron. In this study, ex vivo MRI was employed to investigate alterations in T2 related to Alzheimer’s disease (AD) pathology and other types of neuropathology common in old age, as well as the relationship between T2 alterations and cognition. Cerebral hemispheres were obtained from 371 deceased older adults. Using fast spin-echo imaging with multiple echo times, T2 maps were produced and warped to a study-specific template. Hemispheres underwent neuropathologic examination for identification of AD pathology and other common age-related neuropathologies. Voxelwise linear regression was carried out to detect regions of pathology-related T2 alterations and, in separate analyses, regions in which T2 alterations were linked to antemortem cognitive performance. AD pathology was associated with T2 prolongation in white matter of all lobes and T2 shortening in the basal ganglia and insula. Gross infarcts were associated with T2 prolongation in white matter of all lobes, and in the thalamus and basal ganglia. Hippocampal sclerosis was associated with T2 prolongation in the hippocampus and white matter of the temporal lobe. After controlling for neuropathology, T2 prolongation in the frontal lobe white matter was associated with lower performance in the episodic, semantic, and working memory domains. In addition, voxelwise analysis of in vivo and ex vivo T2 values indicated a positive relationship between the two, though further investigation is necessary to accurately translate findings of the current study to the in vivo case.
Keywords: Neuroimaging, MRI, gross infarct, hippocampal sclerosis, cognition, voxelwise
1 Introduction
The transverse relaxation time constant, T2, describes the decay rate of the transverse component of magnetization due to irreversible dephasing, and is one of the main sources of contrast available in MRI. For H1 imaging at a given field strength, T2 is influenced by a number of factors, including the ratio of free to myelin-bound water molecules (House et al., 2006), and the presence of paramagnetic molecules, such as the iron-laden compounds ferritin and hemosiderin (Haacke et al., 2005). Various types of pathology can alter these tissue properties and consequently change T2 values. For this reason, T2 has the potential to serve as a biomarker for a range of diseases, and studies that investigate the relationships between pathology, cognition, and T2 are warranted.
Alzheimer’s disease (AD) is one such condition for which T2 alterations have been observed in different regions of the brain. Several studies have reported a significant increase in hippocampal T2 in individuals with AD compared to controls (Arfanakis et al., 2007, Kirsch et al., 1992, Laakso et al., 1996, Wang et al., 2004), and this is thought to be attributable to neuronal loss (West et al., 1994) and a resulting increase in the tissue’s water content. However, other studies that examined the hippocampus did not find significant alterations in T2 in individuals with mild to severe cognitive impairment (House et al., 2006) or in histopathologically confirmed cases of AD (House et al., 2008). Outside of the hippocampus, elevated T2 times have been reported in the internal capsule and in the frontal, temporal, and parahippocampal white matter of individuals with clinically or pathologically diagnosed AD and those with memory complaints or mild to severe cognitive impairment, a phenomenon which some attribute to the breakdown of myelin and the resultant increase in the tissue’s free water content (Bartzokis et al., 2003, Besson et al., 1992, House et al., 2006). T2 prolongation has also been reported in the amygdala of individuals with AD (Wang et al., 2004). Significantly reduced T2 times thought to result from iron accumulation have been detected in the temporal lobe gray matter of living individuals reporting memory loss (House et al., 2006). By contrast, a related study of postmortem brain tissue did not find a significant T2 reduction in temporal lobe gray matter in histopathologically confirmed cases of AD (House et al., 2008).
While these findings shed light on a potentially important relation of T2 to AD, the majority of the studies did not have the benefit of a neuropathologic index or diagnosis of AD, and instead relied only on clinical diagnosis. Even in those few studies that combined measurements of T2 with pathologic diagnosis of AD, common coexisting age-related pathologies, such as infarcts, were rarely included in the analyses. Further, we are not aware of prior studies which have examined the clinical significance of T2 alterations by investigating their relation to cognitive status proximate to death, while also controlling for the effects of pathology. Therefore, several gaps in our knowledge of T2 alterations related to AD and other common neuropathologies in the human brain remain to be filled.
To address these issues, it is necessary to combine clinical data with neuropathology and imaging data from the same human brain tissue via one of two methods. One approach involves conducting MRI during life, and then, upon death of those individuals, examining the brain tissue histopathologically. The other approach, the one adopted in the current study, avoids a potentially lengthy delay between MRI and death by subjecting ex vivo human brain specimens to MRI, followed by histopathologic examination. This second approach eliminates the possible confound of additional neuropathology formation between MRI and histopathologic examination, since both procedures are performed postmortem. It also permits assessment of the large number of cerebral hemispheres necessary to examine the range of pathologies across the spectrum of cognitive function.
The purpose of this study was to conduct a cerebrum-wide analysis of T2 alterations associated with AD pathology, Lewy bodies, microscopic infarcts, gross infarcts, and hippocampal sclerosis (HS). We also investigated whether T2 alteration is associated with cognitive function beyond that accounted for by neuropathology. The investigations were carried out by employing ex vivo MRI to map T2 values in human cerebral hemispheres from nearly 375 individuals, followed by histopathological examination of the same specimens. All brain hemispheres were obtained from study participants whose cognition had been assessed proximate to death. By accounting for the aforementioned types of neuropathology in a combined multiple linear regression, it was possible to study the T2 alterations associated with each condition, something that has not been accomplished previously in either ROI-based or voxel-based analyses. Additional voxelwise regression was carried out to assess the contribution of T2 to variation in cognitive measures, while also considering the association of cognition with neuropathologic indices.
2 Materials and Methods
2.1 Ethics Statement
Older adults included in this work were participants in one of two longitudinal clinical-pathologic studies of aging: the Rush Memory and Aging Project (MAP) or the Religious Orders Study (ROS) (Bennett et al., 2012a, Bennett et al., 2012b). All participants provided written informed consent and signed an anatomical gift act. The study was approved by the Institutional Review Board of Rush University Medical Center.
2.2 Brain Hemispheres
In a period of six years, 395 individuals from the MAP and ROS projects who died were considered for inclusion in this study. After a participant’s death, autopsy technicians removed and separated the cerebral hemispheres as previously described (Dawe et al., 2011). One hemisphere per participant was selected for ex vivo imaging, and this hemisphere was immersed in phosphate-buffered 4% formaldehyde solution (prepared from paraformaldehyde) and refrigerated at 4° C in a sealed plastic container within an hour of its removal from the skull.
2.3 Ex Vivo Image Acquisition and Processing
MRI scans occurred at 47 ± 25 days (mean ± standard deviation) postmortem (range 22–235) (Dawe et al., 2009). All scans were performed with the hemisphere immersed in room temperature formaldehyde solution in a clear, sealed acrylic container. Due to the ongoing nature of this imaging study, three different scanners were used to acquire fast spin-echo MRI data with at least two different echo times (TEs): a General Electric Signa scanner (Waukesha, WI, USA) was used from 2006 to 2008, a Siemens Trio (Erlangen, Germany) from 2008 to 2010, and a Philips Achieva (Best, The Netherlands) after 2010. While all three scanners had the same nominal field strength (3 Tesla) and approximately the same scan time (31–35 minutes), other factors varied among the scanners, including details of the fast spin-echo sequence (Table 1). The last two echoes from the Philips scanner (66 ms and 82.5 ms) were discarded in order to maintain a similar range of echo times across all three scanners.
Table 1.
Fast spin-echo sequences from each of three MRI scanners employed in the current study.
| GE Signa 3T | Siemens Trio 3T | Philips Achieva 3T | |
|---|---|---|---|
| Subjects scanned | 95 | 88 | 188 |
| FOV (mm) | 160 × 160 | 160 × 160 | 160 × 160 |
| Acquisition matrix | 256 × 256 | 256 × 256 | 260 × 258 |
| Slice thickness (mm) | 1.5 | 1.5 | 1.5 |
| Native voxel dimensions (mm) | 0.625 × 0.625 × 1.5 | 0.625 × 0.625 × 1.5 | 0.615 × 0.620 × 1.5 |
| Native voxel volume (mm3) | 0.59 | 0.59 | 0.57 |
| Echo Times (ms) | 13, 52 | 12, 35, 58 | 16.5, 33, 49.5, 66, 82.5 |
| TR (ms) | 3600 | 3750 | 4055 |
| ETL | 6 | 2 | 5 |
| NEX | 6 | 4 | 2 |
| Scan time (min) | 31 | 32 | 35 |
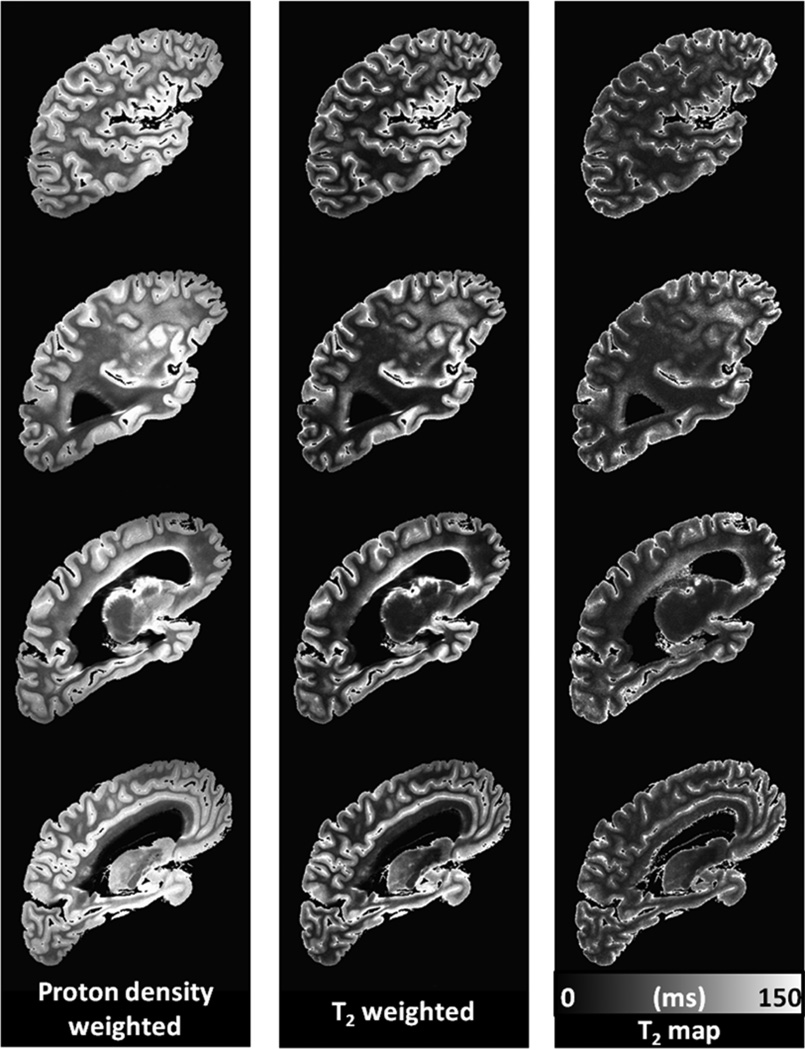
For a given postmortem hemisphere, a T2 image volume was estimated from the set of images generated from the multiple echo times (Fig. 1). This was accomplished by fitting the equation Si = S0 exp(−TEi/T2) to the data using a least squares approach, where Si is the signal measured at TEi, and S0 is the fitted signal extrapolated to a proton density weighting at TE = 0 ms. The resulting S0 and T2 maps were masked at a T2 value of 150 ms to eliminate the long-T2 formaldehyde solution from the T2 image volumes. For hemispheres from the left side of the brain, the slice order was reversed so that they appeared as right hemispheres in all subsequent analyses. Of the 395 hemispheres undergoing MRI, 24 were found to have gross abnormalities, such as tumors or large hemorrhages, precluding accurate spatial normalization. These hemispheres were removed from the analysis, leaving 371 total hemispheres.
Figure 1.
Slices from a typical masked proton density-weighted image volume (TE = 13 ms), a T2-weighted image volume (TE = 52 ms), and estimated T2 map from an individual postmortem cerebral hemisphere, shown in the same orientation and resolution as the natively acquired sagittal slices.
2.4 Study-Specific Template Creation
Of the remaining 371 hemispheres, 30 were used to generate a study-specific template. These 30 hemispheres were chosen to span the range of AD pathology burden (see Section 2.6, Neuropathologic Evaluation), from minimal to severe, so as to avoid bias toward a particular level of AD pathology. Half came from male participants, and the mean ages at death were matched between males and females. The high dimensional warping algorithm of the Automatic Registration Toolbox (ART) (Ardekani et al., 2005) was used to spatially normalize the 30 hemispheric S0 image volumes in an iterative fashion (Joshi et al., 2004). The final template was produced by calculating the mean of the 30 S0 volumes and is visible in Figures 2 through 6, in which it serves as the grayscale underlay.
Figure 2.
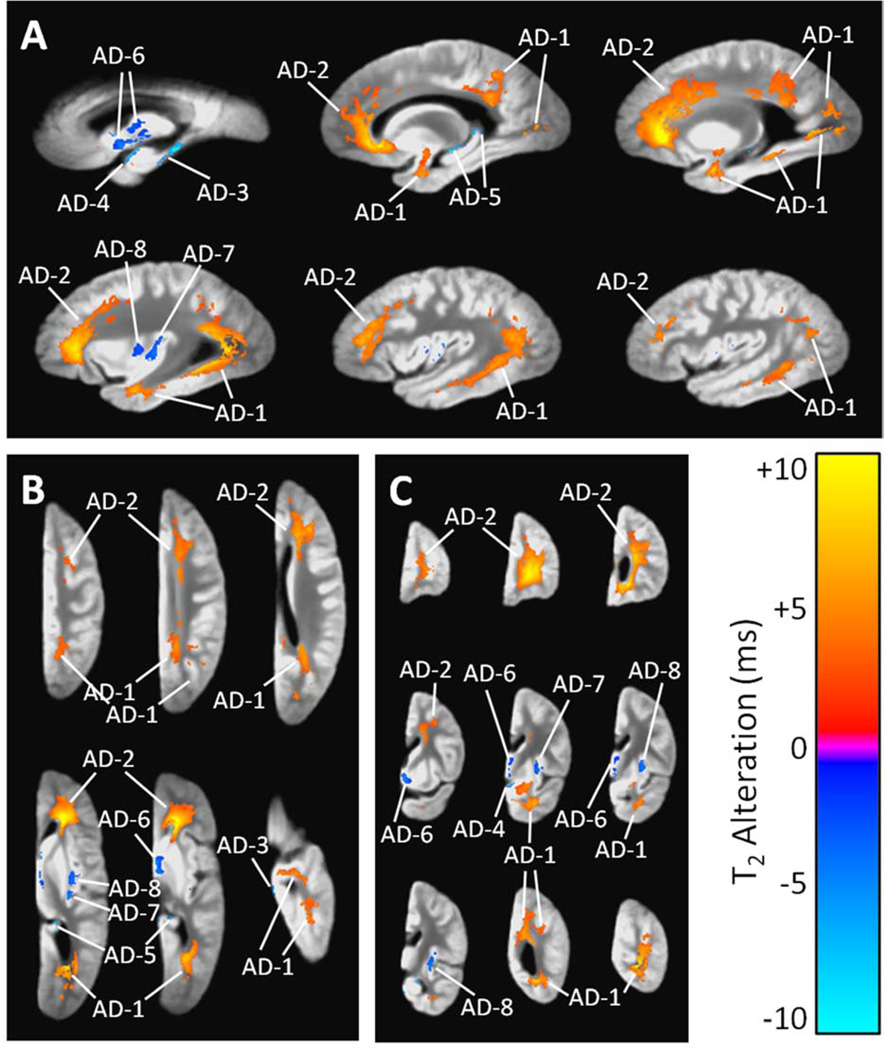
(A) Sagittal, (B) axial, and (C) coronal views showing regions of significant T2 prolongation (orange) and T2 shortening (blue) associated with a high summary score of AD pathology, according to a combined linear regression analysis that simultaneously considered AD pathology, acute and chronic gross infarcts, and hippocampal sclerosis (and covariates). The grayscale underlay is the study-specific template (proton density-weighted). Alphanumeric labels are those cluster identifiers introduced in Table 3.
Figure 6.
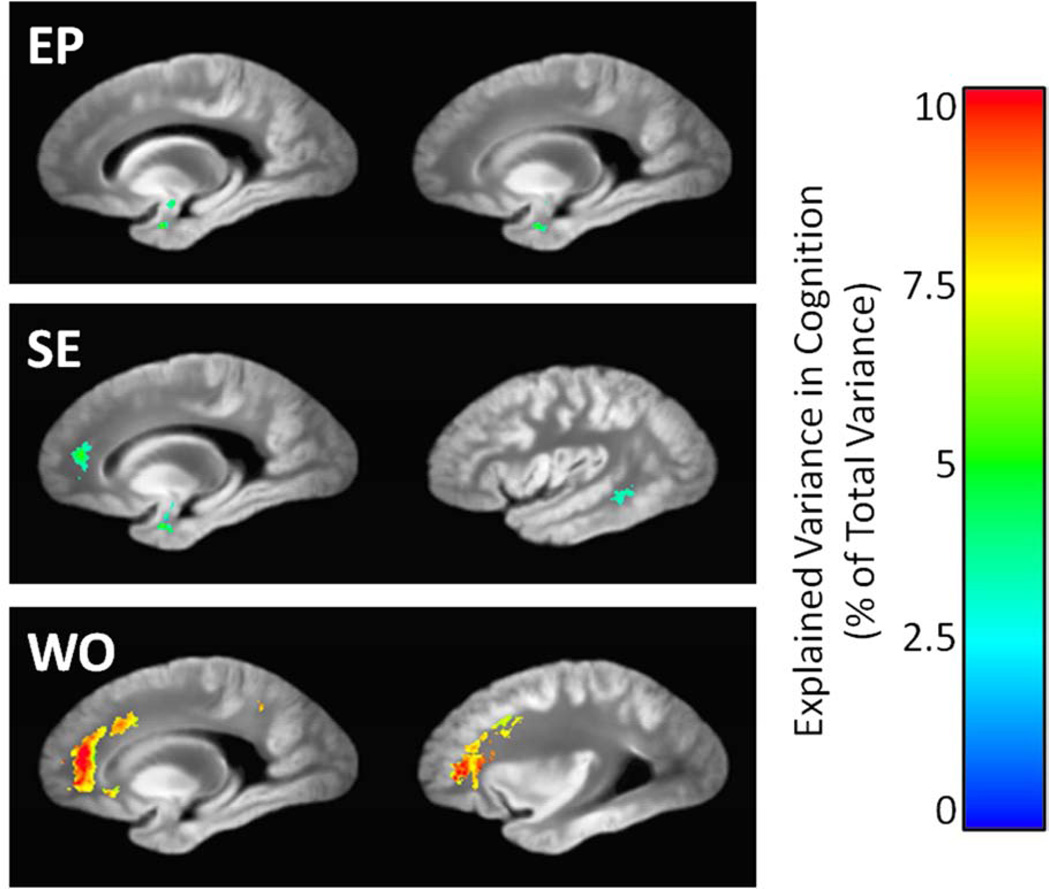
Sagittal views of the regions in which episodic memory (EP), semantic memory (SE), and working memory (WO) deficits were significantly associated with T2 prolongation, according to linear regression models that also considered neuropathology measures and demographic covariates. Colors indicate the amount of additional variance in working memory explained by T2, beyond that accounted for by neuropathology and demographics. The grayscale underlay is the study-specific template (proton density-weighted).
2.5 Spatial Normalization of Individual Hemispheres
Using ART, the S0 volume from each brain hemisphere was warped to match the template. The deformation field map defining this transformation was then applied to that same hemisphere’s T2 volume. Thus, all 371 T2 volumes became spatially normalized to an unbiased template via a high dimensional transformation, facilitating voxelwise analyses.
2.6 Neuropathologic Evaluation
Following MRI, each cerebral hemisphere was placed in a Plexiglas jig and cut coronally into 1-cm thick slabs. Slabs underwent complete macroscopic evaluation and dissection of blocks, including midfrontal, middle temporal, inferior parietal, and entorhinal cortices, hippocampus, anterior basal ganglia, anterior thalamus, and midbrain, including substantia nigra. These regions were embedded in paraffin, cut into sections, and mounted on glass slides. Brain autopsy procedures have been described previously (Schneider et al., 2009).
Neuropathologic assessments were completed by a board-certified neuropathologist blinded to age and all clinical data. Bielschowsky silver stain was used to visualize neuritic plaques, diffuse plaques, and neurofibrillary tangles in the frontal, temporal, parietal, and entorhinal cortices and the hippocampus. Plaque and tangle counts were converted to standardized scores in each area. Summary scores for each marker were then computed by averaging across the five regions. Finally, these three summary scores were averaged to produce a global measure of AD pathology for each hemisphere. Details of the evaluation of AD pathology and computation of the global score have been described previously (Schneider et al., 2007). For descriptive purposes only, a neuropathologic diagnosis of low, intermediate, or high AD pathology was also rendered, based on the Braak score for neurofibrillary pathology and the CERAD estimate of neuritic plaques, as recommended by the NIA-Reagan criteria (The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease, 1997), but modified to exclude clinical diagnosis. Lewy bodies were identified with antibodies to alpha-synuclein (Schneider et al., 2009) and were classified as limbic or neocortical based on recommended guidelines (McKeith, 2006). The brain and slabs were examined for gross cerebral infarcts (infarcts visualized by the naked eye) as previously described (Schneider et al., 2009). For analyses, the total number of gross infarcts was recorded, as well as each infarct’s age (acute, subacute, or chronic). Microscopic infarcts (defined as infarcts seen microscopically but not macroscopically) were counted on 6-micron hematoxylin and eosin-stained routine sections (Arvanitakis et al., 2011) and were also classified by age. Hippocampal sclerosis was defined as substantial neuronal loss and gliosis including the hippocampal CA1 sector, as assessed on 6-micron sections (Dawe et al., 2011).
2.7 Assessment of Cognitive Performance
The two longitudinal studies from which participants originated (MAP and ROS) have 19 cognitive tests in common. Of those, the Mini-Mental State Examination (MMSE) is used for descriptive purposes, and one other test, Complex Ideational Material, is only used in diagnostic classification. The remaining 17 tests assess a broad range of cognitive abilities. The scores from those 17 tests are converted to z-scores based on the mean and standard deviation at baseline, and combined to obtain measures of five cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability. Details of the cognitive tests and the use of combined measures from both cohorts have been previously described (Bennett et al., 2006, Bennett et al., 2009, Wilson et al., 2007).
2.8 Voxelwise Statistical Analysis
Custom software written in Matlab (The Mathworks, Inc., Natick, MA) was used to conduct voxelwise linear regression. First, each type of pathology was considered separately to assess its potential association with T2 in each voxel (i.e., one voxelwise regression for each type of pathology, with T2 as the outcome variable). Subsequently, a combined voxelwise regression was conducted in which those types of pathology found to have significant associations with T2 were simultaneously entered in the model. Each regression model included terms to account for the postmortem interval to MRI (Dawe et al., 2009), the scanner used to acquire images from each hemisphere (GE, Siemens, or Philips), participant’s age at death (Hasan et al., 2010), sex (Kumar et al., 2012), and years of education.
A separate analysis was then performed to investigate the possible association of T2 with cognition. For each of five cognitive domains, a series of regression analyses were carried out with cognitive score as the outcome variable. First, only demographics were included as explanatory variables. The five types of neuropathology were then included, with acute and subacute infarcts (both gross and microscopic) held out of the analysis since most participants likely underwent cognitive assessment prior to sustaining these. Finally, voxelwise T2 was added as an explanatory variable. The explained percentage of variance in cognition was computed for each addition of explanatory variables to the model.
For all voxelwise regression analyses conducted throughout the study, voxels for which the explanatory factor of interest had significant association with the outcome variable (either T2 or cognitive score) were identified by masking the raw p-value maps at a threshold expected to produce a false discovery rate (FDR) of 5% (‘fdr’ tool of the FSL software package, http://www.fmrib.ox.ac.uk/fsl/). To further guard against Type I errors, only voxels belonging to clusters greater than 100 mm3 in volume were ultimately considered to be statistically significant. The FDR-adjusted and clustered p-value maps were used to mask colorized contrast maps, which were then overlaid on the template volume in AFNI for visualization (Cox, 1996).
2.9 Addressing Inter-Scanner Variation in T2
We guarded against spurious findings stemming from systematic variation in T2 among scanners in two ways. First, as mentioned in the preceding section, we included binary explanatory variables in each regression model to code for the scanner used to image each hemisphere, allowing constant, voxelwise offsets in T2 among the three different instruments. Second, we repeated all voxelwise analyses on the largest subgroup of hemispheres imaged on a single scanner (Philips Achieva, 188 hemispheres) and compared these results to the findings of the full analysis. An incidental benefit of the single scanner analysis was a marked reduction in the variance and range of postmortem intervals to MRI compared to the full analysis (mean ± standard deviation of 35 ± 7.1 days, range of 24–58 days), meaning that a potential contribution from this factor was effectively minimized.
2.10 Generalization of Ex Vivo Findings
To explore the potential for extrapolation of ex vivo imaging findings to the in vivo case, we investigated the relationship between in vivo and ex vivo T2 values. T2 maps were calculated for 28 living participants in the MAP and ROS studies who had undergone multi-spin echo MRI in a 3-Tesla scanner (Siemens Trio, Erlangen, Germany) using the following parameters: TE = 20/40/60/80/100 ms, TR = 5.7 s, field of view = 183 × 230 mm, acquisition matrix = 143 × 256, slice thickness = 3 mm, parallel imaging factor = 2, and a single average. These in vivo T2 maps were spatially warped to a common template along with ex vivo T2 maps from 28 age- and sex-matched brain donors. Spherical ROIs were selected in white matter (at least one ROI in each lobe) and in two well-registered subcortical gray matter regions, the putamen and globus pallidus. ROIs were not selected in cortical gray matter due to reduced registration accuracy of cortical areas across subjects. For each voxel, the mean ex vivo T2 was plotted against the corresponding mean in vivo T2. Linear regression was carried out per ROI to assess the relationship between ex vivo and in vivo T2.
3 Results
3.1 Participant Demographics and Neuropathology
Characteristics of the 371 participants/hemispheres are summarized in Table 2. AD (either intermediate or high NIA-Reagan score) was the most commonly observed neuropathology, followed by chronic microscopic and chronic gross infarcts. Hippocampal sclerosis and Lewy bodies were less common, but were still observed in more than 20% of the hemispheres. Subacute and acute infarcts (both microscopic and gross) were relatively rare, each observed in 11% or fewer of the hemispheres.
Table 2.
Selected demographic and neuropathologic characteristics of 371 cerebral hemispheres.
| Total number of subjects, n | 371 |
| Specimen source | |
| --Memory and Aging Project (MAP), n (%) | 229 (62%) |
| --Religious Orders Study (ROS), n (%) | 142 (38%) |
| MRI scanner, n (%) | |
| --GE | 95 (26%) |
| --Siemens | 88 (24%) |
| --Philips | 188 (51%) |
| Age at death, y (SD) | 89.6 (6.2) |
| Sex, n (%) | |
| --Male | 111 (30%) |
| --Female | 260 (70%) |
| Education, y (SD) | 15.9 (3.6) |
| Right hemisphere, n (%) | 175 (47%) |
| Postmortem interval to imaging, days (SD) | 46.9 (25.4) |
| NIA Reagan (AD pathology), n (%) | |
| --None or low | 126 (34%) |
| --Intermediate | 168 (45%) |
| --High | 77 (21%) |
| Lewy bodies, n (%) | |
| --Limbic | 33 (9%) |
| --Neocortical | 42 (11%) |
| --Total, any location | 75 (20%) |
| Gross infarcts, n (%) | |
| --Chronic, only one | 61 (16%) |
| --Chronic, more than one | 67 (18%) |
| --Subacute, only one | 42 (11%) |
| --Subacute, more than one | 11 (3%) |
| --Acute, only one | 15 (4%) |
| --Acute, more than one | 6 (2%) |
| Microscopic infarcts, n (%) | |
| --Chronic, only one | 70 (19%) |
| --Chronic, more than one | 38 (10%) |
| --Subacute, only one | 21 (6%) |
| --Subacute, more than one | 4 (1%) |
| --Acute, only one | 14 (4%) |
| --Acute, more than one | 3 (1%) |
| Hippocampal sclerosis, n (%) | 80 (22%) |
3.2 Association of T2 with Neuropathology
In the voxelwise regression analyses in which only one type of neuropathology was considered at a time, statistically significant associations with T2 were detected for the global summary score of AD pathology, chronic and acute gross infarcts, and hippocampal sclerosis. Therefore, these four types of pathology were entered in a combined regression model for further analysis. No regions with significant T2 alterations were observed in association with subacute gross infarcts, microscopic infarcts of any age, or Lewy bodies, after FDR adjustment of voxelwise p-values.
3.2.1 Alzheimer’s Disease
In the combined regression (multiple types of neuropathology considered simultaneously), the global AD pathology measure was associated with significant T2 alterations in eight separate clusters throughout the hemisphere (Table 3, Fig. 2). The largest two clusters exhibited T2 prolongation and included large portions of the white matter in all four lobes. The remaining six smaller clusters exhibited T2 shortening in the insula, caudate, globus pallidus, the medial portion of the thalamus, and around the edges of the hippocampus and temporal lobe.
Table 3.
Clusters of 100 mm3 or more with statistically significant T2 alterations associated with Alzheimer’s disease, gross infarcts, and hippocampal sclerosis, with all three types of neuropathology considered simultaneously.
| Cluster Identifier |
Principal Location | Volume (mm3) |
Mean T2 Alteration (ms) |
Minimum P-Value (unadjusted) |
|---|---|---|---|---|
| AD-1 a | Temporal, occipital, and parietal WMb | 11,320 | +4.4 | 2.0 × 10−4 |
| AD-2 | Frontal WM | 13,648 | +3.1 | 1.5 × 10−3 |
| AD-3 | Edge of temporal lobe | 114 | −5.8 | 5.0 × 10−4 |
| AD-4 | Edge of temporal lobe | 161 | −5.6 | 2.1 × 10−3 |
| AD-5 | Edge of hippocampus | 133 | −5.4 | 1.3 × 10−3 |
| AD-6 | Caudate, globus pallidus, medial thalamus | 594 | −2.5 | 1.5 × 10−3 |
| AD-7 | Insula | 263 | −2.8 | 2.9 × 10−3 |
| AD-8 | Insula | 311 | −3.1 | 1.5 × 10−3 |
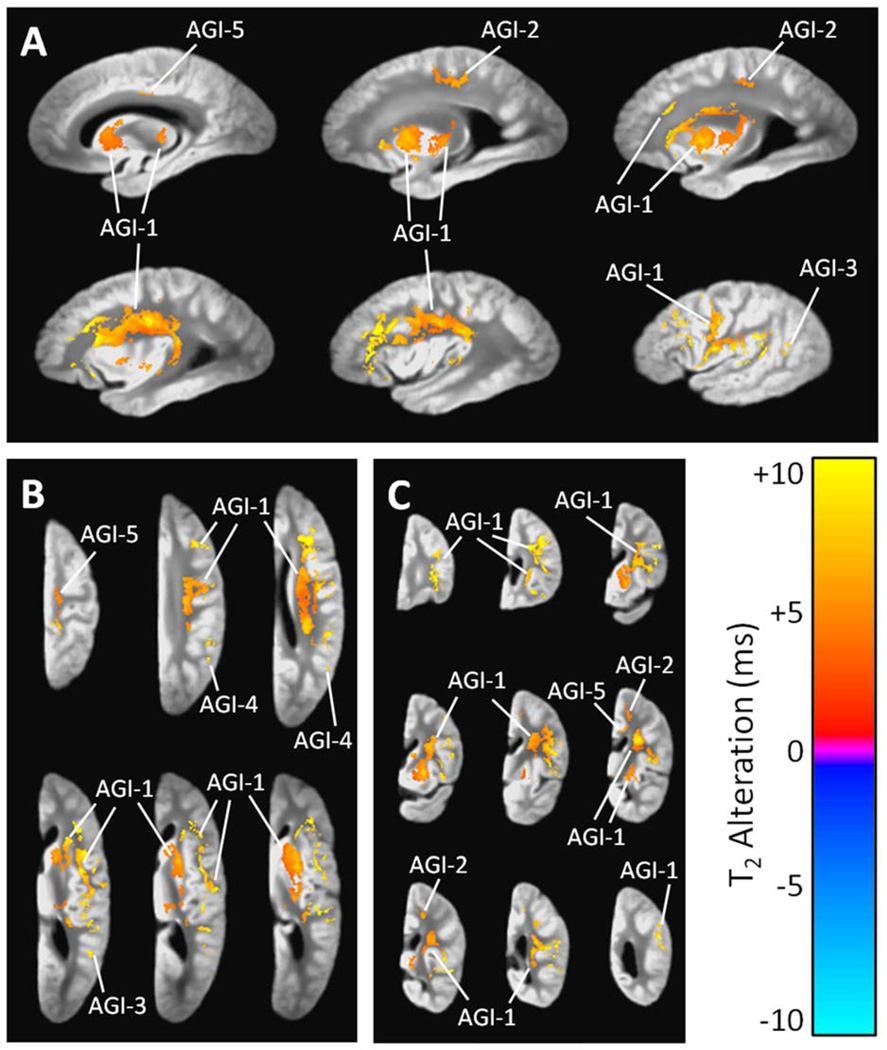
| CGI-1 c | Widespread | 76,716 | +2.7 | 4.3 × 10−3 |
| AGI-1 d | Frontal and parietal WM, putamen | 19,581 | +6.9 | 2.3 × 10−3 |
| AGI-2 | Frontal and parietal WM | 774 | +5.6 | 2.5 × 10−3 |
| AGI-3 | Occipital WM and GMb | 141 | +7.3 | 1.8 × 10−3 |
| AGI-4 | Occipital and parietal WM and GM | 136 | +8.1 | 7.5 × 10−4 |
| AGI-5 | Cingulum | 110 | +4.8 | 7.6 × 10−4 |
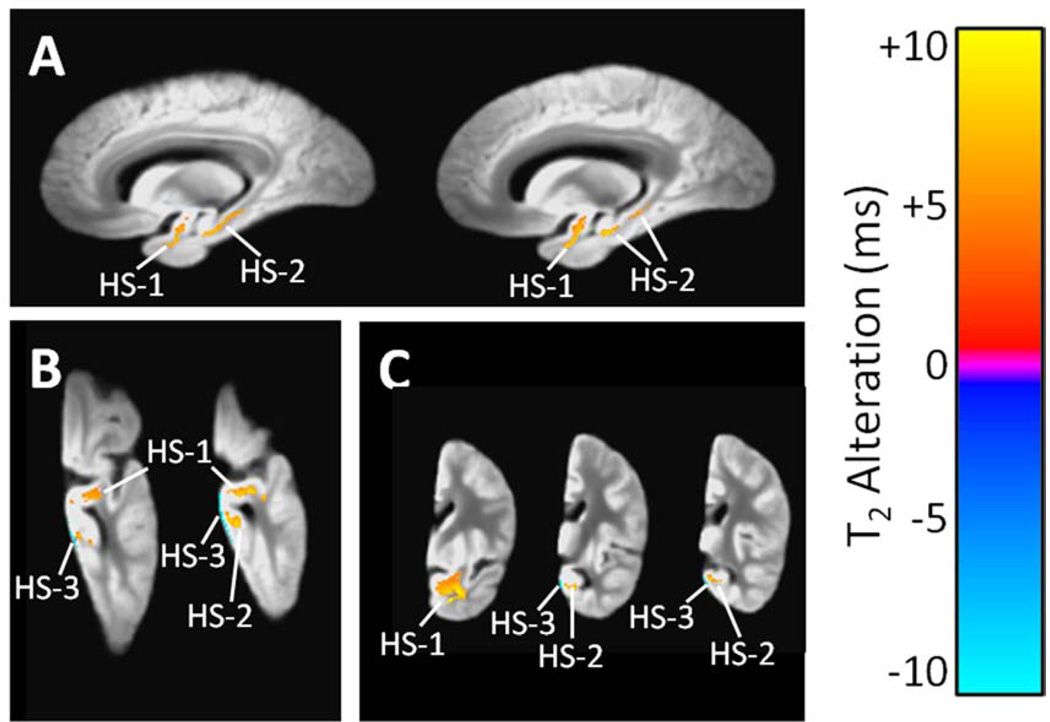
| HS-1 e | Temporal stem WM | 912 | +6.7 | 1.2 × 10−4 |
| HS-2 | Hippocampus and parahippocampal WM | 308 | +6.2 | 1.3 × 10−4 |
| HS-3 | Edge of temporal lobe | 123 | −9.1 | 6.0 × 10−6 |
AD clusters relate to the association of T2 with the global Alzheimer’s pathology score.
WM = white matter, GM = gray matter.
CGI cluster relates to the association of T2 with the number of chronic gross infarcts.
AGI clusters relate to the association of T2 with the number of acute gross infarcts.
HS clusters relate to the association of T2 with the presence of hippocampal sclerosis.
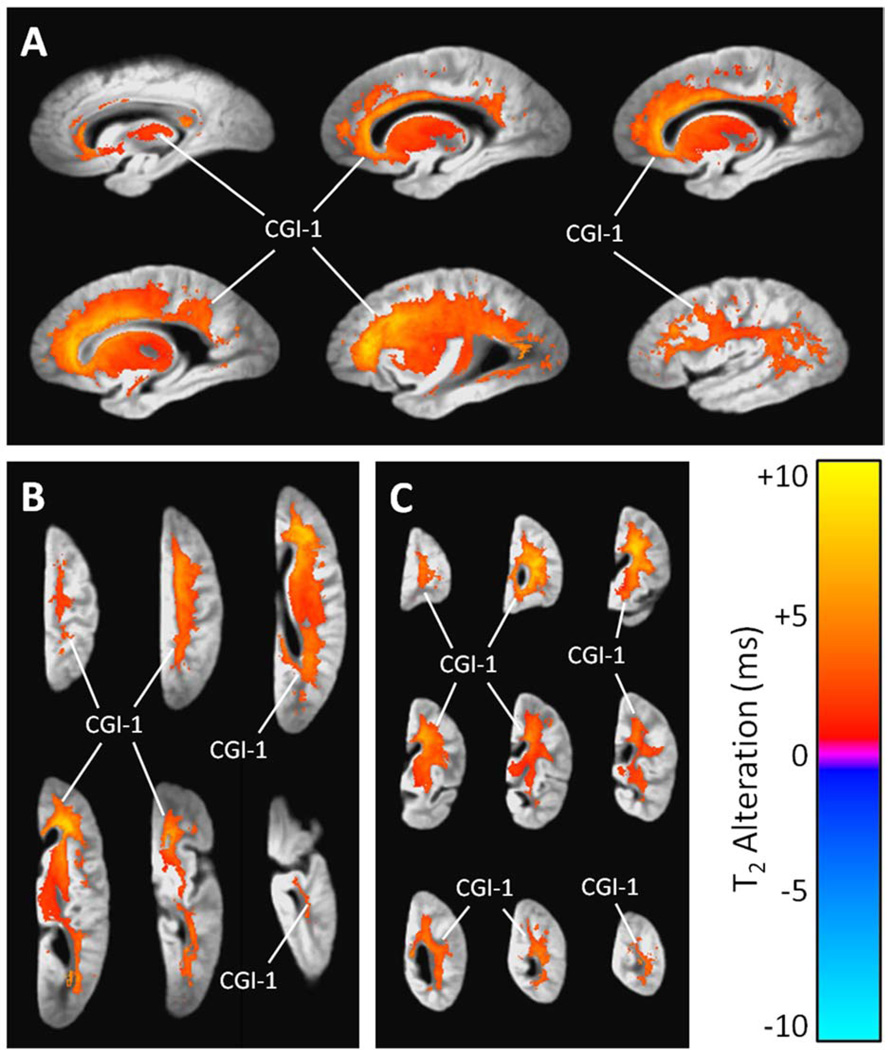
3.2.2 Chronic Gross Infarcts
Chronic gross infarcts were associated with a large cluster of significant T2 prolongation (Table 3, Fig. 3). This single continuous cluster engulfed nearly all of the frontal and parietal lobe white matter, sizable portions of the white matter in the occipital and temporal lobes, the putamen, the lateral globus pallidus, and parts of other regions, including the corpus callosum, thalamus, caudate, and medial globus pallidus.
Figure 3.
(A) Sagittal, (B) axial, and (C) coronal views showing regions of significant T2 prolongation and T2 shortening associated with the number of chronic gross infarcts per cerebral hemisphere, according to a combined linear regression analysis that simultaneously considered AD pathology, acute and chronic gross infarcts, and hippocampal sclerosis (and covariates). The grayscale underlay is the study-specific template (proton density-weighted). Alphanumeric labels are those cluster identifiers introduced in Table 3.
3.2.3 Acute Gross Infarcts
Acute gross infarcts were associated with T2 prolongation in five separate clusters (Table 3, Fig. 4). The largest cluster included sizable portions of the frontal and parietal lobe white matter, with confluence into the superior temporal white matter, putamen, caudate, and posterior portion of the thalamus. The remaining four clusters included a smaller portion of the frontal and parietal lobe white matter, tissue in or near the cingulum, and white and gray matter near the parietal-occipital border.
Figure 4.
(A) Sagittal, (B) axial, and (C) coronal views showing regions of significant T2 prolongation and T2 shortening associated with the number of acute gross infarcts per cerebral hemisphere, according to a combined linear regression analysis that simultaneously considered AD pathology, acute and chronic gross infarcts, and hippocampal sclerosis (and covariates). The grayscale underlay is the study-specific template (proton density-weighted). Alphanumeric labels are those cluster identifiers introduced in Table 3.
3.2.4 Hippocampal Sclerosis
Three clusters of significant T2 alteration were observed in association with hippocampal sclerosis (Table 3, Fig. 5). The two larger clusters exhibited T2 prolongation and included portions of the hippocampus, amygdala, parahippocampal white matter, and other white matter of the temporal lobe. The third cluster exhibited T2 shortening and was located at the inferior edge of the temporal lobe.
Figure 5.
(A) Sagittal, (B) axial, and (C) coronal views region of significant T2 prolongation associated with hippocampal sclerosis, according to a combined linear regression analysis that simultaneously considered AD pathology, acute and chronic gross infarcts, and hippocampal sclerosis (and covariates). The grayscale underlay is the study-specific template (proton density-weighted). Alphanumeric labels are those cluster identifiers introduced in Table 3.
3.3 Clinical Significance of T2 Alterations
For voxelwise regression analyses in which cognition was modeled as a function of T2 and neuropathologic indices, reduced performances in the episodic, semantic, and working memory domains were associated with prolonged values of T2, primarily in white matter. A single cluster of prolonged T2 in the temporal lobe white matter and amygdala (Fig. 6) accounted for 5.5% of the variance in episodic memory scores, beyond that explainable by demographics and the neuropathologic indices (Table 4, bottom three rows). Similarly, four significant clusters of prolonged T2 combined to account for 8.8% of the variance in semantic memory (Table 4). One of these clusters was located in the frontal lobe white matter, while the other three were located in the temporal lobe white matter (Fig. 6). T2 prolongation in a large frontal lobe white matter cluster and a smaller parietal lobe cluster accounted for 7.7% of the variance in the working memory scores (Table 4, Fig. 6). Significant associations of cognition with T2 were not observed in the domains of perceptual speed or visuospatial ability after FDR adjustment of p-values. These results were essentially unchanged after exclusion of those participants who had not undergone cognitive testing within one year of death (resulting n = 290, or 78% of the 371 total participants).
Table 4.
Variance in cognition explained by linear models.
| Cognitive domain | |||||
|---|---|---|---|---|---|
| Factors included in model |
Episodic Memory |
Semantic Memory |
Working Memory |
Perceptual Speed |
Visuospatial Ability |
| Demographics only | 4.7% | 2.2% | 2.7% | 5.5% | 10.6% |
| Demographics + AD | 23.6 | 14.4 | 14.3 | 12.4 | 16.6 |
| Demographics + gross infarcts | 6.8 | 5.0 | 5.1 | 7.6 | 10.7 |
| Demographics + micro infarcts | 5.0 | 2.8 | 3.1 | 7.1 | 10.7 |
| Demographics + HS | 12.5 | 10.4 | 4.7 | 10.1 | 12.4 |
| Demographics + Lewy bodies | 5.7 | 2.9 | 3.4 | 6.1 | 11.5 |
| Demographics + all pathology | 31.8 | 24.0 | 18.2 | 19.6 | 18.2 |
| Demographics + all pathology + ROI T2 f | 37.3 | 32.8 | 25.9 | NA g | NA g |
| Additional variance in cognition explained by ROI T2 | 5.5 | 8.8 | 7.7 | NA g | NA g |
All values are percentages. Each cognitive domain was assessed separately.
ROI T2 means the average value of T2 calculated over all voxels within a cluster detected as having significant association of cognition with T2 for a given domain. T2 for each ROI was included as a separate factor in the model.
Not applicable; there were no clusters detected as having significant associations of cognition with T2 for these domains.
3.4 Single Scanner Analysis
The entire set of voxelwise analyses was repeated using only the 188 hemispheres that were imaged on the Philips Achieva scanner (the largest single scanner subgroup, Table 2), and the results were similar to those of the full 371-hemisphere analysis (Supplementary Table 5). Notably, in the 188-hemisphere analysis, a cluster of AD-associated T2 prolongation was observed within the hippocampus proper, which did not reach the FDR-corrected significance threshold in the full 371-hemisphere analysis. Elsewhere, several clusters of significant T2 alteration from the full 371-hemisphere analysis appeared only as sub-significant trends in the lower-powered 188-hemisphere analysis.
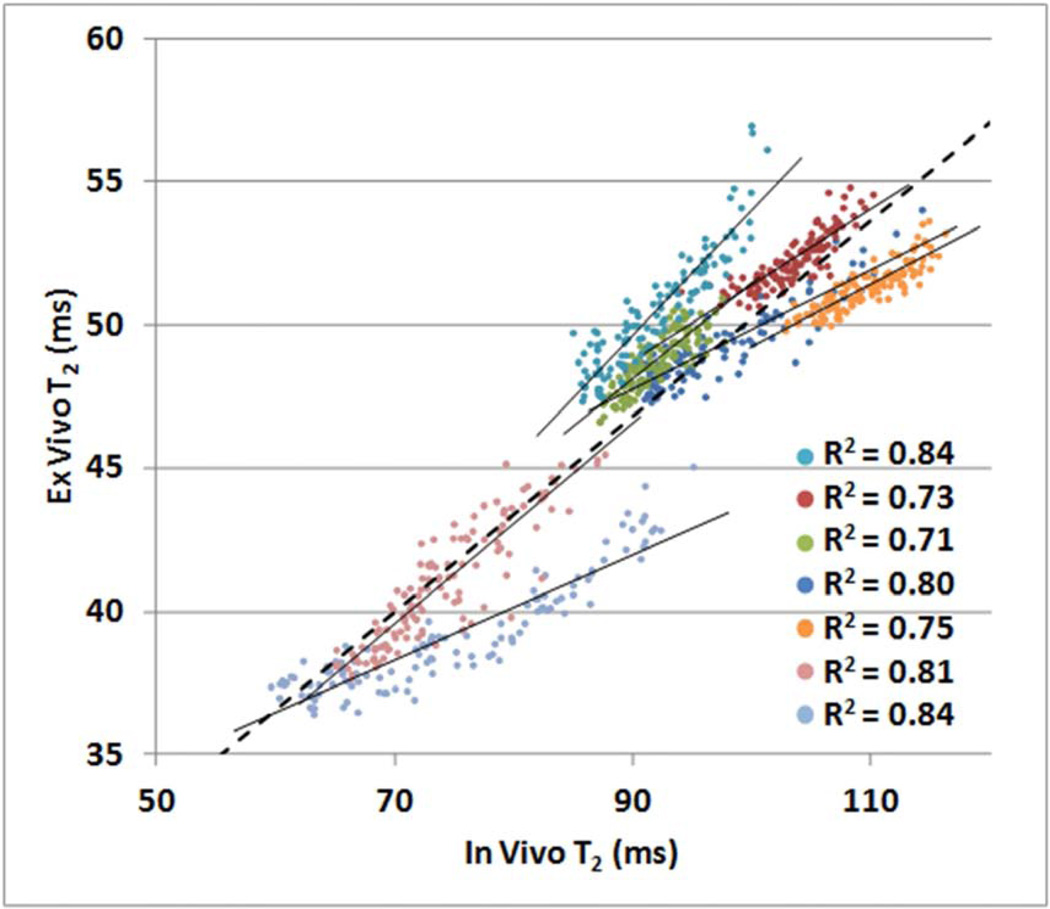
3.5 Generalization of Ex Vivo Findings
Ex vivo T2 values were approximately 50% of the corresponding in vivo values. The relationships between ex vivo and in vivo T2 per ROI are illustrated in Figure 7. Linear relationships with R2 values greater than 0.71 were detected in all cases (p < 10−15). When voxels from all seven regions were pooled in a single analysis, the regression line was described by the equation [ex vivo T2] = 0.34·[in vivo T2] + 16.0 ms, and the R2 value was 0.84 (p < 10−15).
Figure 7.
Ex vivo T2 values versus in vivo T2 values for corresponding voxels within seven regions of interest located in white matter and subcortical gray matter (one color per region). The mean ex vivo T2 value for a given voxel was calculated across 28 ex vivo hemispheres registered to a template. The mean in vivo T2 value for each voxel was calculated across 28 in vivo hemispheres registered to the same template. Slopes for all regression lines were significantly positive (R2 values displayed, p < 10−15 for all).
4 Discussion
The current study builds on previous research of AD-related T2 alterations by combining ex vivo brain MRI data with neuropathologic and antemortem cognitive measures. To our knowledge, this study represents the first cerebrum-wide analysis of T2 effects associated with the histopathology of AD as well as other types of neuropathology commonly found in the brain. In addition, findings regarding associations between cognition and T2 show that the T2 prolongations are clinically relevant.
4.1 T2 Alterations Related to AD
The white matter of the temporal lobe, particularly the temporal stem, exhibited prolonged T2 in association with AD pathology, as detected by the combined regression that also accounted for other types of neuropathology. In some studies that made similar investigations, AD-related prolongation of T2 in the temporal lobe white matter was detected in living individuals (House et al., 2006) and in unfixed postmortem tissue (Besson et al., 1992). In the latter case, the prolongation of T2 was accompanied by a significant increase in the tissue’s total water content, leading the authors to surmise that atrophy of the neural component of white matter is responsible for the change in relaxation times. Wallerian-type degeneration has indeed been observed in the temporal white matter of AD brains subjacent to the most severely diseased portion of the cortex (Englund, 1998). In addition, a multitude of diffusion weighted imaging (DWI) and diffusion tensor imaging (DTI) studies have reported increased diffusivity and decreased diffusion anisotropy in temporal lobe white matter of living individuals with clinically diagnosed AD, both of which indicate a reduction of barriers to diffusion (Arfanakis et al., 2007, Bozzali et al., 2002, Hanyu et al., 1998, Huang et al., 2007, Takahashi et al., 2002, Taoka et al., 2006, Wang et al., 2012, Xie et al., 2006).
T2 prolongation associated with AD was also observed in frontal lobe white matter. This has been reported previously (Bartzokis and Tishler, 2000, Bartzokis et al., 2003, House et al., 2006), though other studies addressing this issue did not detect any significant AD-related T2 alterations on postmortem MR (Besson et al., 1992, House et al., 2008). The diffuse pattern of this T2 prolongation, which was most intense toward the deep portions of the frontal white matter and tapered off toward the cortex, convincingly matches the description of white matter disease (WMD) observed in approximately 60% of AD brains (Brun and Englund, 1986, Englund, 1998). Ex vivo MRI has previously been shown to detect this type of white matter abnormality with relatively high sensitivity and specificity (Fernando et al., 2004), presumably owing to the correlation between T2 and severity of WMD (Englund et al., 1987). Partial loss of myelin or myelin components with a corresponding increase in the tissue’s water content is strongly implicated as the primary biophysical basis of such T2 prolongation (Englund et al., 1988, Roher et al., 2002, Soderberg et al., 1992). Interestingly, the neuropathologic and biochemical signatures of WMD, including glial changes (Sjobeck and Englund, 2003) and alterations in lipid and protein content (Englund et al., 1988, Roher et al., 2002, Soderberg et al., 1992), bear a striking resemblance to those of the transitional zones surrounding complete infarcts, leading several researchers to theorize that the frontal lobe WMD associated with AD may in fact lie on an axis between normal white matter and fully infarcted white matter (Englund et al., 1988, Sjobeck and Englund, 2003). Furthermore, the pattern of WMD often does not correlate with the spatial distribution of gray matter degeneration in AD (Englund et al., 1988, Roher et al., 2002). Taken together, this information suggests that the frontal lobe T2 prolongation observed in the current study may stem from a non-Wallerian mechanism of white matter degeneration, with much evidence pointing toward a vascular etiology (Englund et al., 1988, Englund, 1998, Roher et al., 2002, Sjobeck and Englund, 2003). This white matter degeneration, though common in the AD brain, may be decoupled from the disease’s hallmark gray matter pathology and degeneration, and as such, may contribute independently to cognitive decline (see Section 4.4).
AD-associated T2 prolongation was also widespread in the white matter of the parietal and occipital lobes, with apparent confluence into the posterior portion of the cingulum. To the best of our knowledge, such T2 prolongation in the cingulum has not been reported previously, though a number of DTI studies have shown disruption of diffusion anisotropy and mean diffusivity in the cingulum (especially in the posterior portion) of clinically diagnosed AD patients (Bozzali et al., 2012, Choo et al., 2010, Fellgiebel et al., 2005, Mielke et al., 2009, Takahashi et al., 2002, Zhang et al., 2007). Based on the findings of the current study and supporting DTI evidence from the literature, we surmise that the AD-associated T2 prolongation in parietal and occipital white matter (including cingulum) is brought about by pathologic processes that ultimately lead to an increase in the tissue’s free water content, possibly by the same mechanisms suspected in frontal white matter T2 prolongation. It is notable that deafferentation of cingulum fibers from the medial temporal lobe has recently been suggested as the progenitor of cingulum disruption in AD (Bozzali et al., 2012).
AD-related alteration of hippocampal T2 is alluring as a potential biomarker because of the structure’s important role in the development of the disease and has been reported in some (though not all) previous studies involving clinically diagnosed living participants (Arfanakis et al., 2007, Kirsch et al., 1992, Laakso et al., 1996, Wang et al., 2004). The results of the current study were inconclusive in this area, as hippocampal T2 prolongation was detected as a significant alteration in the 188-hemisphere, single scanner regression analysis, but only as a sub-significant trend in the full, 371-hemisphere analysis utilizing three different scanners. In both cases, the effect was positive in sign (i.e. T2 prolongation), indicating that at 3 T, hippocampal T2 marker of AD would likely stem from neuronal loss rather than a T2-shortening effect, such as an abnormal accumulation of iron.
T2 shortening in association with AD pathology was observed in several relatively small clusters within the cerebral hemisphere. One such cluster included portions of the caudate and globus pallidus. This is a notable finding, given that iron concentrations have been reported to be 78% higher in the globus pallidus of postmortem AD tissue compared to control specimens (Loeffler et al., 1995). Further, ferritin levels in the caudate, putamen, and globus pallidus are elevated in clinically diagnosed AD patients compared to individuals with normal cognition, as indicated by studies employing the field-dependent relaxation rate increase (FDRI) method of iron detection (Bartzokis et al., 1994, Bartzokis et al., 2000).
Significant AD-associated T2 shortening was also observed in the insula. This appears to be a unique finding not previously described in the literature, although decreased insular T2*-weighted signal has been reported in patients with mild cognitive impairment and AD (Rombouts et al., 2007). Plaques and tangles, the pathologic hallmarks of AD, and the accompanying iron species (Smith et al., 1997) could potentially be responsible for the observed insular T2 shortening effect. However, simple calculations show that plaques and tangles alone are unlikely to account for the entire difference in iron concentration in AD brains (House et al., 2008). Instead, it seems that most of the elevation in iron content and consequent T2 shortening of the tissue may stem from the increased iron concentration of the neuropil in AD (Lovell et al., 1998, Watt, 1996).
Finally, we also detected three ribbon-like clusters of AD-associated T2 shortening around the edges of the temporal lobe and hippocampus. Based on their location, shape, and large magnitude of T2 alteration, we speculate that these clusters may be attributable to partial volume effects at the borders of gray matter and non-tissue, rather than a genuine T2 shortening effect.
4.2 T2 Alterations Related to Gross Infarcts
Chronic gross infarcts were associated with the most extensive T2 alterations, by far, of any pathology that was investigated. The large cluster of T2 prolongation can be described as periventricular in nature, with confluence into deep parenchymal white matter. Although all lobes exhibited at least some T2 prolongation in white matter, the frontal lobe was the most severely affected region, both in terms of the magnitude of T2 alteration and the portion of tissue that was affected. The T2 prolongation associated with gross infarcts is not due to the presence of macroscopic fluid-filled spaces, since these were masked out of individual T2 maps, effectively removing them from analyses. Instead, the T2 prolongation detected in association with gross infarcts in the current study may be a manifestation of leukoaraiosis, or white matter rarefaction (observed originally on CT and later extended to MRI) (Inzitari, 2003). The observed pattern of infarct-related T2 prolongation is qualitatively similar to that seen on T2-weighted images of severe leukoaraiosis (Fazekas et al., 1993, Inzitari, 2003). While its underlying pathophysiology remains vague, previous research suggests that leukoaraiosis results from selective neuronal loss due to hypoxic but “incomplete” infarction (Auriel et al., 2011, Fernando et al., 2006). In this regard, it is possible that the infarct-related T2 prolongation shares a similar etiology with the AD-related T2 prolongation in the frontal lobe (see Section 4.1). Alternatively, the widespread T2 prolongation in white matter could be due to Wallerian degeneration following infarction, though the pattern appeared to be global, or diffuse, as opposed to tract-specific. The white matter T2 prolongation observed in association with acute gross infarcts should be interpreted with caution, since only 12 hemispheres (3% of the total sample) had acute gross infarcts without any accompanying chronic gross infarcts.
4.3 T2 Alterations Related to Hippocampal Sclerosis
HS is characterized by severe neuronal loss with gliosis in the cornu ammonis 1 (CA1) subregion of the hippocampus (Dickson et al., 1994), and previous MRI studies have shown a corresponding increase in T2 and T2-weighted signal intensity in sclerotic hippocampi (Berkovic et al., 1991, Bronen et al., 1991, Jackson et al., 1990, Jackson et al., 1993). In the current study, the combined regression analysis revealed significant HS-associated T2 prolongation, with one of the clusters located strikingly close to, if not within, the CA1 subregion. In addition, a cluster of significant T2 prolongation was detected in the anterior portion of the temporal lobe white matter, where there have been at least two reports of tissue abnormalities associated with HS (McMillan et al., 2004, Meiners et al., 2000).
4.4 Clinical Significance of T2 Alterations
To the best of our knowledge, this study is the first to show spatial distributions of T2 alterations associated with different types of neuropathology observed postmortem. These findings suggest that T2 alterations may serve as a useful biomarker of pathology, complementing neuropsychological assessment and other testing techniques to improve the accuracy of clinical diagnosis. In addition to its associations with pathology, T2 prolongation was shown to contribute a substantial percentage of variation in episodic, semantic, and working memory scores among participants – at least 5.5% in each domain (Table 4). These findings imply that the T2 prolongation may reflect underlying pathologic processes not already captured by neuropathologic assessment. Conversely, the lack of significant association between T2 shortening and cognition suggests that any related cognitive deficits were largely explained by the direct measures of neuropathology. Because of the methodology for this portion of the analysis, one concern is that worsening of neuropathology or other age-related events occurring between the final cognitive assessment and death may have affected the prominence of associations between cognition and ex vivo T2. However, this interval was relatively short on average, and results of a secondary analysis that excluded the 22% of participants who had a lag of more than one year between cognitive assessment and death were unremarkable compared to the full analysis.
4.5 Generalization of Ex Vivo Findings
Based on previous work, we anticipated ex vivo T2 values in fixed brain tissue to be approximately 50% of those found in vivo (Dawe et al., 2009), and this was supported by the comparison of 28 in vivo T2 maps against 28 ex vivo T2 maps from matched participants. Furthermore, this comparison revealed positive correlations between in vivo and ex vivo T2 in several brain regions, indicating that findings of the current analysis may potentially be generalized to the in vivo case. As suggested by Figure 7, the precise relationship between ex vivo T2 and in vivo T2 is likely to vary across regions. Yet, because voxelwise analysis allows for spatial variation, regional differences in the in vivo-ex vivo T2 relationship do not preclude the generalization of ex vivo findings to the in vivo case. Additional investigation using in vivo and ex vivo MRI data from the same individuals is necessary to accurately describe the in vivo-ex vivo T2 relationship throughout the brain.
4.6 Strengths and Limitations
This study employed a voxel-based approach, rather than an ROI-based approach, facilitating detection of T2 alterations related to AD and other pathologies throughout the cerebral hemisphere. As such, spatial normalization of individual hemispheres was required. Despite ART’s ranking as one of the top neuroimaging registration tools (Klein et al., 2009), differences in hemisphere anatomy, shape, size, and contrast preclude perfect spatial normalization, potentially contributing to false positives and false negatives, a limitation of the current work.
Three different scanners and pulse sequences were used to acquire T2 data. This was necessary because of the ongoing nature of postmortem MRI data collection – the 371 hemispheres included in the full analysis were imaged over a span of approximately five years. In the voxelwise regression models, we accounted for a possible constant offset in T2 values among the three scanners and pulse sequences, but we did not include any interaction terms to model, for example, scanner-specific disease effects. We recognized that using a single scanner and pulse sequence would be the most reliable method of eliminating scanner-to-scanner variation in T2 values. Therefore, we repeated the entire analysis using only the 188 hemispheres that were imaged on the Philips Achieva scanner (the largest subgroup, see Table 2), and the results were overwhelmingly similar to the full 371-hemisphere analysis.
Finally, laterality was not a primary consideration of the current work, as left and right cerebral hemispheres were combined in the analyses in order to maximize degrees of freedom. Left-right asymmetry in the substrates of pathology, cognition, and T2 alterations could have obscured otherwise significant associations among those factors. Therefore, we repeated the analysis for left and right hemispheres separately. Significant T2 associations with pathology were similar between left and right hemispheres, though less extensive than those revealed by the full analysis, probably due to the lower power. T2 associations with cognition (after controlling for pathology) were rendered largely non-significant in the separate left-right analyses. However, sub-significant trends did show a degree of laterality, especially in the semantic memory domain, suggesting that further investigation in this area is warranted.
Supplementary Material
Acknowledgments
We thank the participants of the Rush Memory and Aging Project and the Religious Orders Study. We also thank the reviewers of this manuscript for their valuable comments.
Funding
This work was supported by the National Institute on Aging [grant numbers P30 AG10161, R01 AG15819, and R01 AG17917] and by the Alzheimer’s Disease Research Fund of the Illinois Department of Public Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J. Neurosci. Methods. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Gui M, Tamhane A, Carew J. Investigating the Medial Temporal Lobe in Alzheimer’s Disease and Mild Cognitive Impairment, with Turboprop Diffusion Tensor Imaging, MRI-volumetry, and T2-relaxometry. Brain Imaging Behav. 2007;1:11–21. [Google Scholar]
- Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann. Neurol. 2011;69:320–327. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriel E, Bornstein NM, Berenyi E, Varkonyi I, Gabor M, Majtenyi K, Szepesi R, Goldberg I, Lampe R, Csiba L. Clinical, radiological and pathological correlates of leukoaraiosis. Acta Neurol. Scand. 2011;123:41–47. doi: 10.1111/j.1600-0404.2010.01341.x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch. Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Cummings J, Holt LE, Hance DB, Henderson VW, Mintz J. In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Arch. Gen. Psychiatry. 2000;57:47–53. doi: 10.1001/archpsyc.57.1.47. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Mintz J, Holt LE, Marx P, Phelan CK, Marder SR. In vivo evaluation of brain iron in Alzheimer's disease and normal subjects using MRI. Biol. Psychiatry. 1994;35:480–487. doi: 10.1016/0006-3223(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA. MRI evaluation of basal ganglia ferritin iron and neurotoxicity in Alzheimer's and Huntingon's disease. Cell. Mol. Biol. (Noisy-le-grand) 2000;46:821–833. [PubMed] [Google Scholar]
- Bennett DA, De Jager PL, Leurgans SE, Schneider JA. Neuropathologic intermediate phenotypes enhance association to Alzheimer susceptibility alleles. Neurology. 2009;72:1495–1503. doi: 10.1212/WNL.0b013e3181a2e87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr. Alzheimer Res. 2012a;9:628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr. Alzheimer Res. 2012b;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovic SF, Andermann F, Olivier A, Ethier R, Melanson D, Robitaille Y, Kuzniecky R, Peters T, Feindel W. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Ann. Neurol. 1991;29:175–182. doi: 10.1002/ana.410290210. [DOI] [PubMed] [Google Scholar]
- Besson JA, Best PV, Skinner ER. Post-mortem proton magnetic resonance spectrometric measures of brain regions in patients with a pathological diagnosis of Alzheimer's disease and multi-infarct dementia. Br. J. Psychiatry. 1992;160:187–190. doi: 10.1192/bjp.160.2.187. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry. 2002;72:742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Giulietti G, Basile B, Serra L, Spano B, Perri R, Giubilei F, Marra C, Caltagirone C, Cercignani M. Damage to the cingulum contributes to alzheimer's disease pathophysiology by deafferentation mechanism. Hum. Brain Mapp. 2012;33:1295–1308. doi: 10.1002/hbm.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronen RA, Cheung G, Charles JT, Kim JH, Spencer DD, Spencer SS, Sze G, McCarthy G. Imaging findings in hippocampal sclerosis: correlation with pathology. AJNR Am. J. Neuroradiol. 1991;12:933–940. [PMC free article] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann. Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Choo IH, Lee DY, Oh JS, Lee JS, Lee DS, Song IC, Youn JC, Kim SG, Kim KW, Jhoo JH, Woo JI. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2010;31:772–779. doi: 10.1016/j.neurobiolaging.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One. 2011;6:e26286. doi: 10.1371/journal.pone.0026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe RJ, Bennett DA, Schneider JA, Vasireddi SK, Arfanakis K. Postmortem MRI of human brain hemispheres: T2 relaxation times during formaldehyde fixation. Magn. Reson. Med. 2009;61:810–818. doi: 10.1002/mrm.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement. Geriatr. Cogn. Disord. 1998;9(Suppl 1):6–12. doi: 10.1159/000051183. [DOI] [PubMed] [Google Scholar]
- Englund E, Brun A, Alling C. White matter changes in dementia of Alzheimer's type. Biochemical and neuropathological correlates. Brain. 1988;111(Pt 6):1425–1439. doi: 10.1093/brain/111.6.1425. [DOI] [PubMed] [Google Scholar]
- Englund E, Brun A, Persson B. Correlations between histopathologic white matter changes and proton MR relaxation times in dementia. Alzheimer Dis. Assoc. Disord. 1987;1:156–170. doi: 10.1097/00002093-198701030-00008. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Muller MJ, Wille P, Dellani PR, Scheurich A, Schmidt LG, Stoeter P. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol. Aging. 2005;26:1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fernando MS, O'Brien JT, Perry RH, English P, Forster G, McMeekin W, Slade JY, Golkhar A, Matthews FE, Barber R, Kalaria RN, Ince PG Neuropathology Group of MRC CFAS. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol. Appl. Neurobiol. 2004;30:385–395. doi: 10.1111/j.1365-2990.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O'Brien JT, Ince PG MRC Cognitive Function and Ageing Neuropathology Study Group. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Sakurai H, Iwamoto T, Takasaki M, Shindo H, Abe K. Diffusion-weighted MR imaging of the hippocampus and temporal white matter in Alzheimer's disease. J. Neurol. Sci. 1998;156:195–200. doi: 10.1016/s0022-510x(98)00043-4. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Walimuni IS, Kramer LA, Frye RE. Human brain atlas-based volumetry and relaxometry: application to healthy development and natural aging. Magn. Reson. Med. 2010;64:1382–1389. doi: 10.1002/mrm.22515. [DOI] [PubMed] [Google Scholar]
- House MJ, St Pierre TG, Foster JK, Martins RN, Clarnette R. Quantitative MR imaging R2 relaxometry in elderly participants reporting memory loss. AJNR Am. J. Neuroradiol. 2006;27:430–439. [PMC free article] [PubMed] [Google Scholar]
- House MJ, St Pierre TG, McLean C. 1.4T study of proton magnetic relaxation rates, iron concentrations, and plaque burden in Alzheimer's disease and control postmortem brain tissue. Magn. Reson. Med. 2008;60:41–52. doi: 10.1002/mrm.21586. [DOI] [PubMed] [Google Scholar]
- Huang J, Friedland RP, Auchus AP. Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am. J. Neuroradiol. 2007;28:1943–1948. doi: 10.3174/ajnr.A0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari D. Leukoaraiosis: an independent risk factor for stroke? Stroke. 2003;34:2067–2071. doi: 10.1161/01.STR.0000080934.68280.82. [DOI] [PubMed] [Google Scholar]
- Jackson GD, Berkovic SF, Duncan JS, Connelly A. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR Am. J. Neuroradiol. 1993;14:753–762. [PMC free article] [PubMed] [Google Scholar]
- Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GC, Bladin PF. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869–1875. doi: 10.1212/wnl.40.12.1869. [DOI] [PubMed] [Google Scholar]
- Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage. 2004;23(Suppl 1):S151–S160. doi: 10.1016/j.neuroimage.2004.07.068. [DOI] [PubMed] [Google Scholar]
- Kirsch SJ, Jacobs RW, Butcher LL, Beatty J. Prolongation of magnetic resonance T2 time in hippocampus of human patients marks the presence and severity of Alzheimer's disease. Neurosci. Lett. 1992;134:187–190. doi: 10.1016/0304-3940(92)90513-7. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Delshad S, Woo MA, Macey PM, Harper RM. Age-related regional brain T2-relaxation changes in healthy adults. J. Magn. Reson. Imaging. 2012;35:300–308. doi: 10.1002/jmri.22831. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Partanen K, Soininen H, Lehtovirta M, Hallikainen M, Hanninen T, Helkala EL, Vainio P, Riekkinen PJS. MR T2 relaxometry in Alzheimer's disease and age-associated memory impairment. Neurobiol. Aging. 1996;17:535–540. doi: 10.1016/0197-4580(96)00036-x. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, Connor JR, Juneau PL, Snyder BS, Kanaley L, DeMaggio AJ, Nguyen H, Brickman CM, LeWitt PA. Transferrin and iron in normal, Alzheimer's disease, and Parkinson's disease brain regions. J. Neurochem. 1995;65:710–724. doi: 10.1046/j.1471-4159.1995.65020710.x. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J. Alzheimers Dis. 2006;9:417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- McMillan AB, Hermann BP, Johnson SC, Hansen RR, Seidenberg M, Meyerand ME. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage. 2004;23:167–174. doi: 10.1016/j.neuroimage.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Meiners LC, van der Grond J, van Rijen PC, Springorum R, de Kort GA, Jansen GH. Proton magnetic resonance spectroscopy of temporal lobe white matter in patients with histologically proven hippocampal sclerosis. J. Magn. Reson. Imaging. 2000;11:25–31. doi: 10.1002/(sici)1522-2586(200001)11:1<25::aid-jmri4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Kozauer NA, Chan KC, George M, Toroney J, Zerrate M, Bandeen-Roche K, Wang MC, Vanzijl P, Pekar JJ, Mori S, Lyketsos CG, Albert M. Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2009;46:47–55. doi: 10.1016/j.neuroimage.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Weiss N, Kokjohn TA, Kuo YM, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, Emmerling M, Goux W, Beach T. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer's disease. Biochemistry. 2002;41:11080–11090. doi: 10.1021/bi026173d. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Scheltens P, Kuijer JP, Barkhof F. Whole brain analysis of T2* weighted baseline FMRI signal in dementia. Hum. Brain Mapp. 2007;28:1313–1317. doi: 10.1002/hbm.20349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann. Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- Sjobeck M, Englund E. Glial levels determine severity of white matter disease in Alzheimer's disease: a neuropathological study of glial changes. Neuropathol. Appl. Neurobiol. 2003;29:159–169. doi: 10.1046/j.1365-2990.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg M, Edlund C, Alafuzoff I, Kristensson K, Dallner G. Lipid composition in different regions of the brain in Alzheimer's disease/senile dementia of Alzheimer's type. J. Neurochem. 1992;59:1646–1653. doi: 10.1111/j.1471-4159.1992.tb10994.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci. Lett. 2002;332:45–48. doi: 10.1016/s0304-3940(02)00914-x. [DOI] [PubMed] [Google Scholar]
- Taoka T, Iwasaki S, Sakamoto M, Nakagawa H, Fukusumi A, Myochin K, Hirohashi S, Hoshida T, Kichikawa K. Diffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer disease: evaluation of the "tract of interest" by diffusion tensor tractography. AJNR Am. J. Neuroradiol. 2006;27:1040–1045. [PMC free article] [PubMed] [Google Scholar]
- The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol. Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- Wang H, Yuan H, Shu L, Xie J, Zhang D. Prolongation of T(2) relaxation times of hippocampus and amygdala in Alzheimer's disease. Neurosci. Lett. 2004;363:150–153. doi: 10.1016/j.neulet.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Wang Y, West JD, Flashman LA, Wishart HA, Santulli RB, Rabin LA, Pare N, Arfanakis K, Saykin AJ. Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim. Biophys. Acta. 2012;1822:423–430. doi: 10.1016/j.bbadis.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. Nuclear microscope analysis in Alzheimer's and Parkinson's disease: A review. Cell. Mol. Biol. (Noisy-le-grand) 1996;42:17–26. [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66:1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.