Abstract
Aims:
The aim of this study was to examine the impacts of N-nitrosodiethylamine (DENA), a potent environment carcinogen on liver tissue of mice which was attenuated by isolated flavonoid and hydro-ethanolic extract of Euphorbia neriifolia (HEEN) leaves.
Materials and Methods:
Carcinogenicity was induced in albino mice by a single oral administration of DENA (50 mg/kg body weight). The HEEN (150 and 400 mg/kg body weight), butylated hydroxyanisole (BHA; 0.5 and 1%) and E. neriifolia flavonoid (ENF; 50 mg/kg body weight) were estimated to examine the possible anti-cancer potential.
Results:
DENA exposed animals showed alterations in normal hepatic histo-architecture, which comprised of necrosis (N), dilated sinusoids and vacuolization of the cells. Mice treated with E. neriifolia lower (ENL) and higher (ENH) dose and ENF before intoxicated with DENA showed that the liver cells were normal, with very little necrosis (Day 31). On the other hand, BHA higher (BHAH) and lower (BHAL) dose failed to diminish the abnormalities caused by the DENA.
Conclusion:
Results of the present study suggests that the ENH and ENF protects the hepatic tissue against DENA-induced hepatic carcinoma. The results could also be expressed in the order of ENH> ENF> ENL> BHAH> BHAL.
Keywords: Euphorbia neriifolia, flavonoid, liver, necrosis, N-nitrosodiethylamine
INTRODUCTION
Cancer is one of the most dreaded diseases of the 20th century and spreading further with continuance and increasing incidence in 21st century. Hepatocarcinoma (HCC) is a major problem not only in developed countries, but also in most undeveloped countries. Hepatocellular carcinoma is considered to be the 5th most common ubiquitous deadliest liver cancer representing up to 83% of all cases and one of the most common lethal pathology world-wide with poor diagnosis.[1] It is one of the most frequent malignant tumors world-wide and a leading cause of cancer related deaths in the world and incidence of over 1 million cases every year <1.25 annually.[2] It accounts for about 90% of all primary liver cancers. HCC, a fatal malignancy represents 4% of all malignant tumors. It is induced by toxic industrial chemicals, and air pollutants as also, food additives and fungal toxins.[3]
N-nitrosodiethylamine (DENA) is an N-Nitroso alkyl compound and was chosen as a carcinogenic model because this well-investigated, classic carcinogen is present in our environment and suggested to increase the generation of reactive oxygen species (ROS) that results oxidative stress or cellular injury.[4,5] DENA is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals.[4,5]
Histopathology, the microscopic study of diseased tissue is an important tool in anatomical pathology, since accurate diagnosis of many diseases usually requires histopathological examination of samples. Several medicinal plants including Euphorbia neriifolia tend to reduce the severity of cancer and prescribed constituents of liver protective herbal drugs have been shown to inhibit DENA-induced hepato-carcinogenicity in experimental animals,[6,7,8,9] but these studies did not report any histological changes during hepatocarcinogenesis induced by DENA. Moreover, no published information is available on the histological study of the use of E. neriifolia flavonoid (ENF) (2-[3,4-dihydroxy-5-methoxy-phenyl]-3,5-dihydroxy-6,7-dimethoxychromen-4-one) and hydro-ethanolic extract of E. neriifolia (HEEN) against hepatic carcinogenesis induced by DENA. Therefore, we examined the chemo preventive efficacy of E. neriifolia and its isolated flavonoid by ameliorating the hepatic carcinoma induced by DENA.
MATERIALS AND METHODS
Chemicals and reagents
DENA (CAS number: 55-18-5) was purchased from Sigma Chemical Co., USA. All other chemicals used for the study were of analytical grade and were purchased from reliable firms.
Preparation of HEEN
E. neriifolia leaves were collected from medicinal garden of Banasthali University, Banasthali and nearby areas of Banasthali (Latitude N-26°24’14.8414”; Longitude E-73°52’9.7194”), in the month of October, 2009 and taxonomically identified with the help of available literature and authenticated by botanist of Krishi Vigyan Kendra, Banasthali University, Banasthali (Rajasthan, India). A voucher specimen was deposited in the herbarium of department of bioscience and Biotechnology, Banasthali University (No. BVH-780141A). Shade dried leaves were powdered soxhlet extracted with 70% (v/v) ethanol and vacuum concentrated to dryness under reduced pressure at 60 ± 1°C. After drying in a hot air oven (40-45°C), it was stored in an air tight container in the refrigerator at 5°C. The residue was designated as HEEN and was used to assess hepato-protective activity.
Isolation of flavonoid from E. neriifolia
Dried leaves of E. neriifolia (500 g) were extracted successively with non-polar to polar to get respective extracts. Flavonoid contents and their presence were determined by the method of Harborne,[10] using quercetin as standard. Out of all extracts dark-brown sticky semi-solid ethanolic extract (48.9 g) contained bulk of flavonoids was used for chromatographic separation. The ethanol extract obtained was concentrated and chromatographed on silica plates by using n-butanol: acetic acid: water (2:2:6) as mobile phase. As a result three spots were resolved and nomenclatured as IF1, IF2 and IF3 having Rf values of 0.60, 0.79 and 0.90 respectively. The Rf of IF2 was coinciding with standard quercetin Rf value that was found to be 0.79. IF2 fraction carefully crystallized, gave solid pale yellow crystal, soluble in water and in organic solvents was designed as ENF. High performance thin layer chromatography and infra-red spectrum of ENF in KBr pellet confirmed the presence of hydroxyl (-OH) group with H-bonded primary alcohol in flavonoids. With the help of 1H NMR and MS the ENF was characterized as 2-(3,4-dihydroxy-5-methoxy- phenyl)-3,5-dihydroxy-6,7-dimethoxychromen-4-one. ENF was used to assess tissue-protective activity.
Experimental animals and treatment regimen
Healthy male Swiss albino mice (Mus musculus L.) procured from Chaudhary Charan Singh Haryana Agricultural University, Hissar (Haryana, India) were housed under standard laboratory conditions of temperature (22 ± 3°C), 50 ± 15% Relative humidity and photoperiod (12:12 h L: D cycle). Animals lead free access to standard food pellet diet (Hindustan Lever Limited, India) and tap water ad libitum. The studies were carried out in accordance with the guidelines given by Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA, Reg. No: IAEC/814 date. 23.01.2010).
After 2 weeks of acclimatization, mice were randomly divided into seven groups of six mice each and were administered orally by gavage. Treatment regimen as follows:
Group I: Normal control,
Group II: Carcinogen control, received distilled water for 21 days and then single dose of DENA (50 mg/kg body weight) and left for 10 days,
Group III: E. neriifolia lower (ENL; 150 mg/kg body weight/day) and
Group IV: E. neriifolia higher (ENH; 400 mg/kg body weight/day) for 21 days, before being intoxicated with DENA, once,
Group V: Butylatedhydroxyanisole lower (BHAL; 0.5% mg/kg body weight/day) and
Group VI: Butylated hydroxyanisole higher (BHAH) (1% mg/kg body weight/day) for 21 days, dissolved in 0.5% acetone, before being intoxicated with DENA, once,
Group VII: ENF (50 mg/kg body weight/day) for 21 days, dissolved in dH2O, before being intoxicated with DENA.
Then, the animals were sacrificed on 10 day after DENA administration and the total duration of the experiment was of 31 days. The dose for DENA (Sigma-N0258-Material Safety Data Sheet, 2003),[6,7] BHA,[11] plant[6,7,12,13,14] and ENF[15] were selected on the basis of LD50 calculated in our own laboratory and other published reports.
Autopsy and isolation of organs
After 31 days of treatment, mice were deprived of food overnight and then on the next day they were sacrificed under light chloroform anaesthesia. After postpartum liver was excised immediately, washed, cleaned and rinsed in ice cold normal saline solution (0.9% NaCl, pH 7.4), until bleached of all the blood and blotted dry on filter paper sheets to remove blood. Proper care was taken to avoid damage of any part of tissue while departing from the body. Small portion of tissue was fixed in buffered formalin (10% formaldehyde diluted using normal saline) for histological studies.
Histopathological studies
Fixation was followed by washing the tissue in running water, dehydrated by graded series of alcohol, cleaned in xylene and stained in hematoxylin and eosin. Paraffin blocks with tissue were prepared and sections of about 3-5 μm in thickness were cut on rotatory microtome, paraffin ribbons with tissue taken on microscopic slides were dehydrated. These tissue sections were mounted and then examined for pathological alterations by light microscope (Model-Motic).[16]
RESULTS
Percentage of tissue carcinogenesis
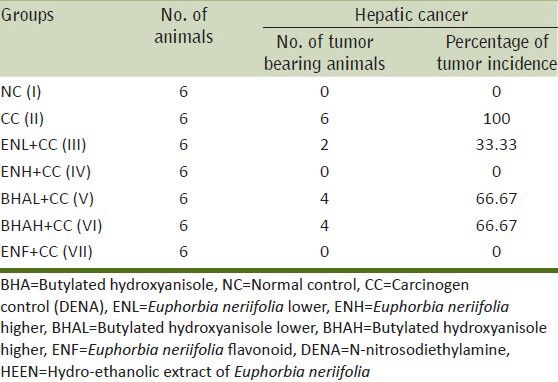
The animals were examined for DENA-induced tumors in the liver. None of the mice from either control or HEEN groups developed liver tumors, whereas all the mice treated with DENA developed 100% liver carcinogenesis [Table 1]. However, pre-treatment with HEEN (at both doses) and ENF before intoxicated with DENA reduced liver tumor incidence to 0-33.33%. These results suggested that HEEN (at both doses) and ENF can prevent DENA-initiated tumor development in the mice liver tissue.
Table 1.
Effect of DENA, HEEN, BHA (at both doses) and isolated flavonoid on DENA-induced hepatocarcinogenesis in Swiss albino mice
Histopathological analysis of the liver tissue
Table 2 depicted the histo-pathological features seen in the liver of mice of different groups. The histological aspects showed normal hepatic tissue after administration of normal diet and water in the experimental duration of 31 days [Figure 1a–c]. Each lobule exhibited normal cellular architecture with thin walled central vein (CV) from which cords of distinct polyhedral hepatic cells (hepatocytes; H) each with well-preserved cytoplasm, prominent nucleus (N) and nucleolus radiated toward the lobule periphery [Figure 1b]. Nuclei were spherical or ovoid, with a regular surface and show considerable variation in size from cell to cell [Figure 1a and b]. Around the periphery of each lobule, portal canal was also present [Figure 1c]. It is a connective tissue septum that carries the branches of hepatic artery, hepatic and portal vein (HV and PV), bile duct and lymphatic vessels.
Table 2.
Histopathological features seen in the liver of mice of different treatment groups
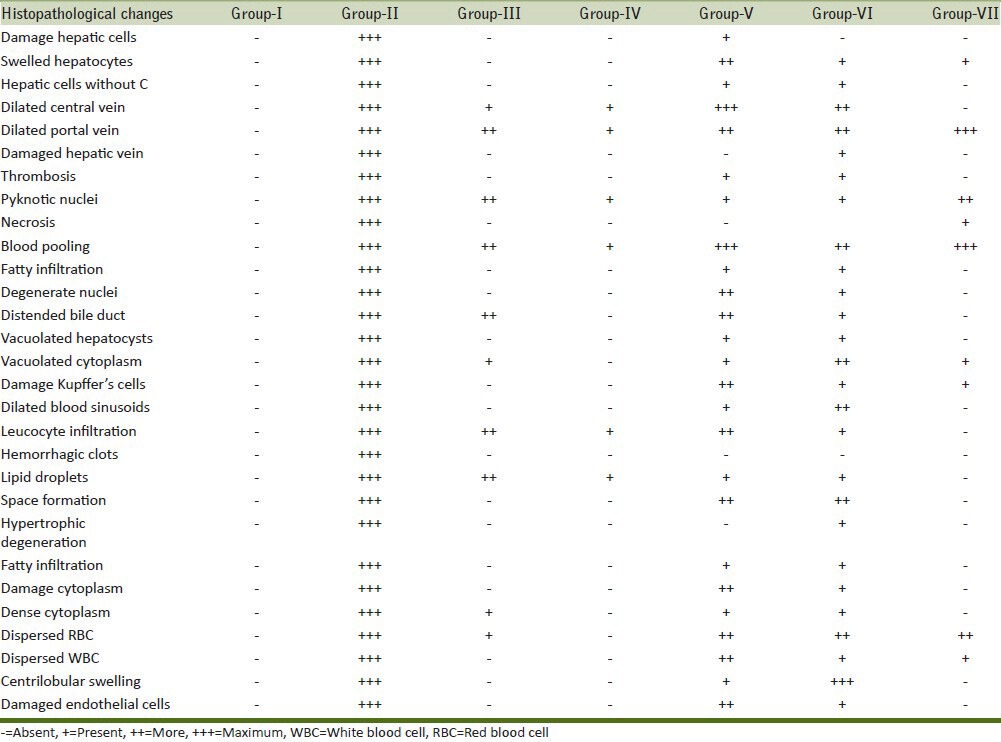
Figure 1.
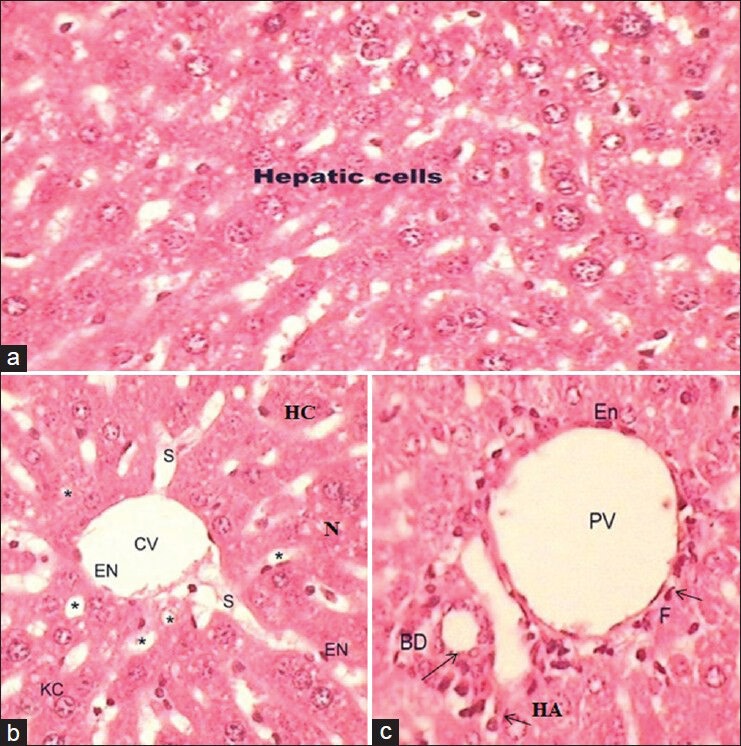
(a-c) Photomicrograph of transverse section of liver (H and E, ×40) of untreated Group I showing the normal hepatic cells, thin walled central vein, hepatic sinusoids (S; *), portal vein, hepatic artery and bile ducts
DENA exposure produced significant tumor thrombi in both hepatic and portal vessels [Figure 1a and b]. The histologic appearance of HCC is also extremely variegated. The tumor cells in nests and thick cords and were separated from one another by thin walled sinusoids(S). Cytologically, the tomor cells bear some resemblance to normal hepatocytes, but are slightly larger, have more irregular and prominent nuclei [Figure 2a]. Many of the tumor cells contain intra-cytoplasmic violaceous, hyaline globules (arrow) that represents proteins produced by the tumor cells [Figure 2a]. The damaged hepatocyte cells with manifestations of extensive cytoplasmic vacuolization, broad patches of necrosis, damaged sinusoidal, massive fatty degeneration, swelling, ballooning degradation, broad infiltration of the lymphocytes, portal tract fibrosis with endothelial swelling and kuffer cells around the CV and the loss of cellular boundaries [Figure 2c and d] were also observed. In this group, the hepatic cords became distorted and the hepatocytes were bloated with large, spherical droplets of fat in a focal and/or diffuse manner in association with the dilatation and congestion of the central and PVs and infiltration of the parenchyma with inflammatory cells [Figure 2c]. These droplets pressed the nucleus toward the periphery giving a signet-ring appearance to the cell [Figure 2b]. Inflammation in the cells, congestion, sinusoids, blood cells pooling in sinusoidal spaces as well as in CV [Figure 2c and d] were also observed in DENA exposed group. Hepatocyte vacuolation and swelling, parenchyma disorganization, dilation of the inter hepatocyte space and hemorrhagic clots [Figure 2e] were also observed when compared with normal control animals (Group I). The nuclei of these cells were pyknotic and shrinked [Half Moon; Figure 2g]. Focal area of necrosis infiltrated with mononuclear cells (Nc) and degenerate nuclei were also seen [Figure 2f]. Space formation and high degree of vacuolation was also seen in liver tissue. Rarely, the bile ductules showed distention and proliferation. Furthermore, necrosis of the hepatocytes was mainly noticed around the portal area. It was observed that DENA caused a heavy destruction of the overall arrangement of liver cells because most of the cells were found in a ruptured state and without cytoplasm.
Figure 2.
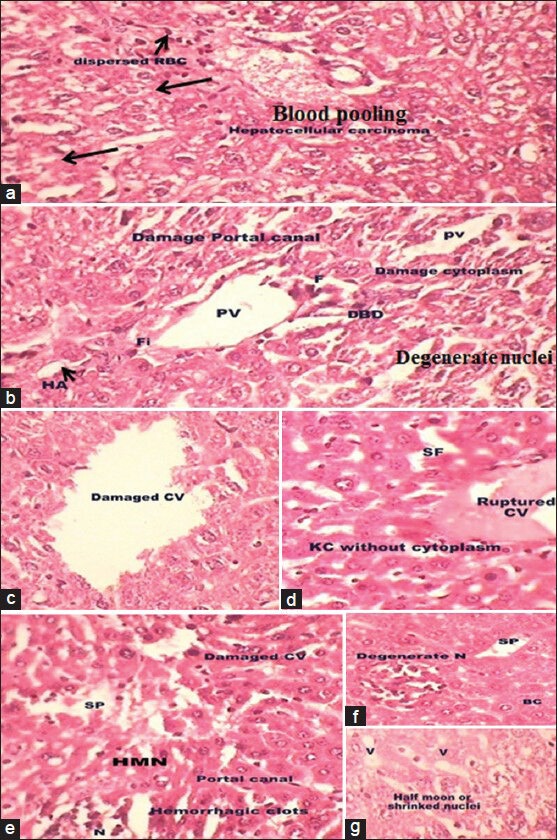
(a-g) Photomicrographs of transverse section of liver of N-nitrosodiethylamine-intoxicated mice (H and E, ×40) showing the distorted liver architecture and arrow indicated tumor cells contain intracytoplasmic violaceous, hyaline globules that represents proteins produced by the tumor cells (a). Damaged portal vein, hepatic artery and bile ducts (b), ruptured central vein (c and d), degenerate nuclei (N; f) with damage hyperchromatic malignant nuclei (HMN; e) and vacuoles (V; g) were also observed
Groups III [Figure 3a] and IV [Figure 3b] in comparison to carcinogen control exposed group (Group II) illustrated that HEEN extract at both doses retained hepatic architecture, recover the pattern of necrosis, fibrosis, hepatocyte vacuolation, fatty changes and hemorrhagic clots, incidence of inflammatory cells infiltration, centrilobular hepatocyte swelling. However, diminutive accumulation of fatty globules, distended bile ductile (DBD), dilated sinusoids, some hepatocytes showing an isokaryosis minimal inflammatory cell infiltration around the portal triads [Figure 3a]. The Group IV showed well-defined structure of hepatocytes with well-defined aggregation of HV and PV and absence of necrosis [Figure 3b].
Figure 3.
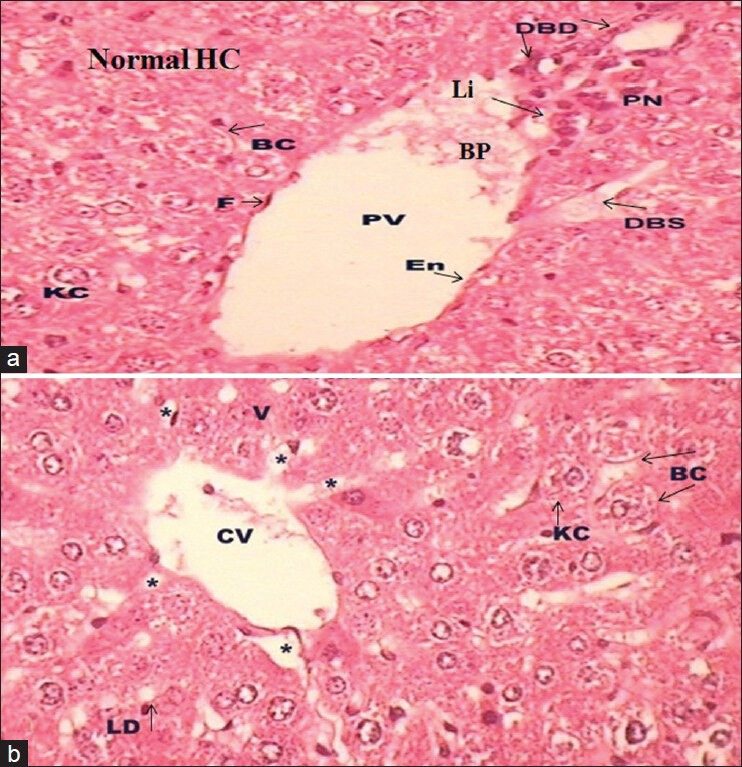
(a-b) Photomicrograph of transverse section of liver (H and E, ×40) of Group III (a) and Group IV (b) showing normal architecture with some centrilobular swelling (a) and some lipid droplets (LD; b)
The liver sections of the mice treated with BHA at 0.5% [Figure 4a and b] before intoxicated with DENA did not show sign of protection whereas at higher dose of 1% [Figure 4c and d] normalize the hepatic cells and CV but failed to demonstrate protective activity as depicted in Figure 4c and d. Showing some neoplastically transformed cells, damaged cytoplasm, dispersed nuclei, dilated sinusoids, large blood pooling (BP) in CV and PV, DBD, lipid droplets (LD), pyknotic nuclei (PN), vacuolated cytoplasm, dense cytoplasm around HV and erythrocytes dispersed in cytoplasm and some hepatocytes showing an isokaryosis minimal inflammatory cell infiltration around the portal triads, many of the tumor cells contain hyaline globules (arrow) that represent protein produced by the tumor cells.
Figure 4.
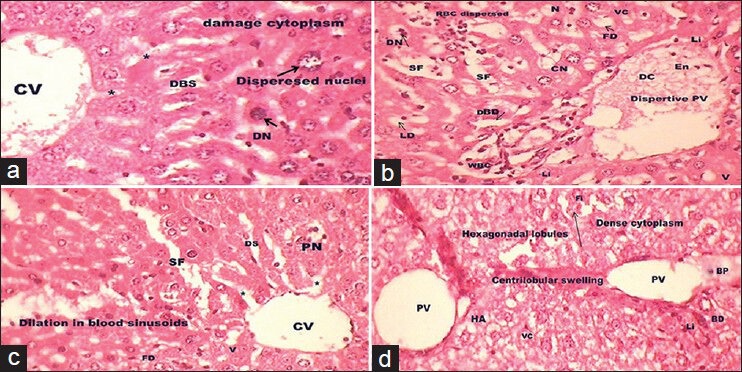
(a-d) A transverse section representation of liver of experimental Group V (a and b) and VI (c and d) showing damage cytoplasm, dispersed nuclei, dilated sinusoids, blood pooling, distended bile ductile, lipid droplets and pyknotic nuclei. Some hepatocytes showing an isokaryosis minimal inflammatory cell infiltration around the portal triads, many of the tumor cells contain hyaline globules (arrow) that represent protein produced by the tumor cells (H and E, ×40)
The ENF (50 mg/kg body weight) treated Group VII [Figure 5] before intoxicated with DENA, showed a normal hepatic architecture with petite BP in PV, LD, PN, centrilobular swelling, dispersed erythrocytes in cytoplasm and some hepatocytes showed an leukocyte inflammatory cell infiltration around the portal triads. The cells were found to be arranged in the form of hepatic cords and the vein and bile canaliculi were also found to be normal to some extent.
Figure 5.
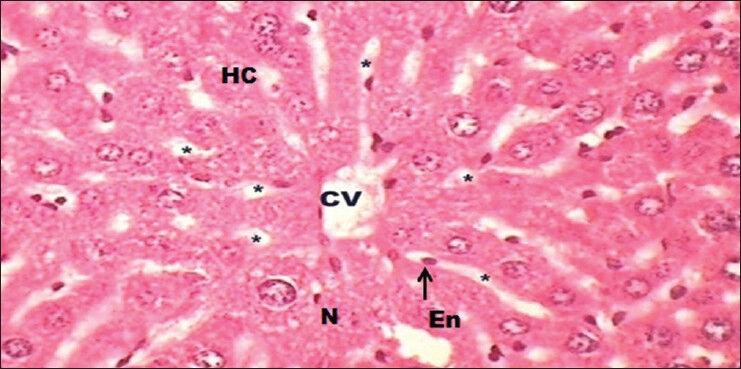
A transverse section representation of liver of Euphorbia neriifolia flavonoid (50 mg/kg/day) pre-treated mice, before intoxicated with N-nitrosodiethylamine at (H and E, ×40) showing normal architecture with recovery
The higher dose (400 mg/kg body weight) of EN and ENF were more effective and were able to recover the injured liver to quite normal form when compared to ENL and BHA (at both doses). Hence, it can be assumed that the hepatoprotective activity by HEEN is dose dependent.
DISCUSSION
Liver plays an important role in the metabolism of drugs and it is the most vulnerable tissue for drug toxicity. According to the reports published by U.S. Food and Drug Administration, more than 900 drugs, toxins and herbs have been reported to cause liver injury. Drugs account for 20-40% of all instances of hepatic failure.[17]
DENA, one of the most important environmental carcinogen and has been reported to cause the generation of ROS resulting in oxidative stress and cellular injury.[4,6,7,12,13,14] As liver is the major site of DENA metabolism, the production of active oxygen species in the liver may be responsible for its carcinogenic effects.[18] They have reported that a cell cycle disturbance induced in DENA-initiated hepatocytes by colchicine gives a growth advantage to putative preneoplastic lesions under conditions of partial hepatectomy and selection pressure and hence that a high incidence of HCCs can be obtained within a short latent period corroborating over present finding.[19]
Observed results depict that the DENA exposed group showed 100% tumor incidence and these results are in agreement with aforementioned findings of previous workers.[20,21] The results are also showed that administration of HEEN (at high dose) and ENF could prevent DENA-induced hepatic carcinogenesis in mice as no malignancies were seen in the animals.
Histopathological examination of liver section of normal mice showed normal hepatic cells with well-defined cytoplasm and nucleus, whereas DENA exposed group showed liver cells were completely damaged and depicted pathological changes such as steatosis, centrilobular necrosis, large vacuolization, hyperchromatic malignant nuclei, hyperplastic nodules and obvious heteromorphism. These results are in confirmation with the findings of previous workers.[20,21,22,23,24] The resulting effect was the production of elevated amounts of malondialdehyde and conjugated dienes, which caused deleterious effects on the membranous components of hepatocytes.
HEEN (at both dose) and ENF pre-treatments before intoxicated with DENA (Groups III, IV and VII) showed the recovery process as indicated by the marked regenerative activity with mild necrosis (Day 31) and the cells became normal. The normalization of histo-architecture of liver damaged by DENA in ENL, ENH and ENF groups might be due to the trapping of ROS directly or indirectly by HEEN and flavonoid. Our results are in agreement with the previous report.[22] Observed results also revealed that BHA (at both dose) alone showed some mild distortion along with necrosis and swelling of the liver tissue as compared with the control group. BHA at both dose levels before intoxicated with DENA tried to revert, but not much extent as by HEEN and ENF group.
The mice treated with the hydro-ethanolic extract and isolated flavonoid before intoxicated with DENA the normal cellular architecture was retained and it is more efficient than the standard BHA (at both dose) group, hence confirming the significant hepato protective effect of extract of HEEN at the dose of 150 and 400 mg/kg body weight and ENF at 50 mg/kg body weight, which is also confirmed by the results of biochemical studies.[6,7]
In this study, E. neriifolia act as antioxidant agent as this plant contained wide range of active ingredients such as sugar, tannins, flavonoids, alkaloids,[7,8,9,25] triterpenoids, tetracyclic triterpene (nerifoliene and euphol), triterpenoidal saponins etc.,[26,27,28] which can inhibit or slow down the severity of cancer.[6,7,8,12,13,14,28,29] These active ingredients especially flavonoids, terpenoids and saponins neutralized free radicals and intermediates of metabolism that are highly reactive since they contain a non-paired electron[30] and to be responsible for the observed protective histological effects. The present study findings provide and validate the scientific evidence to the ethnomedicinal therapeutic use of this plant.
The results presented in this study conclude that hepato-carcinogenesis, which was induced by DENA was effectively inhibited by 70% hydro-ethanolic (v/v) extract of E. neriifolia and by flavonoid isolated from ethanolic extract of E. neriifolia. The result could also be expressed in the order of ENH> ENF> ENL> BHAH> BHAL.
ACKNOWLEDGMENTS
The authors are grateful to acknowledge University Grants Commission for providing financial assistance (Grant/F. No. 37-68/2009; SR). The authors are also thankful to authorities of Banasthali University, Rajasthan for providing necessary facilities to carry out the present study.
Footnotes
Source of Support: Authors are grateful to University Grants Commission for providing financial assistance (Grant/F. No. 37-68/2009; SR) and authorities of Banasthali University, Rajasthan providing necessary facilities to carry out the present study.
Conflict of Interest: None declared.
REFERENCES
- 1.Gao J, Xie L, Yang WS, Zhang W, Gao S, Wang J, et al. Risk factors of hepatocellular carcinoma – Current status and perspectives. Asian Pac J Cancer Prev. 2012;13:743–52. doi: 10.7314/apjcp.2012.13.3.743. [DOI] [PubMed] [Google Scholar]
- 2.Maideen NM, Velayutham R, Manavalan G. Activity of Prosopis cineraria against N-nitrosodiethylamine induced liver tumors by regulating the levels of tumor marker, lipid peroxidation and antioxidants. Asian J Pharm Life Sci. 2012;2:1–9. [Google Scholar]
- 3.Bansal AK, Trivedi R, Soni GL, Bhatnagar D. Hepatic and renal oxidative stress in acute toxicity of N-nitrosodiethylamine in rats. Indian J Exp Biol. 2000;38:916–20. [PubMed] [Google Scholar]
- 4.Sharma V, Janmeda P, Singh L. N-nitrosodiethylamine and carcinogenicity: An over review. Int J Environ Rehabil Conserv. 2012;3:56–67. [Google Scholar]
- 5.1-42. Lyon, France: Int Agency Research Cancer; 1987. IARC. Overall Evaluations of Carcinogenicity. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, Overall evaluation of carcinogenicity: An updating of IARC Monographs. Supplement 7; p. 440. [PubMed] [Google Scholar]
- 6.Pracheta, Sharma V, Paliwal R, Sharma S, Singh L, Janmeda BS, et al. Chemo-protective activity of hydro-ethanolic extract of Euphorbia neriifolia Linn. leaves against DENA-induced liver carcinogenesis in mice. Biol Med. 2011;3:36–44. [Google Scholar]
- 7.Sharma V, Janmeda P, Paliwal R, Sharma S. Antihepatotoxic activity of Euphorbia neriifolia extract against N-nitrosodiethylamine-induced hepatocarcinogenesis in mice. J Chinese Integrat Med. 2012;10:1303–9. doi: 10.3736/jcim20121115. [DOI] [PubMed] [Google Scholar]
- 8.Singh L, Pracheta Cancer: Plant based chemoprevention and therapy: An overview. J Biochem Cell Arch. 2012;12:1–20. [Google Scholar]
- 9.Singh L, Pracheta, Sharma V, Paliwal R. Bio-prospecting anticarcinogenic plants of Rajasthan. J Plant Dev Sci. 2012;4:115–23. [Google Scholar]
- 10.Harborne JB. Phytochemical Methods. 3rd ed. New Delhi: Springer (India) Pvt Ltd; 1998. A Guide to Modern Techniques of Plant Analysis; p. 124. [Google Scholar]
- 11.Bharali R, Tabassum J, Azad MR. Chemomodulatory effect of Moringa oleifera, Lam, on hepatic carcinogen metabolising enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pac J Cancer Prev. 2003;4:131–9. [PubMed] [Google Scholar]
- 12.Pracheta P, Sharma V, Singh L, Paliwal R, Sharma S, Yadav S, et al. Chemopreventive effect of hydroethanolic extract of Euphorbia neriifolia leaves against DENA-induced renal carcinogenesis in mice. Asian Pac J Cancer Prev. 2011;12:677–83. [PubMed] [Google Scholar]
- 13.Sharma V, Pracheta, Paliwal R, Singh L, Sharma V, Sharma SH. Anticarcinogenic potential of Euphorbia neriifolia leaves against N-nitrosodiethylamine-induced nephrotoxicity in mice. J Biochem Cell Arch. 2011;11:393–8. [Google Scholar]
- 14.Sharma V, Janmeda P. Chemopreventive Role of Euphorbia neriifolia (Linn) and its isolated flavonoid against N-nitrosodiethylamine-induced Renal Histopathological damage in male mice. Toxicol Int. 2013;20:101–7. doi: 10.4103/0971-6580.111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan LP, Chen FH, Ling L, Dou PF, Bo H, Zhong MM, et al. Protective effects of total flavonoids of Bidens pilosa L. (TFB) on animal liver injury and liver fibrosis. J Ethnopharmacol. 2008;116:539–46. doi: 10.1016/j.jep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 16.McManus JF, Mowry RW. Histological and Histochemical. New York: Harper and Row; 1965. Staining methods; p. 136. [Google Scholar]
- 17.Soni M, Mohanty PK, Jaliwala YA. Hepatoprotective activity of fruits of Prunus domestica. Int J Pharma Biosci. 2011;2:439–53. [Google Scholar]
- 18.Janani P, Sivakumari K, Parthasarathy C. Hepatoprotective activity of bacoside A against N-nitrosodiethylamine-induced liver toxicity in adult rats. Cell Biol Toxicol. 2009;25:425–34. doi: 10.1007/s10565-008-9096-4. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsumi M, Ohashi K, Tsujiuchi T, Kobayashi E, Kobitsu K, Kitada H, et al. Disturbance of the cell cycle with colchicine enhances the growth advantage of diethylnitrosamine-initiated hepatocytes in rats. Jpn J Cancer Res. 1996;87:5–9. doi: 10.1111/j.1349-7006.1996.tb00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundaresan S, Subramanian P. Evaluation of chemopreventive potential of garlic extract on N-nitrosodiethylamine-induced hepatocarcinoma in rats. Pharm Biol. 2002;40:548–51. [Google Scholar]
- 21.Ismail MF, Ali DA, Fernando A, Abdraboh ME, Gaur RL, Ibrahim WM, et al. Chemoprevention of rat liver toxicity and carcinogenesis by spirulina. Int J Biol Sci. 2009;5:377–87. doi: 10.7150/ijbs.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal AK, Bansal M, Soni G, Bhatnagar D. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact. 2005;156:101–11. doi: 10.1016/j.cbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Sultana S, Ahmed S, Jahangir T. Emblica officinalis and hepatocarcinogenesis: A chemopreventive study in Wistar rats. J Ethnopharmacol. 2008;118:1–6. doi: 10.1016/j.jep.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Zhang CL, Zeng T, Zhao XL, Yu LH, Zhu ZP, Xie KQ. Protective effects of garlic oil on hepatocarcinoma induced by N-nitrosodiethylamine in rats. Int J Biol Sci. 2012;8:363–74. doi: 10.7150/ijbs.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma V, Pracheta Microscopic studies and preliminary pharmacognostic evaluation of Euphorbia neriifolia (Linn.) leaves. Indian J Nat Prod Resour, 2013;4(4):348–57. [Google Scholar]
- 26.Pracheta, Sharma V, Paliwal R, Sharma S. In vitro free radical scavenging and antioxidant potential of ethanolic extract of Euphorbia neriifolia Linn. Int J Pharm Pharm Sci. 2011;3:238–42. [Google Scholar]
- 27.Pracheta, Sharma V, Paliwal R, Sharma S. Preliminary phytochemical screening and In vitro antioxidant potential of hydro-ethanolic extract of Euphorbia neriifolia Linn. Int J Pharm Tech Res. 2011;3:124–32. [Google Scholar]
- 28.Sharma V, Janmeda P, Singh L. A Review on Euphorbia neriifolia (Sehund) Spatulla DD. 2011;1:107–11. [Google Scholar]
- 29.Sharma V, Pracheta Remedial effect of Euphorbia neriifolia leaves and isolated flavonoid against N-Nitrosodiethylamine induced renal-carcinogenesis in mice. Indian J Biochem Bio. 2013;50:521–8. [PubMed] [Google Scholar]
- 30.Krinsky NI. Antioxidant functions of carotenoids. Free Radic Biol Med. 1989;7:617–35. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]


