Abstract
Some epithelia contain cells with multiple, motile cilia that beat in a concerted fashion. New tools and experimental systems have facilitated molecular studies of cilium biogenesis and of the coordinated planar polarization of cilia that leads to their concerted motility. Recent, elegant work by Park and colleagues, using embryonic frog epidermis, demonstrates that Dishevelled (Dvl), a key regulator of both the Wnt/β-catenin and Planar Cell Polarity (PCP) pathways, controls both the docking and planar polarization of ciliary basal bodies.
Introduction
Multiciliated epithelial cells are present in numerous tissues of a wide range of organisms, from embryonic amphibian skin to vertebrate respiratory, oviduct and ependymal epithelia [1]. These cells contain hundreds of motile cilia that beat together to propel substances over the epithelial surface. Cilium biogenesis begins with the generation of basal bodies, the organizing structures at the base of cilia, in the cytoplasm, which then traffic to the apical surface, dock with and anchor to the plasma membrane, and elongate a ciliary axoneme. Each cilium has an intrinsic ultrastructural and functional asymmetry. Concerted ciliary motility is achieved by the co-orientation of cilia structure and direction of beating, both within each cell and between individual ciliated cells [2] and is essential to the physiological functions of ciliated epithelia.
Most of our understanding of multicilated cells comes from extensive electron microscopic analyses that documented the basic steps of ciliogenesis and provided ultrastructural evidence for the planar polarization of ciliary basal bodies and axonemes. The introduction of well-characterized in vitro and in vivo model systems together with improved tools now makes it possible to understand these processes at the molecular level.
The PCP pathway orients cellular structures in multiple systems [3] (Box 1) and is thus a likely candidate for controlling the planar polarized orientation of motile cilia. The PCP protein Dishevelled (Dvl) was previously shown to localize to the apical surface of multiciliated epithelial cells [4]. New work from Park et al. [5], using embryonic frog (Xenopus laevis) epidermis, demonstrates that Dvl, together with Rho GTPase, regulates both the docking and planar polarization of basal bodies. This work both advances our molecular understanding of motile ciliogenesis, and contributes to understanding Dvl, cytoskeletal dynamics and the PCP pathway, all of which are involved in processes key to development and disease.
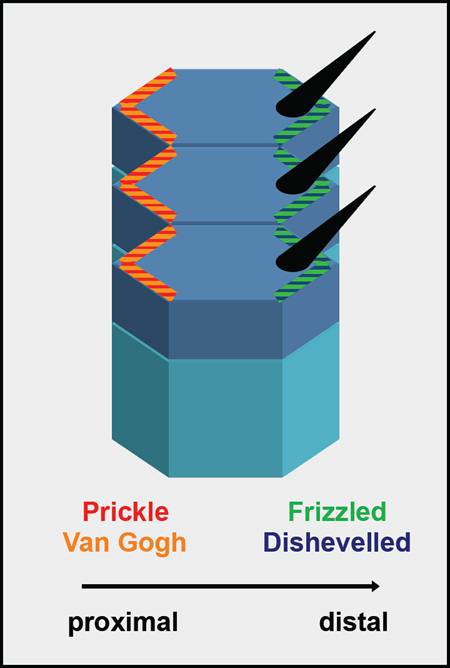
Box 1.
Schematic of the Planar Cell Polarity pathway. This schematic presents the planar polarized fly (Drosophila melanogaster) wing epithelium, where the PCP pathway positions the wing hair (black cones) to the distal side of each cell [16]. PCP proteins are distributed asymmetrically: Dishevelled and Frizzled accumulate on the distal side and Prickle and Van Gogh on the proximal side of cells. These polarized cortical domains are responsible both for aligning the wing hair and communicating polarity information between cells. Similar asymmetric distribution of PCP homologs was observed in the inner ear epithelium [3], suggesting that the pathway and the mechanism are highly conserved.
Dishevelled regulates actin assembly and docking during ciliogenesis
Ciliogenesis occurs through a series of highly conserved steps [6]. Basal bodies form in the cytoplasm, and subsequently one end is thought to associate with a vesicle. This complex then migrates apically, and fusion of the vesicle with the plasma membrane anchors the basal body to the surface (Figure 1a). A massive apical meshwork of actin assembles in ciliating cells. Studies using Cytochalasin D showed that this network is essential for basal body migration [7], suggesting its involvement in the vesicular transport step.
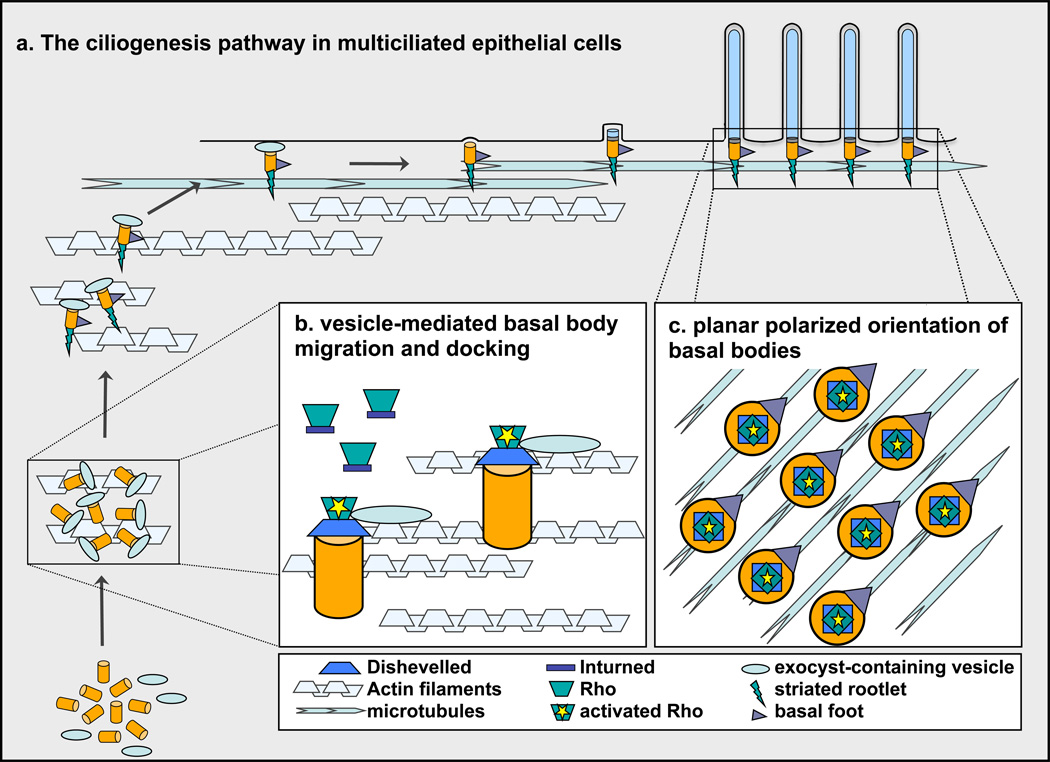
Figure 1.
Dishevelled controls basal body docking and planar polarization during ciliogenesis. (a) Ciliogenesis begins with the generation of basal bodies (orange cylinders) in the cytoplasm, which then associate with vesicles (light blue ovals). The basal body-vesicle complex migrates apically, possibly using actin cables (grey textured lines), which are abundant in the ciliating cytoplasm. Basal bodies dock with the cell surface through vesicular fusion. Basal feet (purple triangles) and striated rootlets (green triangles) assemble onto basal bodies prior to docking with the surface. The ciliary axoneme (long blue cylinders), ensheathed by the plasma membrane, elongates from the basal body after docking. Alignment of basal feet in the direction of ciliary motility shows the planar polarized orientation of cilia, which may result from interaction with a hypothetical, planar polarized microtubule network (blue textured lines). (b) Inturned (blue rectangles) brings Rho GTPase (green trapezoids) to the basal body, then Dvl (blue trapezoids) activates Rho, which is thought to lead to the assembly of actin filaments. Exocyst-containing vesicles may be brought to and join with basal bodies by this localized actin meshwork. (c) Dvl and activated Rho are necessary for the planar polarized orientation of basal bodies, although the mechanism of polarized orientation remains to be uncovered.
Previous work by Park and colleagues found that the PCP effectors Inturned and Fuzzy are involved in the assembly of apical actin filaments during ciliogenesis [4]. They also found that the core PCP protein Dvl localizes in puncta to the apical surface of ciliated epithelial cells [4]. In the current work, the authors tested the role of Dvl in these cells by knocking down the three frog Dvl genes (Dvl1-3) with morpholinos and found that depletion of individual or multiple Dvl proteins results in loss of the apical actin meshwork and trapping of basal bodies in the cytoplasm.
Rho GTPase activation is also required for assembly of the apical actin meshwork in ciliating cells, and Rho inhibitors block ciliogenesis [8]. As Dvl is a known binding partner and activator of Rho in other systems [9], Park et al. hypothesized that basal body docking may involve localization and activation of Rho by basal body-bound Dvl. Consistent with this hypothesis, they showed that while RhoA-GFP accumulates at the apical surface, activated, GTP-bound Rho specifically localizes to basal bodies, and Dvl knockdown leads to the loss of activated Rho from basal bodies without affecting apical enrichment of bulk RhoA. Interestingly, Inturned knockdown delocalizes all RhoA from the apical surface. The authors therefore propose a mechanism in which Inturned localizes and Dvl controls activation of Rho GTPase during basal body docking.
Previous work in ciliated epithelial cells placed Rho activation downstream of Foxj1 [8], a multiciliated cell-specific transcription factor. Basal bodies fail to dock with the apical surface in mouse Foxj1 null airway epithelial cells [10], and similar to cells lacking Dvl, apical actin filaments are absent [8]. It will be interesting to test whether Dvl is somehow regulated by Foxj1, or if it is in a parallel pathway to Rho activation, and whether Dvl phosphorylation regulates its RhoA-activating function.
Park et al. observed that basal bodies in Dvl morphants fail to closely associate with vesicles, pointing to a defect in vesicle-mediated apical transport as the cause for the docking defect. In other polarized tissues, PCP components have been shown to interact with the exocyst complex of the secretory machinery [11, 12]. They therefore examined the exocyst component, Sec8, and found that it localizes to basal bodies in a Dvl-dependent manner. Sec8 is the first identified molecular component of ciliary vesicles, which thus far have only been characterized through electron microscopy. It is worth noting that similar vesicles surround centrioles in monociliated cells during primary cilium biogenesis [6], implying a conserved mechanism. The movement and fusion of basal body-associated vesicles resembles exocytosis [6], suggesting that it also relies on cytoskeleton-based motility. This may explain the importance of apical actin filaments for basal body migration, although this remains to be experimentally confirmed.
The mechanism through which vesicles attach to basal bodies also needs exploration. Dvl may bind Sec8, but it more likely facilitates attachment indirectly, possibly through Rho-dependent actin polymerization needed to bring basal bodies and vesicles into proximity. One candidate for vesicle attachment is the basal body component centriolin [13]. In cells undergoing cytokinesis, centriolin anchors the exocyst complex at the midbody, which is required for secretory vesicle-mediated abscission of daughter cells [14]. Centriolin has been shown to physically interact with Sec8 and other exocyst components, so it would be interesting to test whether this interaction also occurs in ciliated cells.
In sum, the above experiments show that Dvl disruption leads to failure of apical actin meshwork assembly and failure of basal bodies to join with exocyst-containing secretory vesicles. Although it is not directly demonstrated that these events are linked, the simplest interpretation is that Dvl regulates ciliogenesis via Rho GTPase activation to establish the apical actin network necessary for vesicle binding, transport and docking of basal bodies (Figure 1b).
Dishevelled and Rho regulate ciliary PCP
The planar polarized orientation of basal bodies, which is necessary for coordinated ciliary motility, develops in a two-step process [15]. After docking, basal bodies are aligned roughly with the axis of polarity. This orientation is then perfected by directional fluid flow produced by concerted ciliary beating. The planar polarity phenomenon in ciliated epithelia closely resembles other polarized tissues like the fly wing or the mammalian inner ear, where the PCP pathway regulates cellular orientation in a remarkably conserved manner. In these systems, asymmetric membrane localization of Dvl and other core PCP proteins along the axis of polarity is essential to the PCP process (Box 1) [3, 16]. However, in frog epidermal ciliated cells, Dvl localizes to basal bodies, and Vangl2, another core protein of the PCP pathway, localizes to cilia in mouse kidney and airway epithelia [17], so the characteristic asymmetric PCP protein localization may not occur in ciliated epithelia.
Nonetheless, Park et al. demonstrated that Dvl and Rho GTPase are involved in the planar polarized orientation of basal bodies (Figure 1c). Using a clever immunofluorescence-based assay, basal body orientation was observed to be randomized upon overexpression of Xdd1, a dominant negative Dvl deletion mutant that in this system only slightly impairs basal body docking. Expression of dominant negative RhoA-N19 also disrupted basal body alignment. Both interventions also lead to disorganized ciliary beating, underscoring the relationship between coordinated motility and basal body alignment.
Dvl can act through both the Wnt/β-catenin and the PCP pathway, so it will be important to determine which signaling mechanism controls the docking and orientation of basal bodies. Both pathways may be involved and responsible for distinct steps given that Xdd1 expression only slightly affects docking. However, it is also possible that perfect docking is a prerequisite for planar polarized alignment.
Future directions
How basal bodies become physically aligned with the axis of polarity remains unknown. Dvl and Vangl protein distributions suggest that the PCP pathway is active, but may not function by establishing asymmetric cortical domains. It will be important to determine the localization and function of other core PCP components in multiciliated cells to begin to understand how the PCP pathway contributes to ciliary polarity. In addition to discovering factors that orient cilia in individual ciliated cells, the nature of the global polarity cue which aligns ciliated cells with the correct tissue axis needs to be identified. In the fly, a global polarity signal is thought to exist upstream of the core PCP components [16], and this may similarly operate in vertebrate polarized epithelia [18]. Wnt proteins have also been suggested as possible candidates [19], but both need to be tested in ciliated epithelia.
The polarized basal body appendages, the basal foot and striated rootlet, which are known to interact extensively with the apical cytoskeleton [6], also deserve attention. In fly wing cells, apical microtubules organize parallel to the axis of polarity and contribute to PCP [20]. Although a planar polarized cytoskeleton has not been observed in ciliated epithelial cells, such a structure might control polarity through interaction with basal body appendages.
Finally, it will be important to determine the contributions of individual Dvl isoforms for both docking and polarity.
Given the importance of ciliated epithelia in human development and disease [21], there is a substantial need for understanding this process in greater molecular detail. Park and colleagues bring us closer to this goal by identifying Dvl as a regulator of both the docking and polarized orientation of basal bodies. While it is implied that these are sequential but separate steps in ciliogenesis, it is also possible that these two events are not so distinct. By surgically reversing a segment of undifferentiated oviduct, it was demonstrated that polarity is determined prior to differentiation [22]. Furthermore, polarized appendages are already present on nascent basal bodies [6]. Interaction between these appendages and the hypothetical planar polarized apical cytoskeleton during migration may be an early event which could produce the rough alignment of basal bodies seen immediately after docking [15]. Thus, basal body docking and polarization may be closely linked and may both be consequences of a single activity of Dvl.
Acknowledgements
We thank members of the Axelrod Lab for critical reading of the manuscript. J.D.A. is supported by grants from the NIH and E.K.V. is supported by a fellowship award from the National Cancer Institute, DHHS (PHS Grant Number T32-CA09151).
References
- 1.Hagiwara H, et al. Cell biology of normal and abnormal ciliogenesis in the ciliated epithelium. Int Rev Cytol. 2004;234:101–141. doi: 10.1016/S0074-7696(04)34003-9. [DOI] [PubMed] [Google Scholar]
- 2.Boisvieux-Ulrich E, et al. The orientation of ciliary basal bodies in quail oviduct is related to the ciliary beating cycle commencement. Biol Cell. 1985;55:147–150. doi: 10.1111/j.1768-322x.1985.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 3.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 4.Park TJ, et al. Ciliogenesis defects in embryos lacking in turned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 5.Park TJ, et al. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorokin SP. Centriole formation and ciliogenesis. Aspen Emphysema Conf. 1968;11:213–216. [PubMed] [Google Scholar]
- 7.Boisvieux-Ulrich E, et al. Cytochalasin D inhibits basal body migration and ciliary elongation in quail oviduct epithelium. Cell Tissue Res. 1990;259:443–454. doi: 10.1007/BF01740770. [DOI] [PubMed] [Google Scholar]
- 8.Pan J, et al. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci. 2007;120:1868–1876. doi: 10.1242/jcs.005306. [DOI] [PubMed] [Google Scholar]
- 9.Habas R, et al. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 10.Gomperts BN, et al. Foxj1 regulates basal body anchoring to the cytoskeleton of ciliated pulmonary epithelial cells. J Cell Sci. 2004;117:1329–1337. doi: 10.1242/jcs.00978. [DOI] [PubMed] [Google Scholar]
- 11.Classen AK, et al. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, et al. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008 doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vladar EK, Stearns T. Molecular characterization of centriole assembly in ciliated epithelial cells. J Cell Biol. 2007;178:31–42. doi: 10.1083/jcb.200703064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gromley A, et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell B, et al. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 16.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 18.Saburi S, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 19.Qian D, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada Y, et al. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Marshall WF. The cell biological basis of ciliary disease. J Cell Biol. 2008;180:17–21. doi: 10.1083/jcb.200710085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boisvieux-Ulrich E, Sandoz D. Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol Cell. 1991;72:3–14. doi: 10.1016/0248-4900(91)90072-u. [DOI] [PubMed] [Google Scholar]