Abstract
The small intestine plays a fundamentally important role in regulating whole body cholesterol balance and plasma lipoprotein composition. This is articulated through the interplay of a constellation of genes that ultimately determines the net amount of chylomicron cholesterol delivered to the liver. Major advances in our insights into regulation of the cholesterol absorption pathway have been made using genetically manipulated mouse models and agents such as ezetimibe. One unresolved question is how a sustained pharmacological inhibition of intestinal cholesterol synthesis in vivo may affect cholesterol handling by the absorptive cells. Here we show that the lanosterolcyclase inhibitor, Ro 48-8071, when fed to BALB/c mice in a chow diet (20 mg/day/kg body weight), leads to a rapid and sustained inhibition (>50%) of cholesterol synthesis in the whole small intestine. Sterol synthesis was also reduced in the large intestine and stomach. In contrast, hepatic cholesterol synthesis, while markedly suppressed initially, rebounded to higher than baseline rates within 7 days. Whole body cholesterol synthesis, fractional cholesterol absorption, and fecal neutral and acidic sterol excretion were not consistently changed with Ro 48-8071 treatment. There were no discernible effects of this agent on intestinal histology as determined by H&E staining and the level of Ki67, an index of proliferation. The mRNA expression for multiple genes involved in intestinal cholesterol regulation including NPC1L1 was mostly unchanged although there was a marked rise in the mRNA level for the PXR target genes CYP3A11 and CES2A.
Keywords: cholesterol absorption, enterocyte, statins, liver, ezetimibe
1. Introduction
The importance of fully understanding the mechanisms that regulate intestinal cholesterol metabolism resides largely in the fact that the delivery of intestinally-derived cholesterol to the liver has a major impact on intrahepatic cholesterol handling. This is articulated through the interplay of multiple pathways that together dictate cholesterol balance across the liver and profoundly influence plasma lipoprotein composition [1–4]. In addition to being the site for absorbing cholesterol, the small intestine plays a key role in preserving the circulating bile acid pool, and it also actively synthesizes cholesterol and clears low density lipoprotein-cholesterol (LDL-C) from the plasma [5–9]. During the past decade there have been major advances in our understanding, at a molecular level, of how these various processes, particularly the sterol absorption pathway, are regulated. This has been achieved through utilization of genetically manipulated mouse models together with novel agents such as ezetimibe and also plant sterols and stanols [10–21].These endeavors have helped make the pharmacologic control of cholesterol absorption an increasingly attractive target for the management of dyslipidemia in the general population [22].
Despite this progress, the question of how statins, competitive inhibitors of hydroxymethylglutaryl coenzyme A reductase, the rate-limiting enzyme in cholesterol biosynthesis [23], affect intestinal cholesterol homeostasis remains largely unanswered. Several studies in humans suggest that statin monotherapy may cause a compensatory increase in cholesterol absorption [24–26]. However, some of these data are ambiguous or show only marginal changes in the key parameters measured [27, 28].The more immediate but unresolved question concerns the extent to which intestinal cholesterol synthesis is inhibited by statin monotherapy. One study using human enterocytes treated with lovastatin demonstrated a significant inhibition of labeled acetate incorporation into sterols [29]. Another study using a miniature pig model found no effect of two statins on the mRNA level for HMGCoA reductase in small intestine [30]. Unfortunately, the impact of statins on cholesterol synthesis and related pathways in the intestine cannot be investigated in vivo using more conventional animal models such as the mouse or hamster because of their adverse effects in these species, especially at higher doses [31, 32]. Moreover, statin treatment in such models results in a paradoxical upregulation of cholesterol synthesis, at least in the liver [31–33].
Starting from about the time that statin monotherapy emerged as a major therapeutic option for treating hypercholesterolemia in humans, another class of sterol synthesis inhibitors that act more distally than do statins in the biosynthetic pathway was developed and tested to varying degrees in an array of animal models and in vitro systems. These compounds were designed to specifically inhibit 2,3-oxidosqualene:lanosterol cyclase (OSC) which catalyzes the cyclization of monooxidosqualene to lanosterol [32, 34–36]. At their optimum dose, these inhibitors not only potentially suppress cholesterol synthesis, but also increase the generation of 24(S),25-epoxycholesterol, a potent ligand activator of the liver X receptor (LXR) [37]. Hypothetically, this dual mechanism of action should make these compounds potent cholesterol lowering agents. The efficacy and safety of those OSC inhibitors for which published data are available vary considerably [32, 34, 38].
Our exploratory studies with the OSC inhibitor Ro 48-8071 revealed a selective inhibition of cholesterol synthesis, mainly in the small intestine, in the mouse and hamster. This prompted a detailed investigation of the effects of this compound on intestinal cholesterol homeostasis at a biochemical and molecular level, as well as on whole body cholesterol metabolism, principally in BALB/c mice maintained on a conventional rodent chow diet. Together, these data suggest that Ro 48-8071 may be a useful tool for further elucidation of the mechanisms that collectively regulate cellular cholesterol content and flux in the mucosa of the small intestine.
2. Materials and Methods
2.1. Animals and diets
BALB/c mice were generated in our own colony that was originally established using breeding stock from The Jackson Laboratory (Bar Harbor, ME). LDLR-deficient (ldlr−/−) mice and matching ldlr+/+ controls, all on a 129/Sv background, were bred in our facility from breeding stock generously provided by Dr. Joachim Herz at this center [5]. BALB/c mice were the primary model for this project because this was the dominant strain in our laboratory when we started testing Ro 48-8071 and not because of any particular metabolic feature that made this strain a superior choice. Male Golden Syrian hamsters (Mesocricetusauratus) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN). The mice and hamsters were housed in separate rooms with alternating 12-h of light and darkness as described [8, 15]. Except in the study with hamsters, and in two experiments with mice involving stool collections, all animals were group housed. The gender and age of the mice varied in different studies as specified. The hamsters were ~12 weeks old at the time study. In all experiments a cereal-based rodent chow diet (No. 7001, Harlan Teklad, Madison, WI), with an inherent cholesterol content of ~0.02% w/w, was used. This regimen was defined as the basal diet. In one study with BALB/c mice, the dietary cholesterol content was raised to 1.0% w/w. All experimental diets were fed in powder form. Treatment with Ro 48-8071 or with simvastatin was achieved by the inclusion of these agents in the diets of the mice and hamsters which were fed ad libitum in every case. Ro 48-8071 (fumarate salt, MW 564.4) was purchased from either Sigma-Aldrich Corp. (R2278) or Enzo Life Sciences, Inc. (BML-E1342). Crystals of this compound were mixed into a small quantity of the basal diet powder using a mortar and pestle. This premix was then incorporated into the remaining quantity of basal diet using a food mixer (Hobart Corp.). To achieve a dose of ~20 mg/day/kg bw, 125 mg of Ro 48-8071 was incorporated into 1000 g of the basal diet. This formulation was based on an average daily intake of the basal diet of 160 g/day/kg bw by young adult mice of various strains in this laboratory. The dose of Ro 48-8071 in an initial experiment was set at 0, 5, 10, 15, and 20 mg/day/kg bw. Thereafter, it was either 15 or 20 mg/day/kg bw as specified. For the experiment with hamsters, a dose of 20 mg/day/kg bw was used. Ezetimibe (gift from Merck & Co.) was used at a dose of ~20 mg/day/kg bw. Simvastatin (Merck & Co.) was tested at 20 and 200 mg/day/kg bw. These diets were prepared the same way as those containing Ro 48-8071. The period of feeding of the different diets ranged from 5 to 21 days as specified. Body weights were recorded before, during, and at the end of each study. All animals had unrestricted access to their respective diet and water, and were in the fed state at the time of study. All studies were approved by the Institutional Animal Care and Use Committee of The University of Texas Southwestern Medical Center.
2.2. Rates of cholesterol and fatty acid synthesis in small intestine, liver and other organs
These rates were measured in vivo using [3H] water as detailed elsewhere [39]. One h after the mice were administered ~40 mCi of [3H] water intraperitoneally, the liver, whole small intestine, stomach, large intestine, spleen, kidneys, and lungs were removed, rinsed, blotted, and weighed. They were then saponified and the labeled sterols extracted and quantitated as described [39]. The rate of cholesterol synthesis in each organ was calculated as nmol of [3H] water incorporated into sterols/h/g wet weight of tissue. In one study the rate of sterol synthesis in the whole animal was measured by digestion of the entire carcass followed by isolation of the labeled sterols. In this case the rate of synthesis is expressed as the µmol of [3H] water incorporated into sterols/h/100 g bw. In the Ro 48-8071 dose-response experiment, the rate of fatty acid synthesis in the small intestine and liver was also measured [39] and expressed as the µmol of [3H] water incorporated into fatty acids/h/g tissue
2.3. Intestinal cholesterol absorption and rates of fecal neutral sterol and bile acid excretion
The experiments comparing the effects of Ro 48-8071 with those of ezetimibeon multiple parameters of intestinal and hepatic cholesterol metabolism included the measurement of fractional cholesterol absorption, as well as the rates of fecal neutral and acidic sterol excretion. In a separate study, fecal bile acid excretion rates were measured in the same group of mice, initially while they were given the basal diet alone, and again later during treatment with Ro 48-8071. The details of the methods for fecal bile acid and neutral sterol quantitation have been described earlier [39, 40].
2.4. Concentrations of total, unesterified and esterified cholesterol in liver, small intestine, and plasma
Aliquots of liver, plasma, and the whole small intestine were saponified and extracted and their total cholesterol concentrations were measured by gas chromatography using stigmastanol as an internal standard [39]. In some studies, aliquots of liver and the whole small intestine were extracted in chloroform:methanol (2:1 v/v) for the measurement of unesterified and esterified cholesterol concentrations as described [15]. In one study plasma alanine aminotransferase (ALT) activity was measured by a commercial laboratory.
2.5. Histology and immunohistochemistry
The entire small intestine was removed, rinsed with ice-cold phosphate-buffered saline (PBS) and divided into three sections of similar length. A 1cm proximal portion of the second section was fixed in ice-cold 10% neutral buffered formalin before being processed into paraffin blocks according to standard procedures. Tissue sections (5µm) were either stained using hematoxylin and eosin (H&E) for histological analysis, or were used for immunohistochemistry. For Ki67 detection, anti-mouse Ki67 antibody (Vector Laboratory, Burlingame, CA,Cat#VP-K452, 1:300) was used as primary antibody followed by a mouse on mouse block/biotinylated secondary kit (Vector Laboratory, Burlingame, CA,Cat#BMK-2202, 1:250) and Fluoresce in Avid in DCS Tertiary reagent (Vector Laboratory, Burlingame, CA, Cat# A-2011, 1:60).
2.6. Relative mRNA expression analysis
Whole small intestines were flushed with ice-cold PBS, opened longitudinally, and the mucosae were removed by gentle scraping. These scrapings were quickly frozen in liquid nitrogen. Total RNA was isolated and mRNA levels were measured using a quantitative real-time PCR assay as previously described [8]. All analyses were determined by the comparative cycle number at threshold method (User Bulletin No. 2, Perkin- Elmer Life Sciences) with cyclophilin as the internal control. A prior evaluation of multiple housekeeping genes showed cyclophilin to be the best choice because its expression level in the intestine across all treatments was the most constant. Relative mRNA levels in individual animals were determined by expressing the amount of mRNA found relative to that obtained for mice given the basal diet alone, which in each case was arbitrarily set at 1.0. In the study involving the feeding of a high-cholesterol diet, aliquots of liver were taken for the measurement of the mRNA level for two enzymes involved in bile acid synthesis, cholesterol 7α-hydroxylase (CYP7A1) and sterol 27-hydroxylase (CYP27A1). The primer sequences used to measure all mRNA levels are given in Table 1.
Table 1.
Primer sequences used for analyzing mouse intestinal mRNA expression
| Gene | Gene name | Accession | Primer sequence Forward |
Reverse |
|---|---|---|---|---|
| Abca1 | ATP-binding cassette A1 | NM_013454 | CGTTTCCGGGAAGTGTCCTA | TAGAGATGACAAGGAGGATGGA |
| Abcg5 | ATP-binding cassette G5 | NM_031884 | TGGATCCAACACCTCTATGCTAAA | GGCAGGTTTTCTCGATGAACTG |
| Abcg8 | ATP-binding cassette G8 | NM_026180 | TGCCCACCTTCCACATGTC | ATGAAGCCGGCAGTAAGGTAGA |
| Casp3 | Caspase 3 | NM_009810 | CATAAGAGCACTGGAATGTCATCTC | CCCATGAATGTCTCTCTGAGGTT |
| Casp8 | Caspase 8 | NM_009812 | CCTGAGGGAAAGATGTCCTCAA | GTCGTCTTTATTGCTCACGTCATAG |
| Ces2a | Carboxylesterase 2A | NM_133960 | CTCACAGCCGGCCATGT | AGATTCATTTCCTTCGCATCCT |
| Cyp3a11 | Cytochrome P450, family 3, subfamily a, polypeptide 11 | NM_007818 | AAACTGCAGGATGAGATCGATGA | TCCAGGTATTCCATCTCCATCAC |
| Cyp7a1 | Cholesterol 7α-hydroxylase | NM_009696 | AGCAACTAAACAACCTGCCAGTACTA | GTCCGGATATTCAAGGATGCA |
| Cyp27a1 | Sterol 27-hydroxylase | AK004977 | GCCTCACCTATGGGATCTTCA | TCAAAGCCTGACGCAGATG |
| Cyp51 | Sterol 14α-demethylase | NM_020010 | GCCGTGGGCCACATG | TGGAGAGTAAATATGTGGTGGACTT |
| Cyclophilin | Cyclophilin | M60456 | TGGAGAGCACCAAGACAGACA | TGCCGGAGTCGACAATGAT |
| Hmgcs | Hydroxymethylglutaryl coenzyme A synthase | NM_145942 | GCCGTGAACTGGGTCGAA | GCATATATAGCAATGTCTCCTGCAA |
| Idol | Inducible degrader of the LDL-receptor | NM_153789 | TGTGCTATGTGACGAGGCCG | CCTGCACACCTGGTTGAGACA |
| Insig1 | Insulin-induced gene 1 | NM_153526 | TCACAGTGACTGAGCTTCAGCA | TCATCTTCATCACACCCAGGAC |
| Insig2a | Insulin-induced gene 2a | NM_178082 | CCCTCAATGAATGTACTGAAGGATT | TGTGAAGTGAAGCAGACCAATGT |
| Ki67 | Antigen identified by monoclonal antibody Ki 67 | NM_001081117 | CAGGCAGGAAGAGCGGTAACCT | AGAATGCCATGCTGACTCCG |
| Ldlr | Low density lipoprotein receptor | NM_010700 | GAGGAACTGGCGGCTGAA | GTGCTGGATGGGGAGGTCT |
| Mtp | Microsomal triglyceride transfer protein | NM_008642 | CCTACCAGGCCCAACAAGAC | CGCTCAATTTTGCATGTATCC |
| Npc1l1 | Niemann-Pick C1-Like 1 | NM_207242 | TGGACTGGAAGGACCATTTCC | GACAGGTGCCCCGTAGTCA |
| Pcna | Proliferating cell nuclear antigen | NM_011045 | CGAAGGCTTCGACACATACC | GGACATGCTGGTGAGGTTCA |
| Pcsk9 | Proprotein convertase subtilisin/kexin type 9 | NM_153565 | CAGGCGGCCAGTGTCTATG | GCTCCTTGATTTTGCATTCCA |
| Srb1 | Scavenger receptor class B member 1 | NM_016741 | TCCCCATGAACTGTTCTGTGAA | TGCCCGATGCCCTTGA |
| Srebp2 | Sterol regulatory element-binding protein 2 | NM_033218 | GCGTTCTGGAGACCATGGA | CAAAGTTGCTCTGAAAACAAATCA |
2.7. Analysis of data
Values are mean ± SEM for the specified number of animals. GraphPad Prism 6 software (GraphPad, San Diego, CA) was used to perform all statistical analyses. Depending on the design of each experiment, differences between mean values were tested for statistical significance (p< 0.05) by an unpaired two-tailed Student’s t-test, a paired Student’s t-test, or a one-way or two-way analysis of variance with genotype, diet, or time on diet, as factors. Tukey’s post hoc multiple-comparison test for statistical significance was used for all one-way and two-way analyses of variance.
3. Results
In all experiments involving the feeding of diets containing either Ro 48-8071, ezetimibe, simvastatin, or an elevated cholesterol content, the body weights of the mice, and in one study of hamsters, were maintained or increased over the treatment period which generally lasted for 7 to 10 days. In a preliminary study (data not shown) male BALB/c mice fed the diet with Ro 48-8071 for 21 days showed no adverse effects on their body weights or plasma ALT levels.
3.1.Intestinal cholesterol synthesis was unchanged with simvastatin treatment
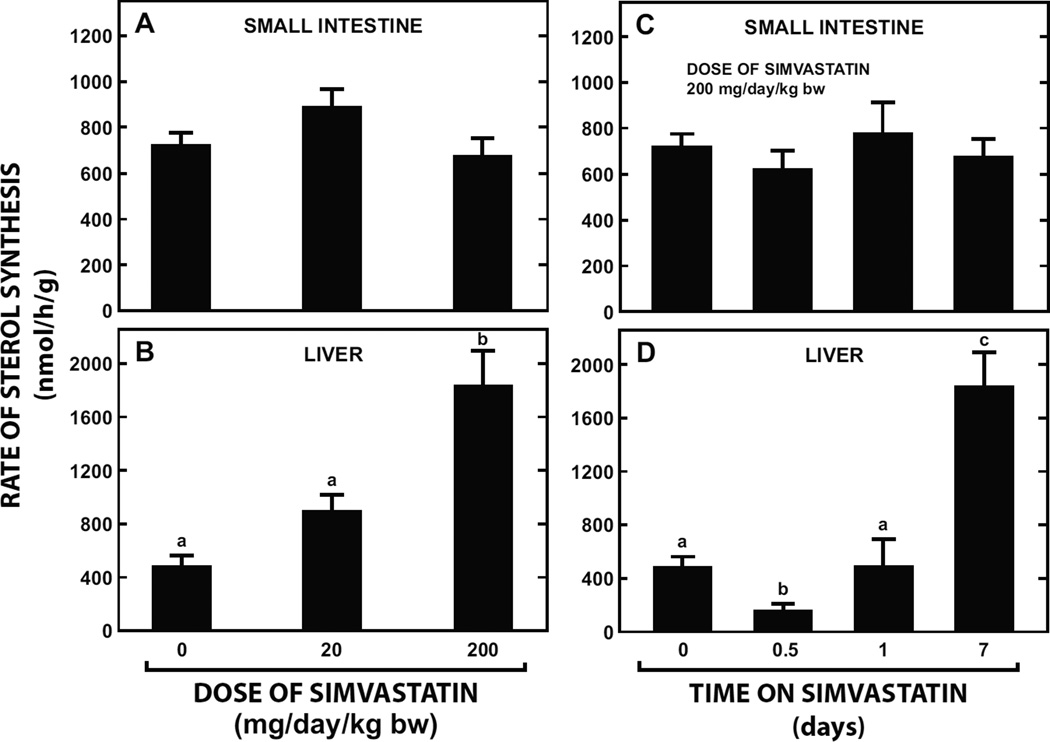
Despite being given a simvastatin dose as high as 200 mg/day/kg bw for as long as 7 days there was no effect on cholesterol synthesis in the small intestine (Fig. 1A and 1C). In contrast, in the liver, after a marked suppression within the first 12 h of commencing treatment, sterol synthesis rebounded to rates higher than those at baseline in the face of continuing simvastatin intake (Fig. 1B and 1D).
Fig. 1.
Effect of varying the dose and time of simvastatin treatment on cholesterol synthesis in the small intestine and liver of mice. In the first study 9-week old male BALB/c mice were fed for 7 days a basal rodent diet containing simvastatin at levels that provided a dose of either 20 or 200 mg/day/kg bw. The control group was fed the basal diet alone. Rates of cholesterol synthesis in the small intestine (A) and liver (B) were measured in vivo using [3H] water as described in Materials and Methods. In a separate study matching male BALB/c mice were fed simvastatin at a fixed dose (200 mg/day/kg bw) for 0.5, 1, or 7 days and used for the measurement of intestinal (C) and hepatic (D) sterol synthesis. All values are the mean ± SEM of data from 5 to 7 animals in each group. Different letters (a, b, c) denote statistically significant (p< 0.05) differences between valuesas determined by one-way analysis of variance (ANOVA) with dose of simvastatin or time of feeding as variables.
3.2.Dose-related inhibition by Ro 48-8071 of cholesterol synthesis in the small intestine but not in the liver
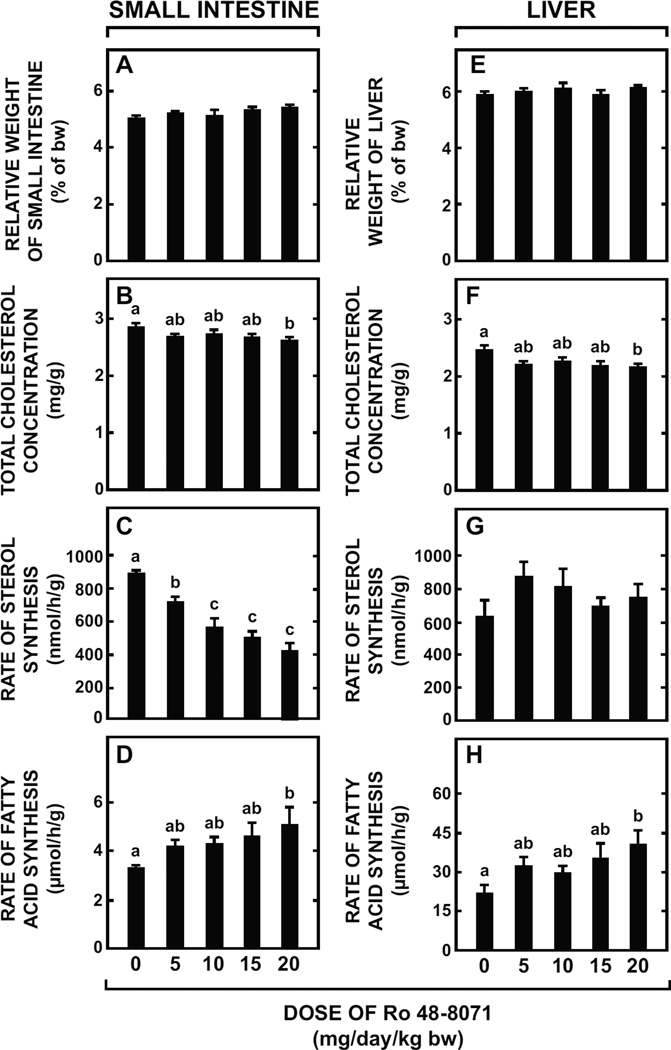
In mice given Ro 48-8071 at doses up to 20 mg/day/kg bw, the weight of the small intestine was unchanged (Fig. 2A), as was the intestinal total cholesterol concentration (Fig. 2B) except at the highest dose where a reduction from a basal value of 2.84 ± 0.04 mg/g to 2.63 ± 0.02 mg/g occurred (p < 0.05). There was a clear dose-related inhibition of intestinal cholesterol synthesis equaling 52% at the highest dose (Fig. 2C). Conversely, intestinal fatty acid synthesis tended to increase with Ro 48-8071 dose (Fig. 2D). Across this same dose range, liver weight was unchanged (Fig. 2E) but there was a clear trend towards lower hepatic total cholesterol concentrations (Fig. 2F). At the highest dose the concentration decreased to 2.16 ± 0.03 mg/g from a baseline value of 2.44 ± 0.04 mg/g (p < 0.05). In most groups given Ro 48-8071, the rate of both cholesterol and fatty acid synthesis in liver (Fig. 2G and 2H, respectively) was marginally elevated but only for fatty acid synthesis at the highest dose was the effect significant (p< 0.05).
Fig. 2.
Effect of varying the dose of Ro 48-8071 on small intestine and liver weights, intestinal and hepatic cholesterol concentrations, and rates of intestinal and hepatic sterol and fatty acid synthesis in mice. Female BALB/c mice at 7 to 8 weeks of age were fed a rodent chow diet containing varying levels of Ro 48-8071 to provide doses of about 5 to 20 mg/day/kg bw over a 7-day feeding period. For the small intestines in these mice, organ weight (A), total cholesterol concentration (B), rate of cholesterol synthesis (C), and rate of fatty acid synthesis (D) were measured as described in Materials and Methods. The same parameters for liver were also measured in these mice (E-H). All values are the mean ± SEM of data from 6 to 8 animals at each dose. Different letters (a, b, c) denote statistically significant (p< 0.05) differences between values as determined by one-way ANOVA with Ro 48-8071 dose as the variable.
3.3. Sustained inhibition by Ro 48-8071 of cholesterol synthesis in the small intestine but not in the liver
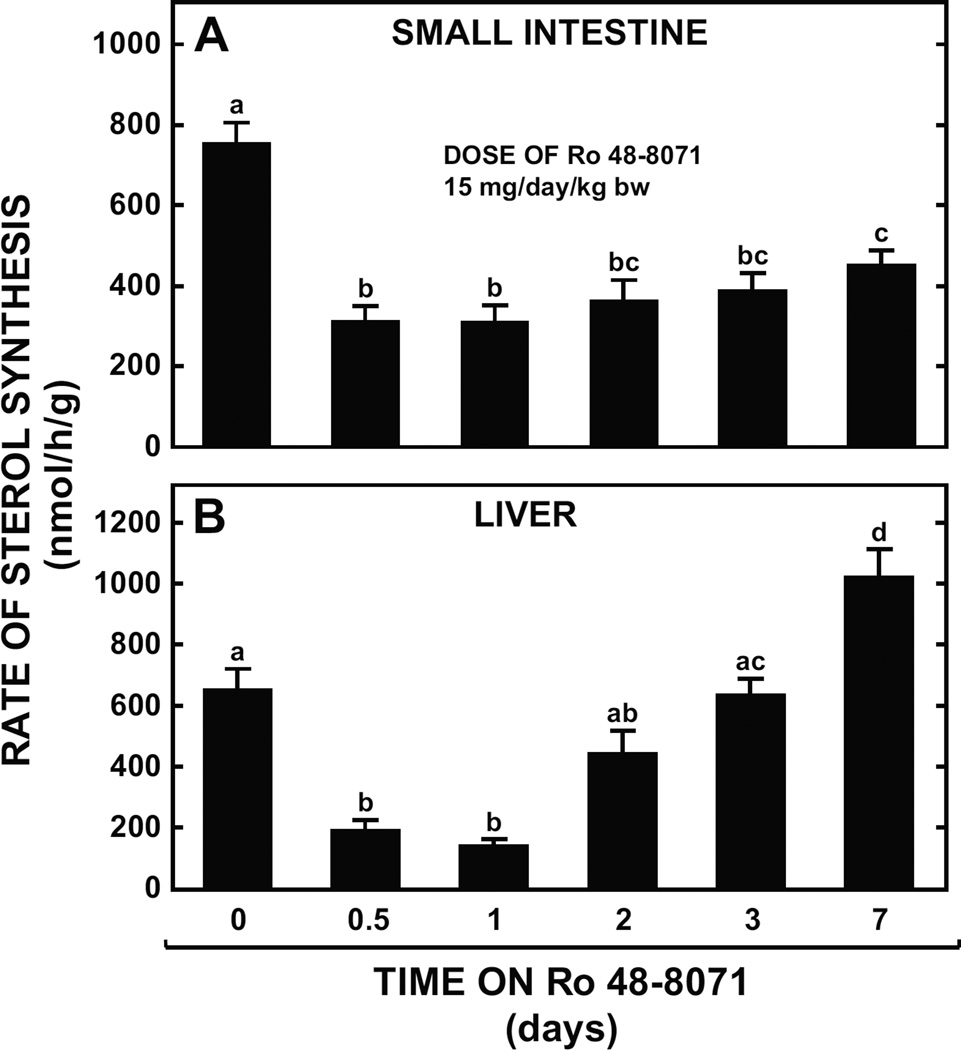
In both the small intestine (Fig. 3A) and in the liver particularly (Fig. 3B), Ro 48-8071 caused a marked inhibition of cholesterol synthesis within the first 24 h of treatment. However, in the case of the liver this effect was lost within 3 days whereas in the small intestine it was still clearly evident after 7 days despite a marginal recovery from the degree of inhibition seen 24 h after the start of treatment. In a related experiment using female mice we found that if, after just 12 h of treatment, the Ro 48-8071-containing diet was replaced with the basal diet alone for the next 12 h, the rate of intestinal cholesterol synthesis rebounded to a value of 1176 ± 60 nmol/h/g which was significantly higher (p< 0.05) than that seen in the female mice fed the basal alone (869 ± 32 nmol/h/g). A comparable rebound in hepatic sterol synthesis at 24h was also found (data not illustrated).
Fig. 3.
Effect of varying period of treatment with Ro 48-8071 on rates of intestinal and hepatic sterol synthesis in mice. Male BALB/c mice at 7 to 9 weeks of age were fed a rodent chow diet containing Ro 48-8071 at one dose (15 mg/day/kg bw) for periods varying from 12h to 7 days. The rate of sterol synthesis in the small intestine (A) and liver (B) was measured in vivo specifically at 12 h, and 1, 2, 3 and 7 days after the start of treatment. All values are the mean ± SEM of data from 6 to 8 animals at each time point. Different letters (a, b, c) denote statistically significant (p< 0.05) differences between values as determined by one-way ANOVA with time on Ro 48-8071 treatment as the variable.
3.4.Prolonged treatment with Ro 48-8071 did not cause discernable pathological changes in the small intestine
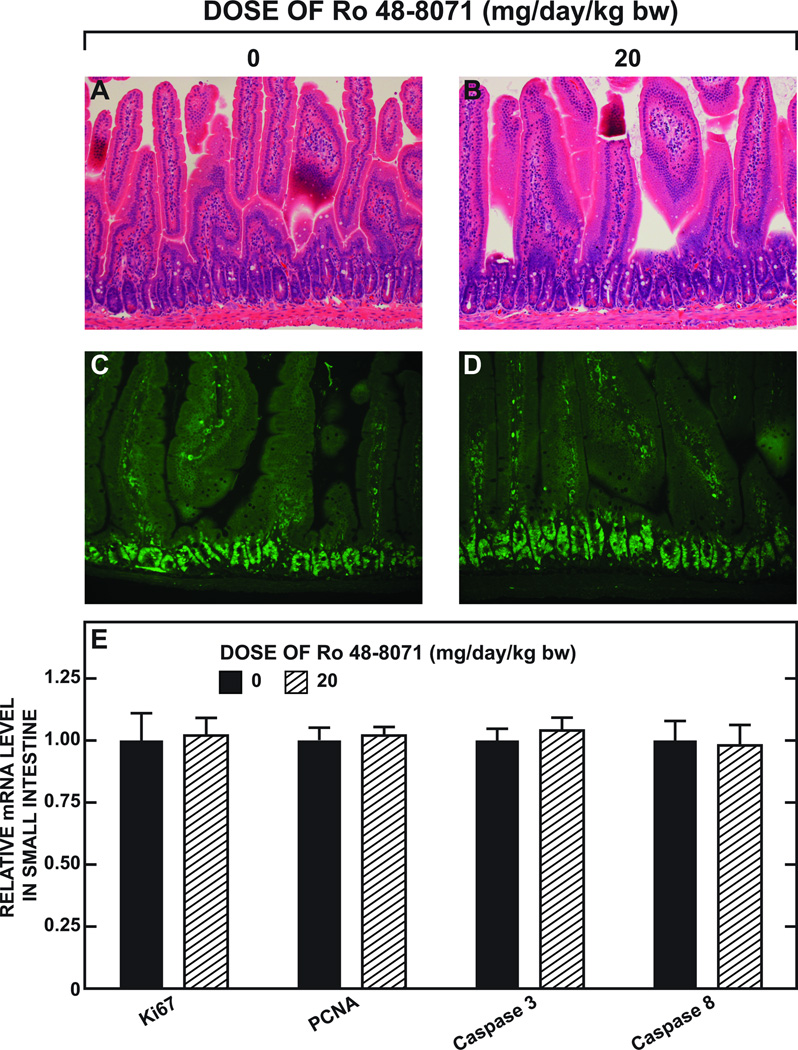
The histology for representative sections of the small intestine from mice given the basal diet alone or containing Ro 48-8071 for 16 days is shown in Fig. 4. There were no discernable differences in general architecture as demonstrated by H&E staining (Fig. 4B vs Fig. 4A), or in the number of proliferating cells, as evaluated by immunohistochemistry using an anti-mouse Ki67 antibody (Fig. 4D vs Fig. 4C). Moreover, the relative mRNA levels in the intestine for markers of proliferation (Ki67 and PCNA), and also for apoptosis (caspase 3 and caspase 4) (Fig. 4E) showed no change with Ro 48-8071 treatment.
Fig. 4.
Histology and immunochemistry of the midsection of the small intestine, and relative levels of expression of mRNA for genes serving as markers for proliferation and apoptosis in the whole small intestine in mice given Ro 48-8071. The results of two separate experiments are shown here. In the first, two matching groups of male BALB/c mice (n=3 each) at 10 to 16 weeks of age were given the basal diet alone or containing Ro 48-8071 (20 mg/day/kg bw) for 16 days. A 1 cm portion of the midsection of the small intestine was taken for histology (H&E staining)(A and B) and for immunohistochemistry (C and D) as described. The second experiment involved the measurement in the mucosae of the whole small intestine of the relative mRNA expression levels of a constellation of genes as fully detailed in Fig. 9. The expression level of mRNA for four of these genes are shown here in panel E. For each gene the level of expression in the mice fed the basal diet alone was arbitrarily set at 1.0. The values here represent the mean ± SEM of data from 6 or 7 mice in each group. None of the values for the mice given Ro 48-8071 are significantly different (p< 0.05) from their corresponding control as determined by an unpaired Student’s t-test.
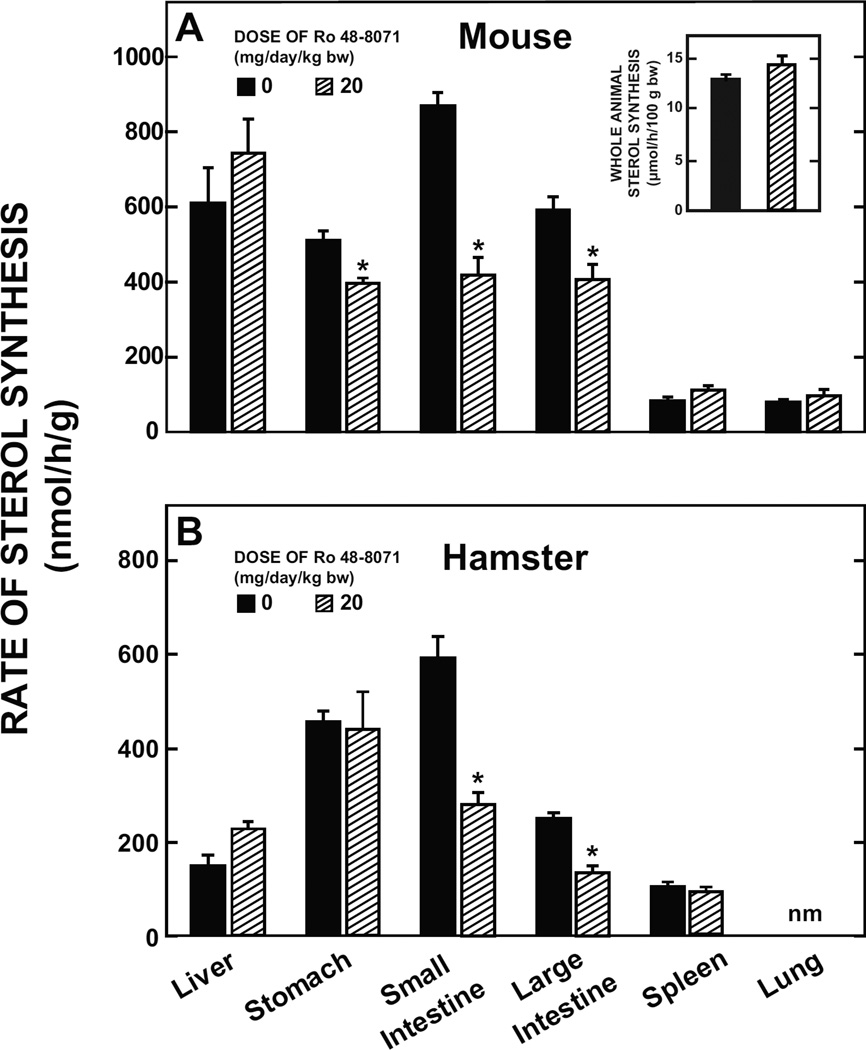
3.5.Suppression of cholesterol synthesis by Ro 48-8071 is confined mainly to the gastrointestinal tract in mice and also hamsters
In BALB/c mice (Fig. 5A) and in Golden Syrian hamsters (Fig. 5B) the suppression of cholesterol synthesis by Ro 48-8071 was most evident in the small and large intestine, with a reduction seen also in the stomach of mice. In both species hepatic cholesterol synthesis changed in the opposite direction to that in the gastrointestinal tract, in keeping with what was found in the earlier experiments in mice (Fig. 2G and Fig 3B). Clearly, this offset the reduction in the amount of newly synthesized cholesterol generated by the gastrointestinal tract because there was no discernable change in whole body cholesterol synthesis as shown by data for the mice (Fig. 5A inset).
Fig. 5.
Rate of sterol synthesis in the liver, various regions of the gastrointestinal tract, and other extrahepatic organs in mice and hamsters given Ro 48-8071. Two separate experiments with mice were carried out. In the first, female BALB/c mice at 7 weeks of age were fed the basal diet alone or containing Ro 48-8071 (20 mg/day/kg bw) for 7 days. Rates of sterol synthesis were measured in 6 organs as described in the preceding experiments. These rates are expressed as the nmol of [3H] water incorporated into sterols/h/g of tissue. In a follow-up study, other female BALB/c mice at 10 weeks of age were fed the same diets as in the first study except for 10 days. These were used specifically for the measurement of whole animal sterol synthesis (data shown in inset). These rates are expressed as the umol of [3H] water incorporated into sterols/h/100 g bw. For the experiment with hamsters, males at approx 12 weeks of age were used and treatment with Ro 48-8071 was for 5 days. All values from both experiments are the mean ± SEM of data from 5 animals per group. The asterisk indicates the value is significantly different (p< 0.05) (by unpaired Student’s t-test) than that for the matching group fed the basal diet alone.nm – not measured.
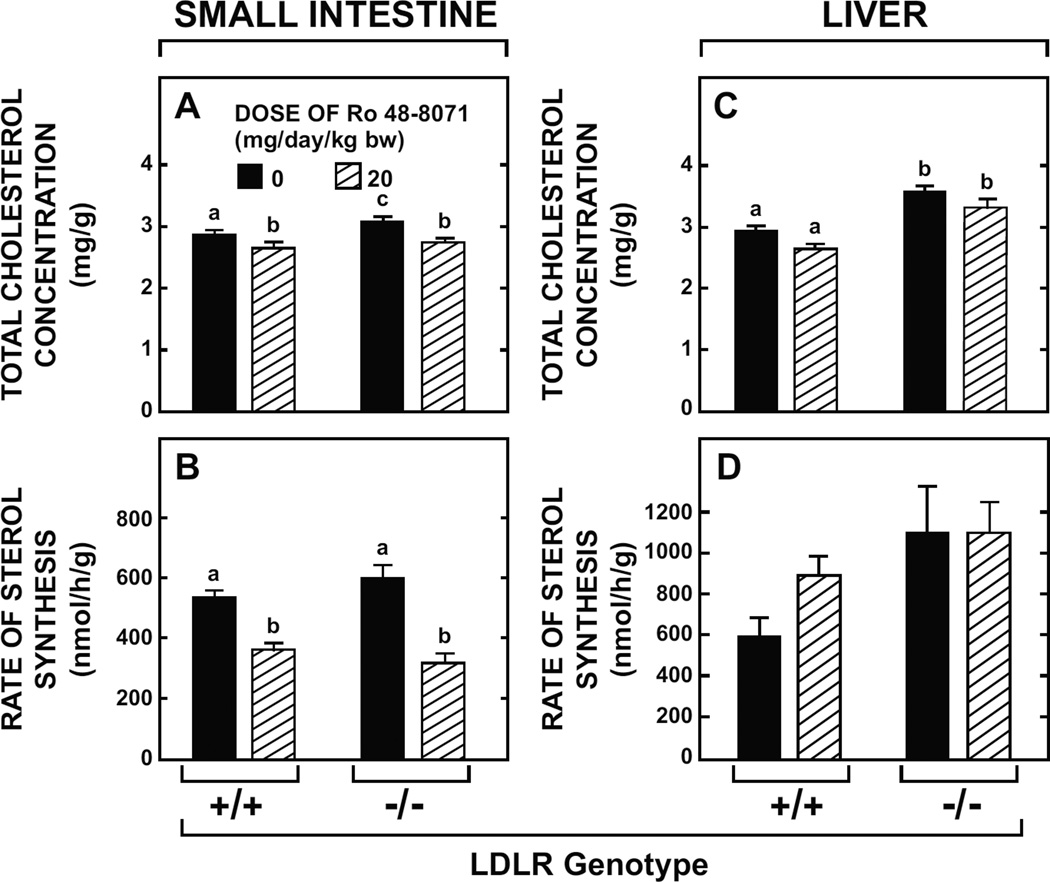
3.6. Effects of Ro 48-8071 in the small intestine and liver of LDL-receptor-deficient mice were the same as in their LDLR+/+ controls
The direction and magnitude of change in the total cholesterol concentration and rate of cholesterol synthesis in the small intestine of ldlr−/− mice given the OSC inhibitor were not different than those found in their matching ldlr+/+ controls (Fig. 6A and Fig. 6B). This was also the case for the liver in these same groups of mice (Fig. 6C and Fig. 6D). Although the data are not shown, there was no reduction in the plasma total cholesterol concentration in mice of either LDLR genotype given Ro 48-8071. For the ldlr+/+ mice on the diet containing the inhibitor the plasma total cholesterol (mg/dl) was 114 ± 5 vs 111 ± 8 in the ldlr+/+ mice fed the basal diet alone. In the treated and untreated ldlr−/− mice the concentrations were 301 ± 16 and 273 ± 14, respectively.
Fig. 6.
Intestinal and hepatic cholesterol concentrations and rates of sterol synthesis in LDLR-deficient mice given Ro 48-8071. Female ldlr−/− mice and matching ldlr+/+ controls (129/Sv background) at 21 to 25 weeks of age were fed a rodent chow diet alone or containing Ro 48-8071 (20mg/day/kg bw) for 10 days. The total cholesterol concentration and rate of sterol synthesis in both the small intestine (A and B) and liver (C and D) were measured as described. All values are the mean ± SEM of data from 6 animals in each group. Different letters (a, b, c) denote statistically significant differences between (p< 0.05) values as determined by two-way ANOVA with genotype and Ro 48-8071 dose as variables.
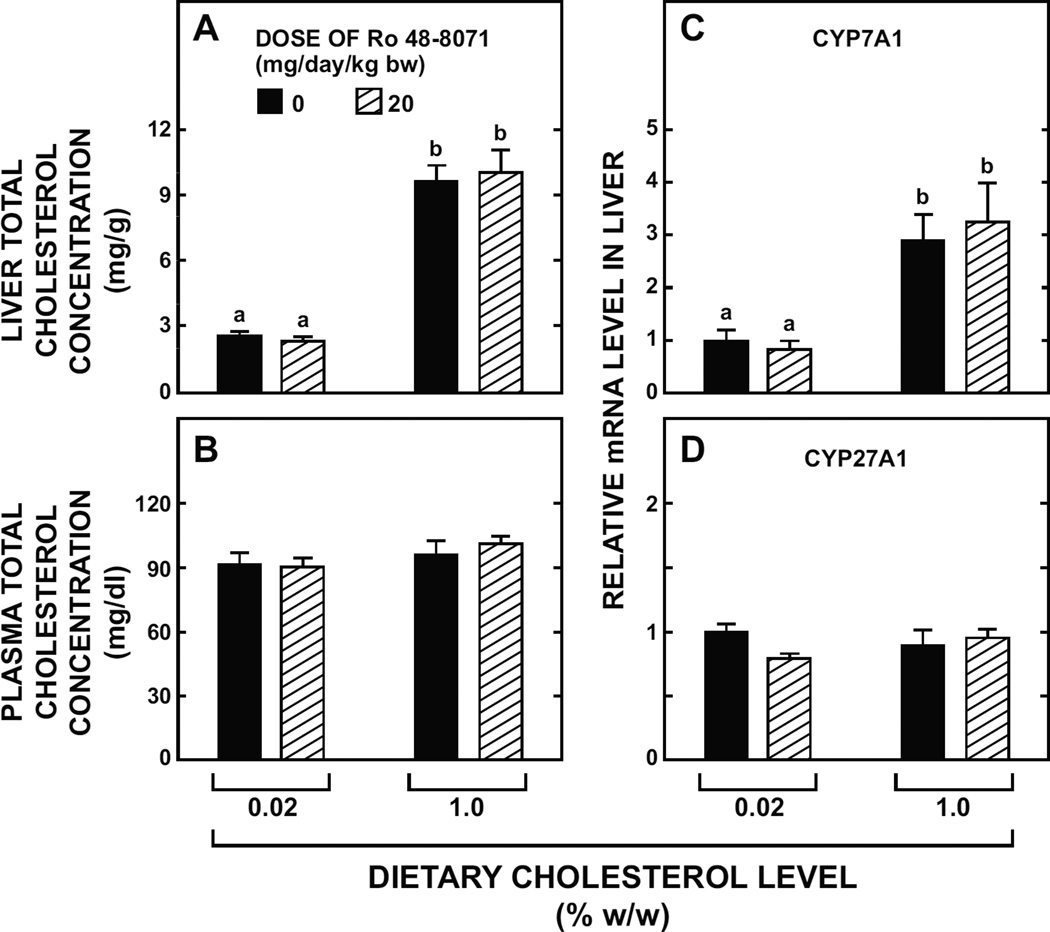
3.7. Hepatic cholesterol accumulation in mice fed a high cholesterol diet is not attenuated by Ro 48-8071
When genetically unmanipulated BALB/c mice were fed the basal diet with substantial cholesterol enrichment their hepatic total cholesterol concentration increased more than 3-fold (Fig. 7A) with no discernable change in their plasma cholesterol concentration (Fig. 7B). The inclusion of Ro 48-8071 in the high cholesterol diet had no effect on the magnitude of change in the level of cholesterol in the liver. As shown in Fig. 7C, the high cholesterol diet clearly raised hepatic mRNA levels for CYP7A1 but Ro 48-8071 had no effect on the magnitude of this increase. In contrast, the mRNA expression level for CYP27A1 was about the same irrespective of treatment (Fig. 7D).
Fig. 7.
Hepatic and plasma cholesterol concentrations and relative level of expression in liver of mRNA for two genes involved in bile acid synthesis in mice fed a high cholesterol diet along with Ro 48-8071. Female BALB/c mice at 10 to 16 weeks of age were fed a rodent chow diet alone or containing added cholesterol, with or without Ro 48-8071 (20mg/day/kg bw) for 18 days. The basal chow diet (inherent cholesterol content 0.02% w/w) was supplemented with powdered cholesterol to a final level of 1.0 % w/w. Hepatic and plasma total cholesterol concentrations and mRNA levels were measured as described. The mRNA levels are expressed relative to those obtained for mice given the basal diet alone which, in each case, were arbitrarily set at 1.0. Values are the mean ± SEM of data from 4 animals per group (low cholesterol) or 5 animals per group (high cholesterol). Different letters (a, b) denote statistically significant (p<0.05) differences between values as determined by two-way ANOVA with dietary cholesterol level and Ro 48-8071 dose as variables.
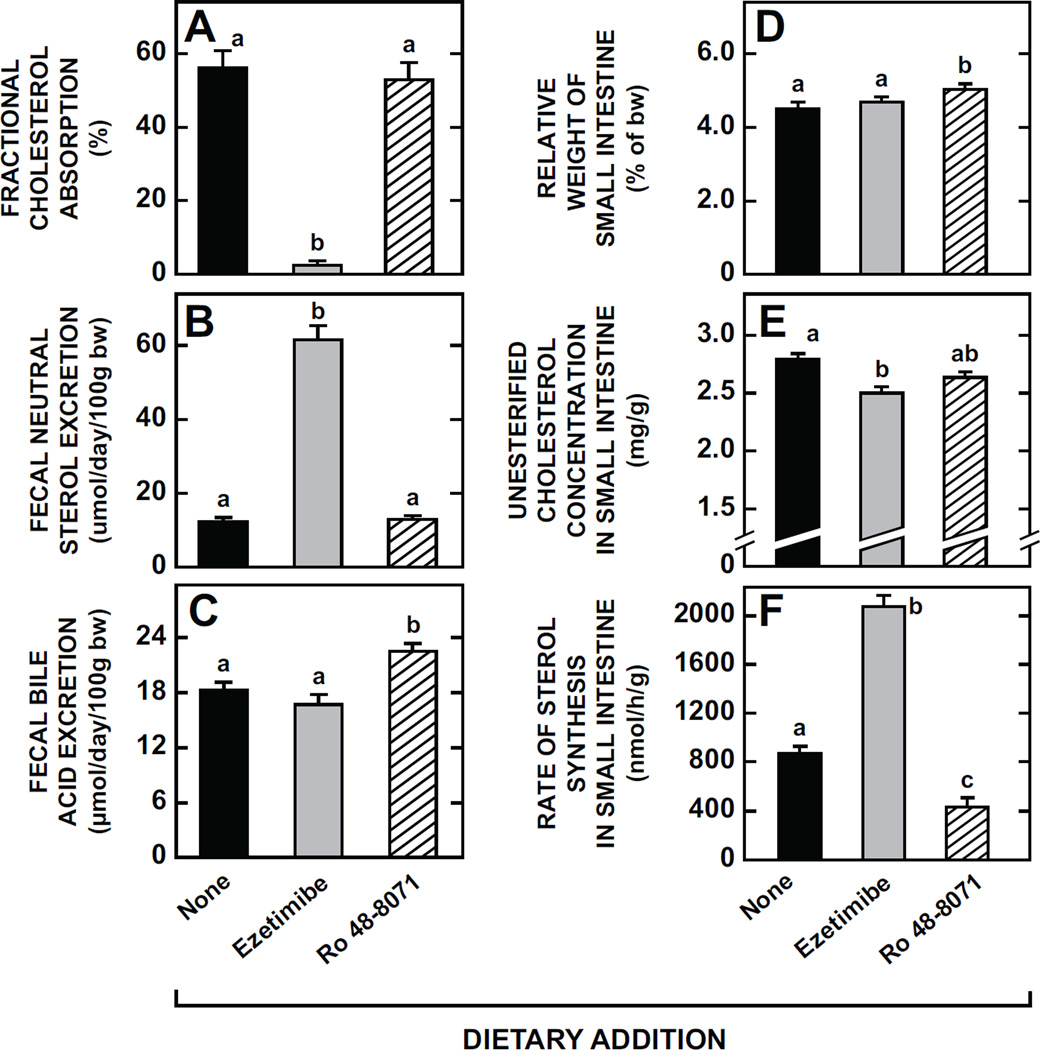
3.8. Comparison of effects of Ro 48-8071 with those of ezetimibe, a potent cholesterol absorption inhibitor, on intestinal cholesterol handling at a biochemical and molecular level
The final set of experiments used ezetimibe as a positive control for helping to better define the quantitative significance of any changes detected in various parameters of intestinal cholesterol handling resulting from treatment with Ro 48-8071. Consistent with its well documented actions, ezetimibe caused a dramatic reduction in fractional cholesterol absorption which remained unchanged in response to treatment with the OSC inhibitor (Fig. 8A). Commensurate with their different impacts on cholesterol absorption, ezetimibe caused a ~4-fold increase in fecal neutral sterol excretion whereas there was no change in this parameter with Ro 48-8071 (Fig. 8B). In contrast, ezetimibe did not significantly change the rate of fecal bile acid excretion but there was a marginal increase (p< 0.05) in this parameter in the mice given the OSC inhibitor (Fig. 8C). The weight of the whole small intestine did not shift with ezetimibe but, a slight increase (p< 0.05) was evident in the mice given Ro 48-8071 (Fig. 8D). Both agents lowered the unesterified cholesterol (UC) concentration in the small intestine (Fig. 8E). Whereas intestinal cholesterol synthesis was suppressed in the mice given the OSC inhibitor, in those on ezetimibe treatment it was increased 2.4-fold (Fig. 8F). The plasma total cholesterol concentrations (mg/dl) in these mice were 89.4 ± 4.6 (no treatment), 82.4 ± 3.9 (ezetimibe), and 102.5 ± 3.0 (Ro 48-8071) (data not illustrated).
Fig. 8.
Parameters of intestinal cholesterol metabolism and rates of neutral sterol and bile acid excretion in mice given either ezetimibe, a cholesterol absorption inhibitor, or Ro 48-8071. The data presented here were derived from four separate groups of experiments, all carried out using female BALB/c mice at 7 to 10 weeks of age. In one study cholesterol absorption (A) and fecal neutral sterol excretion (B) were measured. Separate groups were used for measuring fecal bile acid excretion (C). The third lot of mice was used for determining relative intestine weight (D) and the unesterified cholesterol (UC) concentration in the whole small intestine (E). Another set of mice was used for measuring the rate of intestinal sterol synthesis in vivo (F). In all experiments the dose of both ezetimibe and Ro 48-8071 was 20 mg/day/kg bw. Values are the mean ± SEM of data from 7 to 10 animals per group (A, B, C, F) or 5 animals per group (D, E). Different letters (a, b, c) denote statistically significant (p< 0.05) differences between values using a one-way ANOVA with type of dietary agent as the variable.
Although the fecal bile acid excretion data in Fig. 8C suggested there was a marginal stimulation of bile acid synthesis with Ro 48-8071, this was contrary to the conclusion drawn from the hepatic mRNA levels for CYP7A1 in the cholesterol-fed mice (Fig. 7C). Thus it was decided to conduct another experiment to further explore whether this OSC inhibitor had any effect on bile acid synthesis. This was done by measuring fecal bile acid excretion rates in another set of mice while they were fed only the basal control diet and later after they had been given Ro 48-8071 for 7 to 10- days. The data in Table 2 show unequivocally that bile acid synthesis was unchanged with Ro 48-8071 treatment.
Table 2.
Rate of fecal bile acid excretion in mice before and during treatment with Ro 48-8071
| Parameter | Baseline | Ro 48-8071 |
|---|---|---|
| Body weight (g) | 20.6 ± 0.2 | 21.0 ± 0.2 |
| Stool output (g/day/100g bw) | 4.22 ± 0.11 | 4.27 ± 0.21 |
| Fecal bile acid content h (µmol/g) | 3.72 ± 0.23 | 3.81 ± 0.24 |
| Fecal bile acid excretion (µmol/day/100g bw) | 15.7 ± 1.0 | 16.1 ± 0.8 |
Seven female BALB/c mice at 8 to 10 weeks of age were housed individually and fed a basal rodent chow diet during which time stools were collected over a 3-day period (baseline). These same mice were then given the diet containing Ro 48-8071 (20mg/day/kg bw) for ten days. Over the last three days of this period stools were collected. The rate of fecal bile acid excretion was then measured. Values are the mean ± SEM. A paired Student’s t-test of all data showed no statistically significant (p<0.05) differences in the values for any parameter during the baseline and treatment periods.
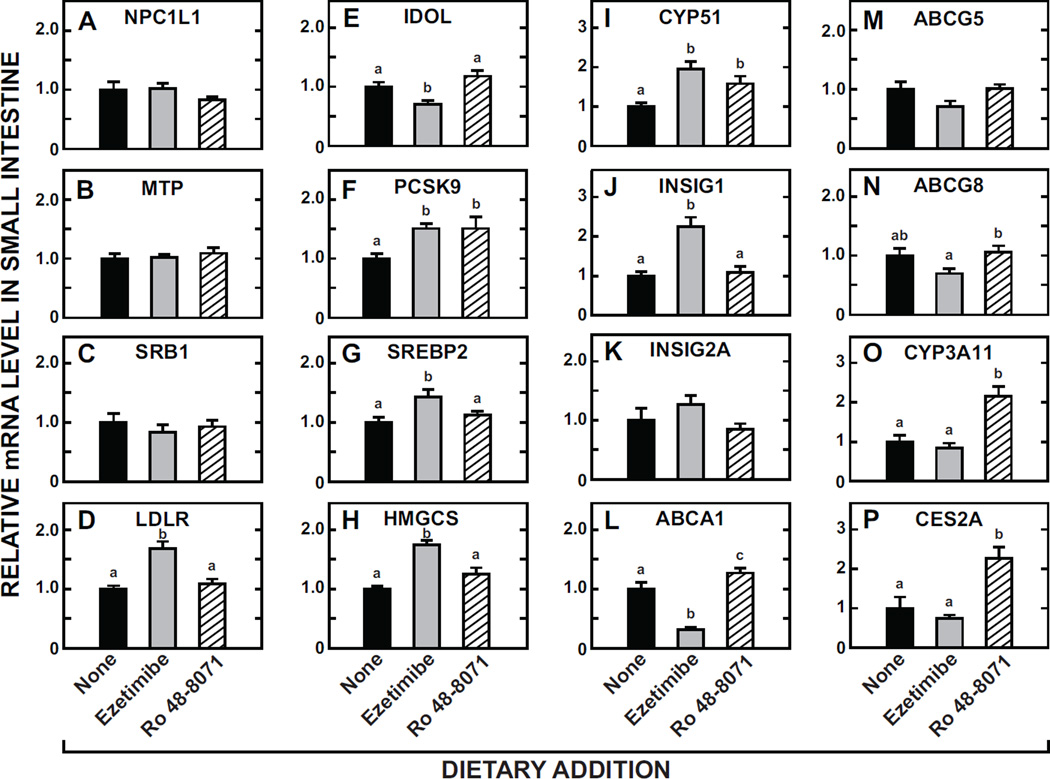
The relative mRNA expression levels (Fig. 9) for a constellation of genes in the small intestines of mice fed the same diets as were evaluated for the experiments described in Fig. 8. Although neither treatment significantly changed the mRNA level for NPC1L1 (A), MTP (B), or SRB1 (C), ezetimibe clearly raised the expression level of the LDLR (D), and also of the transcription factor SREBP2 (G) and multiple of its target genes including PCSK9 (F), HMGCS (H), CYP51 (I), and INSIG1 (J) with a trend towards such an effect for INSIG2A (K) also. Amongst these particular genes only PCSK9 and CYP51 showed a response to Ro 48-8071, and, in both cases there was a significant increase in the mRNA level. For the LXR target genes ABCA1 (L), ABCG5 (M) and ABCG8 (N), the main change was a decisive reduction in the mRNA for ABCA1 with ezetimibe. In sharp contrast, Ro 48-8071, but not ezetimibe, caused marked increases in the mRNA expression level for the PXR target genes CYP3A11 (O) and CES2A (P).
Fig. 9.
Relative levels of expression of mRNA for multiple genes in the small intestine of mice given ezetimibe or Ro 48-8071. The mucosa from the entire small intestine was used. Most, but not all, of the mice used for these analyses were the same as those used for the measurements described in Fig. 8A and Fig. 8B. The mRNA levels were measured as described in Materials and Methods and are expressed relative to those obtained for mice given the basal diet alone, which in each case were arbitrarily set at 1.0. Values are the mean ± SEM of data from 6 or 7 mice per group. Different letters (a, b, c) denote statistically significant (p< 0.05) differences between values using a one-way ANOVA with type of dietary agent as the variable.
4. Discussion
It has been known for decades that the small intestine not only actively synthesizes cholesterol but the rate at which it does so can fluctuate markedly, depending on multiple factors such as dietary cholesterol intake, the size and composition of the bile acid pool, and time of day [40–43]. Furthermore, there are significant regional differences in sterol synthetic activity within the small intestine, as well as along the villus-crypt axis [44]. Hence, the best strategy for initially evaluating the impact of Ro 48-8071 on intestinal cholesterol synthesis, and related parameters, was to make all measurements in the whole organ, in vivo, using genetically normal mice fed a low-cholesterol rodent chow diet. This approach has yielded novel and useful information about the selective action of one type of OSC inhibitor in the mouse.
Broadly, four main conclusions can be drawn from these studies. The first relates to the suppression of cholesterol synthesis. Together the data show that this inhibition is, most evident in the small intestine, sustained for long periods in that organ, selective for the sterol biosynthetic pathway, rapidly reversible, achieved at moderate doses, and not a mouse-specific response. Importantly, treatment with this compound was innocuous. The prolonged inhibition of synthesis in the small intestine by Ro 48-8071 was in contrast to the lack of any consistent effect of simvastatin. It is impressive that the inhibition is sustained given that there is continual sloughing of cells from the mucosa, with the average life span of enterocytes being just 2 to 3 days [45]. However, perhaps this constant renewal of the mucosa explains why a complete inhibition of cholesterol synthesis in the small intestine is not achieved, at least with this particular compound. The action of this OSC inhibitor in the liver was very different than in the gastrointestinal tract and followed a similar pattern to that shown by the liver in the face of treatment with simvastatin. Typically this was characterized by a marked inhibition of hepatic synthesis over the first day of treatment followed by a decisive rebound in synthesis to rates higher than those found before the start of treatment. At least for Ro 48-8071, the rebound in hepatic sterol synthesis was not as dramatic as that reported for various statins in the mouse and other models [31–33]. The data in Fig. 3A might be taken to mean that in the small intestine of the mice given Ro 48-8071 a marginal rebound in sterol synthesis starts to occur before 7 days of treatment. However, the slight increase in the amount of labeled sterol found in the small intestine about 3 days after the start of Ro 48-8071 treatment might reflect the presence of increasing amounts of labeled sterol formed during the rebound of hepatic synthesis that travel from the liver via the bile to the mucosal surface where it is internalized by enterocytes.
There was no detectable change in whole animal cholesterol synthesis with Ro 48-8071 treatment despite its inhibitory action in the stomach, small intestine and large intestine. However, given that in the normal mouse the gastrointestinal tract represents only about 6% of whole body mass, and that there was a trend toward increased cholesterol synthesis in the liver of the treated mice, the lack of change in whole body synthesis was not unexpected. This finding contrasts with that for Ro 0717625 (an OSC inhibitor developed after Ro 48-8071) in the minipig which showed a lowering of the plasma lathosterol to cholesterol ratio with this OSC inhibitor [34]. While this is taken to mean there was a reduction in whole body cholesterol synthesis, it is not possible to discern which organ(s) was making less cholesterol.
The second conclusion concerns the contentious question of whether a sustained inhibition of intestinal cholesterol synthesis leads to a compensatory increase in cholesterol absorption. Such an adaptive response has been purported to occur in humans given statins, although the extent to which intestinal cholesterol synthesis is inhibited by statins in humans is unknown [24, 26]. In the case of the Ro 48-8071- treated mouse, the sustained inhibition of intestinal cholesterol synthesis was not accompanied by any change in either the rate of fecal neutral sterol excretion (Fig. 8B), or the relative mRNA expression level for NPC1L1 in the small intestine (Fig. 9A). However, these findings alone cannot be taken as conclusive evidence that the absolute rate of cholesterol absorption was unchanged by this OSC inhibitor. Often there can be dramatic changes in both fractional cholesterol absorption and fecal neutral sterol excretion with no change in the mRNA level for NPC1L1 as exemplified here by the data for mice given ezetimibe. Providing there are no treatment-induced changes in either the amount of cholesterol or of non-cholesterol sterols entering the intraluminal pool, then fractional cholesterol absorption values can be a reliable barometer of the absolute amount of cholesterol reaching the liver from the lumen of the small bowel. It is unknown whether Ro 48-8071 had an effect on the amount of biliary cholesterol entering the intestinal lumen. However, if this had occurred to an appreciable degree then likely this would have been reflected in an increased rate of fecal neutral sterol excretion. This was unchanged.
Perhaps the best indication that Ro 48-8071 treatment did not cause a compensatory increase in the amount of intestinal cholesterol delivered to the liver was that it did not raise hepatic cholesterol concentrations, even when dietary cholesterol19 intake was increased substantially (Fig 1F, 6C and 7A). In a minipig model fed a lipid-rich diet, treatment with Ro 0717625 decreased postprandial transport of cholesterol in chylomicrons, and also lowered hepatic cholesterol concentrations [34].Thus, while it is not known whether Ro 0717625 has the same inhibitory effect on intestinal cholesterol synthesis as Ro 48-8071, taken together these various findings suggest that this class of inhibitors does not cause a compensatory increase in intestinal cholesterol absorption, and may possibly have the opposite effect. In a newly published study investigating the role of Insig1 and Insig2 in regulating intestinal cholesterol synthesis it was noted that the deletion of these proteins, while causing a dramatic increase in intestinal cholesterol synthesis, did not result in a change in fractional cholesterol absorption. The authors thus concluded that the sterol synthesis and absorption pathways appear not to be co-ordinately regulated [21].
The third conclusion relates to the lack of change in the plasma total cholesterol concentration in the various mouse models treated with Ro 48-8071 in the present studies. This was not surprising given the maintenance of normal rates of cholesterol absorption, fecal neutral sterol excretion, and whole body cholesterol synthesis in these animals. Added to these findings, the present data also show unequivocally that Ro 48-8071 does not consistently change the rate of conversion of cholesterol to bile acids, as judged by the rate of fecal bile acid excretion and the hepatic expression level of mRNA for CYP7A1. These parameters are known to faithfully reflect the rate of bile acid synthesis as shown by earlier studies using various models in this and other laboratories [46–50]. Unlike the mouse, other animal models including the hamster, minipig and squirrel monkey given lipid-rich diets did show reductions in their plasma LDL-cholesterol levels in response to doses of Ro 48-8071 comparable to those used here in the mouse [32]. It is unknown whether the more recently developed OSC inhibitor, Ro 0717625, lowers plasma cholesterol levels in mouse models because it appears that the only published data for this compound are in the minipig [34].
The fourth conclusion centers on the paradoxical changes in the mRNA levels for the LDLR (Fig 9D) and PCSK9 (Fig 9F) in mice given ezetimibe or Ro 48-8071. In the case of ezetimibe, the relative mRNA changes for these two genes in whole intestinal mucosal scrapings closely mirrored those reported for isolated jejunocytes from ezetimibe-treated mice [18]. In both studies the LDLR and PCSK9 mRNA levels increased (under SREBP2 regulation) and IDOL mRNA levels decreased (by diminished LXR activity) consistent with changes in enterocyte sterol flux. However, a different and unexpected result was seen with Ro 48-8071treatment for the two SREBP2 target genes, PCSK9 and CYP51. While SREBP2 and LDLR mRNA levels were unaffected by Ro 48-807, PCSK9 and CYP51 showed enhanced expression suggesting additional, yet unknown, mechanisms of regulation for enterocyte PCSK9 and CYP51. It is noteworthy that in a newly published study using an ezetimibe-treated primate model, an inverse relationship between LDL-cholesterol and plasma PCSK9 levels was found [51].Furthermore, several other studies provide evidence that the expression of PCSK9 mRNA can be regulated by SREBP1c [52, 53], HNF1a [53], and FXR [54] in addition to the conventional SREBP2 pathway in hepatocytes. Whether these pathways are responsible for our observation will require further investigation.
The final point to be made concerns the potential application of the present findings. While the development of OSC inhibitors as agents for treating hypercholesterolemia appears to have waned, new studies point to a possible use of these agents for slowing tumor growth [55]. Be that as it may, the current findings reveal the potential value of Ro 48-8071, and perhaps other OSC inhibitors, as tools for further investigating the mechanisms that regulate intestinal cholesterol homeostasis. A high priority will be to determine whether Ro 0717625 exerts the same inhibitory effect on cholesterol synthesis in the small intestine. Irrespective of the outcome of such studies, another novel approach would be to investigate the effects of combining Ro 48-8071 with other classes of cholesterol-modifying agents. Such strategies may for example be useful in further exploration of the putative transintestinal cholesterol efflux (TICE) pathway [56–59]. Another potential use of Ro 48-8071 relates to the current finding of marked increases in intestinal mRNA levels for CYP3A11 and CES2A, both PXR target genes [60]. CYP3A11, the mouse ortholog of human CYP3A4, is a principal enzyme involved in drug metabolism, and has been implicated in cholesterol metabolism because of its capacity to affect the half-life of some statins [61], and also because it facilitates modification of cholesterol by 4β-hydroxylation [62]. Together these new data, added to those from earlier studies, including one that used Ro 48-8071-treated primary cultured human hepatocytes [63], suggest that further exploration of such actions of this OSC inhibitor, and others, in the small intestine is warranted.
Acknowledgments
This work was supported by the National Institutes of Health [Grants R01HL009610 (S.D.T.), R01DK078952 (J.J.R), and GM07062 (M.A.V.)]. We thank Mario Saucedo, Heather Waddell, and Brian Griffith for excellent technical assistance. The ezetimibe used in these studies was kindly provided by Harry R. Davis, Jr., Ph.D. at Merck& Co., Inc.
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- ABCG5
ATP-binding cassette transporter G5
- ABCG8
ATP-binding cassette transporter G8
- CES2A
carboxylesterase 2A
- CYP3A11
cytochrome P450, family 3, subfamily a, polypeptide 11
- CYP51
sterol 14α-demethylase
- FXR
farnesoid X receptor
- H&E stain
hematoxylin and eosin stain
- HMGCS
hydroxymethylglutaryl coenzyme A synthase
- IDOL
inducible degrader of the LDL-receptor
- INSIG
insulin-induced gene
- LDLR
low density lipoprotein receptor
- MTP
microsomal triglyceride transfer protein
- NPC1L1
Niemann-Pick C1-Like 1
- OSC
2,3- oxidosqualene:lanosterolcyclase
- PCNA
proliferating cell nuclear antigen
- PCSK9
proproteinconvertasesubtilisin/kexin type 9
- PXR
pregnane X receptor
- Ro 48-8071
4′-[6-(Allylmethylamino) hexyloxy]-4-bromo-2′-fluorobenzophenone fumarate (1:1)
- SRB1
scavenger receptor class B member 1
- SREBP
sterol regulatory element-binding protein
- UC
unesterified cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jen-Chieh Chuang, Email: jen-chieh.chuang@utsouthwestern.edu.
Mark A. Valasek, Email: mvalasek@ucsd.edu.
Adam M. Lopez, Email: adam.lopez@utsouthwestern.edu.
Kenneth S. Posey, Email: kenneth.posey@utsouthwestern.edu.
Joyce J. Repa, Email: joyce.repa@utsouthwestern.edu.
References
- 1.Grundy SM. Absorption and metabolism of dietary cholesterol. Annu Rev Nutr. 1983;3:71–96. doi: 10.1146/annurev.nu.03.070183.000443. [DOI] [PubMed] [Google Scholar]
- 2.Stange EF, Dietschy JM. Cholesterol Absorption and Metabolism by the Intestinal Epithelium. In: Danielsson H, Sjövall J, editors. Sterols and Bile Acids. New York: Elsevier Science Publishers; 1985. pp. 121–149. [Google Scholar]
- 3.Turley SD, Dietschy JM. The Metabolism and Excretion of Cholesterol by the Liver. In: Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DA, editors. The Liver: Biology and Pathobiology. New York: Raven Press; 1988. pp. 617–641. [Google Scholar]
- 4.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–2192. [PubMed] [Google Scholar]
- 5.Osono Y, Woollett LA, Herz J, Dietschy JM. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J Clin Invest. 1995;95:1124–1132. doi: 10.1172/JCI117760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turley SD, Spady DK, Dietschy JM. Role of liver in the synthesis of cholesterol and the clearance of low density lipoproteins in the cynomolgus monkey. J Lipid Res. 1995;36:67–79. [PubMed] [Google Scholar]
- 7.Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24:1803–1823. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- 8.Valasek MA, Repa JJ, Quan G, Dietschy JM, Turley SD. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am J Physiol Gastrointest Liver Physiol. 2008;295:G813–G822. doi: 10.1152/ajpgi.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Veen JN, Kruit JK, Havinga R, Baller JF, Chimini G, Lestavel S, et al. Reduced cholesterol absorption upon PPARδ activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res. 2005;46:526–534. doi: 10.1194/jlr.M400400-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Rozner S, Garti N. The activity and absorption relationship of cholesterol and phytosterols. Colloids Surf A Physicochem Eng Asp. 2006:282–283. 435–456. [Google Scholar]
- 12.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, et al. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem. 2006;281:4075–4086. doi: 10.1074/jbc.M510622200. [DOI] [PubMed] [Google Scholar]
- 13.Wang DQ. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–248. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 14.Davis HR, Jr, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter. Biochim Biophys Acta. 2009;1791:679–683. doi: 10.1016/j.bbalip.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Turley SD, Valasek MA, Repa JJ, Dietschy JM. Multiple mechanisms limit the accumulation of unesterified cholesterol in the small intestine of mice deficient in both ACAT2 and ABCA1. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1012–G1022. doi: 10.1152/ajpgi.00190.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams MR, Konaniah E, Cash JG, Hui DY. Use of NBD-cholesterol to identify a minor but NPC1L1-independent cholesterol absorption pathway in mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G164–G169. doi: 10.1152/ajpgi.00392.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol. 2011;73:239–259. doi: 10.1146/annurev-physiol-012110-142233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelking LJ, McFarlane MR, Li CK, Liang G. Blockade of cholesterol absorption by ezetimibe reveals a complex homeostatic network in enterocytes. J Lipid Res. 2012;53:1359–1368. doi: 10.1194/jlr.M027599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurano M, Hara M, Tsuneyama K, Okamoto K, Iso ON, Matsushima T, et al. Modulation of lipid metabolism with the overexpression of NPC1L1 in mouse liver. J Lipid Res. 2012;53:2275–2285. doi: 10.1194/jlr.M026575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen TM, Sawyer JK, Kelley KL, Davis MA, Kent CR, Rudel LL. ACAT2 and ABCG5/G8 are both required for efficient cholesterol absorption in mice: evidence from thoracic lymph duct cannulation. J Lipid Res. 2012;53:1598–1609. doi: 10.1194/jlr.M026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarlane MR, Liang G, Engelking LJ. Insig proteins mediate feedback inhibition of cholesterol synthesis in the intestine. J Biol Chem Epub. 2013 doi: 10.1074/jbc.M113.524041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SD, Gershkovich P, Darlington JW, Wasan KM. Inhibition of cholesterol absorption: targeting the intestine. Pharm Res. 2012;29:3235–3250. doi: 10.1007/s11095-012-0858-6. [DOI] [PubMed] [Google Scholar]
- 23.Sharpe LJ, Brown AJ. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA Reductase (HMGCR) J Biol Chem. 2013;288:18707–18715. doi: 10.1074/jbc.R113.479808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miettinen TA, Gylling H. Synthesis and absorption markers of cholesterol in serum and lipoproteins during a large dose of statin treatment. Eur J Clin Invest. 2003;33:976–982. doi: 10.1046/j.1365-2362.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 25.van Himbergen TM, Matthan NR, Resteghini NA, Otokozawa S, Ai M, Stein EA, et al. Comparison of the effects of maximal dose atorvastatin and rosuvastatin therapy on cholesterol synthesis and absorption markers. J Lipid Res. 2009;50:730–739. doi: 10.1194/jlr.P800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremblay AJ, Lamarche B, Lemelin V, Hoos L, Benjannet S, Seidah NG, et al. Atorvastatin increases intestinal expression of NPC1L1 in hyperlipidemic men. J Lipid Res. 2011;52:558–565. doi: 10.1194/jlr.M011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turley SD. The role of Niemann-Pick C1 - Like 1 (NPC1L1) in intestinal sterol absorption. J Clin Lipidol. 2008;2:S20–S28. doi: 10.1016/j.jacl.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Descamps OS, De Sutter J, Guillaume M, Missault L. Where does the interplay between cholesterol absorption and synthesis in the context of statin and/or ezetimibe treatment stand today? Atherosclerosis. 2011;217:308–321. doi: 10.1016/j.atherosclerosis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Sviridov DD, Pavlov MY, Safonova IG, Repin VS, Smirnov VN. Inhibition of cholesterol synthesis and esterification regulates high density lipoprotein interaction with isolated epithelial cells of human small intestine. J Lipid Res. 1990;31:1821–1830. [PubMed] [Google Scholar]
- 30.Burnett JR, Wilcox LJ, Telford DE, Kleinstiver SJ, Barrett PHR, Newton RS, et al. The magnitude of decrease in hepatic very low density lipoprotein apolipoprotein B secretion is determined by the extent of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition in miniature pigs. Endocrinology. 1999;140:5293–5302. doi: 10.1210/endo.140.11.7150. [DOI] [PubMed] [Google Scholar]
- 31.Bisgaier CL, Essenburg AD, Auerbach BJ, Pape ME, Sekerke CS, Gee A, et al. Attenuation of plasma low density lipoprotein cholesterol by select 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in mice devoid of low density lipoprotein receptors. J Lipid Res. 1997;38:2502–2515. [PubMed] [Google Scholar]
- 32.Morand OH, Aebi JD, Dehmlow H, Ji YH, Gains N, Lengsfeld H, et al. Ro 48-8.071, a new 2,3-oxidosqualene:lanosterol cyclase inhibitor lowering plasma cholesterol in hamsters, squirrel monkeys, and minipigs: comparison to simvastatin. J Lipid Res. 1997;38:373–390. [PubMed] [Google Scholar]
- 33.Fujioka T, Nara F, Tsujita Y, Fukushige J, Fukami M, Kuroda M. The mechanism of lack of hypocholesterolemic effects of pravastatin sodium, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, in rats. Biochim Biophys Acta. 1995;1254:7–12. doi: 10.1016/0005-2760(94)00154-q. [DOI] [PubMed] [Google Scholar]
- 34.Telford DE, Lipson SM, Barrett PH, Sutherland BG, Edwards JY, Aebi JD, et al. A novel inhibitor of oxidosqualene:lanosterol cyclase inhibits very low-density lipoprotein apolipoprotein B100 (apoB100) production and enhances low-density lipoprotein apoB100 catabolism through marked reduction in hepatic cholesterol content. Arterioscler Thromb Vasc Biol. 2005;25:2608–2614. doi: 10.1161/01.ATV.0000189158.28455.94. [DOI] [PubMed] [Google Scholar]
- 35.Mark M, Muller P, Maier R, Eisele B. Effects of a novel 2,3-oxidosqualene cyclase inhibitor on the regulation of cholesterol biosynthesis in HepG2 cells. J Lipid Res. 1996;37:148–158. [PubMed] [Google Scholar]
- 36.Eisele B, Budzinski R, Muller P, Maier R, Mark M. Effects of a novel 2,3-oxidosqualene cyclase inhibitor on cholesterol biosynthesis and lipid metabolism in vivo. J Lipid Res. 1997;38:564–575. [PubMed] [Google Scholar]
- 37.Huff MW, Telford DE. Lord of the rings--the mechanism for oxidosqualene:lanosterol cyclase becomes crystal clear. Trends Pharmacol Sci. 2005;26:335–340. doi: 10.1016/j.tips.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Pyrah IT, Kalinowski A, Jackson D, Davies W, Davis S, Aldridge A, et al. Toxicologic lesions associated with two related inhibitors of oxidosqualene cyclase in the dog and mouse. Toxicol Pathol. 2001;29:174–179. doi: 10.1080/019262301317052440. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz M, Russell DW, Dietschy JM, Turley SD. Marked reduction in bile acid synthesis in cholesterol 7α-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J Lipid Res. 1998;39:1833–1843. [PubMed] [Google Scholar]
- 40.Jones RD, Repa JJ, Russell DW, Dietschy JM, Turley SD. Delineation of biochemical, molecular, and physiological changes accompanying bile acid pool size restoration in Cyp7a1−/− mice fed low levels of cholic acid. Am J Physiol Gastrointest Liver Physiol. 2012;303:G263–G274. doi: 10.1152/ajpgi.00111.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shefer S, Hauser S, Lapar V, Mosbach EH. Diurnal variation of HMG CoA reductase activity in rat intestine. J Lipid Res. 1972;13:571–573. [PubMed] [Google Scholar]
- 42.Andersen JM, Turley SD, Dietschy JM. Relative rates of sterol synthesis in the liver and various extrahepatic tissues of normal and cholesterol-fed rabbits. Relationship to plasma lipoprotein and tissue cholesterol levels. Biochim Biophys Acta. 1982;711:421–430. doi: 10.1016/0005-2760(82)90056-x. [DOI] [PubMed] [Google Scholar]
- 43.Repa JJ, Lund EG, Horton JD, Leitersdorf E, Russell DW, Dietschy JM, et al. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J Biol Chem. 2000;275:39685–39692. doi: 10.1074/jbc.M007653200. [DOI] [PubMed] [Google Scholar]
- 44.Stange EF, Dietschy JM. Absolute rates of cholesterol synthesis in rat intestine in vitro and in vivo: a comparison of different substrates in slices and isolated cells. J Lipid Res. 1983;24:72–82. [PubMed] [Google Scholar]
- 45.Lipkin M. Proliferation and differentiation of gastrointestinal cells in normal and disease states. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1981. pp. 145–167. [Google Scholar]
- 46.Dueland S, Drisko J, Graf L, Machleder D, Lusis AJ, Davis RA. Effect of dietary cholesterol and taurocholate on cholesterol 7α-hydroxylase and hepatic LDL receptors in inbred mice. J Lipid Res. 1993;34:923–931. [PubMed] [Google Scholar]
- 47.Xie CL, Turley SD, Dietschy JM. Centripetal cholesterol flow from the extrahepatic organs through the liver is normal in mice with mutated Niemann-Pick type C protein (NPC1) J Lipid Res. 2000;41:1278–1289. [PubMed] [Google Scholar]
- 48.Post SM, Duez H, Gervois PP, Staels B, Kuipers F, Princen HM. Fibrates suppress bile acid synthesis via peroxisome proliferator-activated receptor-α-mediated downregulation of cholesterol 7α-hydroxylase and sterol 27-hydroxylase expression. Arterioscler Thromb Vasc Biol. 2001;21:1840–1845. doi: 10.1161/hq1101.098228. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz M, Russell DW, Dietschy JM, Turley SD. Alternate pathways of bile acid synthesis in the cholesterol 7α-hydroxylase knockout mouse are not upregulated by either cholesterol or cholestyramine feeding. J Lipid Res. 2001;42:1594–1603. [PubMed] [Google Scholar]
- 50.Erickson SK, Lear SR, Deane S, Dubrac S, Huling SL, Nguyen L, et al. Hypercholesterolemia and changes in lipid and bile acid metabolism in male and female cyp7A1-deficient mice. J Lipid Res. 2003;44:1001–1009. doi: 10.1194/jlr.M200489-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Hentze H, Jensen KK, Chia SM, Johns DG, Shaw RJ, Davis HR, Jr, et al. Inverse relationship between LDL cholesterol and PCSK9 plasma levels in dyslipidemic cynomolgus monkeys: Effects of LDL lowering by ezetimibe in the absence of statins. Atherosclerosis. 2013;231:84–90. doi: 10.1016/j.atherosclerosis.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 52.Costet P, Cariou B, Lambert G, Lalanne F, Lardeux B, Jarnoux AL, et al. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem. 2006;281:6211–6218. doi: 10.1074/jbc.M508582200. [DOI] [PubMed] [Google Scholar]
- 53.Wu M, Dong B, Cao A, Li H, Liu J. Delineation of molecular pathways that regulate hepatic PCSK9 and LDL receptor expression during fasting in normolipidemic hamsters. Atherosclerosis. 2012;224:401–410. doi: 10.1016/j.atherosclerosis.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langhi C, Le May C, Kourimate S, Caron S, Staels B, Krempf M, et al. Activation of the farnesoid X receptor represses PCSK9 expression in human hepatocytes. FEBS Lett. 2008;582:949–955. doi: 10.1016/j.febslet.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 55.Staedler D, Chapuis-Bernasconi C, Dehmlow H, Fischer H, Juillerat-Jeanneret L, Aebi JD. Cytotoxic effects of combination of oxidosqualene cyclase inhibitors with atorvastatin in human cancer cells. J Med Chem. 2012;55:4990–5002. doi: 10.1021/jm300256z. [DOI] [PubMed] [Google Scholar]
- 56.van der Velde AE, Vrins CL, van den Oever K, Kunne C, Oude Elferink RP, Kuipers F, et al. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 57.Brown JM, Bell TA, III, Alger HM, Sawyer JK, Smith TL, Kelley K, et al. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J Biol Chem. 2008;283:10522–10534. doi: 10.1074/jbc.M707659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vrins CL. From blood to gut: direct secretion of cholesterol via transintestinal cholesterol efflux. World J Gastroenterol. 2010;16:5953–5957. doi: 10.3748/wjg.v16.i47.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marshall SM, Kelley KL, Davis MA, Wilson MD, McDaniel AL, Lee RG, et al. Reduction of VLDL secretion decreases cholesterol excretion in Niemann-Pick C1-Like 1 hepatic transgenic mice. PLoS One. 2014;9:e84418. doi: 10.1371/journal.pone.0084418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones RD, Taylor AM, Tong EY, Repa JJ. Carboxylesterases are uniquely expressed among tissues and regulated by nuclear hormone receptors in the mouse. Drug Metab Dispos. 2013;41:40–49. doi: 10.1124/dmd.112.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moron B, Verma AK, Das P, Taavela J, Dafik L, DiRaimondo TR, et al. CYP3A4-catalyzed simvastatin metabolism as a non-invasive marker of small intestinal health in celiac disease. Am J Gastroenterol. 2013;108:1344–1351. doi: 10.1038/ajg.2013.151. [DOI] [PubMed] [Google Scholar]
- 62.Diczfalusy U, Nylen H, Elander P, Bertilsson L. 4β-hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br J Clin Pharmacol. 2011;71:183–189. doi: 10.1111/j.1365-2125.2010.03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duniec-Dmuchowski Z, Fang HL, Strom SC, Ellis E, Runge-Morris M, Kocarek TA. Human pregnane X receptor activation and CYP3A4/CYP2B6 induction by 2,3-oxidosqualene:lanosterol cyclase inhibition. Drug Metab Dispos. 2009;37:900–908. doi: 10.1124/dmd.108.025130. [DOI] [PMC free article] [PubMed] [Google Scholar]