Abstract
Introduction:
Arsenic, an environmental contaminant naturally occurred in groundwater and has been found to be associated with immune-related health problems in humans.
Objective:
In view of increasing risk of arsenic exposure due to occupational and non-occupational settings, the present study has been focused to investigate the protective efficacy of amla against arsenic-induced spleenomegaly in mice.
Results:
Arsenic exposures (3 mg/kg body weight p.o for 30 days) in mice caused an increase production of ROS (76%), lipid peroxidation (84%) and decrease in the levels of superoxide dismutase (53%) and catalase (54%) in spleen as compared to controls. Arsenic exposure to mice also caused a significant increase in caspases-3 activity (2.8 fold) and decreases cell viability (44%), mitochondrial membrane potential (47%) linked with apoptosis assessed by the cell cycle analysis (subG1-28.72%) and annexin V/PI binding in spleen as compared to controls. Simultaneous treatment of arsenic and amla (500 mg/kg body weight p.o for 30 days) in mice decreased the levels of lipid peroxidation (33%), ROS production (24%), activity of caspase-3 (1.4 fold), apoptosis (subG1 12.72%) and increased cell viability (63%), levels superoxide dismutase (80%), catalase (77%) and mitochondrial membrane potential (66%) as compared to mice treated with arsenic alone.
Conclusions:
Results of the present study indicate that the effect of arsenic is mainly due to the depletion of glutathione in liver associated with enhanced oxidative stress that has been found to be protected following simultaneous treatment of arsenic and amla.
Keywords: Amla, apoptosis, arsenic, mice, oxidative stress, spleen
INTRODUCTION
High levels of arsenic (As) in groundwater have caused a great concern, especially in the Indo-Bangladesh region where over a million of people are reported to be suffering from As poisoning. One of the most common sources of As contamination is drinking water, where levels ranges from 0.01 to 4.0 mg arsenic/L.[1] Dietary intake of As through consumption of contaminated food, as well as exposures through medicines and occupational settings, is also reported.[2] Comparing all three oxidation states of As, the trivalent form is considered to be more toxic due to its ability to bind with sulfhydryl groups of proteins and disrupt enzyme activity.[3]
Alterations in the expression of genes involved in immune function have been reported in mouse embryonic cells following exposure to arsenic.[4] Studies have also reported that exposure to As significantly inhibits mitogen-induced lymphocyte proliferation in cells from exposed humans and mice.[5,6] It has been shown that As induces immunosuppression via apoptosis in thymocytes and splenocytes in a dose-dependent manner in epidemiological and experimental studies.[7] Exposure to As during pregnancy was found to be associated with decreased thymus size in children and increased infectious diseases in both mothers and children, indicating an As-related immunosuppression.[8,9] Arsenic exposure has also been found to give rise to reductions in CD4+ cell counts, CD4+:CD8+ cell ratios, and T-regulatory cell levels in adults and children.[10,11,12,13,14] Chronic exposure to As has been shown to be involved in the activation of inflammatory signaling pathways in humans and rodents.[15] Apart from mammals, low levels of As exposure were also seen to cause reduced immune defense responses in birds and fish.[16]
An involvement of oxidative stress in a large number of pathological states (including cancer) has been proven clinically.[17] As toxicity is known to be mediated in great part through the generation of reactive oxygen species (ROS), possibly in combination with changes in host anti-oxidative defenses.[3,18,19] A prime target of As intoxication is mitochondria affected either by ROS generation or through a condensing of the mitochondrial matrix and an opening of permeability transition pores.[20] Irregular apoptosis of immune cells due to As exposure may lead to immune-related disorders that in turn may result in immunodeficiency, autoimmune diseases, or malignancies.[21,22] Recently, we reported that As induced oxidative damage and apoptosis in thymocytes of mice and that these changes were associated with increased oxidative stress.[23] We also found that As accumulated in the liver and impaired its anti-oxidant status and induced alterations in the levels of serum hepatic enzymes associated with hepatic toxicity.[24]
Natural products, mainly those obtained from dietary sources, contain a wide variety of anti-oxidants. Phytoconstituents, an important source of anti-oxidants, are capable of terminat-ing free radical chain reactions.[25] Recently, a number of studies have been carried out to assess the protective potential of these herbal and natural agents against As-induced toxicity and related conditions.[3,20,23] The fruit extract of amla and its individual constituents have shown to impart anti-oxidant, inflammatory, cancer, and microbial effects as well as to appear to induce immuno-modulation in situ.[26] Amla is a rich source of Vitamin C, a water-soluble anti-oxidant that acts as a scavenger of free radicals and plays an important role to protect against lipid damage, protein oxidation, and DNA oxidation.[27] Amla also contains a variety of active constituents like polyphenols, flavones, tannins, anthocyanins, flavonols, ellagic acid, and derivatives that protect against ROS and exhibit a wide range of biological effects.[28] Studies have shown that the aqueous extract of amla induced apoptosis and inhibited lung metastasis by injected murine colon 26-L5 carcinoma cells[29] and B16-BL6 melanoma cells in mice. We recently reported on the protective efficacy of amla against As-induced enhanced oxidative stress and apoptosis in thymocytes and hepatotoxicity in mice.[23,24] Although the anti-oxidant potential of amla and its various constituents has already been reported,[30] not much is known about the immunomodulatory effects of the crude extract of amla and associated outcomes. As whole amla fruit is taken as a dietary supplement therefore, the present study was undertaken to investigate the immunomodulatory role of crude extract of amla during As-induced toxicities in the spleens of mice.
MATERIALS AND METHODS
Chemicals
Sodium arsenite, RNase A, 2’,7’- dichlorofluorescein diacetate (DCFH-DA), 3-(4,5-di-methyl-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), 7-amino-4-trifluoromethylcoumarin (AFC), and all other chemicals were purchased from Sigma (St. Louis, MO, USA). Rhodamine 123 (Rh123) was obtained from Molecular Probes (Eugene, OR, USA), propidium iodide (PI) was bought from Calbiochem (San Diego, CA, USA), and Annexin V-FITC was purchased from Biovision (San Francisco, CA, USA).
Plant material
The ethyl acetate extract of amla contains many phenolics and flavonoids including gallic acid, ellagic acid, isocorilagin, chebulanin and chebulagic acid.[31,32] Therefore, this extract was been selected for use in the present study. Briefly, the fresh fruits of Emblica officinalis were collected from an authentic source; the materials were verified by the Herbarium of Birbal Sahni Institute of Palaeobotany, Lucknow, India (plant identification no-13394). The samples were then allowed to dry at 40°C and then the materials were pulverized and screened through a 60-mesh sieve. Thereafter, the fruit powder (100 g) was extracted three times using 95% ethanol. The combined extracts were then filtered through 0.45 μm Whatman filter paper and the filtrate then evaporated to dryness with a rotary evaporator under reduced pressure. The residue was then suspended in water and extracted successively with diethyl ether and ethyl acetate. The extract was evaporated under reduced pressure to obtain the powdered form of the ethyl acetate fraction. Yields of 5.5% (w/v; in ethyl acetate fraction) were routinely obtained and used for the present study.
Animals and treatment
The study was approved by the institutional animal ethics committee of King George Medical University, Lucknow (No. 121 IAH/Pharma-11), India, and all experiments were carried out in accordance with guidelines set by the committee for the purpose of control and super-vision of experiments on animals (CPCSEA), Ministry of Environment and Forests (Government of India), New Delhi, India. Male Balb/c mice (15 ± 2 g) were obtained from the animal breeding colony of CSIR-Indian Institute of Toxicology Research (Lucknow). Mice were housed in an air-conditioned room at 25 ± 2°C with a 12 h light/dark cycle under standard hygiene conditions and had ad libitum access to a pellet diet and filtered water. The dose of fruit extract of Embilica officinalis and As is based on our previous studies[23,24] and other literature.[33,34,35,36] The study is a part of ongoing research work in our laboratory where we have previously carried out the studies on the doses of As (3 mg/kg BW) and Emblica officinalis (500 mg/kg BW) and further to avoid variations in the study we have selected these doses for the present study.
For the study, the mice were randomly divided into four groups with 10 animals/group:
Group I Mice treated with vehicle (2% gum acacia) for duration of treatment (control)
Group II Mice treated with sodium arsenite (dissolved in distilled water at 3 mg arsenic/kg BW, per os daily for 30 days)
Group III Mice treated with fruit extract of Emblica officinalis (500 mg/kg BW, suspended in 2% gum acacia, per os daily for 30 days)
Group IV Mice co-treated daily with As and Emblica officinalis extract as in Groups II and III.
24 h after the final dosing, all mice were euthanized by cervical dislocation and each spleen isolated. The spleens of three mice/group were cleaned and placed in phosphate-buffered saline (PBS, pH 7.4) and subsequently processed for measures of apoptotic parameters. The remaining spleens in each set were placed in a ice-cold saline solution (0.15 M), blot-dried, weighed, and then immediately processed for use in enzyme/non-enzyme anti-oxidant assays.
Preparation of splenocytes
Each spleen removed at necropsy was made into a single-cell suspension using a 400 μm pore stainless steel mesh.[37] Erythrocytes in the suspension were then lysed with a hypotonic buffered solution and the cells washed with PBS. The remaining cells were suspended in PBS, counted in a hemocytometer and then diluted with PBS to appropriate concentrations for use in the various assays outlined below.
Biochemical parameters
Cell viability
Cell viability was measured by the MTT reduction method Mosman.[38] In brief, cells were seeded at 104 cells/well in a 96-well plate and then a 10 μl MTT (5 mg/ml PBS) solution was added to each well. After the plate was incubated 4 he at 37°C in a CO2 incubator, it was centrifuged (1200 × g, 10 min) and then the supernatant in each well was removed. Thereafter, 100 μl DMSO was added to each well to dissolve the formazan that had formed in the wells and, after 5 min, the absorbance in each well was read and then measured at 530 nm using a Biotek Multiwell plate reader (Biotek Synergy HT, Winooski, VT).
For several of the splenocyte-based assays below, tissue homogenates were required and prepared. Briefly, cells were pelleted by centrifugation (200 × g, 10 min, 4°C) and then re-suspended in PBS. The final lysate was obtained by re-centrifugation (200 × g, 10 min, 4°C) to remove debris. The supernatant was collected and then total protein content measured using the method of Lowry et al.,[39] and bovine serum albumin as a standard.
ROS generation
The generation of ROS was measured using DCFH-DA by flow cytometry as described previously by Wang et al.[40] Single cell suspensions (106 /ml) of splenocytes from control and treated mice were prepared in PBS and incubated with DCFH-DA (100 μM final conc.) at 37°C for 1 hour. Hydrolysis of DCFH-DA leads to formation of fluorescent DCFH that was measured by fluorescence intensity (FL-1, 530 nm) in a BD LSR II flow cytometer (Becton Dickinson, San Jose, CA, USA). All data were analyzed using the ‘Cell Quest’3.3 software (Becton Dickinson). A minimum of 10,000 events per sample were acquired for each analysis.
Lipid peroxidation
To assess intensity of oxidative stress, lipid peroxidation (as reflected in levels of malondialdehyde (MDA) present) was measured using the procedure of Ohkawa et al.[41] The absorbance of each reaction mixture was read at 532 nm using a Cary 300 Bio UV-visible spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Total peroxidation levels were then expressed in μmole MDA/mg protein.
Superoxide dismutase and catalase activity
To assess superoxide dismutase activity in splenocyte homogenates, the assay of Marklund and Marklund[42] was utilized. A unit of activity was defined as the amount of enzyme required for 50% inhibition of pyrogallol auto-oxidation. All results were expressed as U/min/mg protein. Catalase activity in the homogenates was assayed using spectrophoto-metrically by the protocol of Aebi (1984), and using hydrogen peroxide (H2O2) as substrate. All activities were expressed in μmole/min/mg protein.
Caspase-3 activity
Splenocyte caspase-3 activity was measured using kit (Chemi-Con USA). In brief, cells (3.0 × 106 /ml) were placed in lysis buffer and incubated on ice for 10 min. Reaction buffer (10 mM Tris·HCl, 1 mM EDTA, 10 mM dithiothreitol, 5% glycerol) and DEVD-AFC substrate (50 μM) were then added and the mixture then incubated for 2 h at 37°C in the dark. AFC was used as a standard; fluorescence was measured at excitation and emission wavelengths of, respectively, 400 and 505 nm on a microplate reader. Enzyme activity was expressed as nmoles AFC/60 min.
Assay of mitochondrial membrane potential
Mitochondrial membrane potential was assessed by flow cytometry using the procedure of Bai et al.[43] Single cell suspensions (106/ml) of splenocytes from each mouse were incubated with Rh123 (at 5 μg/ml final concentration) at 37°C for 60 min in the dark. Membrane potential was then measured using an FL-1 filter (at fluorescence intensity of 530 nm) in the LSR II flow cytometer. A minimum of 10,000 events per sample were acquired for each analysis.
Analysis of apoptotic DNA
Assays of apoptotic DNA levels were performed using the procedure of Darzynkiewicz et al.[44] Briefly, sets of 106 splenocytes from individual control and treated mice were prepared cell cycle analysis. The cells were washed with PBS and fixed with 70% ethanol. The fixed cells were then again washed with PBS, suspended in phosphate citrate buffer (200 μl, pH 7.8), and incubated for 60 min at room temperature. After centrifugation, the cells were re-suspended in 0.5 ml propidium iodide (100 μg/ml) and 0.5 ml RNAse (50 μg/ml) and incubated a further 30 min in the dark. PI fluorescence was measured using an FL-2 filter (585 nm) in the LSR II flow cytometer. A minimum of 10,000 events per sample were acquired for each analysis.
Assessment of apoptotic and necrotic cell
Apoptotic and necrotic cell distribution among the splenocytes was analyzed via Annexin V binding and PI uptake, following the procedure of Vermes et al.[45] Briefly, sets of 106 splenocytes from individual control and treated mice were each suspended in 1 ml binding buffer (1X), and then a 100 μl aliquot was incubated with 5 μl Annexin V-FITC and 10 μl PI (As provided with kit obtained from Biovision, San Francisco, CA) at room temperature for 15 min in the dark. Thereafter, 400 μl binding buffer (1X) was added to each sample and the associated FITC and PI fluorescence then measured through, respectively, FL-1 (530 nm) and FL-2 (585 nm) filters in the LSR II flow cytometer. A minimum of 10,000 events/sample were acquired each time.
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) followed by a Newman–Keuls test for multiple pair-wise comparisons among the groups. All values were expressed as mean (±SEM). P < 0.05 were considered significant.
RESULTS
Effect on cell viability in splenocytes of mice
The effect of As and co-treatment of As and amla on cell viability in spleen has been presented in Figure 1. Mice exposed to As exhibited a significant decrease in cell viability (44%, P < 0.01) as compared to controls. Co-treatment with As and amla increased the cell viability (63%, P < 0.05) in spleen as compared to those treated with As alone. No significant effect on cell viability was observed in mice treated with amla alone as compared to controls [Figure 1].
Figure 1.
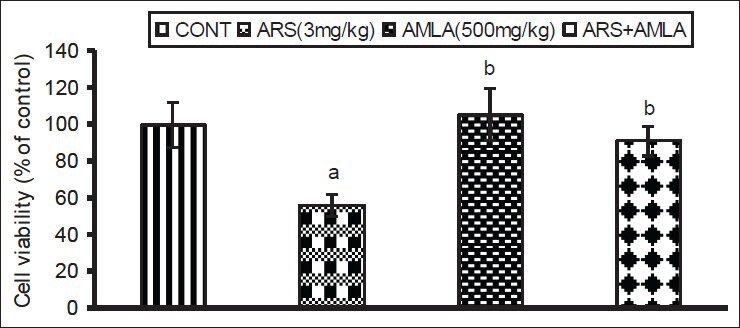
Effect of host treatments on splenocyte viability. Values shown are mean (±SEM) of 5 mice/group. Groups (left to right): control, arsenic only (3 mg/kg), amla only (500 mg/kg) and co-treatment with arsenic and amla. (a) Value significantly differs from control and (b) from arsenic only group (P < 0.05)
Effect on splenocytes ROS generation
Exposure to As caused an increased generation of ROS (76%) in splenocytes as compared to in cells from control mice [Figure 2]. Co-treatment with amla decreased ROS generation (24%) compared to values in cells from mice treated with As alone, suggesting an anti-oxidative free-radical scavenging activity by the extract. No significant effect on ROS production was seen in splenocytes from mice treated with amla alone as compared to controls.
Figure 2.
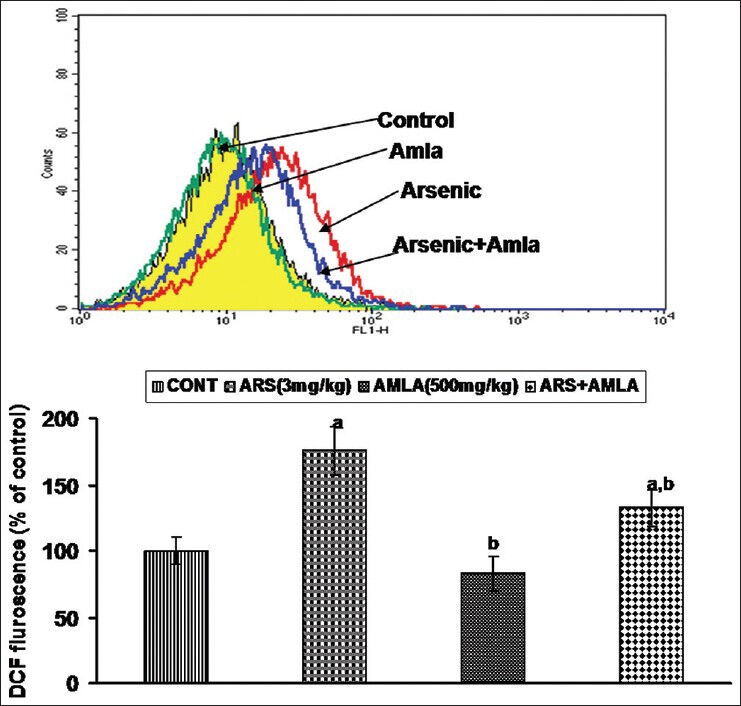
Effect of host treatments on generation of reactive oxygen species in splenocytes. (Top) Cells were incubated with DCFH-DA and fluorescence was measured using a flow cytometer with FL-1 filter. (Bottom) Values shown are mean (±SEM) of 5 mice/group. Groups (left to right): control, arsenic only (3 mg/kg), amla only (500 mg/kg), and co-treatment with arsenic and amla. (a) Value significantly differs from control and (b) from arsenic only group (P < 0.05)
Effect on the lipid peroxidation in splenocytes
The effects of As and co-treatment with amla on lipid peroxidation in splenocytes are shown in Figure 3. Exposure to As caused a significant increase in lipid peroxidation (84%) levels in splenocyte as compared to values in cells from control mice. Co-treatment with amla significantly decreased the level of peroxidation (33%) compared to values in cells of mice treated with As alone. No significant effect on lipid peroxidation levels were seen in cells from mice treated with amla alone as compared to controls.
Figure 3.
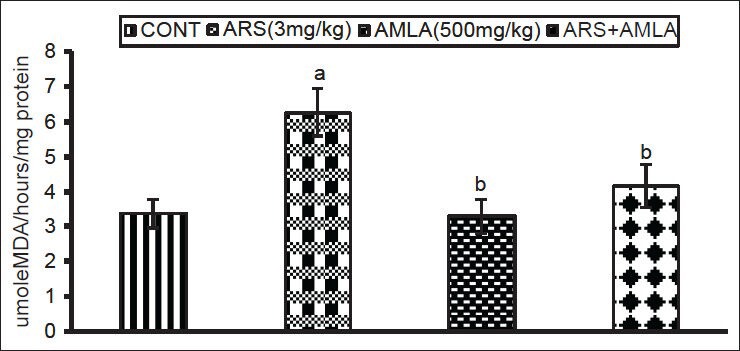
Effect of host treatments on lipid peroxidation levels in splenocytes. Values shown are mean (±SEM) of 5 mice/group. Groups (left to right): control, arsenic only (3 mg/kg), amla only (500 mg/kg) and co-treatment with arsenic and amla. (a) Value significantly differs from control and (b) from arsenic only group (P < 0.05)
Effect on the activity of superoxide dismutase in splenocytes
Exposure to As caused a significant decrease (53%) in splenocyte SOD activity compared to values in cells from control mice. Co-treatment with amla increased the activity (80%) compared to values in cells of the mice treated with As alone. No significant effect on activity was noted in splenocytes of mice treated with amla alone as compared to controls [Figure 4].
Figure 4.
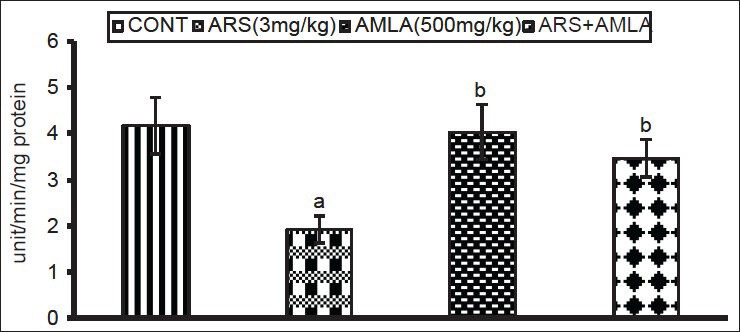
Effect of host treatments on superoxide dismutase activity in splenocytes. Values shown are mean (±SEM) of 5 mice/group. Groups (left to right): control, arsenic only (3 mg/kg), amla only (500 mg/kg) and co-treatment with arsenic and amla. (a) Value significantly differs from control and (b) from arsenic only group (P < 0.05)
Effect on the activity of catalase in splenocytes
Arsenic exposure caused significant decreases (54%) in catalase activity in splenocytes compared to values in cells from control hosts [Figure 5]. Amla co-treatment increased the activity (77%) as compared to values in cells from mice treated with As alone, suggesting a protective effect against oxidative insult. No significant effect on activity was seen in splenocytes of mice treated with amla alone as compared to controls.
Figure 5.
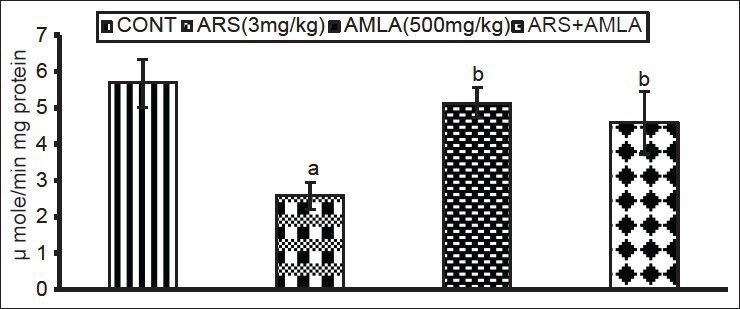
Effect of host treatments on catalase activity in splenocytes. Values shown are mean (± SEM) of 5 mice/group. Groups (left to right): control, arsenic only (3 mg/kg), amla only (500 mg/kg) and co-treatment with arsenic and amla. (a) Value significantly differs from control and (b) from arsenic only group (P < 0.05)
Effect on the mitochondrial membrane depolarization in splenocytes
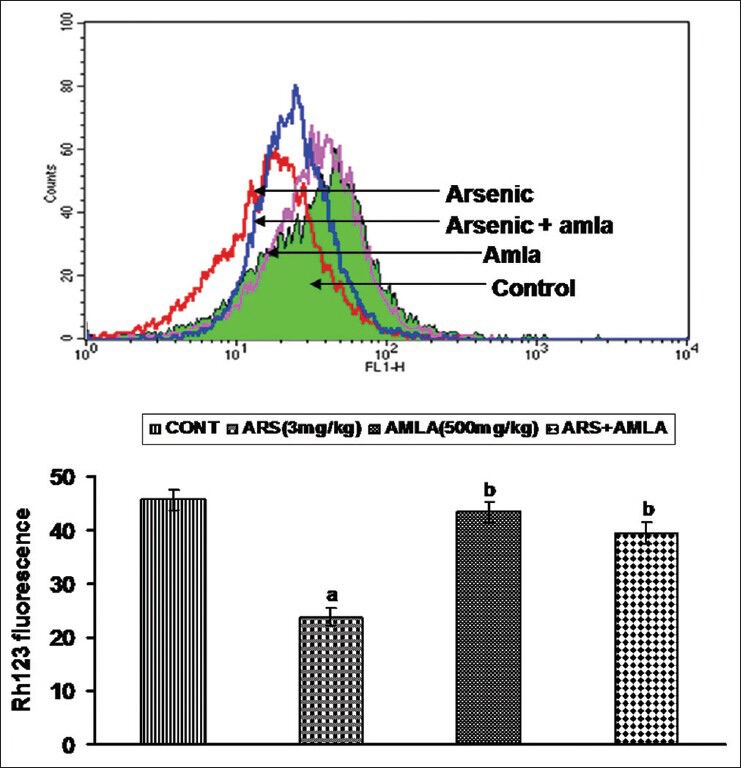
Mice exposed to As displayed decreased mitochondrial membrane depolarization (47%) in their splenocytes as compared to those in cells from controls [Figure 6]. Co-treatment with amla increased the membrane depolarization (66%) compared to values in cells from mice treated with As alone. No significant effect on depolarization was observed in splenocytes from mice treated with amla alone as compared to controls.
Figure 6.
Effect of host treatments on mitochondrial membrane potential in splenocytes. (Top) Cells were incubated with Rh123 and fluorescence was measured using a flow cytometer with FL-1 filter. (Bottom) Values shown are mean (±SEM) of 5 mice/group. Groups (left to right): control, arsenic only (3 mg/kg), amla only (500 mg/kg) and co-treatment arsenic and amla. (a) Value significantly differs from control and (b) from arsenic only group (P < 0.05)
Effect on caspase-3 activity in splenocytes
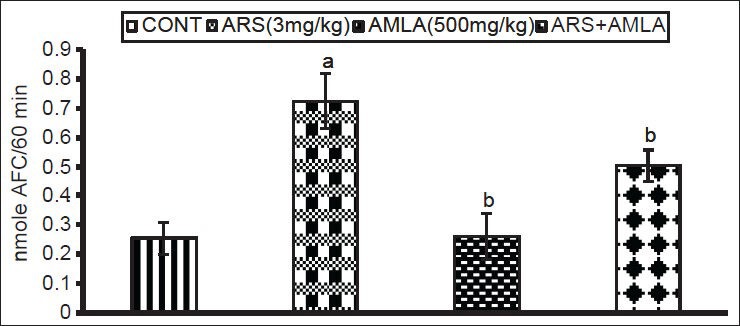
The effects of As and co-treatment with amla on caspase-3 activity in splenocytes from treated mice are presented in [Figure 7]. Exposure to As caused a significant increase in the caspase activity (2.8 fold) in splenocytes as compared to levels noted in cells from control hosts. Amla co-treatment decreased this activity (1.4 fold) in splenocytes as compared to values in cells from mice treated with As alone. No significant effect on caspase activity was noted in splenocytes from mice treated with amla alone as compared to controls.
Figure 7.
Effect of host treatments on caspase-3 activity in splenocytes. Values shown are mean (± SEM) of 5 mice/group. Groups (left to right): control, arsenic only (3 mg/kg), amla only (500 mg/kg) and co-treatment with arsenic and amla. (a) Value significantly differs from control and (b) from arsenic only group (P < 0.05)
Effect on cell cycle in splenocytes
Exposure of mice to As caused an increased number of cells in the sub-G1 portion of the cell cycle, indicating DNA damage (28%) in these hosts’ splenocytes as compared to in the cells from control hosts [Figure 8]. Co-treatment with amla reduced the number of cells in sub-G1 (12%) relative to the numbers seen in mice treated with As alone. No significant effect on cell cycle was noted splenocytes from mice treated with amla alone as compared to controls.
Figure 8.
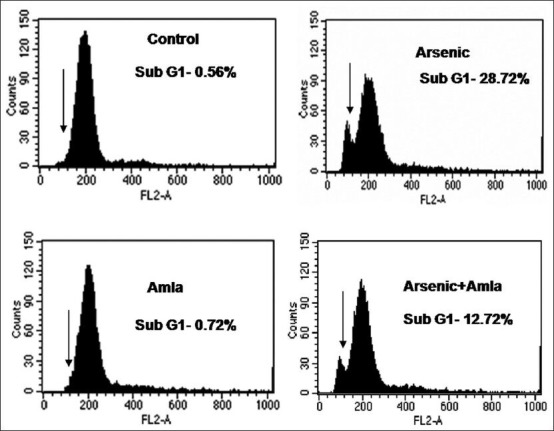
Effect of host treatments on cell cycle progression in splenocytes. Values are presented in histograms as mean (±SEM) of three assays performed independently and indicating sub-G1 cells. Cells were incubated with propidium iodide and fluorescence was measured in a flow cytometer with FL-2 filter
Effect on annexin V/PI binding by splenocytes
Annexin binding was used to reflect numbers of apoptotic and necrotic cells in a given test population. Exposure of As caused an increased in the numbers of necrotic (22%) and apoptotic cells (15%) among all splenocytes as compared to values seen in cells recovered in control mice spleens [Figure 9]. Amla co-treatment decreased the number of necrotic (1.37%) and apoptotic cells (6.02%) relative to levels seen in mice treated with As alone. No significant effect on apoptotic/necrotic cell levels in splenocyte populations were documented as a result of the treatment with amla alone.
Figure 9.
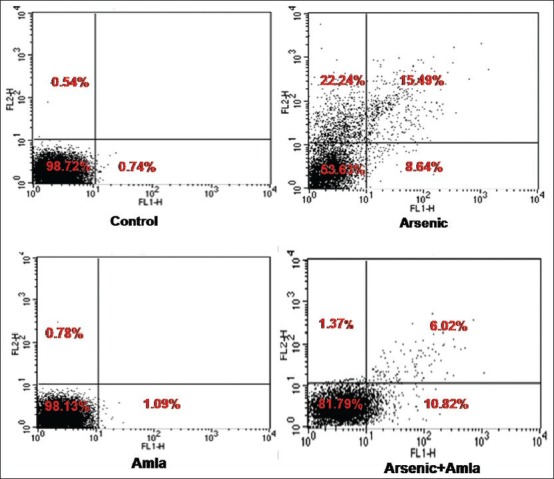
Effect of host treatments on Annexin V/PI dual staining in splenocytes. Values are presented as mean % (±SEM) of cell forms in three assays that were performed independently. Cells in upper right (UR) quadrant = late apoptotic cells; upper left (UL) quadrant = necrotic cells; lower left (LL) and lower right (LR) quadrants = viable and early apoptotic cells, respectively
DISCUSSION
Exposure to As impairs lymphocyte, monocyte and macrophage activity and affects the cellular immune responses, resulting in immunosuppression in mammals.[46,47] Arsenic is a potent immunotoxicant that modulates non-specific immune responses and alters expression of cytokines often in dose and time-dependent manners.[48] Studies have demonstrated that As exposure decreases body weight and impairs anti-oxidant defenses leading to enhanced oxidative damage to biological membranes in part via increased levels of lipid peroxidation, protein carbonyl contents, and reduced anti-oxidant levels in situ.[3,7,23,36] The increased generation of free radicals/ROS associated with enhanced oxidative stress has been implicated in the induction of apoptosis in both patho and physiologic states.[7,36] Such radicals react with biomolecules and so modify protein structure and function and increase the extent of oxidative damage in DNA. Chronic As exposure has been found associated with apoptosis in lymphocytes; this may be an additional under pinning to the documented immunomodulation arising from As exposure.
Arsenic-induced depletion of anti-oxidant defense enzymes, e.g., superoxide dismutase, catalase, and glutathione peroxidase, has also been shown to occur in dose and time-dependent manners.[49] It has been shown that glutathione, the most abundant non-protein thiol-bearing agent in cells, could play an important role in As-induced oxidative stress.[50] Recently, we showed that As treatment resulted in increased generation of ROS and enhanced oxidative stress, outcomes linked with apoptosis in hepatcytes and thymocytes of mice.[23,24] The mechanisms of As toxicity in case of hepatocytes and thymocytes are linked with the enhanced oxidative stress associated with the free radicals generations. In the present study, the decreased viability of splenocytes following exposure to As suggested to us there was likely increased production of ROS and lipid peroxidation, in conjunction with decreased levels of reduced glutathione, activity of superoxide dismutase and catalase, was in line with observations in earlier studies[7,36] and indicative of enhanced oxidative stress in these hosts following exposure to arsenic. The effect on cell viability and on lipid peroxidation following exposure to As is quite high in splenocytes as compare to thymocytes as observed in the earlier study.[23]
Mitochondria are prime targets for As toxicity, either indirectly via ROS accumulation or directly through a condensing of mitochondrial matrices and opening of permeability transition pores (by virtue of the thiol-oxidizing property of some As agents).[20] In either case, the As-induced mitochondrial insult initiates apoptosis.[51] Because decreased MMP results in further generation of ROS, it may be difficult to conclude whether As-induced ROS are the cause or consequence of mitochondrial damage. Studies have reported that As-induced mitochondrial damage in guinea pig liver leads to leakage of super-oxide ions into the cytosol; this suggested to us that here, the NaAsO2 may in fact have been inducing apoptosis by activating intrinsic mitochondrial pathways.[52]
Apoptosis is linked to increased mitochondrial oxidative stress that causes cytochrome c release and consequent activation of caspases and cell death.[53] Arsenic exposure has not only been found to be strongly associated with production of ROS, decreased mitochondrial membrane potential, caspase activation and increased DNA fragmentation, but also with decreased expression of anti (and increased expression of pro) apoptotic proteins linked to apoptosis in normal and transformed cells.[54,55,56,57] Exposure to As is known to result in increased ROS production and also altered expression of genes associated with immune responses[58] in particular those related to T-cell activity.[6] As such, the findings here of decreases in splenocyte cytochrome c oxidase activity and mitochondrial membrane potential, along with the cellular increases in activity of mitochondrial caspase-3 and both the number of cells in sub-G1 portion of cell cycle and those that are apoptotic/necrotic due to exposure to As are consistent with many previous findings.
A number of studies have been carried to investigate the protective efficacy of herbal and/or plant extracts with strong anti-oxidant potential against As-induced oxidative damage and apoptosis.[59,36] Amla is widely accepted as an “immune booster” and possesses multiple pharmacological and immunomodulatory properties[24,36,59] due in part to its content of a wide variety of phenolics and its derivatives. Use of amla to mitigate oxidative stress has been widely accepted.[28] In addition, the metal-binding properties of amla due by many of its constituents have been reported.[36]
Amla has been scientifically studied for its protective role against toxicities associated with exposure to ethanol,[60] carbon tetrachloride, or hexachlorocyclohexane.[61] Studies showed that amla extract could modulate levels of certain of kidney and liver anti-oxidants and helped to restore activities of anti-oxidant enzymes in cyclo-phosphamide-treated animals.[62] The extract of amla has also been reported to enhance cyto-protection and to reduce levels of apoptosis and DNA fragmentation in cells exposed to toxicants.[63] The extract has also been found to impart protection against metal-induced clastogenicity, including that by chromium (CrVI).[59] Further, CrVI-induced apoptosis, DNA fragmentation and immunosuppression were ameliorated by treatment with amla.[59] We recently noted immunomodulatory and anti-oxidative potentials of amla against As-induced oxidative stress and apoptosis in the thymocytes of exposed mice.[23] In the present study, changes in levels of lipid peroxidation and reduced glutathione, and in superoxide dismutase and catalase activities following exposure to As were found to be mitigated/inhibited by co-treatment with amla, suggesting a protective effect. This effect was also reflected by the impact of the extract against As-induced changes in mitochondrial membrane potential, caspase-3 activity, and numbers of sub-G1, apoptotic, and/or necrotic splenocytes.
CONCLUSIONS
The results of this study clearly indicated that while arsenic-induced free radical generation and enhanced oxidative stress, leading to apoptosis/necrosis among splenocytes of mice, co-treatment with amla mitigated/prevented many of these effects. Taken together, the findings here suggest to us that amla could provide a new approach for combating arsenic-induced immunotoxicity. Further studies are required to understand the detailed mechanisms of any potential immunoprotection being afforded by this particular extract.
ACKNOWLEDGMENTS
The authors are thankful to the Head, Department of Pharmacology at King George Medical University, Lucknow, for his interest in the study. The authors also thank Dr. P. C. Choudhury, Professor, at the Government Ayurvedic College and Hospital, Lucknow. Manish Kumar Singh is grateful to the Indian Council of Medical Research, New Delhi, for the research fellowship award. Technical support by Mr. Veerendra Kumar Saini is also acknowledged.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37:1921–42. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Environmental Health Criteria 224. 2nd ed. Geneva: World Health Organization; 2001. WHO (World Health Organization). Arsenic and Arsenic Compounds. Inter-organization Programm for the Sound Management of Chemicals. [Google Scholar]
- 3.Yadav RS, Sankhwar ML, Shukla RK, Chandra R, Pant AB, Islam F, et al. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol Appl Pharmacol. 2009;240:367–76. doi: 10.1016/j.taap.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ Health Perspect. 2008;116:524–31. doi: 10.1289/ehp.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vega L, Montes de Oca P, Saavedra R, Ostrosky-Wegman P. Helper T cell subpopulations from women are more susceptible to the toxic effect of sodium arsenite in vitro. Toxicology. 2004;199:121–8. doi: 10.1016/j.tox.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Chouly C, Morzadec C, Bonvalet M, Galibert MD, Fardel O, Vernhet L. Inorganic arsenic alters expression of immune and stress response genes in activated primary human T lymphocytes. Mol Immunol. 2011;48:956–65. doi: 10.1016/j.molimm.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Stepnik M, Stañczyk M, Arkusz J, Lewiñska D. Assessment of apoptosis in thymocytes and splenocytes from mice exposed to arsenate in drinking water: Cytotoxic effects of arsenate on the cells in vitro. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2005;40:369–84. doi: 10.1081/ese-200045629. [DOI] [PubMed] [Google Scholar]
- 8.Moore SE, Prentice AM, Wagatsuma Y, Fulford AJ, Collinson AC, Raqib R, et al. Early-life nutritional and environmental determinants of thymic size in infants born in rural Bangladesh. Acta Paediatr. 2009;98:1168–75. doi: 10.1111/j.1651-2227.2009.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman A, Vahter M, Ekström EC, Persson LÅ. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ Health Perspect. 2011;119:719–24. doi: 10.1289/ehp.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mc-Collum G, Keng PC, States JC, McCabe MJ., Jr Arsenite delays progression through each cell cycle phase and induces apoptosis following G2/M arrest in U937 myeloid leukemia cells. J Pharmacol Exp Ther. 2005;313:877–87. doi: 10.1124/jpet.104.080713. [DOI] [PubMed] [Google Scholar]
- 11.Tenorio EP, Saavedra R. Differential effect of sodium arsenite during the activation of human CD4+and CD8+T lymphocytes. Int Immunopharmacol. 2005;5:1853–69. doi: 10.1016/j.intimp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Soto-Peña GA, Luna AL, Acosta-Saavedra L, Conde P, López-Carrillo L, Cebrián ME, et al. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. FASEB J. 2006;20:779–81. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- 13.Biswas R, Ghosh P, Banerjee N, Das JK, Sau T, Banerjee A, et al. Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Human Exp Toxicol. 2008;27:381–6. doi: 10.1177/0960327108094607. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Castro B, Doníz-Padilla LM, Salgado-Bustamante M, Rocha D, Ortiz-Pérez MD, Jiménez-Capdeville ME, et al. Effect of arsenic on regulatory T cells. J Clin Immunol. 2009;29:461–9. doi: 10.1007/s10875-009-9280-1. [DOI] [PubMed] [Google Scholar]
- 15.Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal M, Naraharisetti SB, Dandapat S, Degen GH, Malik JK. Perturbations in immune responses induced by concurrent subchronic exposure to arsenic and endosulfan. Toxicology. 2008;251:51–60. doi: 10.1016/j.tox.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 17.Pillai CK, Pillai KS. Antioxidants in health. Indian J Physiol Pharmacol. 2002;46:1–5. [PubMed] [Google Scholar]
- 18.Vahter M. Effects of arsenic on maternal and fetal health. Annu Rev Nutr. 2009;29:381–99. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury R, Chatterjee R, Giri AK, Mandal C, Chaudhuri K. Arsenic-induced cell proliferation is associated with enhanced ROS generation, ERK signaling and CyclinA expression. Toxicol Lett. 2010;198:263–71. doi: 10.1016/j.toxlet.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51:257–81. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Ling YH, Jiang JD, Holland JF, Perez-Soler R. Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol Pharmacol. 2002;62:529–38. doi: 10.1124/mol.62.3.529. [DOI] [PubMed] [Google Scholar]
- 22.Patra PH, Bandyopadhyay S, Bandyopadhyay MC, Mandal TK. Immunotoxic and genotoxic potential of arsenic and its chemicals species in goats. Toxicol Int. 2013;20:6–10. doi: 10.4103/0971-6580.111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh MK, Yadav SS, Gupta V, Khattri S. Immunomodulatory role of Emblica officinalis in arsenic induced oxidative damage and apoptosis in thymocytes of mice. BMC Complement Altern Med. 2013;13:193. doi: 10.1186/1472-6882-13-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh MK, Dwivedi S, Yadav SS, Sharma P, Khattri S. Arsenic-induced hepatic toxicity and its attenuation by fruit extract of Emblica-officinalis (Amla) in mice. Indian J Clin Biochem. 2014;29:29–37. doi: 10.1007/s12291-013-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oluwaseun AA, Ganiyu O. Antioxidant properties of methanolic extracts of mistletoes (Viscum album) from cocoa and cashew trees in Nigeria. Afr J Biotechnol. 2008;7:3138–42. [Google Scholar]
- 26.Ihantola-Vormisto A, Summanen J, Kankaanranta H, Vuorela H, Asmawi ZM, Moilanen E. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 1997;63:518–24. doi: 10.1055/s-2006-957754. [DOI] [PubMed] [Google Scholar]
- 27.Riccioni G, Bucciarelli T, Mancini B, Di Ilio C, Capra V, D’Orazio N. The Role of the antioxidant vitamin supplementation in the prevention of cardiovascular diseases. Expert Opin Investig Drugs. 2007;16:25–32. doi: 10.1517/13543784.16.1.25. [DOI] [PubMed] [Google Scholar]
- 28.Poltanov EA, Shikov AN, Dorman HJ, Pozharitskaya ON, Makarov VG, Tikhonov VP, et al. Chemical and antioxidant evaluation of Indian gooseberry (Emblica officinalis Gaertn., syn. Phyllanthus emblica L.) supplements Phytother Res. 2009;23:1309–15. doi: 10.1002/ptr.2775. [DOI] [PubMed] [Google Scholar]
- 29.Ogasawara M, Matsunaga T, Suzuki H. Differential effects of antioxidants on the in vitro invasion, growth and lung metastasis of murine colon cancer cells. Biol Pharm Bull. 2007;30:200–4. doi: 10.1248/bpb.30.200. [DOI] [PubMed] [Google Scholar]
- 30.Suresh K, Vasudevan DM. Augmentation of murine natural killer cell and antibody dependent cellular cytotoxicity activities by Phyllanthus emblica, a new immunomodulator. J Ethnopharmacol. 1994;44:55–60. doi: 10.1016/0378-8741(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Cui C, Zhao M, Wang J, Luo W, Yang B, et al. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their anti-oxidant activities. Food Chem. 2008;109:909–15. doi: 10.1016/j.foodchem.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 32.Luo W, Zhao M, Yang B, Shen G, Rao G. Identification of bioactive compounds in Phllenthus emblica L. fruit and their free radical scavenging activities. Food Chem. 2009;114:499–504. [Google Scholar]
- 33.Schulz H, Nagymajtényi L, Institoris L, Papp A, Siroki O. A study on behavioral, neurotoxicological, and immunotoxicological effects of subchronic arsenic treatment in rats. J Toxicol Environ Health A. 2002;65:1181–93. doi: 10.1080/152873902760125390. [DOI] [PubMed] [Google Scholar]
- 34.Khandelwal S, Shukla LJ, Shanker R. Modulation of acute Cadmium toxicity by Emblica officinalis fruit in rat. Indian J Exp Biol. 2002;40:564–70. [PubMed] [Google Scholar]
- 35.Suja RS, Nair AM, Sujith S, Preethy J, Deepa AK. Evaluation of immunomodulatory potential of Emblica officinalis fruit pulp extract in mice. Indian J Anim Res. 2009;43:103–6. [Google Scholar]
- 36.Sharma A, Sharma MK, Kumar M. Modulatory role of Emblica officinalis fruit extract against arsenic induced oxidative stress in Swiss albino mice. Chem Biol Interact. 2009;180:20–30. doi: 10.1016/j.cbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Gao S, Wang Y, Zhang P, Dong Y, Li B. Subacute oral exposure to dibromoacetic acid induced immunotoxicity and apoptosis in the spleen and thymus of mice. Toxicol Sci. 2008;105:331–41. doi: 10.1093/toxsci/kfn139. [DOI] [PubMed] [Google Scholar]
- 38.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1988;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 39.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:263–75. [PubMed] [Google Scholar]
- 40.Wang JF, Jerrells TR, Spitzer JJ. Decreased production of reactive oxygen intermediates is an early event during in vitro apoptosis of rat thymocytes. Free Radic Biol Med. 1996;20:533–42. doi: 10.1016/0891-5849(95)02085-3. [DOI] [PubMed] [Google Scholar]
- 41.Ohkawa H, Osishi N, Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 42.Marklund S, Marklund G. Involvement of superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 43.Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem. 1999;274:26217–24. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 44.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: Analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 45.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 46.Burchiel SW, Mitchell LA, Lauer FT, Sun X, McDonald JD, Hudson LG, et al. Immunotoxicity and biodistribution analysis of arsenic trioxide in C57Bl/6 mice following a 2-wk inhalation exposure. Toxicol Appl Pharmacol. 2009;241:253–9. doi: 10.1016/j.taap.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Q, Lauer FT, Liu KJ, Hudson LG, Burchiel SW. Low-dose synergistic immunosuppression of T-dependent antibody responses by polycyclic aromatic hydrocarbons and arsenic in C57BL/6J murine spleen cells. Toxicol Appl Pharmacol. 2010;245:344–51. doi: 10.1016/j.taap.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das S, Pan D, Bera AK, Rana T, Bhattacharya D, Bandyapadyay S, et al. Sodium arsenite mediated immune-disruption through alteration of transcription profile of cytokines in chicken splenocytes under in vitro system. Mol Biol Rep. 2011;38:171–6. doi: 10.1007/s11033-010-0091-5. [DOI] [PubMed] [Google Scholar]
- 49.Mishra D, Mehta A, Flora SJ. Reversal of arsenic-induced hepatic aoptosis with combined administration of DMSA and its analogues in guinea pigs: Role of glutathione and linked enzymes. Chem Res Toxicol. 2008;21:400–7. doi: 10.1021/tx700315a. [DOI] [PubMed] [Google Scholar]
- 50.Habib A, Hayashi T, Sato KK, Hata A, Ikebe M, Rahman F, et al. Effectiveness of arsenic mitigation program in Bangladesh--relationship between arsenic concentrations in well water and urine. Osaka City Med J. 2007;53:97–103. [PubMed] [Google Scholar]
- 51.Pulido MD, Parrish AR. Metal-induced apoptosis: Mechanisms. Mutat Res. 2003;533:227–41. doi: 10.1016/j.mrfmmm.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 53.Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25:17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Hossain K, Akhand AA, Kato M, Du J, Takeda K, Wu J, et al. Arsenite induces apoptosis of murine T lymphocytes through membrane raft-linked signaling for activation of c-Jun amino-terminal kinase. J Immunol. 2000;165:4290–7. doi: 10.4049/jimmunol.165.8.4290. [DOI] [PubMed] [Google Scholar]
- 55.Li D, Morimoto K, Takeshita T, Lu Y. Formamidopyrimidine-DNA glycosylase enhances arsenic-induced DNA strand-breaks in PHA-stimulated and unstimulated human lymphocytes. Environ Health Perspect. 2001;109:523–6. doi: 10.1289/ehp.01109523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maeda H, Hori S, Nishitoh H, Ichijo H, Ogawa O, Kakehi Y, et al. Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res. 2001;61:5432–40. [PubMed] [Google Scholar]
- 57.Banerjee N, Banerjee M, Ganguly S, Bandyopadhyay S, Das JK, Bandyopadhay A, et al. Arsenic-induced mitochondrial instability leading to programmed cell death in the exposed individuals. Toxicology. 2008;246:101–11. doi: 10.1016/j.tox.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 58.Flora S, Bhatt K, Mehta A. Arsenic moiety in gallium arsenide is responsible for neuronal apoptosis and behavioral alterations in rats. Toxicol Appl Pharmacol. 2009;240:236–44. doi: 10.1016/j.taap.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Sai-Ram M, Neetu D, Yogesh B, Anju B, Dipti P, Pauline T, et al. Cyto-protective and immunomodulating properties of Amla (Emblica officinalis) on lymphocytes: An in-vitro study. J Ethnopharmacol. 2002;81:5–10. doi: 10.1016/s0378-8741(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 60.Pramyothin P, Samosorn P, Poungshompoo S, Chaichantipyuth C. The protective effects of Phyllanthus emblica Linn. extract on ethanol induced rat hepatic injury. J Ethnopharmacol. 2006;107:361–4. doi: 10.1016/j.jep.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 61.Anilkumar KR, Nagaraj NS, Santhanam K. Reduction of hexachlorocyclohexane-induced oxidative stress and cytotoxicity in rat liver by Emblica officinalis Gaertn. Indian J Exp Biol. 2007;45:450–4. [PubMed] [Google Scholar]
- 62.Haque R, Bin-Hafeez B, Ahmad I, Parvez S, Pandey S, Raisuddin S. Protective effects of Emblica officinalis Gaertn in cyclophosphamide-treated mice. Human Exp Toxicol. 2001;20:643–50. doi: 10.1191/096032701718890568. [DOI] [PubMed] [Google Scholar]
- 63.Bandyopadhyay SK, Pakrashi SC, Pakrashi A. The role of antioxidant activity of Phyllanthus emblica fruits on prevention from indomethacin induced gastric ulcer. J Ethnopharmacol. 2000;70:171–6. doi: 10.1016/s0378-8741(99)00146-4. [DOI] [PubMed] [Google Scholar]


