Abstract
Objective
This study had three goals, to examine: 1) the frequency of atypical development, consistent with the broader autism phenotype, in high-risk infant siblings of children with ASD, 2) the age at which atypical development is first evident, and 3) which developmental domains are affected.
Method
A prospective longitudinal design was used to compare 294 high-risk infants and 116 low-risk infants. Participants were tested at 6, 12, 18, 24, and 36 months of age. At the final visit, outcome was classified as ASD, Typical Development (TD), or Non-TD (defined as elevated ADOS score, low Mullen scores, or both).
Results
28% of the high-risk group was classified as Non-TD at 36 months of age. Growth curve models demonstrated that the Non-TD group could not be distinguished from the other groups at 6 months of age, but differed significantly from the Low-Risk TD group by 12 months on multiple measures. The Non-TD group demonstrated atypical development in cognitive, motor, language, and social domains, with differences particularly prominent in social-communication.
Conclusions
These results demonstrate that features of atypical development, consistent with the broader autism phenotype, are detectable by the first birthday and affect development in multiple domains. This highlights the necessity for close developmental surveillance of infant siblings of children with ASD, along with implementation of appropriate interventions as needed.
Keywords: Autism Spectrum Disorder, broader autism phenotype, siblings, social-communication, infancy
Introduction
The broader autism phenotype (BAP) is a constellation of subclinical characteristics that are seen at elevated rates in family members of children with autism spectrum disorder (ASD).1 It is generally agreed that the BAP encompasses features related to the core diagnostic domains of ASD, such as language delays and deficits, social difficulties, and rigidity of personality or behavior.2–3 Most previous studies have examined the BAP in parents and school-age siblings of children with ASD;2–3 few have investigated BAP features in infancy and toddlerhood, so it is not clear when these differences in behavior first develop and can be detected.
For questions that require precise timing of onset, prospective studies provide an optimal experimental design, because they do not rely solely on parent report, which can be subject to recall errors and other biases. In the past decade, prospective studies of high-risk infants have proliferated. The most common participants at increased risk for ASD studied thus far are later-born siblings of children with ASD. Such infant sibling study designs often compare high-risk samples to low-risk infants with no family history of ASD. While several dozen such studies have been published, most focus on describing the early development and predictive early risk signs of infants who ultimately develop ASD.4–5 Other infant sibling studies have reported differences between high- and low-risk groups in a variety of domains, including eye contact, joint attention, and nonverbal reasoning, but did not follow the infants long enough to know whether these differences are early signs of ASD or may instead index other types of atypical outcomes, including the BAP.6–9
Only a few infant sibling studies have specifically focused on describing early signs of the BAP.10–15 These investigations follow participants until age 3, determine which children develop ASD, and remove them from the larger high-risk group prior to analyses (since, by definition, the BAP and ASD are mutually exclusive). Several studies, most involving small samples, have found significant differences between high-risk non-ASD groups and low-risk controls early in life, on tasks of response to joint attention at 14 months (n=8)10 and social referencing at 18 months (n=30),11 as well as on parent report measures of temperament as early as 7 months (n=12).12 Early differences in parent-reported temperament in high-risk siblings without ASD have also been reported in a much larger sample at 24 months of age (n=104).13 In a comprehensive study examining multiple domains of development, 40 high-risk siblings without ASD outcomes were, as a group, below average in expressive and receptive language, overall IQ, adaptive behavior, and social communication skills at 18–27 months.14 Additionally, parents reported social impairments on a questionnaire by 13 months of age. A recent large study followed 170 high-risk children, none of whom were diagnosed as having ASD at age 3 years.15 A cluster analysis identified a subgroup (19% of the high-risk sample) that had elevated scores on the Autism Observation Scale for Infants at 12 months of age. At age 3, this cluster demonstrated lower scores than low-risk controls on independent social-communication and cognitive measures. Taken together, these and other studies strongly suggest that behavioral and developmental features consistent with the BAP emerge early in life.
Most published sibling studies have been cross-sectional and/or focused on whether group differences are evident at a single age. Only one study thus far has examined longitudinal trajectories of development, following a cohort of 37 high-risk children from 4 months to 7 years of age.16 At 7, the researchers split their high-risk group into two subgroups, one with BAP features (40%) and one without, and then examined their cognitive and language trajectories in the preschool years (4–54 months) using growth curve analysis. They found that language scores were different for the BAP group as early as 14 months, but that cognitive scores did not differentiate the group from the low-risk controls at any age. The current study took a similar approach, examining development longitudinally from 6 to 36 months in high- and low-risk infants (n=294 and n=116, respectively) and looking for the earliest inflection point at which the trajectories diverge from one of typical to atypical development. The current study is the largest sample to date that examines BAP features longitudinally. We focus on several domains of early development: social-communication, language, nonverbal cognitive, and fine motor abilities.
The studies reviewed above have taken one of two approaches when studying the BAP. Some have studied all children in the high-risk group, after excluding those with an ASD outcome, looking for differences from low-risk infants.14 Others have classified an “atypical” outcome group, using varying criteria at varying outcome ages, and then examined whether this “atypical” subgroup differs from low-risk controls at earlier ages than when the groups were defined.10,12,16 This latter approach is the one used in the current study. It is clear that there is substantial heterogeneity within the high-risk group; virtually all previous studies find that atypical development or BAP-like features are present in only a subset of siblings of children with ASD.2–3,17 Therefore, studying all high-risk siblings without ASD outcomes risks the possibility of obscuring potential differences that may be evident in a subgroup. Using a definition similar to other recent investigations,10,12 we identified a group of high-risk children with non-typical developmental outcomes at 36 months of age. We then used growth curve analysis to examine when non-typical development could first be detected. We studied multiple areas of development, extending more broadly than the BAP (e.g., social-communication, but also cognition and motor skills), to examine in what domains non-typical development was evident.
Method
Participants
The sample reported in this paper was drawn from a larger longitudinal study of infant siblings of children with ASD (High-Risk group) or children with typical development (Low-Risk group), recruited at two sites (university1 and university2) during two phases of grant funding (2003–2008 and 2008–2013). The sole inclusion criterion for the High-Risk group was status as a younger sibling of a child with ASD. Diagnosis of the affected older sibling was confirmed by meeting ASD criteria on both the Autism Diagnostic Observation Schedule (ADOS) and the Social Communication Questionnaire (SCQ).18–19 Exclusion criteria for the High-Risk group included birth before 36 weeks of gestation and a known genetic disorder (e.g., Fragile X syndrome) in the older affected sibling. The primary inclusion criterion for the Low-Risk group was status as a younger sibling of a child (or children) with typical development. Low-risk status of all older siblings was confirmed by an intake screening questionnaire and scores below the ASD range on the SCQ. Exclusion criteria for the Low-Risk group were birth before 36 weeks of gestation, developmental, learning, or medical conditions in any older sibling, and ASD in any first-, second-, or third-degree relatives. All participants with complete data at the 36-month outcome visit were included.
Participants were enrolled before 18 months of age (age at enrollment M = 6.7 months, SD = 5.2 months; 76% were enrolled by 9 months or earlier). Depending upon age of study entry, data were collected at up to five ages: 6, 12, 18, 24, and 36 months. At the 36-month visit, participants were classified into one of three algorithmically-defined outcome groups: ASD, Typical Development (TD), and Non-Typical Development (Non-TD). See Table 1 for algorithmic group definitions, which were developed by the Baby Siblings Research Consortium, a network of researchers studying very young children at risk for ASD (Chawarska et al., unpublished data, November 2013).
Table 1.
Algorithmic group outcome definitions
| Outcome Classification |
Criteria |
|---|---|
| ASD | At or above the ASD cutoff of the ADOS and Meets DSM-IV-TR criteria for Autistic Disorder or PDD-NOS |
| Typical Development | Does not meet criteria for ASD classification and No more than one Mullen subtest ≥ 1.5 SD below mean and No Mullen subtest ≥ 2 SD below mean and ADOS > 3 points below ASD cutoff |
| Non-Typical Development | Does not meet criteria for ASD classification and Two or more Mullen subtests ≥ 1.5 SD below mean and/or One or more Mullen subtests ≥ 2 SD below mean and/or ADOS ≤ 3 points below ASD cutoff |
Note: ASD = autism spectrum disorder; ADOS = Autism Diagnostic Observation Schedule; PDD-NOS = pervasive developmental disorder not otherwise specified.
Given this paper’s focus on the BAP, which by definition is a characteristic of family members of a child with ASD, the small groups of Low-Risk participants with ASD (n=4) or Non-TD (n=27) outcomes were not included in analyses. The final sample with complete 36-month data included in the study were 51 participants classified with ASD (17.4% of the High-Risk group; n=8 females), 83 with Non-TD outcomes (28.2% of the High-Risk group; n=32 females), and 276 with TD outcomes, who were further stratified into High-Risk TD (n=160; n=90 females) and Low-Risk TD (n=116; n=53 females). Of the 83 participants in the Non-TD sample, 66 were classified into this group because of elevated ADOS alone, 9 were classified because of low Mullen scores alone (8 had at least one Mullen scale that was ≥ 2 SD below mean and 1 had two or more Mullen scales that were ≥ 1.5 SD below mean), and 8 were classified as Non-TD because of both elevated ADOS and low Mullen (7 had at least one Mullen scale that was ≥ 2 SD below mean and 1 had two or more Mullen scales that were ≥ 1.5 SD below mean).
Measures
The study was conducted under the approval of both sites’ IRBs. Infants were assessed by examiners unaware of group membership.
Autism Diagnostic Observation Schedule.18
This is a semi-structured standardized interaction and observation that measures symptoms of autism. It has two empirically derived cutoffs, one for ASD and one for Autistic Disorder. Since data collection occurred prior to the publication of newer ADOS algorithms, the Communication+Social Total algorithm score was used.19 Psychometric studies report high inter-rater reliability and agreement in diagnostic classification (autism v. non-spectrum). The ADOS was used to confirm older sibling diagnosis and to determine infant outcome at 36 months of age (see Table 1).
Mullen Scales of Early Learning.20
This is a standardized developmental test for children birth to 68 months. Four subscales were administered: Fine Motor, Visual Reception, Expressive Language, and Receptive Language. Scores are expressed in raw score points, which can also be converted to T-scores and age equivalents using published normative data. An overall score, the Early Learning Composite, is also obtained. The Mullen subscales have excellent internal consistency (median 0.91) and test-retest reliability (median 0.84). This test was used to measure cognitive functioning at each visit and determine outcome status at 36 months. Ongoing administration and scoring fidelity procedures were implemented to insure that there were minimal cross-examiner and cross-site differences.
Examiner Rated Social Engagement
At the end of the session, examiners rated three behaviors using a 3-point scale (1 = rare, 2 = occasional, 3 = frequent): 1) frequency of eye contact, 2) frequency of shared affect, and 3) overall social responsiveness. These three scores were summed to create a social engagement composite score (ranging from 3 to 9). In a previous study, this measure was able to distinguish infants with typical versus atypical development by 12 months of age.21
Clinical Best Estimate (CBE) outcome classification
At the end of the 36-month visit, examiners classified each child into one of six CBE categories: ASD, BAP, Behavior Problems, Global Developmental Delay, Speech-Language Problems, or Typical Development. In contrast to the algorithmic groups (ASD, TD, Non-TD) that were empirically determined for the current analyses, the CBE classifications were clinically defined. Children classified with ASD met DSM-IV-TR criteria for Autistic Disorder or PDDNOS. Children classified as BAP displayed social-communication difficulties that were judged to be below ASD threshold. Children classified with Behavior Problems displayed issues such as high activity level or oppositional or defiant behavior, beyond what would be expected for developmental level. Children classified clinically with Global Developmental Delay had low scores across multiple cognitive and motor domains. Children classified as having Speech-Language Problems displayed immature speech patterns or low language levels in isolation (no accompanying social or cognitive difficulties). All other participants were classified with Typical Development.
Statistical Analysis
Mixed-effects linear models were used to estimate patterns of change in Mullen raw scores and test whether group was related to the initial level or rate of change in these variables.22 All core models included fixed effects for group (ASD, Non-TD, High-Risk TD, and Low-Risk TD), the linear effect of age (centered at 6 months), and the interaction between group and age. To account for the correlated nature of the data, the core models included two random effects for child-specific intercepts and slopes. Additional fixed terms (for the quadratic effect of age, the interaction of the quadratic effect of age with group, gender, phase, site, etc.) were also added to the core model and tested. These terms were retained in the models only if they were significant. For the models with a significant quadratic effect of age, we also included random effects for the quadratic age. For some of those models, there was little variability left in the intercepts, so only the child-specific slopes were retained. A similar modeling strategy was used to analyze the Examiner Rated Social Engagement composite scores (with age centered at 6 months) and ADOS social-communication scores (with age centered at 18 months). Further details on the mixed-effects models are presented in Supplement 1, available online.
All tests were two-sided, with α = 0.05. Residual analyses and graphical diagnostics determined that the model assumptions were adequately met. Analyses were implemented using PROC MIXED in SAS Version 9.3.23
Results
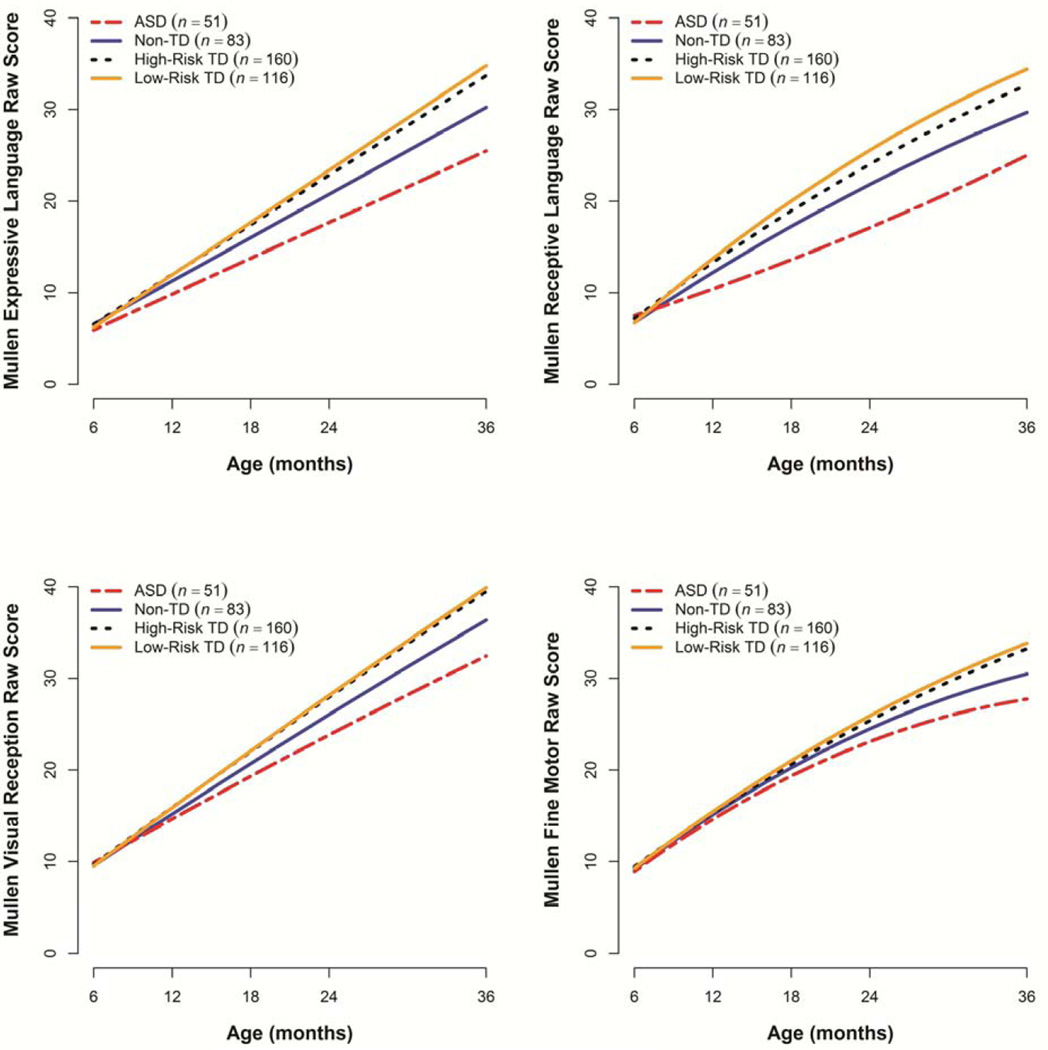
Table 2, Table S1, available online, and Figure 1 summarize the results of the mixed-effects models for Mullen raw scores. At baseline (6 months of age), all four groups had comparable values on all four scales. The Low-Risk TD group demonstrated a sharp increase in raw scores with age on all Mullen scales. The High-Risk TD group had significantly slower growth over time than the Low-Risk TD group on the Expressive and Receptive Language scales, but not on the Visual Reception and Fine Motor scales. At 36 months, the two TD groups had comparable Visual Reception and Fine Motor scores, but the High-Risk TD group showed significantly lower levels of Expressive Language (by 1.1 point) and Receptive Language (by 1.7 points). The ASD group showed a significantly slower rate of change than both TD groups on all four scales and was significantly different from both groups by 12 months of age. Of primary interest for this paper, the Non-TD group’s performance was intermediate between the ASD and both TD groups. The Non-TD group had lower rates of growth than both TD groups, resulting in significant differences from them by 12 months of age on all scales except Fine Motor. The differences from both TD groups were modest at 12 months (differences from Non-TD ranged from 0.3 to 1.5 points across scales for Low-Risk TD and 0.2 to 1.1 points for High-Risk TD) but amplified over time (at 36 months, differences from Non-TD ranged from 3.4 to 4.7 points in Low-Risk TD and 2.8 to 3.5 points in High-Risk TD).
Table 2.
Parameter estimates (SE) for the mixed-effects regression models predicting Mullen raw scores, examiner rated social engagement, and Autism Diagnostic Observation Schedule (ADOS) social-communication scores
| Mullen Scale | Examiner Rated Social Engagement |
ADOS Social- Communication |
||||
|---|---|---|---|---|---|---|
| Model term | EL | RL | VR | FM | ||
| Estimated trajectory for Low-Risk TD group | ||||||
| Baseline | 6.24 (.15)*** | 6.69 (.22)*** | 9.52 (.18)*** | 9.28 (.18)*** | 7.87 (.18)*** | 2.42 (.26)*** |
| Linear age effect (year) | 11.42 (.18)*** | 14.81 (.55) *** | 12.84 (.30) *** | 12.98 (.39)*** | 1.13 (.34)** | −.48 (.82) |
| Quadratic age effect (year) | - | −1.49 (.21)*** | −.28 (.10)** | −1.26 (.16)*** | −.29 (.11)* | −.08 (.49) |
| Estimated difference between ASD and Low-Risk TD group | ||||||
| Baseline | −.30 (.29) | .82 (.42) | .35 (.32) | −.35 (.35) | −.23 (.37) | 8.46 (.70)*** |
| Linear age effect (year) | −3.60 (.34)*** | −9.32 (1.02)*** | −3.11 (.38)*** | −.63 (.73) | −2.12 (.59)*** | −6.83 (2.51)** |
| Quadratic age effect (year) | - | 2.09 (.39)*** | - | −.67 (.30)* | −.46 (.20)* | 5.94 (1.56)*** |
| Estimated difference between Non-TD and Low-Risk TD groups | ||||||
| Baseline | .31 (.24) | .04 (.35) | .03 (.27) | −.13 (.29) | −.37 (.31) | 2.24 (.39)*** |
| Linear age effect (year) | −1.94 (.28)*** | −3.43 (.86)*** | −1.42 (.32)*** | −.17 (.61) | −.53 (.51) | −1.33 (1.23) |
| Quadratic age effect (year) | - | .61 (.33) | - | −.45 (.25) | −.13 (.17) | 1.75 (.73)* |
| Estimated difference between High-Risk TD and Low-Risk TD groups | ||||||
| Baseline | .32 (.21) | .49 (.31) | .15 (.23) | .15 (.26) | −.26 (.29) | 1.10 (.34)** |
| Linear age effect (year) | −.56 (.23)* | −2.03 (.73)** | −.22 (.27) | −.67 (.52) | −.38 (.47) | −2.02 (1.05) |
| Quadratic age effect (year) | - | .46 (.27) | - | .15 (.21) | −.17 (.15) | 1.01 (.62) |
Note: Baseline is 18 months for ADOS and 6 months for all other variables. ASD = autism spectrum disorder; EL = Expressive Language; FM = Fine Motor; RL = Receptive Language; SE = standard error; TD = typically developing; VR = Visual Reception.
p < .05,
p < .01,
p < .001
Figure 1.
Estimated trajectories for Mullen scales. ASD = autism spectrum disorder; TD = typically developing.
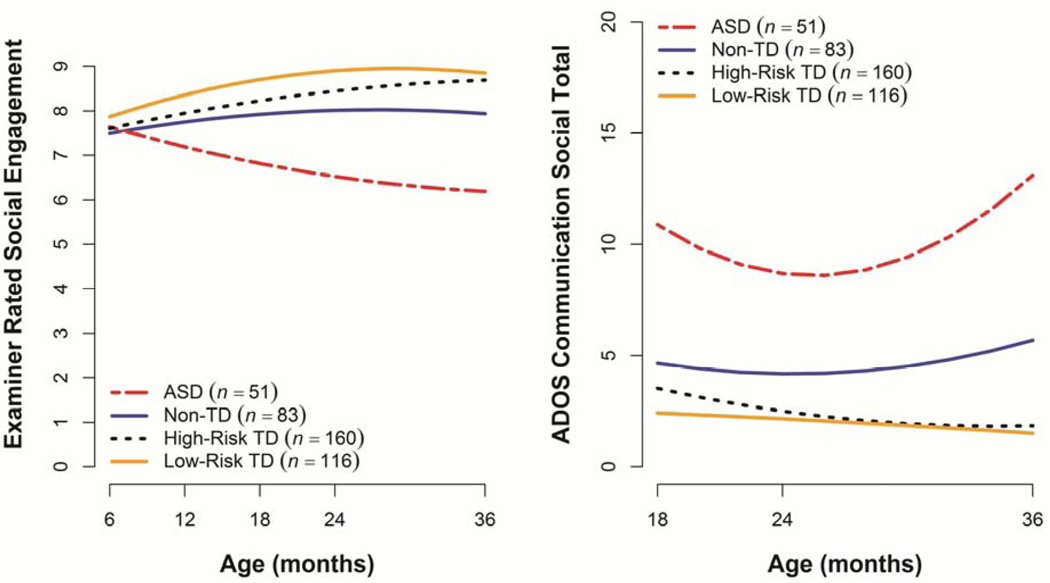
At 6 months of age, all four groups had similar Examiner Rated Social Engagement composite scores (see Table 2 and Figure 2). The two TD groups exhibited significant growth over time and the ASD group showed a sharp decrease in scores with age. The Non-TD group had a flat trajectory, with significant differences from the Low-Risk TD group evident starting at 12 months and resulting in 36-month scores that were significantly lower over time than both TD groups (by about 1 point) but higher than the ASD group (by about 2 points).
Figure 2.
Estimated trajectories for Examiner Rated Social Engagement composite and Autism Diagnostic Observation Schedule (ADOS) social-communication algorithm score. ASD = autism spectrum disorder; TD = typically developing.
At 18 months (the first visit in which the ADOS was administered), there were significant group differences on the social-communication algorithm score, with the Low-Risk TD group demonstrating lower scores than the High-Risk TD (by 1 point), Non-TD (by 2 points), and ASD (by 8 points) groups (see Table 2 and Figure 2). The Low-Risk TD group demonstrated a stable trajectory over time, while the High-Risk TD group exhibited a slight decrease over time. The Non-TD group showed a significant quadratic effect of age. At 36 months, the two TD groups had comparable scores (1.5 and 1.9, respectively), while the Non-TD and ASD group showed significantly higher scores (estimated values 5.7 and 13.1, respectively). Again, as with the Mullen, the scores and longitudinal trajectories of the Non-TD group fell intermediate between the TD and ASD groups.
Table 3 depicts the correspondence between the empirically-derived algorithmic classifications (ASD, TD, Non-TD) and clinical judgment (CBE outcome classification) at 36 months. The perfect correspondence between the two classifications for the ASD group is secondary to the algorithmic definition, which requires a clinical diagnosis of ASD. The Non-TD group had a significantly higher rate of classifications of BAP, Behavior Problems, Global Developmental Delay, and Speech-Language Problems and significantly lower rate of Typical Development classifications than both the High-Risk and Low-Risk TD groups (Fisher’s exact test, p < .001). The most common clinical classification for the Non-TD group was BAP, with over a third of the sample falling in this category. Three Non-TD participants received a CBE rating of ASD but did not meet the algorithmic criteria (e.g., did not have an ADOS score over the ASD cutoff), resulting in their classification as Non-TD. Interestingly, almost 40% of the Non-TD group was judged by examiners to have a CBE outcome of typical development, despite the elevated ADOS scores or lowered Mullen scores that classified them empirically into the Non-TD group.
Table 3.
Clinical Best Estimate classifications at 36 months by algorithmic group
| Clinical Best Estimate | ASD (n = 51) |
Non-TD (n = 83) |
High-Risk TD (n = 160) |
Low-Risk TD (n = 116) |
|---|---|---|---|---|
| Autism Spectrum Disorder | 51 (100%) | 3 (4%) | 0 (0%) | 0 (0%) |
| Broader Autism Phenotype | 0 (0%) | 29 (35%) | 10 (6%) | 0 (0%) |
| Behavior Problems | 0 (0%) | 8 (10%) | 7 (4%) | 2 (2%) |
| Global Developmental Delay | 0 (0%) | 5 (6%) | 2 (1%) | 0 (0%) |
| Speech-Language Problems | 0 (0%) | 6 (7%) | 14 (9%) | 3 (3%) |
| Typical Development | 0 (0%) | 32 (39%) | 127 (79%) | 111 (96%) |
Note: ASD = autism spectrum disorder; TD = typically developing
In secondary analyses, we added to the core models and tested terms for gender, site, phase, and, for those models with significant gender effects, the interactions between gender and group and gender and age. There was no phase effect and site was significant only in the model predicting receptive language (the university2 sample scored 0.4 points higher than the university1 sample, p < .05, but the difference was so small that it is unlikely to be clinically meaningful). Gender was a significant predictor for all Mullen scales except Receptive Language, with girls demonstrating slightly higher Visual Reception scores than boys (0.5 points, p < .05). For Expressive Language and Fine Motor, there was a significant gender by group interaction, driven by girls in the ASD group, who scored lower than boys on these scales, while girls in the other three groups scored about half a point higher than boys on the same scales. There were no gender, phase, or site effects in the model predicting ADOS social-communication score. For the Examiner Rated Social Engagement composite, there was a significant gender by group interaction, driven again by the girls in the ASD group, who scored 2 points lower than the boys (p < .001), while girls in the other three groups scored similarly to same-group boys. For this variable, there was a phase effect, with phase 2 children scoring about 0.2 points higher than phase 1 children (p = .03). The interaction between gender and age was not significant in any of the models considered.
Discussion
This study focused on developmental aspects of the BAP, exploring the frequency of non-typical development in high-risk infant siblings, the age at which atypical development was first evident, and which developmental domains were affected. We found that 28% of the high-risk cohort demonstrated atypical development (not including ASD) at 36 months of age, as defined by elevated ADOS scores (within 3 points of the ASD cutoff), low Mullen scores, or both. Working backwards from this age, we used growth curve models to determine when these differences in development could first be detected. On the Mullen Scales of Early Learning and the Examiner Ratings of Social Engagement, the Non-TD group was not distinguishable from any other group at 6 months, but differed significantly from the Low-Risk TD group by 12 months of age, deviating from typical development as early as the group with ASD. At 18 months, the earliest age at which the ADOS was administered, the Non-TD group was already obtaining significantly higher scores than the Low-Risk TD group.
The aspects of atypical development that distinguished the Non-TD group from the Low-Risk group occurred in all domains assessed in this study (cognition, motor, language, and social development) but were most prominent in the social-communication domain. Ninety percent of the Non-TD group demonstrated social-communication difficulties (as defined by an ADOS score within 3 points of the ASD cutoff), including reduced eye contact, infrequent social initiations with unfamiliar people, repetitive vocalizations, and delayed onset of gestures, speech, and play. Isolated language and cognitive delays (e.g., low Mullen scores alone) were rare, seen in only 10% of the Non-TD group, as previous studies have also found.24 When such delays were evident, they occurred in combination with elevated ADOS scores. Thus, the vast majority of the Non-TD group demonstrated the kinds of social-communication features that have been previously described in older siblings as consistent with the BAP. Interestingly, almost 40% of the Non-TD group was given a CBE of typical development by examiners, despite such elevated ADOS scores. We plan to further examine this subgroup to better understand what may lead to a clinical judgment of typicality, despite non-typical scores. An item analysis of the ADOS, for example, may reveal that high scores on certain items are not considered particularly concerning by clinicians, leading to a CBE of typical development, while high scores on other items (perhaps eye contact) are judged as consistent with the BAP.
One of the primary gaps in the literature motivating this research was the paucity of studies of BAP-like phenomena in very young siblings, with most previous investigations conducted on school-age siblings and parents. This results in a need to “translate” the types of deficits seen at older ages, and instruments used to measure them, into those appropriate for earlier stages of development. Some of this translation was straightforward, when the same instrument used with older siblings and parents could also be employed with this young age group (e.g., the ADOS; see the comprehensive review by Sucksmith and colleagues3 for a list of previous studies and measures used). It was not clear at the start of this study whether the Mullen Scales would adequately index any cognitive delays that might be apparent. The findings here demonstrate that general developmental delays can occasionally be seen in very young siblings and that the Mullen Scales can detect them.
In future follow-up studies as our sample reaches school-age, we plan to examine what proportion of the High-Risk children meet definitions of the BAP used previously in older samples.1 Many definitions include behavioral features not seen in infancy or measured by our tasks, such as peer problems, pragmatic language difficulties, rigid inflexible behavior, anxiety, and depression. It is possible that the rate of atypical development will increase over time and that some children in the High-Risk TD group, who did not show atypicalities at 36 months or did not meet cutoffs for the Non-TD definition, may be identified with a BAP-like phenotype as they are followed longitudinally into the school years. Previous longitudinal studies have in fact reported a significant increase in the number of high-risk siblings identified with BAP-related difficulties at age 7 compared to the preschool years.25–27
The results reported here are largely consistent with a recently published study that used a different type of prospective design.28 This research team analyzed the Avon Longitudinal sample, a very large community-ascertained general population sample, which followed children from before birth to age 11, obtaining parent reports of development (including a measure of ASD traits) at multiple ages. Bolton and colleagues found that parents reported differences in development within the first year of life that not only predicted later diagnoses of ASD, but also a wider, subthreshold range of autistic-like behaviors potentially consistent with the BAP.28
A question that often arises is whether siblings like those in the Non-TD group, who have delays that are sub-threshold to ASD, should receive early intervention services or whether their delays will lessen over time without treatment. There are not as yet any well-controlled intervention studies that can help answer this question, so we must turn to other sources. One answer to the question comes from the law involving early intervention services for children under three, the Individuals with Disabilities Education Act (IDEA), Part C, which states that young children with delays and those who are at high risk for developmental delays are entitled to assessments and intervention services. Thus, good clinical practice suggests that when children are showing atypical development they and their families should be provided with information about the child’s difficulties, clinical reports when practical, and referrals to local Part C service providers. The second response to this question about early intervention for BAP-like features comes from two long-term longitudinal studies of infant siblings, both of which demonstrated that early lagging trajectories continue after the preschool period and do not “catch up” to typically developing groups.16,28
What types of intervention should be provided to this wide-ranging group of children? Certainly, no one approach or modality can be expected to fit a group whose difficulties range from severe oppositionality and hyperactivity, to mild-to-moderate intellectual impairment, to subthreshold symptoms of ASD. Intervention approaches need to be chosen based on each child’s profile of strengths and weaknesses and each family’s goals and priorities. However, there are a range of choices available to early intervention professionals from a range of disciplines. Empirically supported, manualized, parent-implemented interventions for toddlers and preschoolers with behavior disorders, general delayed development, social-communicative symptoms related to autism, and difficulties with expressive communication are represented in the literature and many of these can be carried out by professionals from a variety of disciplines.29–31
We will continue to follow our sample as they reach school age, to examine whether developmental difficulties identified at age 3 persist and whether new difficulties (e.g., learning disorders, anxiety, etc.) emerge over time. It is critical to better understand the long-term functional consequences of the early developmental patterns identified in the current study. The ultimate goal of this program of research is to determine whether monitoring and identification in the preschool years could be used to provide appropriate interventions that would reduce the number of high-risk siblings who display later difficulties.
Supplementary Material
Clinical Guidance.
Close to half of younger siblings of children with ASD develop in an atypical fashion. In the current study, 17% developed ASD and another 28% showed delays or deficits in other areas of development or behavior.
Differences in development are detectable using standardized assessment instruments by 12 months of age in many children.
The most common development differences seen in younger siblings of children with ASD are delays in social-communication development (including reduced eye contact, extreme shyness with unfamiliar people, and delayed onset of gestures and speech). Some younger siblings also show delays in cognitive and motor abilities, as well as behavioral problems.
Close developmental surveillance of infant siblings of children with ASD is necessary, along with implementation of appropriate interventions as needed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Ozonoff, Young, Hutman, Johnson, Miller, Rogers, Schwichtenberg, Steinfeld, and Iosif, and Ms. Belding, Ms. M. Hill and Ms. A. Hill report no biomedical financial disclosures or potential conflicts of interest to report
Supplemental material cited in this article is available online.
Contributor Information
Sally Ozonoff, University of California.
Gregory S. Young, University of California.
Ashleigh Belding, University of California.
Monique Hill, University of California.
Alesha Hill, University of California.
Ted Hutman, University of California – Los Angeles.
Scott Johnson, University of California – Los Angeles.
Meghan Miller, University of California.
Sally J. Rogers, University of California.
A.J. Schwichtenberg, Purdue University.
Marybeth Steinfeld, University of California.
Ana-Maria Iosif, University of California.
References
- 1.Bolton P, Macdonald H, Pickles A, et al. A case-control family history study of autism. J Child Psychol Psychiatry. 1994;35(5):877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 2.Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: The phenotype in relatives. J Autism Dev Disorder. 1998;28(5):369–392. doi: 10.1023/a:1026048320785. [DOI] [PubMed] [Google Scholar]
- 3.Sucksmith E, Roth I, Hoekstra RA. Autistic traits below the clinical threshold: Reexamining the broader autism phenotype in the 21st century. Neuropsychol Rev. 2011;21(4):360–389. doi: 10.1007/s11065-011-9183-9. [DOI] [PubMed] [Google Scholar]
- 4.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. J Child Psychol Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- 5.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23(2):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Bedford R, Elsabbagh M, Gliga T, et al. Precursors to social and communication difficulties in infants at-risk for autism: Gaze following and attentional engagement. J Autism Dev Disord. 2012;42(10):2208–2218. doi: 10.1007/s10803-012-1450-y. [DOI] [PubMed] [Google Scholar]
- 7.Bhat AN, Galloway JC, Landa RJ. Social and non-social visual attention patterns and associative learning in infants at risk for autism. J Child Psychol Psychiatry. 2010;51(9):989–997. doi: 10.1111/j.1469-7610.2010.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merin N, Young GS, Ozonoff S, Rogers SJ. Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. J Autism Dev Disord. 2007;37(1):108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- 9.Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social-communicative and cognitive development of younger siblings of children with autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161(4):384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, Landa R. Response to joint attention in toddlers at risk for autism spectrum disorder: A prospective study. J Autism Dev Disord. 2007;37(1):37–48. doi: 10.1007/s10803-006-0335-3. [DOI] [PubMed] [Google Scholar]
- 11.Cornew L, Dobkins KR, Akshoomoff N, McCleery JP, Carver LJ. Atypical social referencing in infant siblings of children with autism spectrum disorders. J Autism and Dev Disord. 2012;42(12):2611–2621. doi: 10.1007/s10803-012-1518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifford SM, Hudry K, Elsabbagh M, Charman T, Johnson MH. Temperament in the first 2 years of life in infants at high-risk for autism spectrum disorders. J Autism Dev Disord. 2013;43(3):673–686. doi: 10.1007/s10803-012-1612-y. [DOI] [PubMed] [Google Scholar]
- 13.Garon N, Bryson SE, Zwaigenbaum L, et al. Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. J Abnorm Child Psychol. 2009;37(1):59–78. doi: 10.1007/s10802-008-9258-0. [DOI] [PubMed] [Google Scholar]
- 14.Toth K, Dawson G, Meltzoff AN, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. J Autism Dev Disord. 2007;37(1):145–157. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgiades S, Szatmari P, Zwaigenbaum L, et al. A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder. JAMA Psychiatry. 2013;70(1):42–48. doi: 10.1001/2013.jamapsychiatry.1. [DOI] [PubMed] [Google Scholar]
- 16.Gamliel I, Yirmiya N, Jaffe DH, Manor O, Sigman M. Developmental trajectories in siblings of children with autism: Cognition and language from 4 months to 7 years. J Autism Dev Disord. 2009;39(8):1131–1144. doi: 10.1007/s10803-009-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messinger D, Young GS, Ozonoff S, et al. Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. J Am Acad Child and Adolesc Psychiatry. 2013;52(3):300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule— Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 19.Rutter M, Bailey A, Lord C. Social communication questionnaire: manual. Western Psychological Services. 2003 [Google Scholar]
- 20.Mullen EM. Mullen scales of early learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 21.Ozonoff S, Iosif A, Baguio F, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Ch Adol Psychiat. 2010;49:258–268. [PMC free article] [PubMed] [Google Scholar]
- 22.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 23.SAS Institute, Inc. SAS/STAT Version 9.3. Cary, NC, USA: 2002–2010. [Google Scholar]
- 24.Szatmari P, Jones MB, Tuff L, et al. Lack of cognitive impairment in first-degree relatives of children with pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 1993;32(6):1264–1273. doi: 10.1097/00004583-199311000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Gamliel I, Yirmiya N, Sigman M. The development of young siblings of children with autism from 4 to 54 months. J Autism Dev Disord. 2007;37(1):171–183. doi: 10.1007/s10803-006-0341-5. [DOI] [PubMed] [Google Scholar]
- 26.Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. J Child Psychol Psychiatry. 2006;47(5):511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 27.Yirmiya N, Gamliel I, Shaked M, Sigman M. Cognitive and verbal abilities of 24-to 36- month-old siblings of children with autism. J Autism Dev Disord. 2007;37(2):218–229. doi: 10.1007/s10803-006-0163-5. [DOI] [PubMed] [Google Scholar]
- 28.Bolton PF, Golding J, Emond A, Steer CD. Autism spectrum disorder and autistic traits in the Avon Longitudinal Study of parents and children: Precursors and early signs. J Am Acad Child Adolesc Psychiatry. 2012;51(3):249–260. doi: 10.1016/j.jaac.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Wallace KS, Rogers SJ. Intervening in infancy: Implications for autism spectrum disorders. J Child Psychol Psychiatry. 2010;51(12):1300–1320. doi: 10.1111/j.1469-7610.2010.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers SJ, Vismara L. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37(1):8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webster-Stratton CH, Reid MJ, Beauchaine T. Combining parent and child training for young children with ADHD. J Clin Child Adolesc Psychol. 2011;40(2):191–203. doi: 10.1080/15374416.2011.546044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.