Abstract
The collection of blood samples from laboratory rats requires the use of bleeding techniques that provide quality samples of sufficient volume for analysis without injury to the animal. Retro-orbital bleeding (ROB) is a phlebotomy technique that can yield high-quality samples of adequate volume, but it has been criticized for its potential to cause injury. To evaluate the injury-causing potential of their refined ROB method using a lateral approach, the authors retrospectively reviewed ROB procedures carried out in their colony during an 18-month period and found that 0.6% of these procedures were associated with ocular injury. The authors also compared the quality of blood samples collected by ROB and by saphenous phlebotomy and found that ROB yielded samples of better quality. The authors conclude that, when done using a lateral approach and by an experienced technician, ROB is humane and safe and provides blood samples of adequate volume and quality for analysis.
Obtaining blood samples of sufficient volume and quality is important in many scientific studies. If the collected samples are of insufficient volume or compromised quality, collection of additional samples might be required to meet experimental objectives, which is contrary to the ‘reduction’ principle of the 3Rs. In addition, hemolyzed or otherwise degraded blood samples might compromise scientific data.
In laboratory rats, blood samples may be obtained from the saphenous vein, tail vein, retro-orbital venous plexus, mandibular vessels or jugular vein; all of these sites have advantages and disadvantages. Although it remains in common use, retro-orbital bleeding (ROB) in rats has been discouraged because it has the potential to cause ocular complications in the animal during and after the procedure1. Some animal care committees do not allow ROB as a primary phlebotomy method. But ROB has the advantages of being easily taught, being easily performed by a single technician and providing quality blood samples of adequate volume.
We have refined the ROB technique used with rats in our laboratory by using the lateral approach only, rather than the medial approach. We designed and carried out two studies to evaluate its safety and its ability to yield adequate samples. First, we retrospectively assessed the incidence of ocular injury associated with >1,800 ROB procedures done at our institution during an 18-month period. Second, we compared the volume and quality of blood samples obtained by various phlebotomy techniques. We found that, when done using a lateral approach and by an experienced technician, ROB is safe and can provide blood samples of adequate volume and quality for analysis.
METHODS
Rats
The rats used in these studies were from the WAG/ RijCmcr strain derived from Wistar rats. They are maintained in accordance with the US National Institutes of Health’s guidelines for animal studies under standard conditions, are fed standard chow and are used in protocols approved by the IACUC at the Medical College of Wisconsin, which is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. The rats are observed daily, including weekends and holidays, by our personnel and by personnel at the Biomedical Resource Center (BRC). Rats that have health issues, including ocular problems such as redness, drainage, corneal abrasions, infection and swelling, are reported by the BRC animal technicians to the veterinary staff, and all such reports are recorded in the BRC health record database.
Refined ROB technique
We use ROB for ongoing approved protocols2. All ROB procedures are carried out by one of three trained technicians in the Radiation Biology suite at the BRC animal facility. The more junior technicians were trained by the most senior. Trainees practice on rats that are scheduled to be euthanized until they are confident with the procedure.
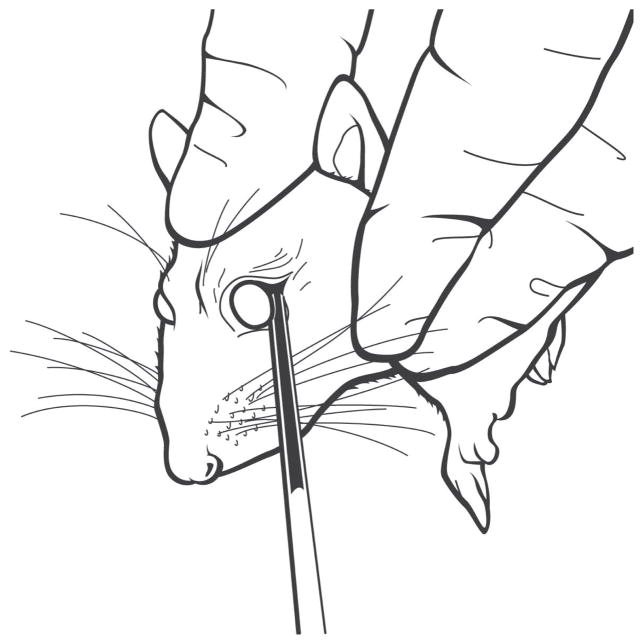
ROB procedures are done using the following method. A sterile glass tube with an outer diameter of 3 mm is cut to a length of 7 in, and the cut ends are ground to have flat edges. The rat is lightly anesthetized with isoflurane, and the eyelid is pulled back to proptose the eye. The glass tube is placed at the lateral canthus and is oriented toward the back of the head at an angle of 45° to the sagittal and coronal planes. It is gently turned with pressure against the orbital bone just in front of the zygomatic arch until blood flows from capillaries that drain the orbital sinus into the superficial temporal vein. The tube is pulled back and blood is collected into it by capillary action (Fig. 1). Blood flow is facilitated by gentle jugular vein pressure. The entire procedure is done by one technician.
FIGURE 1.
Diagram of the lateral approach ROB procedure. The glass tube is shown partially filled with blood.
Ocular complications of ROB
To determine the incidence of ocular complications after ROB, we identified all survival ROB procedures carried out in the Radiation Biology suite at the BRC animal facility between August 2011 and February 2013. Survival ROB procedures are those after which the rat survived a minimum of 3 d; we did not include ROB procedures done at time of animal euthanasia. We found that 472 rats had undergone ROB procedures that met these criteria. The rats’ ages ranged from 5 to 60 weeks, and they included both males and females. About half of the rats had undergone partial or total body irradiation to induce late radiation injury to kidney and or lung. Next, we consulted the BRC health record database to determine how many of these rats had been reported to have ocular complications during the same time period.
Comparison of phlebotomy techniques
To compare the volume and quality of samples obtained by various phlebotomy techniques, we collected blood from eight male rats using first saphenous, then retro-orbital and finally terminal intracardiac bleeding. These rats were not irradiated or treated by any toxic agent. We attempted to obtain 1 ml of blood by each method.
One BRC staff member performed the saphenous bleeds, a procedure with which she had experience. Each unanesthetized rat was manually restrained in a conical plastic bag, and the fur was plucked from its lower leg and thigh. The leg was wiped with ethanol and the saphenous vein was punctured with a 23-gauge needle. The vein was massaged and blood collected into a 2-ml conical tube. ROB was performed as described above by the same experienced Radiation Biology technician who had done most of the ROB procedures that were evaluated in our retrospective analysis. The BRC veterinary staff performed the cardiac bleeds. Each rat was anesthetized with isoflurane and euthanized by cervical dislocation. Its thorax was then opened and blood was taken directly from the heart with a 3-ml syringe and a 22-gauge needle.
We recorded the sample volume obtained using each procedure and tested all the blood samples for alteration by hemolysis, which we assessed using the serum potassium level3, and for blood urea nitrogen (BUN) concentration, because this is a major endpoint for renal injury. Samples were sent to the Clinical Pathology laboratories of the Marshfield Clinic (Marshfield, WI) for testing.
Statistical analysis
Data are reported as mean ± standard deviation (s.d.), unless otherwise stated. Continuous variables were compared using t-tests. Discrete endpoints were tested by Fisher exact tests. Differences in variance were calculated using the F statistic.
RESULTS
Ocular complications of ROB
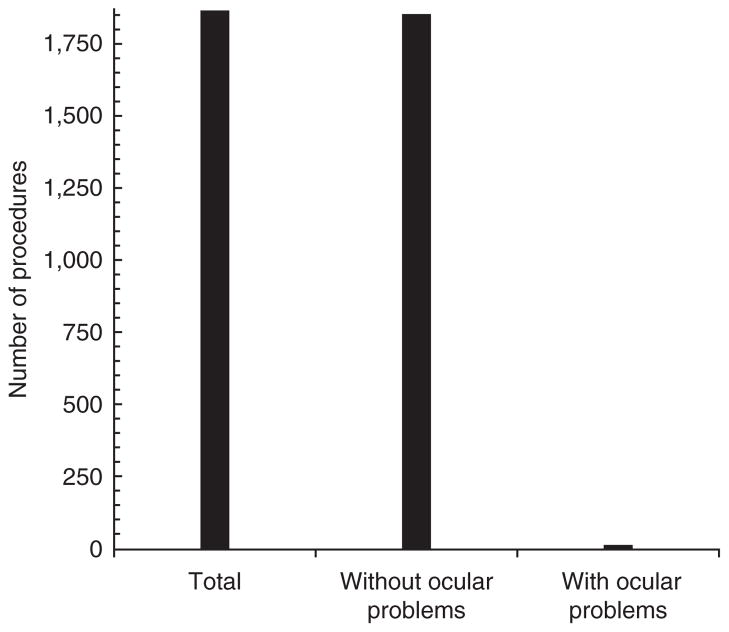
During the 18-month period from August 2011 to February 2013, 1,869 survival ROB procedures were done on 472 rats. Each rat underwent up to nine survival ROB procedures. During this time period, 185 rats were reported to have medical issues, 18 of which had ocular problems. Six of those 18 rats had never undergone phlebotomy, leaving 12 rats that had ocular problems associated with phlebotomy, including corneal lesions, enophthalmia and exophthalmia. Each of the 12 rats had undergone between two and eight ROB procedures at the time that they were reported to have ocular problems. Only three rats were euthanized because of ocular problems alone. The remaining nine rats were euthanized at various time points after undergoing one or more ROB procedure, when they reached a study endpoint. The incidence of ocular complications of ROB is thus 12 of 1,869 survival procedures (0.6%; Fig. 2).
FIGURE 2.
Of 1,869 survival ROB procedures done between August 2011 and February 2013 at our institution, 12 procedures were associated with ocular problems.
Comparison of phlebotomy techniques
Saphenous blood collection yielded lower sample volumes (0.3 ± 0.2 ml) than did ROB or cardiac bleeding (0.9 ± 0.1 ml and 1.4 ± 0.9 ml, respectively; Table 1). The probability of obtaining a 1-ml sample was much higher for ROB than for saphenous collection (P = 0.002 by Fisher exact test).
TABLE 1.
Volume and levels of potassium and BUN of samples collected by saphenous bleeding, ROB and cardiac bleeding
| Rat | Volume (ml) | Potassium (mEq/l) | BUN (mg/dl) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Saph | ROB | Cardiac | Saph | ROB | Cardiac | Saph | ROB | Cardiac | |
| 1 | 0.2 | 1 | 2 | – | 5.3 | 4.4 | 24 | 25 | 25 |
| 2 | 0.2 | 1 | 0.5 | – | 5.4 | 4.6 | 22 | 22 | 22 |
| 3 | 0.1 | 1 | 0.6 | – | 5 | 5.9 | – | 22 | 25 |
| 4 | 0.4 | 1 | 2 | 7.9 | 6.1 | 5.9 | 21 | 22 | 22 |
| 5 | 0.6 | 1 | 1.5 | 7 | 5.9 | 5.1 | 21 | 22 | 23 |
| 6 | 0.4 | 1 | 3 | 11.5 | 5.6 | 4.9 | 33 | 24 | 23 |
| 7 | 0.15 | 0.6 | 0.75 | – | 6 | 5.5 | 52 | 19 | 19 |
| 8 | 0.15 | 0.9 | 0.75 | 20 | 6.4 | 5.4 | 33 | 20 | 20 |
Saph, saphenous. –, insufficient volume for analysis.
Saphenous blood collection also resulted in greater hemolysis as indicated by potassium levels (11.6 ± 5.9 mEq/l) than did ROB or cardiac bleeding (5.7 ± 0.5 mEq/l and 5.2 ± 0.6 mEq/l, respectively; Table 1). The mean serum potassium level in samples obtained by the saphenous technique was significantly higher than that in samples obtained by ROB (P = 0.01). The normal serum potassium level in rats is 4–6 mEq/l (ref. 4).
The normal BUN concentration in our rats is 17–25 mg/dl (ref. 5). BUN levels were elevated in three of the eight samples obtained by the saphenous technique but were within the normal range in all samples obtained by ROB or cardiac bleeding. The mean BUN concentration of the saphenous bleed samples (29.4 ± 11.3 mg/dl) was higher than that of the ROB or cardiac samples (22.0 ± 1.9 mg/dl and 22.4 ± 2.1 mg/dl, respectively; P = 0.13 for saphenous versus ROB samples; Table 1), and the variance in BUN values was much greater for the saphenous samples (F statistic P < 0.001).
DISCUSSION
We designed and carried out two studies to evaluate the safety of ROB and its ability to yield blood samples of adequate volume and quality for analysis. We found that, when done using a lateral approach and by an experienced technician, ROB was safe and provided blood samples of adequate volume and quality for analysis.
First, we retrospectively assessed the incidence of ocular injury associated with 1,869 ROB procedures done at our institution during an 18-month period. Ocular complications were associated with only 12 of these procedures (0.6%), a low incidence that reflects the safety of the ROB technique. We also note that ocular problems were reported in six rats that had not undergone ROB.
Second, we compared the volume and quality of blood samples obtained by various phlebotomy techniques: saphenous bleeding, ROB and cardiac bleeding. ROB yielded samples of adequate volume without hemolysis or artifactual elevation in BUN. A previous study reported a similar lack of hemolysis using the ROB technique compared with tail vein incision or cardiac puncture and affirmed that ROB is the method of choice for rat phlebotomy6. We noted that the ROB procedure was also faster than saphenous phlebotomy (B.L.F. and M.M., unpublished observations), consistent with published reports7. BUN levels were normal in cardiac blood samples from all eight rats, confirming that they had normal kidney function. The elevation in BUN levels in three of eight saphenous blood samples seems to have resulted from hemolysis of the samples that interfered with the colorimetric assay for BUN. The false elevation of BUN concentration in saphenous blood samples from normal animals constitutes a compromise of sample quality, which could lead investigators to collect samples from additional animals, contrary to the 3Rs principle of reduction in animal numbers. We also note that the volumes of blood samples obtained by saphenous bleeding were very low, which could also lead to the use of additional animals.
Ideally, phlebotomy would be completely innocuous, with no ill effects to the animal, as well as efficient. In the latter regard, ROB is quite advantageous, as it requires only one technician and samples may be collected from many animals in quick succession without compromising their integrity. There are potential disadvantages to ROB, however, as there are with all phlebotomy techniques. Rats may be lethargic immediately after the ROB procedure, although this behavior might result from anesthesia alone, as reported previously8,9. Tissue injury, including hemorrhage and inflammation, has been documented by histological study in rats that underwent orbital puncture10. These changes were noted 4 d after the procedure and were resolved by 28 d after the procedure. We did not carry out histological analysis of the phlebotomy sites in our studies; however, we feel that any phlebotomy technique might result in transient histological hemorrhage and inflammation and that these changes should not form the basis for selecting a specific technique. A major criticism of ROB is its potential to cause damage when carried out by an unskilled operator11, yet this is true for any bleeding procedure done by an unskilled technician. Our senior technician has provided careful and repeated instruction to more junior technicians to ensure that phlebotomy procedures are safe and humane. Our technique uses a lateral approach, never medial. This is in contrast to some published methods of ROB12. The lateral technique might be better than the medial, but our data do not enable us to make that comparison. Instead of speculating which technique might result in the most harm if carried out by an untrained technician, a more prudent approach is to evaluate the likelihood of a technique causing damage when carried out by skilled individuals with appropriate training. Our data show a very low incidence of ocular injury associated with ROB using a lateral approach.
Our studies had several limitations that may limit the conclusions that we can draw from them. The first study was retrospective. In the second study, only eight rats were included and different personnel carried out the three different phlebotomy procedures. Nonetheless, our method of ROB had a low incidence of ocular complications, and ROB samples were of good quality and sufficient volume for analysis.
Acknowledgments
These studies were supported in part by resources at the Zablocki VA Medical Center (Milwaukee, WI) and by grants from the US National Institutes of Health to J.E.B., to M.M. and to J.E.M.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Diehl KH, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- 2.Lenarczyk M, et al. Cardiac injury following 10 gy total body irradiation: indirect role of effects on abdominal organs. Radiat Res. 2013;180:247–258. doi: 10.1667/RR3292.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank JJ, Bermes EW, Bickel MJ, Watkins BF. Effect of in vitro hemolysis on chemical values for serum. Clin Chem. 1978;24:1966–1970. [PubMed] [Google Scholar]
- 4.Stender RN, Engler WJ, Braun TM, Hankenson FC. Establishment of blood analyte intervals for laboratory mice and rats by use of a portable clinical analyzer. J Am Assoc Lab Anim Sci. 2007;46:47–52. [PubMed] [Google Scholar]
- 5.Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res. 2011;175:29–36. doi: 10.1667/RR2400.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suber RL, Kodell RL. The effect of three phlebotomy techniques on hematological and clinical chemical evaluation in sprague-dawley rats. Vet Clin Pathol. 1985;14:23–30. doi: 10.1111/j.1939-165x.1985.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 7.van Herck H, et al. Orbital sinus blood sampling in rats: effects upon selected behavioural variables. Lab Anim. 2000;34:10–19. doi: 10.1258/002367700780577993. [DOI] [PubMed] [Google Scholar]
- 8.van Herck H, et al. Endocrine stress response in rats subjected to singular orbital puncture while under diethyl-ether anaesthesia. Lab Anim. 1991;25:325–329. doi: 10.1258/002367791780809931. [DOI] [PubMed] [Google Scholar]
- 9.van Herck H, et al. Orbital bleeding in rats while under diethylether anaesthesia does not influence telemetrically determined heart rate, body temperature, locomotor and eating activity when compared with anaesthesia alone. Lab Anim. 1997;31:271–278. doi: 10.1258/002367797780596284. [DOI] [PubMed] [Google Scholar]
- 10.van Herck H, et al. Histological changes in the orbital region of rats after orbital puncture. Lab Anim. 1992;26:53–58. doi: 10.1258/002367792780809048. [DOI] [PubMed] [Google Scholar]
- 11.van Herck H, et al. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Lab Anim. 1998;32:377–386. doi: 10.1258/002367798780599794. [DOI] [PubMed] [Google Scholar]
- 12.Fitzner Toft M, Petersen MH, Dragsted N, Hansen AK. The impact of different blood sampling methods on laboratory rats under different types of anaesthesia. Lab Anim. 2006;40:261–274. doi: 10.1258/002367706777611433. [DOI] [PubMed] [Google Scholar]