Abstract
Context:
To explore genotoxicity in bidi rollers occupationally exposed to bidi tobacco dust.
Aims:
To assess the extent of genotoxicity of tobacco dust to bidi rollers of Jabalpur, Madhya Pradesh, India and cytotoxicity of bidi tobacco extract.
Settings and Design:
Blood samples from 31 bidi rollers and 30 controls taken after written informed consent were analyzed for chromosome aberrations (CA) and comet assay.
Materials and Methods:
Genotoxicity was studied by CA in cultured peripheral blood lymphocytes of bidi rollers and the deoxyribonucleic acid (DNA) damage studies were done by comet assay of their blood. The toxicity of bidi tobacco extract to normal human lymphocytes was studied by MMT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay as drop in viability.
Statistical Analysis Used:
Student's t-test and DMRT.
Results:
There is a general trend of increase in CA% of both in exposed and control groups with age, but in every group the bidi rollers have a significantly higher CA% than the controls. The CA % is also directly related to exposure. The comet assay findings reveal that the mean comet length and tail length increases with exposure time. The toxicity of bidi tobacco extract (TE) to normal human lymphocytes was tested in vitro by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay at 2 h of incubation. The trend of drop in viability with increasing concentrations of TE was clearly evident from the data from four donors in spite of their individual differences in viability.
Conclusions:
The results obtained in this investigation indicate that bidi rollers seem to be facing the occupational hazard of genotoxicity due to handling bidi tobacco and inhalation of tobacco dust. They should be advised to work under well-ventilated conditions.
Keywords: Chromosome aberration, comet assay, genotoxicity in bidi rollers, MTT assay, tobacco dust
INTRODUCTION
Tobacco (Nicotiana tobacum; Family-Solanaceae) is a major cash crop grown widely in India and many farmers with small holdings work for as well as grow tobacco for wealthy multinational companies. Tobacco production provides employment and livelihood to above 6 million tobacco farmers, 4.4 million bidi rollers, and more than 2 lakh Tendu leaf (Diospyros melonoxylon) pluckers in India. Bidi rollers and Tendu pluckers comprising women and children are among the most exploited working groups in the country earning bare minimum wages and unable to access healthcare facilities[1] and they work in ill-ventilated and confined environments mostly from their homes.
It is well-known that tobacco contains a variety of toxic substances such as nicotine, nitrosamines, polycyclic aromatic hydrocarbons, formaldehyde, hydrogen, etc., which are released into the ambient air during the processing of bidis. Nicotine released from the tobacco leaves can be absorbed in body tissues including skin, respiratory epithelium, and mucous membrane of the mouth. The carcinogenetic potential of tobacco is well-known.[2,3] Umadevi et al.,[3] evaluated the cytogenetic effects of exposure to tobacco dust in male tobacco factory workers and age- and sex-matched controls by evaluating chromosomal aberrations (CA) in peripheral blood lymphocytes. A statistically significant increase was observed in the frequency of CA in nonsmoking and smoking groups of workers when compared to their respective controls. An increase in the frequency of CA was also observed with increase in years of service in the exposed group. Tobacco dust can also cause occupational asthma[4] and it has also been reported to cause respiratory tract diseases such as wheezing, dyspnea, and rhinitis.[5,6] It may also cause nausea, dizziness, and vomiting. Thus, bidi rollers (which are mostly women) are exposed to tobacco constituents through the cutaneous route or through inhalation of tobacco dust.[7] On an average each roller makes 500-1,000 bidis and handles 225450 g of tobacco/day,[8] so they are exposed to a good amount of tobacco dust which may be having cumulative effects on their genetic material with the years of exposure.
Genotoxic studies in bidi workers of this region have not been done although it is a major center for bidi making. Thus, the present investigation was undertaken to study the genotoxic effects of bidi rolling as an occupational hazard with the objectives to study the chromosomal and deoxyribonucleic acid (DNA) damage caused in them.
MATERIALS AND METHODS
Sample collection and blood culture
Peripheral blood samples intended for lymphocyte culture and alkaline comet assay were taken from 31 healthy female bidi rollers and 30 healthy volunteers as controls ranging from 25 to 65 years and were age-matched for comparison of the results with their written informed consent prior to their inclusion in this study. The selected bidi rollers and controls were neither smokers nor did they indulge in tobacco chewing. All the subjects filled in a pro forma in which they gave information about their age, exposure (duration of work), addictions, medications, and illness, if any. The study was approved by the institutional ethical committee. The age of the rollers ranged from 25 to 68 years and their occupational exposure ranged from 13 to 58 years. The peripheral blood samples were collected by brachial venipuncture in sterile heparinized vials and blood culture was done according to the method of Moorhead et al.[9]
Chromosome aberrations
For CA% analysis blood was cultured using TC199 (Hi Media) medium supplemented with fetal calf serum (Hi Media), with phytohemagglutinin (PHA; 10 μg/ml) as a mitogen. After arresting metaphase with colchicine (10 μg/ml), slides were prepared according to the standard air drying/hypotonic/Giemsa technique and analyzed for CA (chromatid breaks, aneuploidy, and dicentric and acentric fragments). Hundred, well-spread metaphase plates were scored for each donor. The data was statistically analyzed by the application of t-test.
Comet assay
The comet assay was performed by Trevigen's (USA) Comet Assay™ Silver Kit. The method was originally given by Singh et al.,[10] It involved following steps.
In brief, blood was combined with low melting (LM) agarose in a ratio of 1:10 and 75 μl of this mixture was spread over sample area on the comet slide. After drying at 4°C the slides were immersed in precooled lysis solution for 40 min. They were then immersed in freshly prepared alkaline unwinding solution (pH > 13). The slides were removed and immersed in electrophoresis buffer (pH > 13). The electrophoresis run was for 30 min at 25 V and then stained by silver staining method. Scoring was done by Comet Score 15 software to get data of comet length, tail length, and tail moment. Data from 50 cells of each donor was collected and was compared to that of controls. t-test was applied to find the significance of differences in the parameters studied between controls and bidi rollers.
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay
Preparation of tobacco extract used for toxicity testing
Tobacco was obtained from bidi rollers and pulverized. TE was prepared in a weight/volume of 1:10 by the standard method of Sheldon et al.[11] The extract was prepared by defatting the material with diethyl ether (boiling point 34°C). A 1:5 weight/volume extract was prepared by stirring the defatted material in phosphate buffered saline (PBS) for 72 h at 4°C. The extract was centrifuged and the supernatant was dialyzed for 48 h against PBS and after that for 24 h against distilled water. Subsequently, the supernatant was filtered under sterile conditions. The filtered extract was stored at −20°C and designated as 100% aqueous extract of smokeless tobacco.[12]
The 100% aqueous TE was appropriately diluted with PBS under sterile conditions to give 3, 6, 12.5, 25, and 50% solutions of TE.
Isolation of lymphocytes from whole blood
Blood (3 ml) from healthy male volunteer donors of 24-27 age groups was collected in sterile heparinized vials. This was diluted with an equal volume of PBS (1 ×). Two milliliter of HiSep™ Lymphocyte Separation Medium (LSM) 1077 (Hi Media) was transferred aseptically into a centrifuge tube. This was then carefully overlaid with 6 ml of diluted blood. It was centrifuged at 400 × g at room temperature (RT) for 20 min. Erythrocytes were sedimented and the lymphocytes formed a layer above the Hi Sep layer. Most of the supernatant was aspirated out and then the lymphocyte layer along with half of the Hi Sep layer was carefully aspirated into a separate centrifuge tube. It was then given two washes with isotonic PBS. The cells were counted in a hemocytometer and appropriately diluted in TC 199 medium (Hi Media) supplemented with fetal bovine serum to give a final concentration of 5 × 105 cells/ml.
MTT assay
Aliquots (180 μl) of the prepared lymphocyte suspension (5 × 105 cells/ml) were seeded into a 96-well polystyrene tissue culture plate in six replicates. One row for each donor containing only medium and cells served as a control. Aliquots of 20 μl of the TE (3, 6, 12.5, 25, and 50% solutions) were added to the wells. Each concentration was tested in six replicates. The experiment was performed with four different healthy donors of the same age and socioeconomic status. The plate was incubated for 2 h at 37°C. Cells incubated in culture medium alone served as a control for cell viability which was taken as 100%. After incubation, 20 μl aliquots of MTT solution (5 mg/ml in PBS) were added to each well and reincubated for 2 h at 37°C followed by low centrifugation at 800 rpm for 5 min. Then 100 μl of supernatant was removed and 60 μl of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystals followed by incubation at 37°C for 30 min. The culture plates were then placed in an enzyme-linked immunosorbent assay (ELISA) microplate reader and absorbance was read at 600 nm. The amount of color produced was directly proportional to the number of viable cells. Before the procedure, the optical density (OD) of the empty microplate wells was also noted. OD of various concentrations of TE was noted and the final OD was calculated after making the due adjustment for these two factors. All assays were performed in six replicates per dose.
Cell viability rate was calculated as the % of MTT absorption as follows:
% survival = (Mean experimental absorbance/Mean control absorbance) × 100.
RESULTS
CA
A scan of the metaphase plates of the subjects and the controls indicated that there was a general trend of increase in CA% with age both in the nonexposed (control groups) and the exposed groups (bidi rollers) [Table 1]. Some of the aberrations such as extra acentric fragment and dicentric chromosome are shown in Figures 1 and 2. The CA% increased uniformly with age in the control group. In general the bidi rollers had a greater percentage of CA than the controls of the same age groups. However, the CA% in the 50-55 years age group of bidi rollers showed a lesser value than the 45-50 years age group. In each group the increase in CA% in bidi rollers was significant (P < 0.01) when compared to the CA% of the controls of the same group. The CA% was also related to the duration of exposure [Table 2].
Table 1.
Effect of age on chromosome aberrations (CA%) in bidi rollers
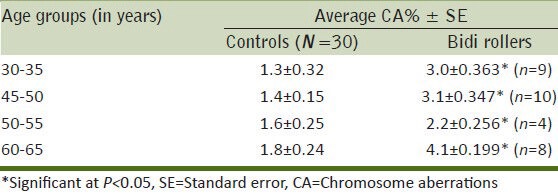
Figure 1.
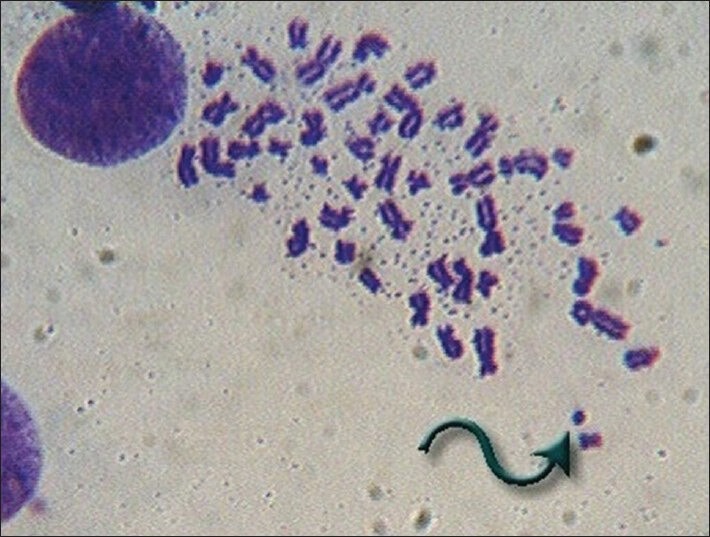
Acentric fragment
Figure 2.
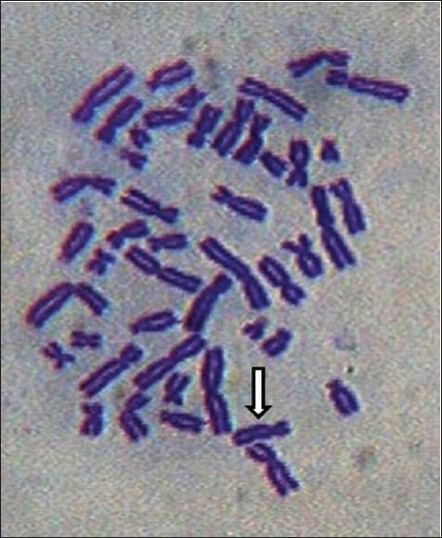
Dicentric chromosome
Table 2.
Effect of duration of exposure to tobacco dust on CA% in bidi rollers

Comet assay
The data for comet assay parameters was collected by the scan of the comet slides by CometScore 15 software. The observations for comet lengths and tail lengths of the exposed groups and controls are presented in Table 3 and Figure 3.
Table 3.
Effect of duration of exposure (years) to tobacco dust on comet length and tail length in bidi rollers
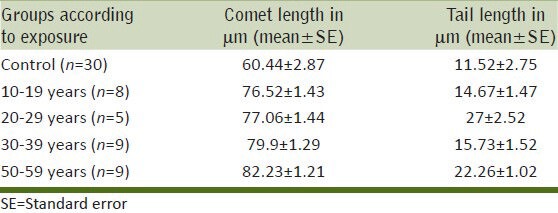
Figure 3.
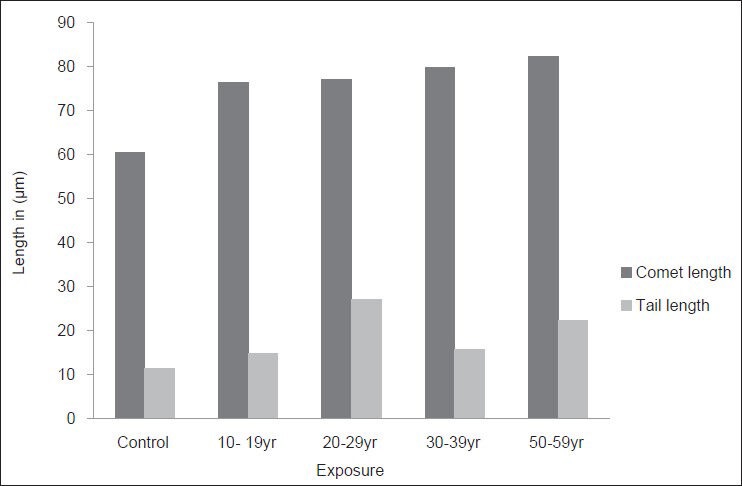
Effect of duration of exposure (years) to tobacco dust on comet length and tail length in bidi rollers
Data indicated that the average comet length 60.44 ± 2.87 μm of control was less than the comet length of all the bidi roller groups. In bidi rollers the length of the comets increased with the duration of the exposure. It was the minimum (76.52 ± 1.43 μm) in the 10-19 year exposure group, it gradually increased in the 20-29 year group and 30-39 year group to reach a maximum of 82.23 ± 1.21 μm in the 50-59 year group. Thus, the comet length showed a positive correlation with the duration of exposure. The increase in each group was significant as compared to the comet length of the control group (P < 0.001).
The mean tail length in the control group was 11.52 ± 2.75 μm. In the exposed groups (of bidi rollers) it was minimum in 10–19 year group (14.67 ± 1.47 μm). The tail length also increased with duration of exposure and reached a level of 22.26 ± 1.02 μm in the 50-59 year group. However, in the 20-29 year group it showed a level of 27.1 ± 2.52 μm. The increase in the tail length was significant as compared to that of the controls (P < 0.001).
The length of the comets increases with age both in bidi rollers and controls, although in each group the bidi rollers have significantly greater comet length, tail length, and tail movement than their respective controls [Table 4] [Figures 4 and 5].
Table 4.
Effect of age on comet length, tail length, and tail movement in bidi rollers and controls
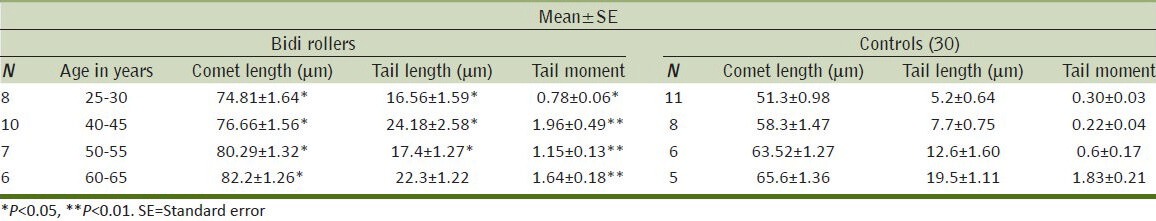
Figure 4.
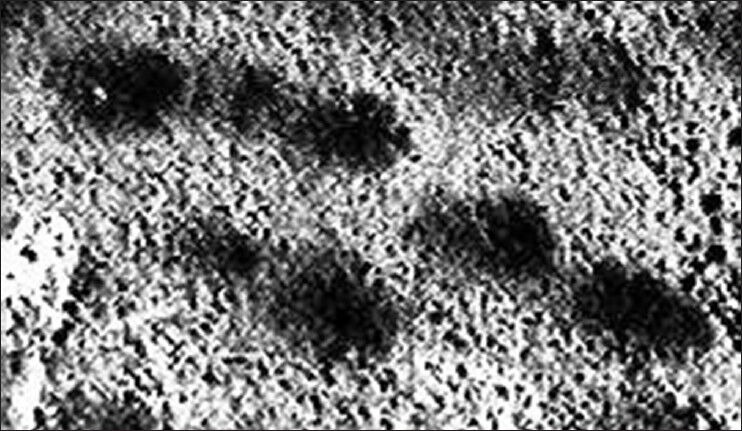
Comets of a bidi roller
Figure 5.
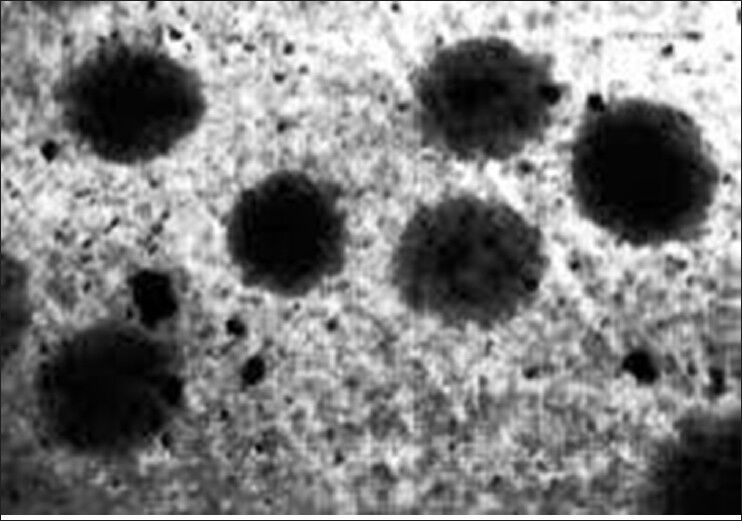
Comets of control
Evaluation of toxicity of TE by MTT assay
The six replicates of viability data of lymphocytes from four donors after being treated with various concentrations of TE was recorded and statistically analyzed by applying one way ANOVA and the differences in the means (of survival values) were found to be significant due to the treatments given. The data were further analyzed by applying Duncan's multiple range test. [Table 5].
Table 5.
Effect of various concentrations of tobacco extract on mean % viability of lymphocytes
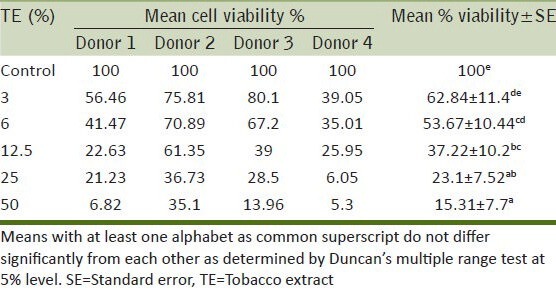
It is clear from Figure 6 that if the viability of the controls (lymphocytes without treatment with TE) was taken to be 100%, the viability dropped as the TE concentration increased. At 3% the mean viability was 62.84%, it decreased gradually as the TE concentrations increased. At 50% TE it reached a minimum viability of 15.31%.
Figure 6.
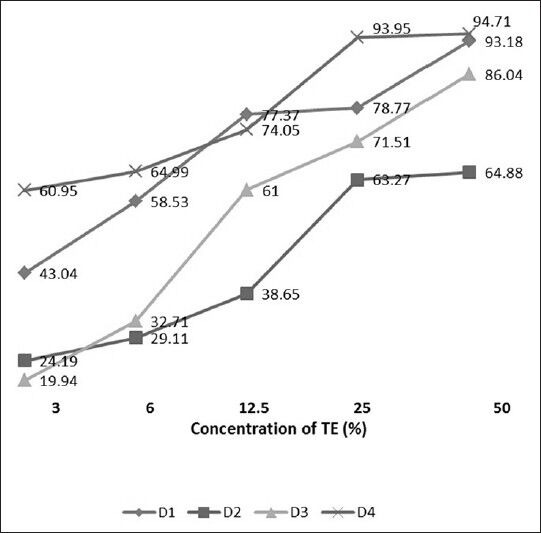
Comparative analysis of effect of different concentrations (3, 6, 12.5, 25, and 50%) of tobacco extract (TE) on drop in cell viability count among four donors (D1, D2, D3, and D4)
The gradual drop in cell viability in all the four donors tested is given in Figure 6. Thus, the toxicity of TE to lymphocytes showed a dose response relationship at a constant incubation time period of 2 h.
DISCUSSION
Bidi (the Indian equivalent of a cheap cigarette) rolling is a common cottage industry in Madhya Pradesh (India). The bidi rollers are from low socioeconomic strata and often work in ill-ventilated confined environments in their dwellings. So they inhale a lot of tobacco dust which leads to many systemic diseases and this is also harmful for the integrity of their chromosomes and DNA.[3,6]
CA studies
In the present study, the frequency of the total CA of the bidi rollers was found to be 3.1% as compared to 1.5% in controls. Similar findings were also reported by Mahimkar and Bhisey[2] who found a significant increase in CA in bidi tobacco processors due to occupational exposure. Yadav and Thakur[13] worked on the genotoxic effects of bidi smoking and found a significant increase in the CA% as compared to that in the nonsmokers. Increase in CA% of smokers was also found by a Nordic study group.[14] Increased CA% in peripheral blood lymphocytes of smokers as compared to those of nonsmokers was found by Kopjar et al.[15] However, Battershill et al.,[16] expresses the view that a significant increase is found in the biomarkers of genotoxicity only in people smoking excess of 20-30 cigarettes/day. Harmful effects on chromosome integrity have been found due to tobacco chewing by a number of workers. Patel et al.,[17] found a significant increase in the CA% of chewers over controls. Similar findings were obtained by Chadha and Yadav[18] who studied the genotoxic effects of Gutka.
Comet assay
As this aspect has not been worked out for the bidi makers of this region, the present study of assessment of DNA damage by comet assay was taken up. The comet length and tail length data reveal that both these parameters (which are a measure of DNA damage) increase with duration of exposure.
The tail length increase was proportional to the increase in duration of exposure. The average tail length of nonexposed controls was found to be 11.52 ± 2.75 μm. This finding is similar to that of Garaj-Vrhovac et al.,[19] who found the control tail length to be 13.5 μm. They found the tail length in the exposed group of cigarette factory workers with an average exposure of 19.5 years to be 14.34 ± 0.77 μm. This is similar to our finding of the average tail length of 14.67 ± 1.47 μm in the 10–19 year exposed group. Shukla et al.,[20] showed that the tail lengths were smaller in bidi rollers who worked in ill-ventilated place as compared to those who work in confined places. Kopjar et al.,[15] used the comet assay for a general assessment of DNA damage in healthy smokers as compared to nonsmokers and they found that the smokers had an increased comet tail length, tail movement, and larger number of long-tailed nuclei than the nonsmokers.
There is no evidence of work on toxicity of bidi TE on human lymphocytes by MTT assay. However, cytotoxicity studies of cigarette smoke condensates were carried out in human lymphoblast TK6 cells by Richter et al.,[21] through MTT assay. They found that maximum reduction in viability (50%) was caused by condensed smoke extract from in BUR (100% Burley tobacco cigarette) followed by 20% in non-menthol cigarette with a charcoal filter (CHAR) and 10% in full flavor, non-menthol cigarette (FF).
Our findings on cytotoxicity by MTT assay are supported by the observations of the above workers.
ACKNOWLEDGEMENT
The authors are grateful to UGC (India) for funding the research through a major research project [F. No. 39-643/2010(SR) dated 10-01-2011]. The authors also wish to thank the Principal St. Aloysius College (autonomous) Cantt, Jabalpur, India for providing the necessary facilities for the research.
Footnotes
Source of Support: UGC (India) for funding the research through a major research project [F. No. 39-643/2010(SR) dated 10-01-2011].
Conflict of Interest: None declared.
REFERENCES
- 1.Karabi MM, Bhavna MB. New Delhi, India: Organization: Voluntary Health Association of India; 2010. Tobacco and poverty research and capacity building: Narrative Report. [Google Scholar]
- 2.Mahimkar MB, Bhisey RA. Occupational exposure to bidi tobacco increases chromosomal aberrations in tobacco processors. Mutat Res. 1995;334:139–44. doi: 10.1016/0165-1161(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 3.Umadevi B, Swama M, Padmavathi P, Jyothi A, Reddy PP. Cytogenetic effects in workers occupationally exposed to tobacco dust. Mutat Res. 2003;535:147–54. doi: 10.1016/s1383-5718(02)00291-7. [DOI] [PubMed] [Google Scholar]
- 4.Haber H, Raber W, Vetter N. Bronchial asthmatic disease associated with tobacco dust: An occupational lung disease. Wien Klin Wochenschr. 2004;116:38–9. [PubMed] [Google Scholar]
- 5.Uitti J, Nordam H, Huuskonen MS, Roto P, Husman K, Reiman M, et al. Respiratory health of cigar factory workers. Occup Environ Med. 1998;5:834–9. doi: 10.1136/oem.55.12.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhisey RA, Bagwe AN, Mahimkar MB, Buch SC. Biological monitoring of bidi industry workers occupationally exposed to tobacco. Toxicol Lett. 1999;108:259–65. doi: 10.1016/s0378-4274(99)00097-1. [DOI] [PubMed] [Google Scholar]
- 7.Poonam P, Khanna A, Jain SK. Evaluation of genotoxicity in Bidi Rollers occupationally exposed to tobacco dust. National J Life Sci. 2010;7:89–93. [Google Scholar]
- 8.Bhisey RA, Govekar RB. Biological monitoring of bidi rollers with respect to genotoxic hazards of occupational tobacco exposure. Mutat Res. 1991;261:139–47. doi: 10.1016/0165-1218(91)90060-y. [DOI] [PubMed] [Google Scholar]
- 9.Moorhead PS, Nowell PC, Mellman WJ, Battips DM, Hunderford A. Chromosome preparations of leucocytes cultured from human peripheral blood. Exp Cell Res. 1960;20:613–6. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- 10.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 11.Sheldon JM, Lowel RG, Mathews KP. Philadelphia: Saunders; 1967. Manual of clinical allergy; pp. 507–31. [Google Scholar]
- 12.Gao XP, Akhter SR, Ikezaki H, Hong D, Rubinstein I. Dexamethasone attenuates acute macromolecular efflux increase evoked by smokeless tobacco extract. J Appl Physiol. 1999;87:619–25. doi: 10.1152/jappl.1999.87.2.619. [DOI] [PubMed] [Google Scholar]
- 13.Yadav JS, Thakur S. Cytogenetic damage in bidi smokers. Nicotine Tob Res. 2000;2:97–103. doi: 10.1080/14622200050011367. [DOI] [PubMed] [Google Scholar]
- 14.Nordic study group on the health risk of chromosome damage. A Nordic database on somatic chromosome damage in humans. Mutat Res. 1990;241:325–37. [PubMed] [Google Scholar]
- 15.Kopjar N, Zeljezic D, Garaj-Vrhovac V. Evaluation of DNA damage in white blood cells of healthy human volunteers using the alkaline comet assay and the chromosome aberration test. Acta Biochim Pol. 2006;53:321–36. [PubMed] [Google Scholar]
- 16.Battershill JM, Burnett K, Bull S. Factors affecting the incidence of genotoxicity biomarkers in peripheral blood lymphocytes: Impact on design of biomonitoring studies. Mutagenesis. 2008;23:423–37. doi: 10.1093/mutage/gen040. [DOI] [PubMed] [Google Scholar]
- 17.Patel BP, Trivedi PJ, Brahmbhatt MM, Shukla SN, Shah PM, Bakshi SR, et al. Micronuclei and chromosomal aberrations in healthy tobacco chewers and controls: A study from Gujrat, India. [Accessed on 2013 Sept 24];Arch. 2009 17:1–2. Available from: http://www.doiserbianbrs/img/doi/0354-7310/2009/0354-73100902007P.pdf . [Google Scholar]
- 18.Chadda P, Yadav JS. Studies on the Genotoxicity of Gutkha. Genet. 2011;11:277–82. [Google Scholar]
- 19.Vera GV, Goran G, Vlatka B. Alkaline comet assay as a biomarker of DNA-damage encountered in workers engaged in cigarette manufacturing. Periodicum Biologorum. 2009;111:85–90. [Google Scholar]
- 20.Shukla P, Khanna A, Jain SK. Working condition: A key factor in increasing occupational hazard among bidi rollers: A population health research with respect to DNA damage. Indian J Occup Environ Med. 2011;15:139–41. doi: 10.4103/0019-5278.93206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richter PA, Li AP, Polzin G, Roy SK. Cytotoxicity of eight cigarette smoke condensates in three test systems: Comparisons between assays and condensates. Regul Toxicol Pharmacol. 2010;58:428–36. doi: 10.1016/j.yrtph.2010.08.009. [DOI] [PubMed] [Google Scholar]


