Abstract
Objectives:
The antimutagenic effect of caffeine is evaluated against ethyl methanesulfonate (EMS)-induced mutation rate in Drosophila.
Materials and Methods:
The mutation rate is evaluated using wing mosaic assay. In transheterozygous larvae, multiple wing hair (mwh 0.3-3) and flare (flr 3-38.8) genes were used as markers of the extent of mutagenicity.
Results:
The results at 0.5 and 1.0 mM EMS concentration at both 48 ± 4 and 72 ± 4 h have shown consistent increase in mutation rate, which was being measured as frequency of clone formation per 105 cells. Toxicity of caffeine at 5 mM concentration was parallel to that of distilled water alone. At 0.5 mM EMS concentration at 42 ± 4 and 72 ± 4 h, Drosophila larvae mutation rate was significantly increased. Although caffeine prevented mutation rate in all pre, post, and combined treatment, it was more significant in pretreatment experiments where it was found to be effective in reducing the genotoxicity of EMS. However, the concentration of caffeine as recommended in dietary allowance did not induce the frequency of mutant clones in somatic mutation and recombination test (SMART) recorded.
Conclusion:
This study shows that caffeine significantly reduced the genotoxicity induced by EMS. However, the limitation in completely abolishing genotoxicity induced by EMS as observed at the dietary allowance of caffeine makes it interesting for further in-depth study. Further studies on the molecular mechanism of antigenotoxic effect of caffeine will also be interesting.
Keywords: Antimutagenicity, caffeine, drosophila melanogaster, ethyl methanesulfonate
INTRODUCTION
The consumption of antimutagens has been suggested as an effective preventive measure for possible occurrence of deleterious effects resulting from exposure to number of mutagenic and carcinogenic agents in environment.[1,2,3] Many of the compounds with known antimutagenic, antcarcinogenic, and antioxidant properties are naturally present in fruits, vegetables, spices, coffee, tea, and so forth.[1,4] Hence natural products can be perceived as potential source of inhibitors of mutagenesis and carcinogenesis caused by environmental substances. Thereby gradual intake of such natural products could be protective to animals in disguise.
Coffee is the most widely consumed natural beverage by the people around the world. It contains caffeine as major bioactive constituent along with caffeic acid, chlorogenic acid, kahweol palmiate, and cafestol palmiate as trace amounts. Caffeine acts as neurostimulator and exerts protective effect against genotoxic/carcinogenic activity of environmental chemicals in in vitro and in vivo assay system.[1,5,6,7,8] These elaborate findings indicate caffeine is a chemopreventive drug against mutagens and carcinogens.
Several studies have been reported during recent years on genotoxic and antigenotoxic properties of caffeine. It acts as double-edged sword, as an antigenotoxic,[9,10] antioxidant,[9,11,12] and genotoxic molecule.[13] Notwithstanding the aforementioned reports, somatic mutation and recombination test (SMART) has been assumed as the most effective way to assess the antigenotoxicity of natural compounds. There are no reports on antigenotoxicity of pure caffeine (CAF) in multiple wing hair (mwh) and flr3 Drosophila larvae barring a lone report being published by Abrahm[14] on coffee powder using Drosophila larvae. Therefore, we made an attempt to evaluate the antimutagenicity of pure caffeine in Drosophila larvae. Hence, this study may be regarded as an important step forward toward understanding the protective effect of caffeine in different mode of treatments against ethyl methanesulfonate (EMS)-induced mutation in Drosophila larvae.
MATERIALS AND METHODS
Chemicals
EMS (CAS No. 62-50.0) was purchased from Sigma Co., St. Louis, USA, sodium chloride, gum arabic, glycerol, and chloral hydrate from Himedia Chemicals, Mumbai, India. Distilled water served as a negative control and 0.1 mM EMS was used as a positive control.
Strains
Two Drosophila melanogaster strains were used: The mwhs strain with genetic constitution mwh/mwh and the flare strain with genetic constitution flr3/In (3LR) TM3, Bds. The transheterozygous larvae were obtained by crossing ORR: Mwh/mwh males and ORR: Flr3/TM3 females and were obtained from Agarkar Institute, Pune. The more detailed information on the genetic symbols and descriptions can be found in the work of Lindsley and Zimm.[15] The tests were performed as described in Graf et al.[16]
Drosophila SMART test
The SMART was essentially performed as described by Graf et al.[16] For this assay, the following cross of D. melanogaster flies was used: ORR (1); ORR (2); flr3/In (3LR) TM3, Bds virgin females were crossed with mwh males (flies that were kindly provided by Agarkar Institute, Pune). The first strain is characterized by constitutively high cytochrome P-450 activity. The markers mwh and flr3 (misshapen, flare-like hairs) are recessive wing-hair mutations located on the third chromosome at 0.3 and 38.8, respectively. This test is able to detect a wide spectrum of genetic alterations including point mutations, deletions, unbalanced half-translocation and mitotic recombination, chromosomal loss, and non-disjunction as described in Graf et al.[16]
Transheterozygous larvae were obtained by parental crosses between flr3 virgin females and mwh males. Eggs were collected from this cross during 8-h period in culture bottles containing fresh standard Drosophila medium (wheat powder, jaggery, agar agar, propionic acid, and water cooked). After 72 h, third instar larvae were floated off with tap water and transferred to plastic vials containing 1.5 g of Drosopila instant medium rehydrated with 9 ml of freshly prepared test solutions (mutagens, mutagens plus extracts, distilled water, and EMS used at positive control at 0.1 mM). For each treatment group in a total of 4000 larvae, 200 in each vial were used. The larvae were fed on this medium until pupation of the surviving larvae. All the experiments were carried out at 24 ± 1°C and at ~60% relative humidity.
Preparation and analysis of wings
The crossing procedure is distinguished phenotypically based on the TM3 and Bds marker. Marker-heterozygous flies (mwh/flr3) and balancer-heterozygous (mwh/TM3, Bds) genotypes were mounted on slides with Faure's solutions (30 g gum arabic, 30 ml glycerol, 50 g chloral hydrate, and 50 ml distilled water). Both the dorsal and ventral surfaces of the wings were analyzed under a microscope at 400× magnification for the presence of clones of cells showing malformed wing hairs, i.e. occurrence of small single spots consisting of one or two mwh cells, large single spots consisting of three or more cells, and twin spots consisting of adjacent mwh and flr3 cells.[16] Single spots can be produced by somatic point mutation, chromosome aberration, deletion, or mitotic recombination; twin spots originate exclusively from mitotic recombination. To determine the recombinogenic activity, the frequencies of mwh clones on the marker-heterozygous wings are compared with the frequencies of mwh clones on the balancer-heterozygous wings. The difference in mwh clone frequency is a direct measure of the proportion of recombination.[17]
Statistical analysis
For the statistical assessment of genotoxicity, the frequencies of each type of spot per fly were compared pairwise with the corresponding negative control; for the antigenotoxicity of amifostine, the frequencies of each type of spot per fly were compared pairwise with the corresponding dose of 8 μg/ml fotemustine. A multiple-decision procedure was used to decide whether a result is positive, weakly positive, inconclusive, or negative.[18,19] For the statistical calculations, the conditional binominal test according to Kastenbaum and Bowman[20] was used with P = 0.05 significance levels. The frequency of clone formation was calculated.[19,21] Based on clone induction frequencies per 105 cells, the recombinogenic activity was calculated as follows: Mutation frequencies (FM) = frequencies clones mwh/TM3 flies/frequencies clones mwh/flr3 flies; recombination frequencies (FR) = 1 − FM. Frequencies of total spots (FT) = total spots in mwh/flr3 flies spots/number of flies; mutation = FT × FM; recombination = FT × FR.[22,23] Based on the control-corrected spot frequencies per 105 cells, the percentage of amifostine inhibition was calculated as follows: (fotemustine alone − amifostine plus fotemustine/fotemustine alone) × 10014.
RESULTS AND DISCUSSION
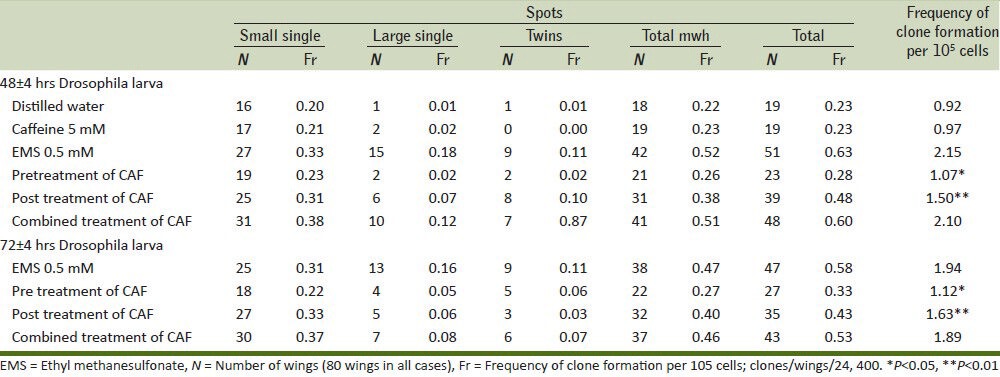
Many chemoprotective agents are found in the dietary material,[10] and these dietary sources containing phytochemicals have a positive bearing effect on ill health. Caffeine is one such phytochemicals that may reduce mutagenicity caused by mutagens in different ways: (1) competition with the nucleophilic sites on DNA for an electrophilic mutagen, (2) inhibition of promutagen bioactivation by blocking oxidation process, and (3) reaction with the electrophilic metabolites of a promutagen. Mechanisms one and three might be involved when direct acting mutagens like EMS interacts with DNA. Caffeine along with minor constituents like caffeic acid, chlorogenic acid, kahweol palimiate, and cafestol palmiate present in coffee may possibly play a crucial role in preventing the deleterious interaction between DNA and EMS. Caffeine can also block the binding of activated carcinogens to DNA, thus reducing the formation of DNA adducts.[24] The present investigation revealed that all the three doses of EMS had significantly increased the number of small, large, twin, and total spots tested either at both in 48 or 72 h larvae as compared with distilled water group [Table 1, Figure 1]. Based on these results, we have chosen 0.5 mM of EMS to conduct experiment for 48 ± 4 and 72 ± 4 h larvae. Caffeine was administered in pre, post, and combined treatment doses at 0.5 mM to EMS-treated Drosophila. All the three ways of caffeine treatment have inhibited the effect of EMS. It is notable that pretreatment of caffeine in both 48 ± 4 and 72 ± 4 h had significantly decreased number of twin spots, total spots, and frequency of clones per 105 cells compared with distilled water along with post and combined treatment doses [Table 2].
Table 1.
Comparison of wing spots with caffeine and different doses of EMS at 48±4 and 72±4 h in Drosophila larva
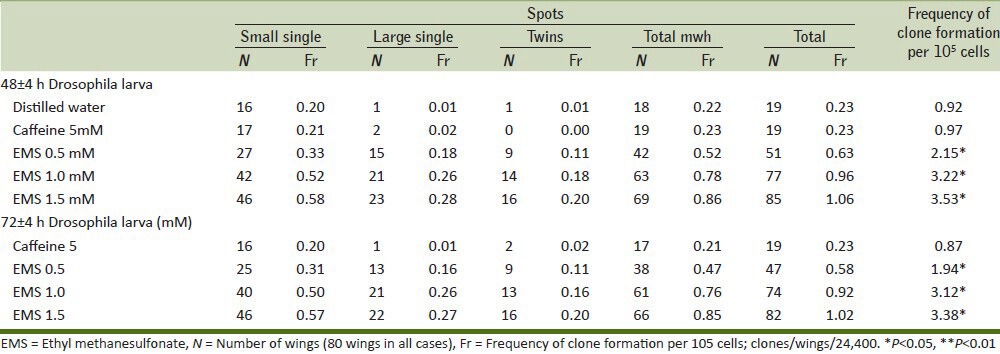
Figure 1.
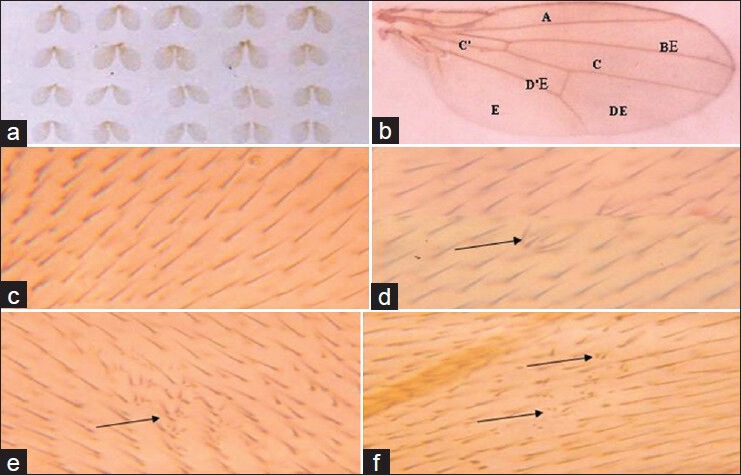
Effects of EMS on wings cells of Drosophila melanogaster. (a) Mounted wings, (b) Wing showing region a-e for scoring spots, (c) Normal trichomes of the wing, (d) Mwh spot with two-cell affected, (e) Mwh spots with more than two cells affected, and (f) Twin spot
Table 2.
Effect of pre, post, and combined treatment of caffeine against EMS-induced mutation rate at 42±4 and 72±4 h in Drosophila larvae
Our reports have confirmed the previous reports on in vivo antigenotoxicity of coffee in Drosophila and mice.[4,5,6,14] However, the concentration of caffeine is of critical importance because high doses of caffeine induce apoptosis and low concentration can act as antioxidant.[25,26,27] Furthermore, this study has demonstrated the suitability of non-mammalian in vivo assay for obtaining qualitative and quantitative data on antigenotoxic compounds. Our results were interesting when investigated through different ways in which the caffeine could interfere in vivo on the effect of genotoxic agent.
CONCLUSION
Pretreatment of caffeine significantly reduces the frequency of clones as compared with post and combined treatment in both 48 ± 4 and 72 ± 4 h larvae against EMS. These results suggest that the caffeine has antigenotoxic factors. As detected in the DNA repair test it is also involved in the antirecombinogenic activity.
ACKNOWLEDGMENTS
The authors are grateful to The Chairman, Department of Post Graduate (PG) Studies and Research in Applied Zoology, Kuvempu University, Karnataka. The authors thank Prof. M. Krishnamurthy, Mangalore University, Manglore, for permitting to learn the techniques in his laboratory under the supervision of Shilet Mathew and they also acknowledge Kuvempu University for partial financial assistance in conducting the research program. DBL thank Jain University for the constant encouragement and support given to the progress in research.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ferguson LR. Antimutagens as cancer chemoprevntive agent in the diet. Mutat Res. 1994;307:395–410. doi: 10.1016/0027-5107(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 2.Mitscher LA, Telikepalli H, McGhee E, Shankel DM. Natural antimutagenic agents. Mutat Res. 1996;350:143–52. doi: 10.1016/0027-5107(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 3.Ikken Y, Morales P, Martínez A, Marín ML, Haza AI, Cambero MI. Antimutagenic effect of fruit and vegetable ethanolic extracts against n-nitrosamines evaluated by the ames test. J Agric Food Chem. 1999;47:3257–64. doi: 10.1021/jf990166n. [DOI] [PubMed] [Google Scholar]
- 4.Abrahm SK. Inhibitory effects of coffee in the genotoxcity of carcinogens in mice. Mutat Res. 1991;262:109–14. doi: 10.1016/0165-7992(91)90115-k. [DOI] [PubMed] [Google Scholar]
- 5.Abrahm SK. Inhibition of in vivo genotoxcity by coffee. Food Chem Toxicol. 1989;27:787–92. doi: 10.1016/0278-6915(89)90109-9. [DOI] [PubMed] [Google Scholar]
- 6.Aeschbacher HU, Jaccaud E. Inhibition by coffee of nitrosourea-mediated DNA damage in mice. Food Chem Toxicol. 1990;28:633–7. doi: 10.1016/0278-6915(90)90171-i. [DOI] [PubMed] [Google Scholar]
- 7.Coffee, tea, mate, methylxanthines and methylglyoxal. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 27 February to 6 March 1990. IARC Monogr Eval Carcinog Risks Hum. 1991:513–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Stavric B. An update on research with coffee/caffeine (1989-1990) Food Chem Toxicol. 1992;30:533–55. doi: 10.1016/0278-6915(92)90106-u. [DOI] [PubMed] [Google Scholar]
- 9.Devasayagam TP, Kesavan PC. Radioprotective and antioxidant action of caffeine: Mechanistic considerations. Indian J Exp Biol. 1996;34:291–7. [PubMed] [Google Scholar]
- 10.Wattenberg LW. Chemoprevention of carcinogenesis by minor dietary constituents: Symposium introduction. Pharm Bull. 1998;36:S6–7. [Google Scholar]
- 11.Shi X, Dalal NS, Jain AC. Antioxidant behavior of caffeine: Efficient scavenging of hydroxyl radicals. Food Chem Toxicol. 1991;29:1–6. doi: 10.1016/0278-6915(91)90056-d. [DOI] [PubMed] [Google Scholar]
- 12.Devasayagam TP, Kamath JP, Mohan H, Kesavan PC. Caffeine as an antioxidant: Inhibition of lipid peroxidation induced by reactive oxygen species. Biochem Biophys Acta. 1996;1282:63–70. doi: 10.1016/0005-2736(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 13.Mohr U, Emura M, Riebe-Imre M. Experimental studies on carcinogenicty and mutagenicity of caffeine. In: Garattini S, editor. New York: Caffeine, Coffee and Health, Revan; 1993. pp. 359–78. [Google Scholar]
- 14.Abrahm SK. Antigenotoxcity of coffee in the Drosophila assay for somatic mutation and recombination. Mutagenesis. 1994;9:383–6. doi: 10.1093/mutage/9.4.383. [DOI] [PubMed] [Google Scholar]
- 15.Lindsley DL, Zimm GG, editors. San Diego: Academic Press; 1992. The Genome of Drosophila melanogaster; p. 1133. [Google Scholar]
- 16.Graf U, Würgler FE, Katz AJ, Frei H, Juon H, Hall CB, Kale PG. Somatic mutation and recombination test in Drosophila melanogaster. Mutat Res. 1984;271:59–67. doi: 10.1002/em.2860060206. [DOI] [PubMed] [Google Scholar]
- 17.Frei H, Clements J, Howe D, Würgler FE. The genotoxicity of anti-cancer drug mitoxantrone in somatic and germ cells of Drosophila melanogaster. Mutat Res. 1992;279:21–33. doi: 10.1016/0165-1218(92)90262-x. [DOI] [PubMed] [Google Scholar]
- 18.Frei H, Würgler FE. Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutat Res. 1988;203:297–308. doi: 10.1016/0165-1161(88)90019-2. [DOI] [PubMed] [Google Scholar]
- 19.Frei H, Würgler FE. Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination test (SMART) in Drosophila. Mutat Res. 1995;334:247–58. doi: 10.1016/0165-1161(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 20.Kastenbaum MA, Bowman KO. Tables for determining the statistical significance of mutation frequencies. Mutat Res. 1970;9:527–49. doi: 10.1016/0027-5107(70)90038-2. [DOI] [PubMed] [Google Scholar]
- 21.Moraga AA, Graf U. Genotoxicity testing of antiparasitic nitrofurans in the Drosophila wing somatic mutation and recombination test. Mutagenesis. 1989;4:105–10. doi: 10.1093/mutage/4.2.105. [DOI] [PubMed] [Google Scholar]
- 22.Santos JH, Graf U, Reguly ML, Rodrigues de Andrade HH. The synergistic effects of vanillin on recombination predominate over its antimutagenic action in relation to MMC-induced lesions in somatic cells of Drosophila melanogaster. Mutat Res. 1999;444:355–65. doi: 10.1016/s1383-5718(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 23.Sinigaglia M, Lehmann M, Baumgardt P, do Amaral VS, Dihl RR, Reguly ML, et al. Vanillin as a modulator agent in SMART test: Inhibition in the steps that precede N-methyl-Nnitrosourea-, N-ethyl-N-nitrosourea-, ethylmethanesulphonate- and bleomycin-genotoxicity. Mutat Res. 2006;607:225–30. doi: 10.1016/j.mrgentox.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Garcia SB, Novelli M, Wright NA. The clonal origin and clonal evolution of epithelial tumours. Int J Exp Pathol. 2000;81:89–116. doi: 10.1046/j.1365-2613.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egner PA, Wang JB, Zhu YR, Zhang BC, Wu Y, Zhang QN, et al. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc Nat Acad Sci U S A. 2001;98:14601–6. doi: 10.1073/pnas.251536898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández MJ, López A, Santa-Maria A. Apoptosis induced by different doses of caffeine on Chinese hamster ovary cells. J Appl Toxicol. 2003;23:221–4. doi: 10.1002/jat.910. [DOI] [PubMed] [Google Scholar]
- 27.He Z, Ma WY, Hashimoto T, Bode AM, Yang CS, Dong Z. Induction of apoptosis by caffeine is medicated by the p53, Bax, and caspase 3 pathway. Cancer Res. 2003;63:4396–401. [PubMed] [Google Scholar]


