Abstract
This study aimed to assess the impacts of ultraviolet-B (UV-B) radiation over a 28-day period on the levels of pigments of Umbilicaria aprina and Bryum argenteum growing in field. The depletion of stratospheric ozone is most prominent over Antarctica, which receives more UV-B radiation than most other parts of the planet. Although UV-B radiation adversely affects all flora, Antarctic plants are better equipped to survive the damaging effects of UV-B owing to defenses provided by UV-B absorbing compounds and other screening pigments. The UV-B radiations and daily average ozone values were measured by sun photometer and the photosynthetic pigments were analyzed by the standard spectrophotometric methods of exposed and unexposed selected plants. The daily average atmospheric ozone values were recorded from 5 January to 2 February 2008. The maximum daily average for ozone (310.7 Dobson Units (DU)) was recorded on 10 January 2008. On that day, average UV-B spectral irradiances were 0.016, 0.071, and 0.186 W m-2 at wavelengths of 305, 312, and 320 nm, respectively. The minimum daily average ozone value (278.6 DU) was recorded on 31 January 2008. On that day, average UV-B spectral irradiances were 0.018, 0.085, and 0.210 W m-2 at wavelengths of 305, 312, and 320 nm, respectively. Our results concludes that following prolonged UV-B exposure, total chlorophyll levels decreased gradually in both species, whereas levels of UV-B absorbing compounds, phenolics, and carotenoids gradually increased.
Keywords: Carotenoids, phenolics, total chlorophyll, UV-B absorbing compounds, UV-B radiation
INTRODUCTION
The stratospheric ozone layer prevents most UV radiation from penetrating into the earth's surface despite permitting penetration of a limited amount of UV radiation. The first observation of a drastic reduction in total ozone over Halley Bay in Antarctica was reported by Farman et al.,[1] and later confirmed by other researchers.[2,3,4] Chlorofluorocarbons (CFCs) and some other air pollutants that diffuse into the ozone layer are responsible for the destruction of the ozone layer, which increases the amount of UV radiation that reaches the earth's surface and adversely affects most living organisms. The potential effects of UV-B radiation on phototrophic organisms may be broadly classified as (a) changes in photosynthesis and growth,[5,6] (b) increased investment in the synthesis and accumulation of UV-B absorbing or screening compounds,[7,8] and (c) DNA damage, repair, and photoreactivation.[9]
Continental Antarctic vegetation is sparse and cryptogamic in nature, with lichens and mosses constituting the dominant flora.[10,11] Lichens, an important component of Antarctic ecosystem, grow in compact turves, mats, and small moss cushions, all of which enable them to collect and retain more water. Moss growths are restricted to melted lakes, streams, and other locations where free water is available.[12]
The responses of photosynthetic pigments in plant leaves or thalli to UV-B exposure often differ between studies, with different experiments showing either an increase,[13] no change,[14,15] or a decrease[16,17,18,19] in chlorophyll concentrations. Phenolic compounds protect plants from exposure to UV-B, most likely by contributing to the decrease in active oxygen species by acting as antioxidants.[20,21,22] Here, we report the results of field experiments that examined total levels of chlorophyll, carotenoids, UV-B absorbing compounds, and phenolics in Umbilicaria aprina and Bryum argenteum with and without UV-B exposure at Schirmacher Oasis in East Antarctica.
MATERIALS AND METHODS
Selection of sites
Experiments involving U. aprina were conducted at sites east of the “Maitri” Indian Research Station (70°45.877'S, 011°43.400'E). Experiments involving B. argenteum were conducted at sites near the banks of Priyadarshni Lake (70°45.908'S, 011°43.507'E), Schirmacher Oasis. These sites were selected on the basis of availability of plant specimens. The UV filter-frame field experiments with moss (B. argenteum) and lichen (U. aprina) were conducted for 28 continuous days, beginning on 5 January 2008 and ending on 2 February 2008.
Selection of plant species
Both U. aprina and B. argenteum grow naturally within the “Maitri” Schirmacher Oasis region, along with other cryptogamic vegetation. U. aprina, the most commonly occurring foliose lichen in the Schirmacher Oasis region, is attached to rocks by a central umbilicus. The size of the thallus varies from a few millimeters to about 16 cm in diameter. The B. argenteum moss we studied were small to medium sized, with a height ranging from 1 to 25 mm, and were also growing naturally on moist and rocky sites near the melting ice areas of Priyadarshni Lake. Both of the plants selected for field experiments were growing in such a way that they were exposed to light uniformly and we could install UV filter frames on flat surfaces to prevent the perturbation of environmental conditions other than UV-B irradiation.
UV filter frames
The UV filter frames (30.5 cm length × 30.5 cm width × 30.5 cm height) each comprised iron stands covered by a Plexiglas acrylic sheet, to ensure that plants received photosynthetically active radiation, but not UV-B radiation. The acrylic sheet absorbs 98% of total-UV radiation. Each acrylic sheet was 3-mm thick, with a surface area of 30.5 cm2. The sides of UV filter frames were perforated using a drill bit (37 mm diameter) to create uniform holes to ensure a free flow and exchange of moisture and gases between the environment and the space enclosed by the frames. The holes cover 10% of the total area of the UV filter frames so that moisture and all other environmental parameters besides UV-B irradiation remain identical with and without UV-B exposure. Cubical frames were established over the selected plants at five different sites to control UV-B exposure.
Analyses of pigments
Pigments were analyzed from plants grown without a UV - B filter frame as well as plants covered with UV - B filter frame to prevent exposure to UV-B. Plant samples were harvested at 7-d intervals from 5 January to 2 February 2008, washed with double-distilled water, and blotted dry using Whatman filter paper No. 1 before their fresh weights were recorded. Standard methodologies were used to estimate pigment contents of both species under the two different conditions.
Photosynthetic pigments (total chlorophyll and carotenoids) analyses
Plant samples were homogenized using a pestle and mortar in 80% (v/v) acetone. The ratio of fresh tissue to volume of acetone used for extraction is 10:1 (w/v). Samples were maintained at 4°C throughout the extraction period and centrifuged at a speed corresponding to approximately 25,000g for 15 min in a refrigerated centrifuge. The supernatant solutions from centrifuged samples were filtered through Whatman filter paper No. 1 and their absorbances at 663, 645, 480, and 510 nm were determined using a Systronics UV-VIS spectrophotometer-117. The chlorophyll content was calculated from absorbance values at 663 and 645 nm.[23] The carotenoid content was calculated from absorbance values at 480 and 510 nm using a previously described method of Parsons et al.[24]
Photoprotective pigments (UV-B absorbing compounds and phenolics) analyses
Levels of UV - B absorbing compounds were assessed as described previously.[25] Plant samples were placed in 25 ml Erlenmeyer flasks containing 5 ml of acidified methanol (MeOH: HCl: H2O, 90:1:1 (v/v)), and the contents of the flask were heated to 60°C and stirred at that temperature for 10 min before cooling to room temperature over 15 min and filtering through 90 μm filters. Concentrations of soluble UV-B absorbing compounds were estimated by measuring the absorbance of the filtrate at 300 nm using a spectrophotometer.
To assay for levels of phenolics, a 10% (w/v) homogenate was prepared in a methanolic HCl solvent (50% methanol, 0.05% concentrated HCl, approximate pH of 3.5) at room temperature. Extracts were acidified to an approximate pH of 1.0 by the addition of HCl (1 M). The precipitate was allowed to settle for 15 h in the dark and filtered through Whatman No. 5 filter paper. The absorbance of the supernatant solution was measured at 280 nm as described previously.[26]
Measurement of ozone and UV-B radiation
The UV-B radiation at 305, 312, and 320 nm wasand data on the thickness of the ozone layer were measured at selected sites using a sun photometer (Microtop II 4701, Solar Light) over a 28-day period beginning on 5 January 2008 and ending on 2 February 2008.
Statistical analysis
The statistical analysis was performed by using graph pad Prism 3.0 and five replicates for the experiment were taken. Two-way analysis of variance (ANOVA) was used for pigments of exposed and unexposed experimental plants. The differences between treatments were considered significant at the P < 0.05 level.
RESULTS
UV-B radiation and ozone levels
Over the 28-day period when ozone levels were measured (5 January–2 February 2008), the average ozone level over the “Maitri” Schirmacher Oasis region was 295.4 DU [Figure 1a]. The maximum daily average ozone value, recorded on 10 January 2008, was 310.7 DU. On the same day, UV-B spectral irradiances of 0.016, 0.071, and 0.186 W m−2 were recorded at wavelengths of 305, 312, and 320 nm, respectively. The minimum daily average ozone value, recorded on 31 January 2008, was 278.6 DU. On the same day, UV-B spectral irradiances of 0.018, 0.085, and 0.210 were recorded at wavelengths of 305, 312, and 320 nm, respectively.
Figure 1.
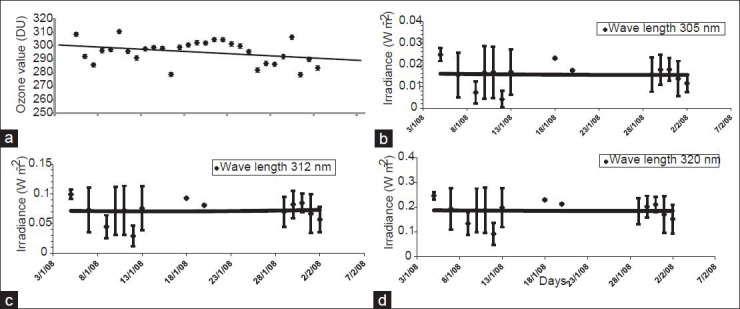
Fluctuations in environmental parameters in the “Maitri” Schirmacher Oasis region over the experimental period. (a) Average atmospheric ozone concentrations. (b) Changes in daily average spectral irradiance at 305 nm. (c) Changes in daily average spectral irradiance at 312 nm. (d) Changes in daily average spectral irradiance at 320 nm
Throughout the study period, values for the daily average UV-B radiation above the selected sites were 0.016, 0.071, and 0.186 W m− 2 at wavelengths of 305, 312, and 320 nm, respectively [Figure 1b–d]. Of all of the daily average measurements of UV-B levels made, the highest were recorded on 5 January 2008, with values of 0.025, 0.10, and 0.25 W m− 2 at wavelengths of 305, 312, and 320 nm, respectively. At that time, average ozone values were 308.6 DU. The minimum daily average UV-B values, recorded on 12 January 2008, were 0.004, 0.028, and 0.091 W m− 2 at wavelengths of 305, 312, and 320 nm, respectively. At that time, average ozone values were 291.1 DU.
Photosynthetic pigments (total chlorophyll and carotenoids)
For both species, total chlorophyll concentrations decreased gradually throughout the period of exposure to UV-B. In contrast, carotenoid concentrations of both species increased gradually over the same period under the similar conditions. A significant decrease in total chlorophyll (P < 0.02) was observed for both species after 28 days of exposure to UV-B [Figure 2], although slight decreases measured after 7, 14, and 21 days of exposure were not statistically significant. In both species, a significant increase in carotenoid concentrations (P < 0.001) was found after 28 days of exposure to UV-B, although increases measured after 7, 14, and 21 days of exposure were not statistically significant [Figure 3]. There were no significant changes in total levels of chlorophyll and carotenoids for either species when plants were not exposed to UV-B.
Figure 2.
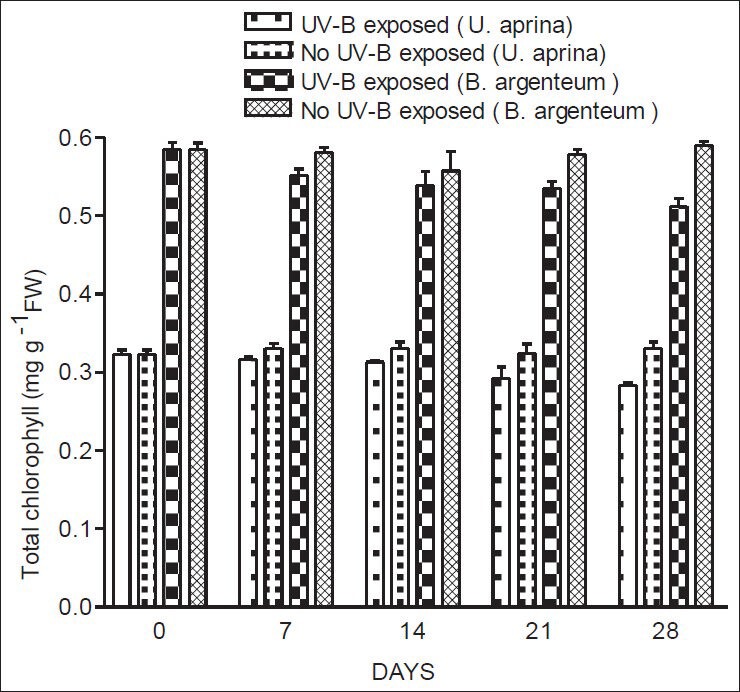
Concentrations of total chlorophyll in U. aprina and B. argenteum with and without exposure to UV-B at Schirmacher Oasis in East Antarctica
Figure 3.
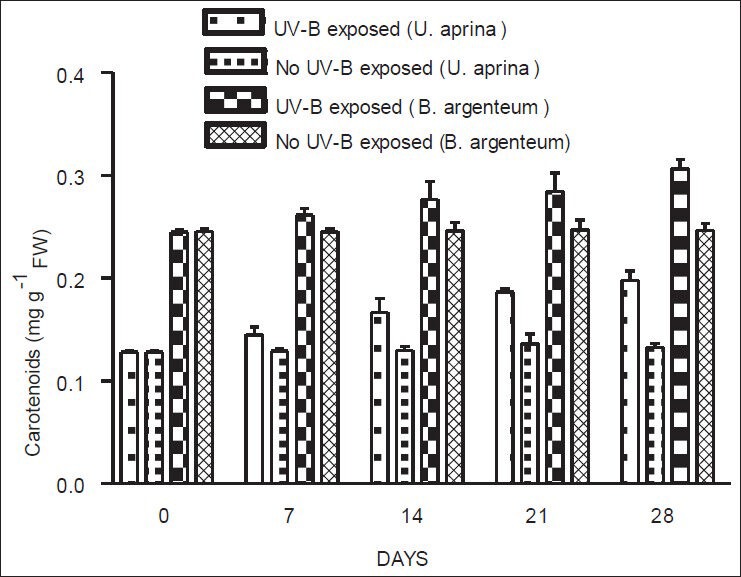
Concentrations of carotenoids in U. aprina and B. argenteum with and without exposure to UV-B at Schirmacher Oasis in East Antarctica
Photoprotective pigments (UV-B absorbing compounds and phenolics)
In both species, levels of UV - B absorbing compounds and phenolics both increased gradually following exposure to UV-B. The increase in UV - B absorbing compounds of both species was significant (P < 0.05) after 28 days of continuous UV-B exposure [Figure 4]. In U. aprina, a significant increase (P < 0.02) in phenolics was recorded on day 21 under UV-B-exposed conditions [Figure 5]. However, in B. argenteum, a significant increase in phenolics (P < 0.001) was only evident at the end of the experiment (after 28 days of UV-B exposure) [Figure 5]. No significant changes in UV-B absorbing compounds and phenolics were observed without exposure to UV-B.
Figure 4.
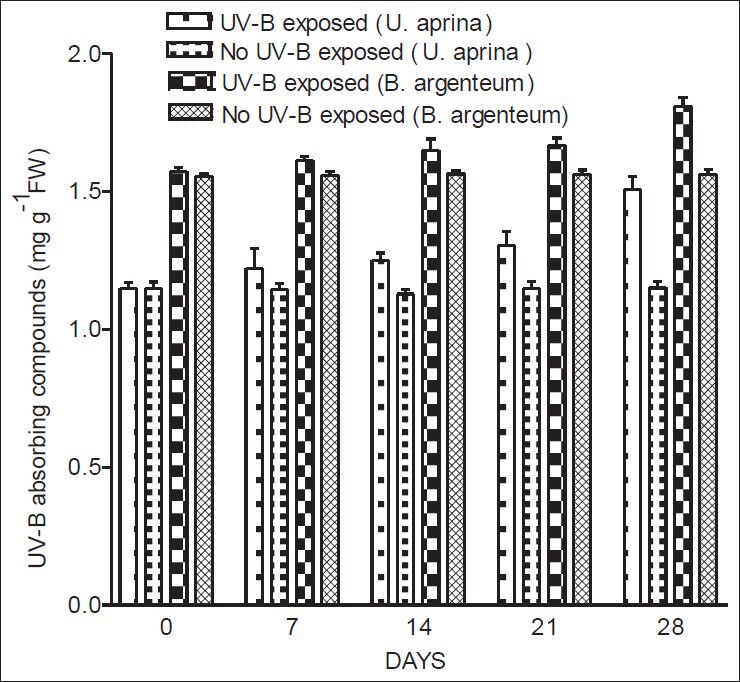
Concentrations of UV-B absorbing compounds in U. aprina and B. argenteum with and without exposure to UV-B at Schirmacher Oasis in East Antarctica
Figure 5.
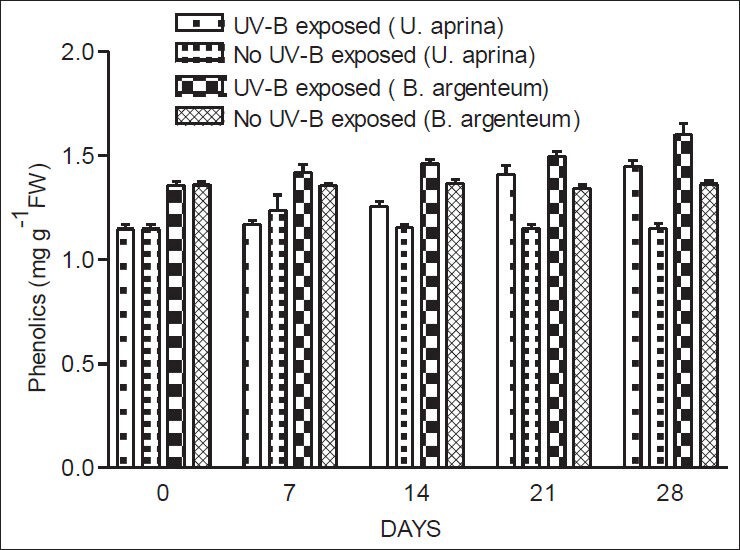
Concentrations of phenolics in U. aprina and B. argenteum with and without exposure to UV-B at Schirmacher Oasis in East Antarctica
DISCUSSION
Our study demonstrated that changes in UV-B radiation altered the pigmentation of B. argenteum and U. aprina. It shows that the total chlorophyll concentration of both plants decreases gradually following exposure to UV-B for a 28-day period. Conversely, carotenoid concentrations in B. argenteum and U. aprina increased gradually over the same time and under identical conditions. Robinson et al.,[18] reported lower concentrations of chlorophyll in Grimmia antarctici under near-ambient UV radiation and correspondingly high relative concentration of carotenoids under reduced UV radiation at Windmill Islands of east Antarctica. For Sanionia uncinata and Cephaloziella varians, carotenoid contents increased under naturally elevated UV-B radiation, although the total chlorophyll concentration was not affected by ozone depletion over a 4-6 week in situ study.[27] Nevertheless, many workers have reported no effect of UV-B radiation on the chlorophyll concentration of plants.[28,29,30,31] For instance, a study similar to ours, involving the lichens Lobaria pulmonaria and Xanthoria aureola (also known as Xanthoria ectaneoides), documented no significant reduction in either chlorophyll a or chlorophyll b at different UV-B levels under laboratory conditions.[32] Similarly, exposure of the lichen Turgidosculum complicatulum to various combinations of UV radiation had no significant effect on levels of chlorophyll, carotenoids, and UV-absorbing compounds or photosystem II efficiency.[33]
Throughout the plant kingdom, UV-B absorbing pigments play essential roles in the absorption of biologically damaging UV-B radiation, whereas transmitting essential photosynthetically active radiation.[7] For example, concentrations of UV-B screening pigments in C. varians and S. uncinata were positively correlated with daily doses of UV-B radiation at Rothera Point over a 4-6 week in situ study.[27] De la Rosa et al.,[34] reported that induction of several phenolics takes place in silver birch (Betula pendula) by UV-B radiation and their concentration was dependent on UV-B daily time-integrated irradiance. Analysis of the response to UV-B radiation over a season (November 1999-March 2000) revealed higher concentrations of UV - B absorbing compounds in the two cosmopolitan moss species Bryum pseudotriquetrum and Ceratodon purpureus but lower concentrations of UV - B absorbing compounds in Schistidium antarctici. Another study, conducted on Svalbard, observed no changes in UV-B absorbing compounds in high Arctic tundra plant in response to UV-B radiation.[35] Several other studies on the effects of UV on plants suggested that lichens contain a variety of polyphenolic compounds, including usnic acid, parietin, and melanin, with strong capacities to absorb UV radiation and that might contribute to photoprotection mechanisms in lichen.[36,37,38,39] Our results also shows that UV - B absorbing compounds and phenolics in B. argenteum and U. aprina increases gradually on the exposure of UV-B and protects the plants from the damaging effects of UV-B radiation.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the National Centre for Antarctic and Ocean Research (NCAOR, Goa), Ministry of Earth Sciences, New Delhi, India.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Farman JC, Gardiner BG, Shanklin JD. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature. 1985;315:207–10. [Google Scholar]
- 2.Bodeker GE, Connor BJ, Liley JB, Matthews WA. The global mass of ozone: 1978 − 1998. Geophys Res Lett. 2001;28:2819–22. [Google Scholar]
- 3.McKenzie RL, Bjorn LO, Bais A, Ilyasd M. Change in biologically active ultraviolet radiation reaching the Earth's surfaces. Photochem Photobiol Sci. 2003;2:5–15. doi: 10.1039/b211155c. [DOI] [PubMed] [Google Scholar]
- 4.Switzerland, Geneva: 2007. WMO. Scientific assessment of ozone depletion: Global ozone research and monitoring project-report No. 50, 2006. [Google Scholar]
- 5.Xiong FS, Day TA. Effect of solar ultraviolet-B radiation during springtime ozone depletion on photosynthesis and biomass production of Antarctic vascular plants. Plant Physiol. 2001;125:738–51. doi: 10.1104/pp.125.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruhland CT, Xiong FS, Clark WD, Day TA. The influence of Ultraviolet-B radiation on growth, hydroxycinnamic acids and flavonoids of Deschampsia antarctica during spring time ozone depletion in Antarctica. Photochem Photobiol. 2005;81:1086–93. doi: 10.1562/2004-09-18-RA-321. [DOI] [PubMed] [Google Scholar]
- 7.Cockell CS, Knowland J. Ultraviolet radiation screening compounds. Biol Rev Camb Philos Soc. 1999;74:311–45. doi: 10.1017/s0006323199005356. [DOI] [PubMed] [Google Scholar]
- 8.Searles P, Flint SD, Caldwell MM. A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia. 2001;127:1–10. doi: 10.1007/s004420000592. [DOI] [PubMed] [Google Scholar]
- 9.Lud D, Buma AG, Van de Poll WH, Moerdijk TC, Huiskes AH. DNA damage and photosynthetic performance in the Antarctic terrestrial alga Prasiola crispa antarctica (Chlorophyta) under manipulated UV-B radiation. J Phycol. 2001;37:459–67. [Google Scholar]
- 10.Smith RIL. Terrestrial plant biology of the sub-antarctic and antarctic. In: Laws RM, editor. Antarctic Ecology. London: Academic Press; 1984. pp. 61–162. [Google Scholar]
- 11.Singh J, Dubey AK, Singh RP. Antarctic terrestrial ecosystem and role of pigments in enhanced UV-B radiations. Rev Environ Sci Biotech. 2011;10:63–7. [Google Scholar]
- 12.Melick DR, Hovendon M, Seppelt R. Vegetation patterns in relation to climatic and endogenous changes in Wilkes Land, continental Antarctica. J Ecol. 1997;85:43–56. [Google Scholar]
- 13.Niemi R, Martikainen P, Silvola J, Wulff A, Turtola S, Holopainen T. Responses of two Sphagnum moss species and Eriophorum vaginatum to enhanced UV-B in a summer of low UV intensity. New Phytol. 2002a;156:509–15. doi: 10.1046/j.1469-8137.2002.00532.x. [DOI] [PubMed] [Google Scholar]
- 14.Niemi R, Martikainen P, Silvola J, Wulff A, Turtola S, Holopainen T. Elevated UV-B radiation alters fluxes of methane and carbondioxide in peatland microcosms. Glob Change Biol. 2002b;8:361–71. [Google Scholar]
- 15.Lappalainen NM, Huttunen S, Suokanerva H. Acclimation of the pleurocarpous moss Pleurozium schreberi (Britt.) Mitt. to enhanced ultraviolet radiation in-situ. Glob Change Biol. 2008;14:321–33. [Google Scholar]
- 16.Gehrke C. Effects of enhanced UV-B radiation on production-related properties of a Sphagnum fuscum dominated sub-arctic bog. Funct Ecol. 1998;12:940–7. [Google Scholar]
- 17.Gehrke C. Impacts of enhanced ultraviolet-B radiation on mosses in a subarctic heath ecosystem. Ecol. 1999;80:1844–51. [Google Scholar]
- 18.Robinson SA, Turnbull JD, Lovelock CE. Impact of changes in natural ultraviolet radiation on pigment composition, physiological and morphological charecterastics of the Antarctic moss, Grimmia antarctici. Glob Change Biol. 2005;11:476–89. [Google Scholar]
- 19.Newsham KK, Geissler PA, Nicolson MJ, Peat HJ, Lewis-Smith RI. Sequential reduction of UV-B radiation in the field alters the pigmentation of an Antarctic leafy liverwort. Environ Exp Bot. 2005;54:22–32. [Google Scholar]
- 20.Husain SR, Cillard J, Cillard P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry. 1987;26:2489–91. [Google Scholar]
- 21.Markham KR, Tanner GJ, Caasi-Lit M, Whitecross MI, Nayudu M, Mitchell KA. Possible protective role for 3, 4-dihydroxyflavones induced by enhanced UV-B in a UV-tolerant rice cultivar. Phytochemistry. 1998;49:1913–9. [Google Scholar]
- 22.Bjerke JW, Lerfall K, Elvebakk A. Effects of ultraviolet radiation and PAR on the content of usnic and divaricatic acids in two arctic-alpine lichens. Photochem Photobiol Sci. 2002;1:678–85. doi: 10.1039/b203399b. [DOI] [PubMed] [Google Scholar]
- 23.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenyl oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons TR, Maita Y, Lalli CM. Oxford: Pergamon; 1984. A manual of chemical and biological methods for seawater analysis. [Google Scholar]
- 25.Ruhland CT, Day TA. Size and longevity of seed banks in Antarctica and the influence of ultraviolet-B radiation on survivorship, growth and pigment concentrations of Colobanthus quitensis seedlings. Environ Exp Bot. 2001;45:143–54. doi: 10.1016/s0098-8472(00)00089-7. [DOI] [PubMed] [Google Scholar]
- 26.Pirie A, Mullins MG. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissue treated with sucrose nitrate and abscisic acid. Plant Physiol. 1976;58:468–72. doi: 10.1104/pp.58.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newsham KK, Hodgson DA, Murray AW, Peat HJ, Lewis Smith RI. Response of two Antarctic bryophytes to stratospheric ozone depletion. Glob Change Biol. 2002;8:1–12. [Google Scholar]
- 28.Day TA, Ruhland CT, Grobe CW, Xiong F. Growth and reduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia. 1999;199:24–35. doi: 10.1007/s004420050757. [DOI] [PubMed] [Google Scholar]
- 29.Lud D, Moerdijk T, Van de Poll W, Buma AG, Huiskes AH. DNA damage and photosynthesis in Antarctic and Arctic Sanionia uncinata (Hedw.) Loeske under ambient and enhanced levels of UV-B radiation. Plant Cell Environ. 2002;25:1579–89. [Google Scholar]
- 30.Singh J, Gautam S, Pant AB. Effect of UV-B radiation on UV absorbing compounds and pigments of moss and lichen of Schirmacher Oasis region, East Antarctica. Cell Mol Biol. 2012;58:76–80. [PubMed] [Google Scholar]
- 31.Newsham KK. UV-B radiation arising from stratospheric ozone depletion influences the pigmentation of the moss Andreaea regularis. Oecologia. 2003;135:327–31. doi: 10.1007/s00442-003-1191-x. [DOI] [PubMed] [Google Scholar]
- 32.Larsson P, Vecerova K, Cempirkova H, Solhaug KA, Gauslaa Y. Does UV-B influence biomass growth in lichen deficient in sun-screening pigment? Environ Exp Bot. 2009;67:215–21. [Google Scholar]
- 33.Lud D, Huiskes A, Moerdijk T, Rozema J. The effects of altered levels of UV-B radiation on an Antarctic grass and lichen. Plant Ecol. 2001b;154:89–99. [Google Scholar]
- 34.de la Rosa TM, Julkunen-Tiitto R, Lehto T, Aphalo PJ. Secondory metabolics and nutrient concentrations in silver bitch seedling under five levels of daily UV-B exposure and two relative nutrient addition rates. New Phytol. 2001;150:121–31. [Google Scholar]
- 35.Rozema J, Boelen P, Solheim B, Zielke M, Buskens A, Doorenbosch M, et al. Stratospheric ozone depletion: High arctic tundra plant growth on Svalbard is not affected by enhanced UV-B after 7 years of UV-B supplementation in the field. Plant Ecol. 2006;182:121–35. [Google Scholar]
- 36.Solhaug KA, Gauslaa Y. Photosynthates stimulate the UV-B induced fungal anthraquinone synthesis in the foliose lichen Xanthoria parietina. Plant Cell Environ. 2004;27:167–76. [Google Scholar]
- 37.Gauslaa Y, McEvoy M. Seasonal changes in solar radiation drive acclimation of the sun-screening compounds parietin in the lichen Xanthoria parietina. Basic Appl Ecol. 2005;6:75–82. [Google Scholar]
- 38.McEvoy M, Nybakken L, Solhaug KA, Gauslaa Y. UV trigger the synthesis of the widely distributed secondary compounds usnic acid. Mycol Pro. 2006;5:221–9. [Google Scholar]
- 39.Nybakken L, Julkunen-Tiitto R. UV-B induced usnic acid in reindeer lichens. Lichenologist. 2006;38:477–85. [Google Scholar]


