Abstract
Introduction:
The purpose of this study was to examine the effect of different extraction media, including culture media, as well as storage times on the elution of monomers from modern dental composites.
Materials and Methods:
Four contemporary composite materials were tested: (a) Clearfil Majesty Esthetic (Kuraray), (b) Esthet X (DENTSPLY), (c) Filtek Silorane (3M ESPE), and (d) Admira (Voco). Forty-eight specimens were made. The specimens were stored in 1 ml of (a) artificial saliva, (b) Dulbecco's Modified Eagle Medium (DMEM), (c) DMEM plus 10% fetal bovine serum (FBS), and (d) ethanol 75%. The specimens were analyzed after 24 hours and after 1 week of storage. HPLC Liquid Chromatography was performed to analyze the extracted solutions. The statistical package SPSS 18 was used for the statistical analysis of the results.
Results:
All the materials tested released monomers that were consistent with the base composition of their resin matrix. Bisphenol-A (BPA) was detected in Clearfil Esthetic and EsthetX when ethanol 75% was used for storage. TEGDMA was released at a faster rate compared to the other monomers with most of the monomer eluted in the first 24 hours. The effect of storage solution and storage time on the elution of the same monomers varied between materials.
Conclusions:
There was a significant effect of time, storage solution, and material on the elution of the detectable unbound monomers. Unbound monomers were detected in culture media, which may lead to false-negative results in cytotoxicity tests of resin composite materials. BPA was detected in two of the tested materials.
Keywords: High-performance liquid chromatography, monomer elution, resin composite restorations, storage solution, storage time
INTRODUCTION
The increasing demand for aesthetic dental restorations and the associated decline in the use of amalgam restorations has resulted in an increase in the use of resin composites in dental practice. Nevertheless, concerns regarding their biocompatibility and clinical safety related to the release of low molecular weight components, such as monomers and Bis-Phenol A (BPA) from the resin matrix still remain.[1]
Several studies have investigated the biocompability of these materials and they have shown that substances released from dental composites can cause significant cytotoxic and genotoxic effects.[2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19] It has been reported that all the components of the composite can leach out of the polymerized material.[8] The most cytotoxic and genotoxic substances released are the unbound monomers of the resin matrix, which are either basic monomers, such as Bisphenol A-Glycidyl Methacrylate (BisGMA), Urethane Dimethacrylate (UDMA), and Ethoxylated Bisphenol-A Dimethacrylate (BisEMA), or co-monomers that are used as diluents, e.g., Tetraethyleneglycol Dimethacrylate (TEGDMA) and 2-Hydroxyethyl Methacrylate (HEMA).[8,20,21,22,23,24,25,26]
More recently, another substance that concerns the scientific society is BPA, which is one of a family of endocrine-disrupting chemicals (EDCs). It shares similarities in structure, metabolism, and action with diethylstilbestrol (DES), a known human teratogen and carcinogen.[27] It is believed to be a degradation product of BisDMA and it has been proven to have estrogenic effects, having adverse effects on the reproductive system of female mice.[28] The current picture appears somewhat confusing as to the extent to which dental material may be a significant source of BPA. Research has shown that BPA is released from a number of resin-based dental materials.[4,29,30] However, where BPA release has been detected, the amounts involved have been very low and well within the Tolerable Daily Intake (TDI) of 0.05 mg/kg bw/day set by the EFSA. A systematic review on pit and fissure sealants published in 2008 stated that: “The evidence suggests that patients are not at risk for exposure to BPA from the use of dental sealants.”[31] However, this recommendation is primarily based on the toxic effects of BPA and more research is needed to determine whether or not the human exposure to very low physiological levels of BPA associated with certain dental materials can cause estrogenic adverse effects.
Furthermore, the amount of components released from dental composite resins and the type of extraction media play an important role in the biocompatibility and toxicity testing of dental materials and can significantly affect the assay results.[32] In previous cytotoxicity studies, a variety of extraction media have been used such as culture medium,[33,34,35] distilled water,[36,37] saline,[32] and acetone plus ethanol in saline.[38] However, only few studies compare the effect of different extraction techniques.[32,39,40] In the majority of studies testing monomer, elution from dental composite extraction media such as water or saliva are mainly used to represent the oral environment. According to Ferracane, solvents that are somewhere between the more aggressive organic solvents and water can be considered to be highly representative of the oral environment, while according to the US FDA an ethanol 75% solution is a food/oral simulating liquid that can be considered clinically relevant.[2] However, for the assessment of the cytotoxicity of these materials, culture media are usually used. It was interesting to see therefore the amount of monomers eluted, if any, in culture media as well as in common storage media used in extraction studies.
The aim of this study was to identify and quantify the elution of monomers and BPA from four contemporary resin composites, representative of the current resin matrix technologies using High-Performance Liquid Chromatography (HPLC), and to evaluate the effect of different extraction media and storage periods.
MATERIALS AND METHODS
For the purpose of this study, the materials selected were representatives of the available resin composite systems. Specifically, the following dental composites were used: (a) a Bis-GMA nano- hybrid composite; Clearfil Majesty Esthetic, Kuraray, (b) a TEGDMA nano-hybrid composite; Esthet-X, DENSPLY, (c) an ormocer composite; Admira, Voco, and (d) a low shrinkage silorane composite; Filtek Silorane, 3M ESPE. The details of the composition of the materials and the manufacturers’ names are given in Table 1. Four extraction media were used, which were (a) artificial saliva, (b) Dulbecco's Modified Eagle's Medium (DMEM), (c) DMEM with 10% fetal bovine serum (FBS), and (d) ethanol 75%.
Table 1.
Composition of the tested materials and manufacturers information
Specimen fabrication
Discs of the composite resin were made by placing the material into a prefabricated metal mould (6 mm diameter and 2 mm thickness). The surface was covered with a transparent strip, pressed with a glass plate and light cured for 40 seconds (Henry Schein LED Light 800). Three discs of each composite were prepared for each solution. A total number of 48 polymerized disks of the four composites were prepared for immersion into the four different extraction media. In order to correlate the amount of composite used for the fabrication of discs to that used for clinical restorations, a typical Mesio-Occlusal-Distal (MOD) cavity was prepared on a phantom molar tooth and restored with resin composite. The phantom tooth was measured before preparation and after restoration. The weight of each disk was measured and found to be approximately the same as that of the MOD composite restoration.
Each disk was immersed in 1 ml of extraction medium and placed in an incubator at 37°C. After 24 hours, all the extraction media were removed from the vials and transferred to different vials for analysis with HPLC. The extraction media were renewed with 1 ml solution in each vial. The reloaded vials were placed again into the pre-conditioned laboratory incubator and kept for another six days to complete 1 week from the beginning of the study. After 1 week, the extraction media were removed and transferred into new vials for HPLC analysis. The limit of quantification was set to 1 μg/ml for UDMA, 0.5 μg/ml for TEGDMA, 1 μg/ml for BisGMA, 1 μg/ml for BISEMA, and 0.5 μg/ml for BPA according to a methodology previously described by Polydorou et al.[4]
Development of HPLC method
A mixture of pure specimens of the monomers BisGMA, UDMA mixture of isomers, TEGDMA, BISEMA, and BPA (Sigma Aldrich) were analyzed by reverse phase HPLC Chromatograph (Waters 2695), with the mobile phase-Acetonitrile in water to run from 0% till 100% so as to find the right concentration of the mobile phase and the right volume of the injection in order to separate the peaks formatted from the monomers in different retention times. The conditions that gave a good separation of the peaks of the tested monomers were as follows:
Column: Steel column (ZorbaxEclipse 5u XDB-C8), 150 mm length, 4.6 mm in diameter, and particle size of 5 μm
Mobilephase: CH3CN 45%/H2O 55%
Flowspeed: 1 ml/min
Detection: UV: 205 nm
Injection: 25 μL loop at constant room temperature.
Since the manufacturers of the Filtek Silorane were not able to provide us with the pure monomer for the material, un-polymerized Filtek Silorane was left in Acetonitrile solution for 24 hours in order to identify any eluted elements.
Calibration curves
In order to quantify each monomer, several known concentrations of each monomer in Acetonitrile solution were prepared and analyzed by HPLC. Calibration standard curves of peak areas vs. monomer concentration for each monomer were produced. For all monomers, a linear curve was produced at known concentrations of 0 to 0.5 mg/ml. A calibration curve was not produced for the silorane extract due to lack of information regarding the chemistry of that monomer and only the retention time was recorded for identification purposes.
Statistical analysis
For the statistical analysis of the results, the statistical package SPSS 18 was used. One way ANOVA and post hoc Tukey's test were performed to identify any significant differences between the materials, storage media, and storage times.
RESULTS
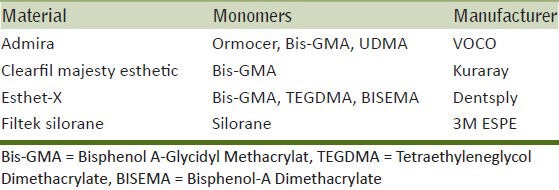
The retention times for each monomer, including the silorane extract, are given in Figure 1. TEGDMA eluted with the faster rate compared to the other monomers.
Figure 1.
Retention times of monomers
All data were analyzed with respect to the different solutions and times. The average amount of monomer elution in μg/ml and standard deviation (SD) were calculated for each group, extraction medium, and storage time [Tables 2–5]. One way Analysis of Variance with Post Hoc tests (Tukey's test) showed that there was a statistically significant difference between the extraction media and the storage times (P < 0.05).
Table 2.
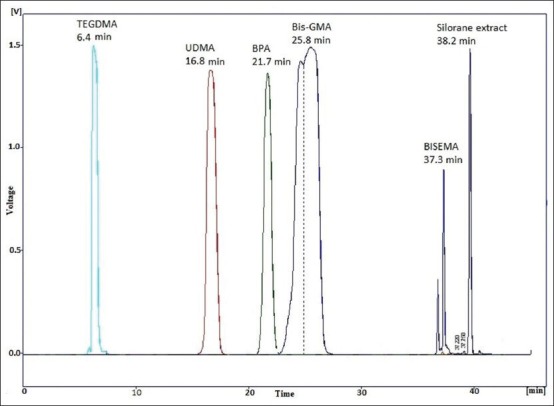
Amount of monomer eluted (μg/ml) and SD after storage in different solutions and times for Admira
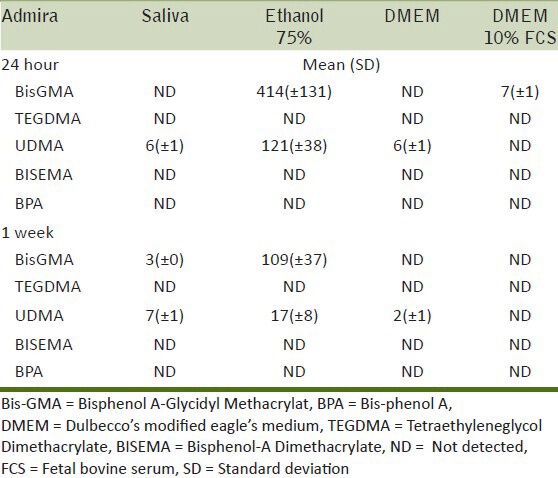
Table 5.
Amount of monomer eluted (μg/ml) and SD after storage in different solutions and times for Filtek Silorane
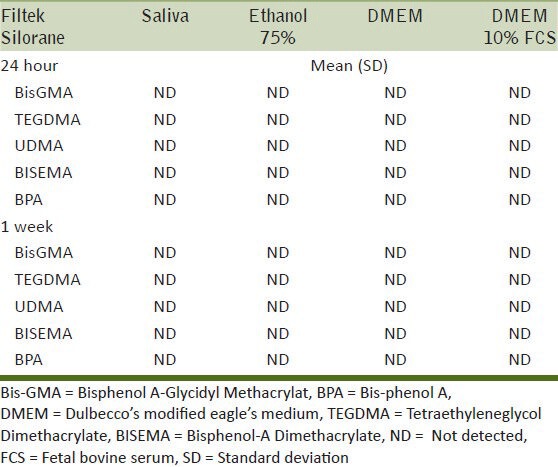
Table 3.
Amount of monomer eluted (μg/ml) and SD after storage in different solutions and times for Clearfil Majesty
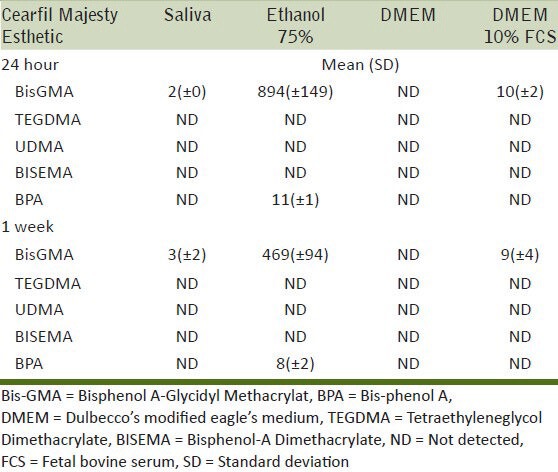
Table 4.
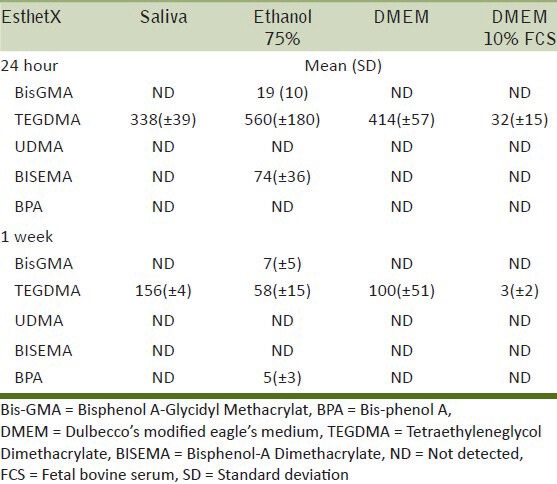
Amount of monomer eluted (μg/ml) and SD after storage in different solutions and times for EsthetX
For the silorane material, none of the tested monomers were detected. However, for the same material, an unknown substance was eluted at 38 minutes after 24 hours of storage in the ethanol 75% solution [Figure 2]. The peak at 38 minutes was consistent with that detected when HPLC of the unpolymerized Filtek Silorane material was performed. Since no information of the monomer was available, it was assumed that the substance released at 38 min must be related to the silorane monomer.
Figure 2.
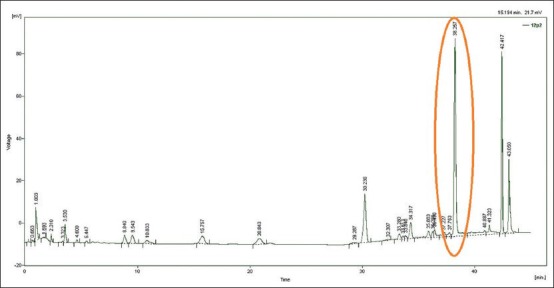
Elution of substances from Filtek Silorane material after storage for 24 hours in ethanol 75% solution. The peak at 42 minutes corresponds to the ethanol peak but the peak at 38 minutes is the same as the peak eluted from the unset Filtek Silorane material
DISCUSSION
The purpose of the present study was to evaluate the effect of four extraction media and of two different storage times on the release of unbound monomers and BPA from four contemporary commercial resin composite materials using HPLC.[6,41] The results obtained demonstrated that time, solution, and material significantly affected the release of the detected monomers.
The results of this study showed that ethanol 75% induced significantly more release of monomers compared to the others solutions. One reason for this increased release of monomers in the ethanol 75% extraction medium can be justified by the fact that ethanol has a solubility parameter similar to the monomers of dental composites,[4,42] which allows it to penetrate the matrix of the composite and swell the polymerized network facilitating the liberation of unreacted and unbound monomers.[2] The high release of Bis-GMA monomer detected in the Clearfil Majesty Esthetic material was in agreement with previous studies.[3,5,43] Bis-GMA is a rigid and highly viscous monomer with a high glass transition temperature, which result in a lower degree of conversion of this monomer during polymerization and more free monomer is able to leach out from the polymerized composite.[44]
Esthet-X released significantly higher amounts of TEGDMA monomer, which was found within the first 24 hours, while Bis-GMA was released in significantly smaller amounts. This is in accordance with the statement of Tanaka et al.,[45] that small molecular weight monomers such as TEGDMA have higher mobility and polarity that enable it to elute faster than other large molecules. With regards to the storage solutions, saliva and DMEM also showed a significant amount of released TEGDMA, suggesting that the solubility parameter of these solvents are very close to TEGDMA. However, a significantly lower release of TEGDMA was detected from DMEM 10% FCS solution. This could be due to the degradation of TEGDMA by various enzymes present in the serum. It has also been reported that TEGDMA binds to albumin that is contained in the serum and consequently intensifies the peak of albumin in the chromatogram, while the concentration of TEGDMA appears to decrease during storage.[39]
Regarding the silorane-based composite, there was no elution of any of the detectable basic monomers or co-monomers from this material. This would appear to be consistent with the matrix of the silorane composite not containing any of the traditional di-methacrylate resins. These results are consistent with another study where a silorane composite was immersed in water and ethanol and no monomers were detected that are used in the di-methacrylate-based composites except silorane monomers.[46] Nevertheless, a peak was detected in the HPLC trace for the ethanol 75%, which would appear to correspond to a component in the matrix of this composite and it might reasonably be speculated that this is due to the release of some of the silorane monomer.
The Admiraormocer dental composite exhibited a quite high release of monomers, especially in the ethanol 75% extraction media, which however decreased with time.
It was interesting to see that BPA was detected in two of the tested composites. This is in agreement with other studies that have reported the release of BPA from dental composites.[4,28,47] The amount of BPA detected varied according to the materials, the solution, and the storage time. The material that gave the highest amount of BPA release was Clearfil Majesty Esthetic, when eluted in ethanol 75% solution. According to a risk assessment on BPA published in January 2007 by the European Foods Standards Agency (EFSA), the TDI for BPA was set at 0.05 mm/kg body weight (bw).[48] The amount of BPA recorded in this study is well below that amount. The fact that the weight of the specimens corresponded to that of an average MOD restoration on a molar tooth indicates that in order to reach the TDI for BPA, a significant number of composite restorations would have to be placed in the mouth simultaneously to approach the TDI and even then it is only for one day and after an extended exposure to ethanol. However, these findings must be interpreted with care as at the moment there is no strong evidence as to whether or not human exposure, even to very low physiological levels of BPA associated with certain dental materials, can cause estrogenic adverse effects and further research is required. Nevertheless, it is important to know that BPA, even in trace amounts, can be released from some contemporary composite systems.
Although all the composites examined released components from their matrix after they had been cured, there are some differences among the materials with the amount of the released monomers. When focusing on the release from ethanol 75%, Clearfil Majesty Esthetic showed the highest release of BISGMA after 24 hours, followed by Admira and Estet-X, which showed a very low release. In contrast, Admira showed a significant release of UDMA, and TEGDMA was only released in relatively large amounts from Esthet-X. The Filtek Silorane only released a compound that is probably a silorane monomer. Regarding BPA, Clearfil Majesty Esthetic showed the highest release after 24 hours, followed by Estet-X which showed a release after 1 week. Also, the type of storage solution had a different effect on each material. For example, Clearfil Majesty Esthetic showed release of BisGMA in the saliva solution, which was not the case for Admira or Esthet-X, while DMEM 10% FCS caused the release of the same monomer from the Admira and Clearfil Majesty Esthetic but not from Esthet-X. It is evident that the type and amount of monomer released from each composite is quite distinctively different and is directly related to the composition of the matrix. Thus, in the context of clinical significance, biocompatibility, and possible adverse reactions associated with these composites, it is important to appreciate that the trigger for this will be a function of the type of composite to which the patient is exposed.
In order to allay patients’ fears of potential adverse reactions to monomer or BPA release from composite resins, whether justified or not, regulatory bodies and manufacturers will need to consider careful approaches that will reduce or better still eliminate exposure of patients to these eluates. This will ensure that dental materials will make no contribution to the body burden of these products.
CONCLUSIONS
It was concluded that the type of storage solutions, the storage times, and the material tested had a significant effect on the elution of the detectable unbound monomers. Unbound monomers were also detected in culture media, a finding that is important to take into consideration incytotoxicity testing of dental materials as it may lead to false-negative results. Bis-Phenol A was found in two of the tested materials, but this was related to the storage solution as it was only detected in the ethanol 75% solution.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bakopoulou A, Papadopoulos T, Garefis P. Molecular toxicology of substances released from resin-based dental restorative materials. Int J Mol Sci. 2009;10:3861–99. doi: 10.3390/ijms10093861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferracane JL. Elution of leachable components from composites. J Oral Rehabil. 1994;21:441–52. doi: 10.1111/j.1365-2842.1994.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 3.Polydorou O, Hammad M, Konig A, Hellwig E, Kummerer K. Release of monomers from different core build-up materials. Dent Mater. 2009;25:1090–5. doi: 10.1016/j.dental.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Polydorou O, Konig A, Hellwig E, Kummerer K. Long-term release of monomers from modern dental-composite materials. Eur J Oral Sci. 2009;117:68–75. doi: 10.1111/j.1600-0722.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 5.Sideridou ID, Achilias DS. Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J Biomed Mater Res B Appl Biomater. 2005;74:617–26. doi: 10.1002/jbm.b.30252. [DOI] [PubMed] [Google Scholar]
- 6.Spahl W, Budzikiewicz H, Geurtsen W. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J Dent. 1998;26:137–45. doi: 10.1016/s0300-5712(96)00086-3. [DOI] [PubMed] [Google Scholar]
- 7.Geurtsen W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med. 2000;11:333–55. doi: 10.1177/10454411000110030401. [DOI] [PubMed] [Google Scholar]
- 8.Geurtsen W. Substances released from dental resin composites and glass ionomer cements. Eur J Oral Sci. 1998;106:687–95. doi: 10.1046/j.0909-8836.1998.eos10602ii04.x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta SK, Saxena P, Pant VA, Pant AB. Release and toxicity of dental resin composite. Toxicol Int. 2012;19:225–34. doi: 10.4103/0971-6580.103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuelsen JT, Dahl JE, Karlsson S, Morisbak E, Becher R. Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation of the MAP-kinases p38, JNK and ERK. Dent Mater. 2007;23:34–9. doi: 10.1016/j.dental.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Stanislawski L, Lefeuvre M, Bourd K, Soheili-Majd E, Goldberg M, Perianin A. TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J Biomed Mater Res A. 2003;66:476–82. doi: 10.1002/jbm.a.10600. [DOI] [PubMed] [Google Scholar]
- 12.Schweikl H, Hiller KA, Eckhardt A, Bolay C, Spagnuolo G, Stempfl T, et al. Differential gene expression involved in oxidative stress response caused by triethylene glycol dimethacrylate. Biomaterials. 2008;29:1377–87. doi: 10.1016/j.biomaterials.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Engelmann J, Leyhausen G, Leibfritz D, Geurtsen W. Metabolic effects of dental resin components in vitro detected by NMR spectroscopy. J Dent Res. 2001;80:869–75. doi: 10.1177/00220345010800030501. [DOI] [PubMed] [Google Scholar]
- 14.About I, Camps J, Mitsiadis TA, Bottero MJ, Butler W, Franquin JC. Influence of resinous monomers on the differentiation in vitro of human pulp cells into odontoblasts. J Biomed Mater Res. 2002;63:418–23. doi: 10.1002/jbm.10253. [DOI] [PubMed] [Google Scholar]
- 15.Noda M, Wataha JC, Kaga M, Lockwood PE, Volkmann KR, Sano H. Components of dentinal adhesives modulate heat shock protein 72 expression in heat-stressed THP-1 human monocytes at sublethal concentrations. J Dent Res. 2002;81:265–9. doi: 10.1177/154405910208100408. [DOI] [PubMed] [Google Scholar]
- 16.Kawai K, Tsuchitani Y. Effects of resin composite components on glucosyltransferase of cariogenic bacterium. J Biomed Mater Res. 2000;51:123–7. doi: 10.1002/(sici)1097-4636(200007)51:1<123::aid-jbm16>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Hansel C, Leyhausen G, Mai UE, Geurtsen W. Effects of various resin composite (co) monomers and extracts on two caries-associated micro-organisms in vitro. J Dent Res. 1998;77:60–7. doi: 10.1177/00220345980770010601. [DOI] [PubMed] [Google Scholar]
- 18.Engelmann J, Janke V, Volk J, Leyhausen G, von Neuhoff N, Schlegelberger B, et al. Effects of BisGMA on glutathione metabolism and apoptosis in human gingival fibroblasts in vitro. Biomaterials. 2004;25:4573–80. doi: 10.1016/j.biomaterials.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 19.Imazato S, Horikawa D, Nishida M, Ebisu S. Effects of monomers eluted from dental resin restoratives on osteoblast-like cells. J Biomed Mater Res B Appl Biomater. 2009;88:378–86. doi: 10.1002/jbm.b.31067. [DOI] [PubMed] [Google Scholar]
- 20.Schweikl H, Schmalz G, Spruss T. The induction of micronuclei in vitro by unpolymerized resin monomers. J Dent Res. 2001;80:1615–20. doi: 10.1177/00220345010800070401. [DOI] [PubMed] [Google Scholar]
- 21.Hanks CT, Strawn SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991;70:1450–5. doi: 10.1177/00220345910700111201. [DOI] [PubMed] [Google Scholar]
- 22.Ratanasathien S, Wataha JC, Hanks CT, Dennison JB. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J Dent Res. 1995;74:1602–6. doi: 10.1177/00220345950740091601. [DOI] [PubMed] [Google Scholar]
- 23.Becher R, Kopperud HM, Al RH, Samuelsen JT, Morisbak E, Dahlman HJ, et al. Pattern of cell death after in vitro exposure to GDMA, TEGDMA, HEMA and two compomer extracts. Dent Mater. 2006;22:630–40. doi: 10.1016/j.dental.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Reichl FX, Simon S, Esters M, Seiss M, Kehe K, Kleinsasser N, et al. Cytotoxicity of dental composite (co) monomers and the amalgam component Hg (2+) in human gingival fibroblasts. Arch Toxicol. 2006;80:465–72. doi: 10.1007/s00204-006-0073-5. [DOI] [PubMed] [Google Scholar]
- 25.Moharamzadeh K, Van Noort R, Brook IM, Scutt AM. Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent Mater. 2007;23:40–4. doi: 10.1016/j.dental.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 26.Issa Y, Watts DC, Brunton PA, Waters CM, Duxbury AJ. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent Mater. 2004;20:12–20. doi: 10.1016/s0109-5641(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Jonathan N, Steinmetz R. The emerging story of bisphenol a. Trends Endocrinol Metab. 1998;9:124–8. doi: 10.1016/s1043-2760(98)00029-0. [DOI] [PubMed] [Google Scholar]
- 28.Al-Hiyasat AS, Darmani H, Elbetieha AM. Leached components from dental composites and their effects on fertility of female mice. Eur J Oral Sci. 2004;112:267–72. doi: 10.1111/j.1600-0722.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmalz G, Preiss A, Arenholt-Bindslev D. Bisphenol-A content of resin monomers and related degradation products. Clin Oral Investig. 1999;3:114–9. doi: 10.1007/s007840050088. [DOI] [PubMed] [Google Scholar]
- 30.Polydorou O, Beiter J, Konig A, Hellwig E, Kummerer K. Effect of bleaching on the elution of monomers from modern dental composite materials. Dent Mater. 2009;25:254–60. doi: 10.1016/j.dental.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Azarpazhooh A, Main PA. Is there a risk of harm or toxicity in the placement of pit and fissure sealant materials? A systematic review. J Can Dent Assoc. 2008;74:179–83. [PubMed] [Google Scholar]
- 32.Hanks CT, Anderson M, Craig RG. Cytotoxic effects of dental cements on two cell culture systems. J Oral Pathol. 1981;10:101–12. doi: 10.1111/j.1600-0714.1981.tb01255.x. [DOI] [PubMed] [Google Scholar]
- 33.Bouillaguet S, Shaw L, Gonzalez L, Wataha JC, Krejci I. Long-term cytotoxicity of resin-based dental restorative materials. J Oral Rehabil. 2002;29:7–13. doi: 10.1046/j.1365-2842.2002.00804.x. [DOI] [PubMed] [Google Scholar]
- 34.Soheili Majd E, Goldberg M, Stanislawski L. In vitro effects of ascorbate and Trolox on the biocompatibility of dental restorative materials. Biomaterials. 2003;24:3–9. doi: 10.1016/s0142-9612(02)00221-1. [DOI] [PubMed] [Google Scholar]
- 35.Huang FM, Chang YC. Cytotoxicity of resin-based restorative materials on human pulp cell cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:361–5. doi: 10.1067/moe.2002.126341. [DOI] [PubMed] [Google Scholar]
- 36.Geurtsen W, Spahl W, Muller K, Leyhausen G. Aqueous extracts from dentin adhesives contain cytotoxic chemicals. J Biomed Mater Res. 1999;48:772–7. doi: 10.1002/(sici)1097-4636(1999)48:6<772::aid-jbm2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 37.Pelka M, Danzl C, Distler W, Petschelt A. A new screening test for toxicity testing of dental materials. J Dent. 2000;28:341–5. doi: 10.1016/s0300-5712(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 38.Eick JD, Kostoryz EL, Rozzi SM, Jacobs DW, Oxman JD, Chappelow CC, et al. In vitro biocompatibility of oxirane/polyol dental composites with promising physical properties. Dent Mater. 2002;18:413–21. doi: 10.1016/s0109-5641(01)00071-9. [DOI] [PubMed] [Google Scholar]
- 39.Moharamzadeh K, Van Noort R, Brook IM, Scutt AM. HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J Mater Sci Mater Med. 2007;18:133–7. doi: 10.1007/s10856-006-0671-z. [DOI] [PubMed] [Google Scholar]
- 40.Polydorou O, Huberty C, Wolkewitz M, Bolek R, Hellwig E, Kummerer K. The effect of storage medium on the elution of monomers from composite materials. J Biomed Mater Res B Appl Biomater. 2012;100:68–74. doi: 10.1002/jbm.b.31923. [DOI] [PubMed] [Google Scholar]
- 41.Lee SY, Huang HM, Lin CY, Shih YH. Leached components from dental composites in oral simulating fluids and the resultant composite strengths. J Oral Rehabil. 1998;25:575–88. doi: 10.1046/j.1365-2842.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu W, McKinney JE. Influence of chemicals on wear of dental composites. J Dent Res. 1982;61:1180–3. doi: 10.1177/00220345820610101501. [DOI] [PubMed] [Google Scholar]
- 43.Polydorou O, Trittler R, Hellwig E, Kummerer K. Elution of monomers from two conventional dental composite materials. Dent Mater. 2007;23:1535–41. doi: 10.1016/j.dental.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials. 2002;23:1819–29. doi: 10.1016/s0142-9612(01)00308-8. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka N, Taira M, Wakasa K, Shintani H, Yamaki M. Cutting effectiveness and wear of carbide burs on eight machinable ceramics and bovine dentin. Dental Materials Dent Mater. 1991;7:247–53. doi: 10.1016/S0109-5641(05)80023-5. [DOI] [PubMed] [Google Scholar]
- 46.Kopperud HM, Schmidt M, Kleven IS. Elution of substances from a silorane-based dental composite. Eur J Oral Sci. 2010;118:100–2. doi: 10.1111/j.1600-0722.2009.00697.x. [DOI] [PubMed] [Google Scholar]
- 47.Pulgar R, Olea-Serrano MF, Novillo-Fertrell A, Rivas A, Pazos P, Pedraza V, et al. Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ Health Perspect. 2000;108:21–7. doi: 10.1289/ehp.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. [Last accessed on 2012]. Available from: http://www.efsa.europa.eu/en/ceftopics/topic/bisphenol.htm.EFSA .


