Abstract
The involvement of lymphocytes in skin wound healing has not been studied extensively. The current study shows that CD4 and CD8 cells are present in significant numbers in skin wounds with peak levels at days 5–10 and 7–10 respectively. Both subsets expressed inflammatory and/or regulatory cytokines. To examine the function of CD4 and CD8 lymphocytes in tissue repair, wound healing was examined in mice deficient for either CD4 or CD8 cells. Wounds in CD4 deficient mice exhibited an initial delayed infiltration of CD8 cells followed by a relative increase in CD8 cells at day 10 and thereafter. Wounds in CD4 deficient mice also displayed up-regulated expression of IL1β, IL-6, IL-17, IFN-γ, CXCL-1 and down-regulated expression of IL-4 as compared to wild type mice. In contrast, wounds in CD8 deficient mice showed significantly decreased infiltration of CD4+ cells, neutrophils, and macrophages along with down-regulated expression of IL1β, IL-6, TNF-α, CXCL-1, CCL-2 and up-regulated expression of IL-4 as compared to wild type mice. Despite these significant changes in cytokine expression and inflammatory cell infiltrate, the rate of wound closure, wound breaking strength, collagen content, and angiogenesis in either CD4 or CD8 deficiency showed no significant difference from that of wild type mice. The results suggest that, despite being present and involved in wound inflammation, neither CD4+ nor CD8+ cells play critical roles in the healing process of skin wounds. Further studies are needed to investigate whether these cells might play critical roles in wounds that experience stress such as ischemia or infection.
Keywords: CD4, CD8, skin, wound
Introduction
Skin wound healing is a dynamic multifaceted process that begins with a homeostasis/inflammatory phase involving platelet aggregation and recruitment of inflammatory cells. This phase is followed by a proliferation phase that involves migration and proliferation of keratinocytes, fibroblasts, and endothelial cells which results in re-epithelialization and granulation tissue formation. A final remodeling phase involves the regression of many of the newly formed capillaries and collagen reorganization. The healing process is orchestrated by well-regulated interactions of cells, including keratinocytes, fibroblasts, endothelial cells, and inflammatory cells, with extracellular matrix molecules and growth factors/cytokines (1–3).
Lymphocytes, especially CD4 helper and CD8 cytotoxic T lymphocytes, are critical immune cells in both innate and adaptive immunity. The first investigations of the role of lymphocytes in wound healing began after early in vitro studies showed that lymphocytes secreted soluble factors which regulate fibroblast migration, replication, and collagen synthesis (4–7). A histological study showed that both CD4 and CD8 subsets were present in mouse incisional skin wounds and implanted sponges at days 5 to 10 post-wounding (8). Later studies examined the role of lymphocyte subsets in wound healing by using cytotoxic antibodies to deplete Thy1.2 cells (assumed to deplete total T cells) (9, 10), or to specifically deplete CD4 and/or CD8 cells (10–12). Wound healing has also been examined in T cell deficient nude mice (13, 14) as well as in mice that have increased numbers of T cells in wounds (15). A recent study showed that CCL17 transgenic mice exhibit accelerated skin wound healing compared to wild type mice; the wounds of the CCL17 transgenic strain also contained significantly more fibroblasts and nerve growth factor positive lymphocytes than those of wild type (15). These studies have provided somewhat conflicting results, although in general they suggested that the T lymphocyte or its subsets might be involved in regulation of collagen deposition or wound breaking strength. Further studies have shown that, similar to mice, human skin wounds also contain CD4 and CD8 lymphocytes (16, 17). However, there have been no experiments studying how the absence of T lymphocytic subsets might influence wound closure, soluble mediator expression, inflammatory cell infiltration, and angiogenesis in the wound healing process. The current study demonstrates that the specific deficiency of CD4 or CD8 lymphocytes significantly changes the infiltration of inflammatory cells and the profiles of cytokine expression in skin wounds. Interestingly, these changes did not impair wound closure, nor result in alterations of wound breaking strength, collagen deposition, or vascularity.
Materials and Methods
Animals and wound model
In our preliminary study, 8 week old female BALB/c mice (Harlan Inc., Indianapolis, IN) were used. For the rest of experiments, 8 week old female CD4 deficient mice (B6.129S2-CD4tm1Mak/J), CD8 deficient mice (B6.129S2-CD8atm1Mak/J) (Jackson lab, Bar Harbor, MA) were used. Since both CD4 and CD8 deficient mice were on the C57BL/6 background, C57BL/6 mice (Jackson lab) were used as wild type control. All CD4 deficient, CD8 deficient, and C57BL/6 mice were housed in the same environment in either Jackson lab or our animal facility. Using a standard biopsy punch, six 3mm or two 8mm full thickness excisional wounds were made on shaved dorsal skin under ketamine (100mf/Kg) and xylazine (5mg/Kg) anesthesia. At various time points after wounding, the wounds were photographed and wound sizes were determined by software AxioVision (ZEISS, Oberkochen, Germany). Three mm wound tissues were removed and stored in either RNAlater (Sigma, St. Louis, MO), or OCT compound for future processing, kept frozen until hydroxyproline analysis. Eight mm wounds were used for examination of breaking strength. All animal procedures were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
Indirect immunofluorescence
Wounds were harvested, embedded in OCT compound, and stored at −80°C until sectioned. Eight µm frozen sections were prepared and fixed in cold acetone for 10 min. Sections were blocked with 10% goat serum and then incubated with rat anti-mouse CD4 (0.3125µg/ml), CD8 (0.3125µg/ml), CD31 (0.3125µg/ml), Gr-1 (for neutrophil staining, 0.5µg/ml) (BD Bioscience, San Jose, CA), rat anti-mouse CD68 (for macrophage staining, 0.5µg/ml, AbD Serotec, Oxford, UK), rabbit anti-mouse ki67 (to identify proliferating cells, 2µg/ml, Abcam, Cambridge, MA, USA) or rat/rabbit IgG as negative control for 45 min followed by Alexa fluor 488 goat anti-rat IgG or goat anti-rat/rabbit Alexa fluor 594 IgG (Invitrogen, Carlsbad, CA). All procedures were performed at room temperature. Results were observed using a fluorescence microscope. Positively stained cells in the wounds and wound margins were counted and the average number per 20x field was calculated. To quantify the density of CD31 stained blood vessels, the total area of the wound bed and CD31-positive area were measured using ImageJ (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, http://rsb.info.nih.gov/ij/). The vessel density was then expressed as the percent CD31 positive area in the wound bed.
Flow cytometry and CD4+ and CD8+ cell sorting
Seven days after wounding, 18 skin wounds from three mice were harvested using a 5mm punch-biopsy instrument. A single-cell suspension was obtained using a tissue dispersion method (18). For control cells, spleens from unwounded mice were removed, minced, and passed through a 70-mm cell strainer to make single cell suspensions. Red blood cells were lysed using a RBC lysis buffer (eBioscience, San Diego, CA). Cell suspensions from both wound and spleen were then incubated with FITC conjugated rat anti-mouse CD4, PE-conjugated anti-mouse CD8, and APC-conjugated anti-mouse CD11a (eBioscience) for 30min on ice. All antibodies were used at the concentration of 0.5µg/ml. After washing, the CD4&CD11a or CD8&CD11a double stained cells were analyzed and sorted using a Moflo high speed cell sorter (Dako cytomation, Glostrup, Denmark). Dead cells were gated out by 7-AAD staining. 3,000–10,000 sorted CD8+ and CD4+ cells were obtained from the wounds. Cells-Ct kit (Invitrogen) was used to prepare cDNA for cytokine real time PCR.
Real time PCR
Total RNA was extracted from skin wounds of CD4 deficient, CD8 deficient, and wild type mice using TriZol (Invitrogen). RNA samples were treated with DNAse I, and subjected to reverse transcription using a Retro-script kit (Invitrogen). mRNA expression of IL-1β, IL-4, IL-6, IL-10, IL-13, IL-17, IFN-γ, TNF-α, TGF-β, CCL-2 (MCP-1) and CXCL-1 (MIP-2α or KC) was examined using a real time PCR system (StepOne Plus, Applied Biosystems, Carlsbad, CA) that employs SYBR Green PCR mix and gene specific primers. All primer sequences except CXCL-1 were previously described (19). The forward primer for CXCL-1 was: 5’-GCTTGAAGGTGTTGCCCTCAG-3’, and the reverse primer was 5’-AGAAGCCAGCGTTCACCAGAC-3’. mRNA expression in normal wild type mice skin was used as baseline. GAPDH was used for normalization. cDNAs used for IL-10, IL-17, IFN-γ, and TGF-β1 cytokine mRNA expression in CD4 or CD8 cells isolated from day 7 wounds were obtained by using a Cells-Ct kit (Invitrogen).
Breaking strength
Breaking strength of 8 mm wounds from CD4 deficient, CD8 deficient and wild type mice was examined at day 15 post-wounding using a motorized tensiometer (Mark-10, Copiague, NY) as described previously (20). Two wounds per mouse were subjected to analysis and the average was recorded as the wound breaking strength for that individual animal. Normal skin from 5 unwounded mice was also tested.
Collagen content quantification
Wounds from day 14 after injury were removed, weighed, and stored at −80°C until analysis. Each wound was hydrolyzed in 0.25ml of 6N HCl overnight at 95°C for 20 hours. The hydroxyproline content was then analyzed using a hydroxyproline assay kit (QuickZyme Biosciences, Leiden, Netherlands). Results were expressed as nM/mg tissue.
Statistical analyses
Results were expressed as means ± standard deviations. A two-way ANOVA followed by a Tukey’s multiple comparisons test or t test was used for statistical analysis. Analyses were completed using GraphPad Prism (GraphPad Software, San Diego, CA). p values less than 0.05 were considered statistically significant.
Results
CD4+ and CD8+ lymphocytes migrate into skin wounds and produce cytokines
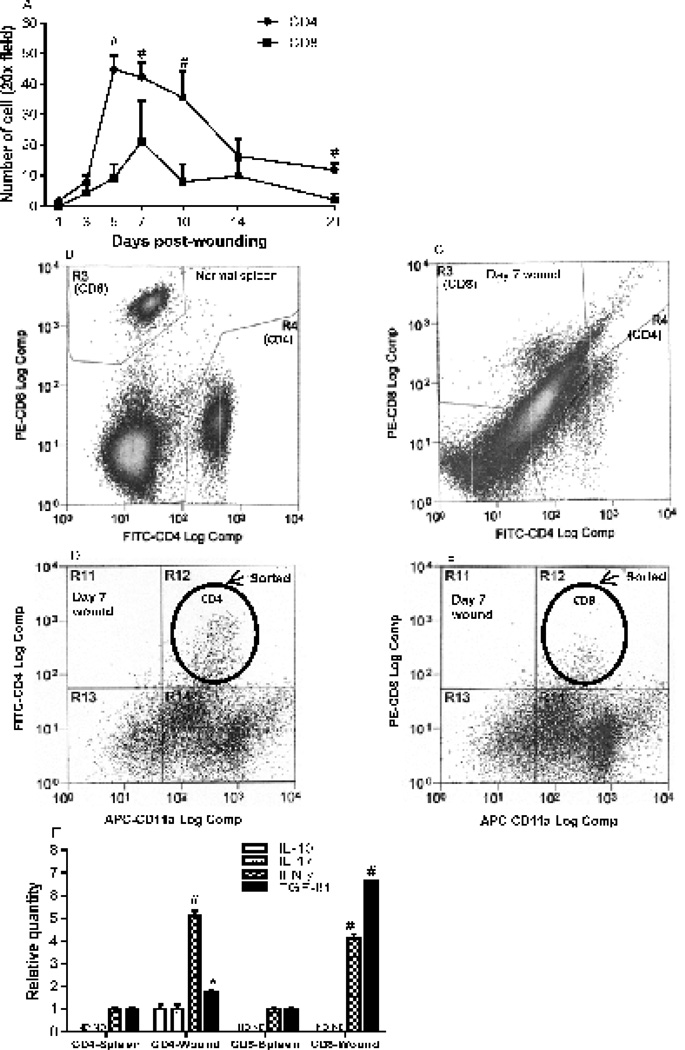
Although the presence of CD4 and CD8 lymphocytes in wounds has been previously reported (8, 16), the dynamic infiltration of these immune cells over time has not been demonstrated. CD4+ cells started to appear at day 1 after wounding, and the number of these cells then gradually increased and peaked at day 5 (44.7±10.5/20x field). After day 10, the numbers dramatically decreased, although CD4+ cells were still present in significant numbers (11.8x±4.7) at day 21 (Fig. 1A). CD8+ cells did not appear in the wound bed until day 3. CD8+ cell numbers peaked at day 7 (21.3±13.5), and decreased markedly by day 21 (2.0±2.0) (Fig. 1A). The numbers of CD4+ cells at days 5, 7, 10 and 21 were significantly higher than those of CD8 cells (p<0.01, Fig. 1A). Since CD4+ and CD8+ cells are both present in significant numbers in the wounds, we used cell sorting to isolate these subsets of T cells from day 7 skin wounds (Fig. 1C, D and E) and spleens of unwounded mice (Fig. 1B). mRNA expression of regulatory or inflammatory cytokines (IL-10, IL-17, IFN-γ, and TGF-β1) by each T cell subset was examined using real time PCR. The results showed that naïve CD4+ cells from spleen expressed IFN-γ, and TGF-β1, but not IL-10 and IL-17. However, CD4+cells from day 7 wounds expressed significantly higher levels of these cytokine than CD4+ cells from spleen (Fig. 1F). Similar results were observed in CD8+ cells except CD8+ cells did not express IL-10 and IL-17 from either spleen or wounds (Fig. 1F). This study demonstrates that CD4 and CD8 lymphocytes are significantly present during the healing process in mouse skin wounds and express regulatory or inflammatory cytokines within the wound.
Figure 1.
Dynamic infiltration of CD4 and CD8 lymphocytes in skin wounds and their expression of cytokines. A) Numbers of positively stained CD4 and CD8 cells in the wounds and wound margins. N=5 in each group. # p<0.01 compared to CD8+ cells, two-way ANOVA followed by a Tukey’s multiple comparisons test was used. B&C) Flow cytometry analyses of CD4+ (gate R4) and CD8+ (gate R3) cells in spleen and day 7 wounds, respectively. Dead cells were gated out using 7-AAD staining. D&E) Representative flow cytometry demonstrating CD4+/CD11a+ or CD8+/CD11a+ (gate 12) populations in wounds. F) Relative mRNA expression of IL-10, IL-17, IFN-γ and TGF-β1 in CD4 and CD8 cells isolated from day 7 wounds. N=3 in each group. * p<0.05, # p<0.01 compared to spleen samples by t test. ND: not detectable. Error bars = standard deviation.
The absence of CD4 and CD8 lymphocytes does not impair skin wound closure
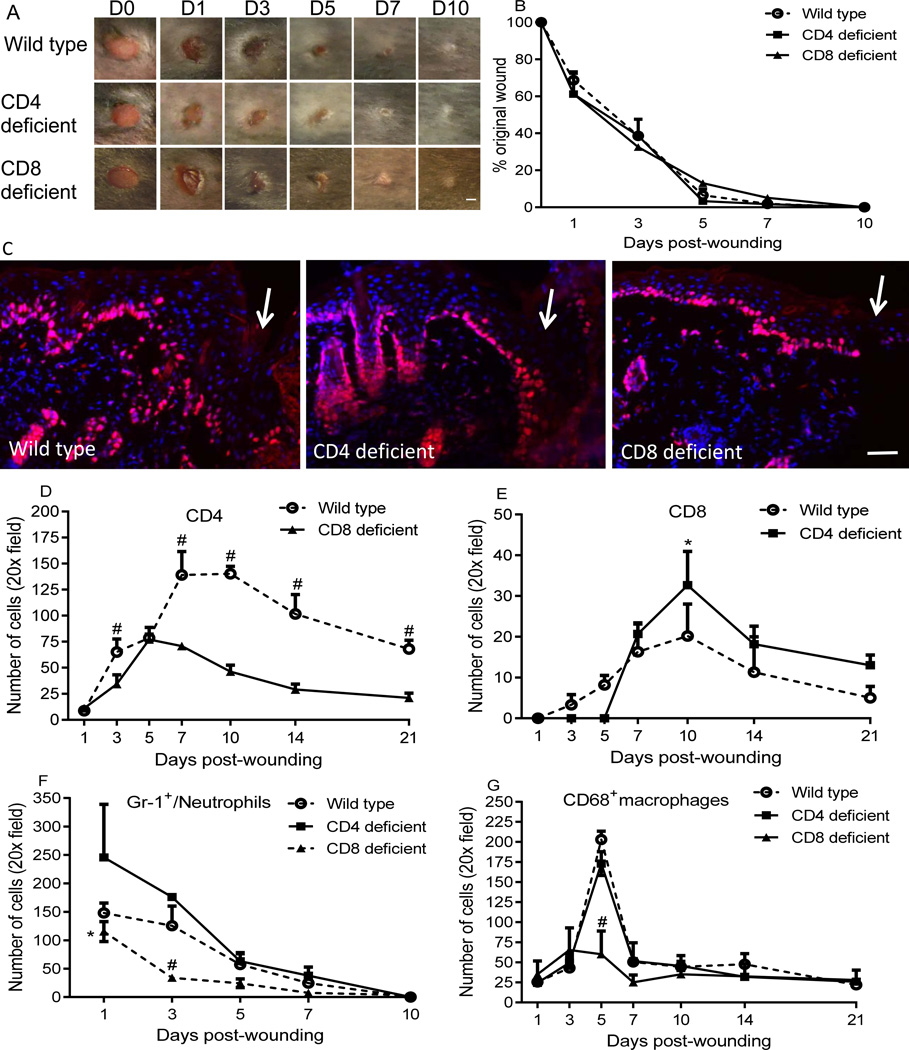
To examine whether the absence of CD4+ or CD8+ cells would affect wound closure, wound healing kinetics were examined in circular 3mm full thickness wounds in CD4 and CD8 deficient mice. No significant changes in wound closure were observed in either CD4 or CD8 deficient mice as compared to wild type mice (Fig. 2A&B). Similar results were obtained in a larger 8mm wound model (data not shown). Therefore, the absence of either CD4+ or CD8+ lymphocytes cells does not appear to significantly impair skin wound closure. Furthermore, no difference in the number of ki67+ proliferating keratinocytes at the wound margins was apparent among the groups at days 1, 3, or 5 (Fig. 2C, day 3 shown). These results further support the idea that CD4 or CD8 deficiency does not impede wound closure.
Figure 2.
CD4 or CD8 lymphocyte deficiency does not impair wound closure, but results in altered CD8+, CD4+, neutrophils and/or macrophage infiltration in the wounds. A) Representative photos of wounds photographed at various time points after injury. B) Percent of the original wound size. N=5 in each group; scale bar=1mm. C) Representative photomicrographs demonstrating immunohistochemical detection of the proliferation marker ki67 at the wound margin at day 3 (days 1 and 5 not shown). Arrows indicate wound edges. Red: ki67, blue: DAPI stained nuclei. Scale bar=100µm. D&E) Time course of numbers of CD4+ and CD8+ cells in the wounds of wild type/CD8 deficient mice, and wild type /CD4 deficient mice respectively. # p<0.001 compared to CD8 deficient mice, * p<0.05 compared to wild type. F&G) Time course of numbers of neutrophils and macrophages. * p<0.05 and # p<0.01 compared to CD4 deficient and wild type mice. Two-way ANOVA followed by a Tukey’s multiple comparisons test was used. Error bars = standard deviation.
The absence of CD4 and CD8 lymphocytes alters lymphocytic infiltration into wounds
In wild type mice, CD4+ and CD8+ cells account for about 60% and 20% of the total lymphocytes in lymph nodes, respectively. However, CD8+ cells in CD4 deficient mice account for about 77% of the total lymphocytes (21), and CD4+ cells in CD8 deficient mice account for approximately 73 % of the total lymphocyte (22). To determine if CD4 or CD8 deficiency would change infiltration of CD8+ and CD4+ cells into wounds, the numbers of CD4+ and CD8+ cells in the wounds of wild type, CD4 deficient, and CD8 deficient mice were counted. Despite the predominance of CD4+ cells in CD8 deficient mice (22), there were significantly fewer CD4+ cells in the wounds of CD8 deficient mice than in wounds of wild type mice at days 3, 7, 10, 14, and 21 after injury (p<0.001, Fig. 2D&Fig.s1A). In wild type mice, CD8 cells were present from day 3. In contrast, in CD4 deficient mice, CD8+ cells did not appear until 7 days after wounding (Fig. 2E). After day 7, more CD8+ cells were observed in CD4 deficient mice than in wild type mice, especially at day 10 (p<0.05, Fig. 2E&Fig.s1B). When BALB/c and C57BL/6 mice were compared, the general pattern of CD4+ and CD8+ cell infiltration was similar (Fig 1A & Fig. 2D&E). However, higher absolute levels of CD4+ and CD8+ cells were observed in the wounds of C57BL/6 mice when compared to BALB/c mice (Fig 1A & Fig. 2D&E). Together, the data show that although CD4 or CD8 deficiency does not influence wound closure, each deficiency significantly affects the infiltration of CD8 or CD4 cells into wounds.
The absence of CD4 and CD8 lymphocytes influences neutrophil and/or macrophage infiltration in the wounds
At day 1 after injury, the number of neutrophils in wounds of CD4 deficient mice was higher than that of wild type mice, although this difference was not statistically significant (Fig. 2F&Fig.s1C). In contrast, wounds of CD8 deficient mice exhibited significantly lower numbers of neutrophils than those of wild type and CD4 deficient mice at days 1 and 3 after wounding (p<0.05 and 0.01 respectively, Fig. 2F). Similar to neutrophils, the number of CD68 macrophages in wounds CD8 deficient mice was also significantly less than wild type and CD4 deficient mice (p<0.001, Fig. 2G&Fig.s1D). To exclude the possibility that pre-existing systemic cellular deficiencies might be responsible for the decreased neutrophils and macrophages in the wounds of CD8 deficient mice, a peripheral differential white blood cell (WBC) count was performed. CD8 deficient and wild type mice were found to have similar levels of peripheral neutrophils and monocytes (8.5% vs. 8.4% neutrophils/total WBC, 0.9% vs. 1.1% monocytes/total WBC, CD8 deficient vs. wild type, respectively, p>0.05). Together, this data suggests that the loss of CD8+ cells does not alter peripheral levels of neutrophils or monocytes, yet results in decreased infiltration of neutrophils and macrophages into skin wounds.
The absence of CD4 and CD8 lymphocytes changes cytokine expression
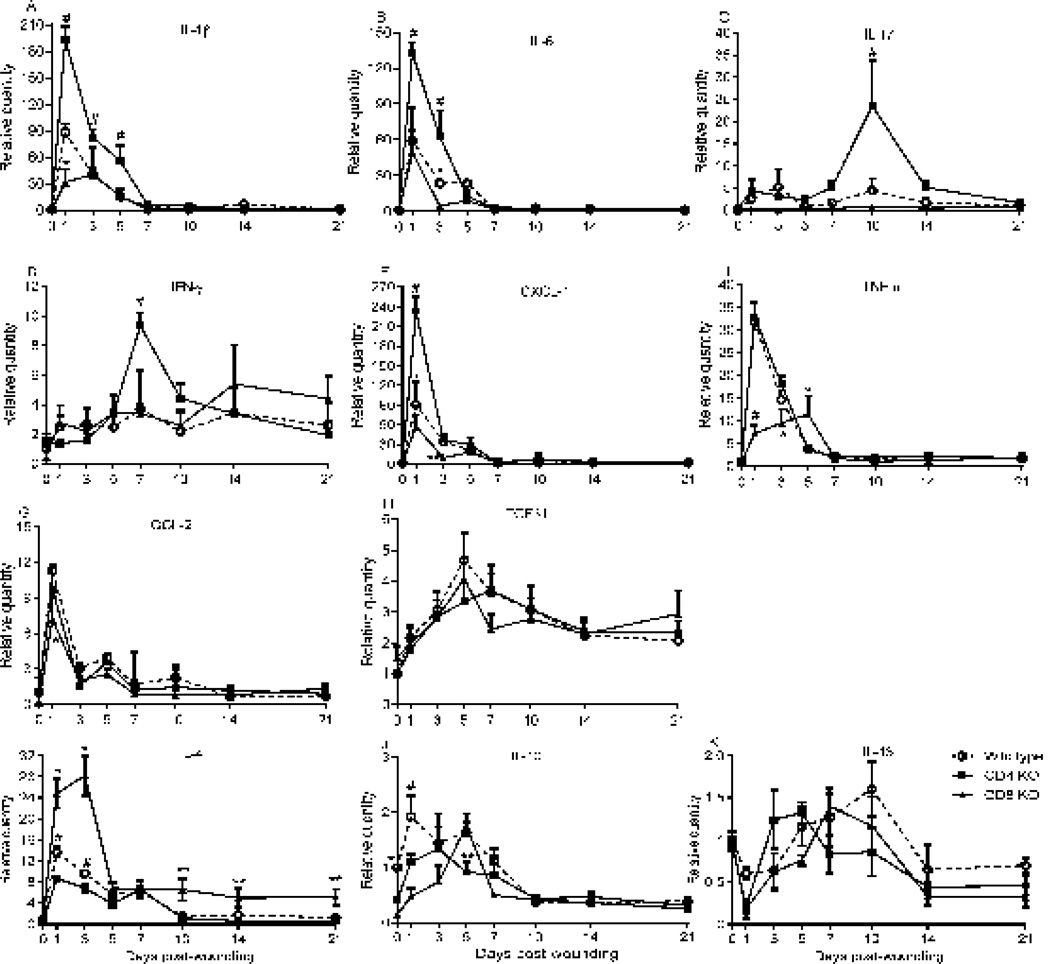
The effect of the absence of CD4+ or CD8+ cells on cytokine mRNA expression in skin wounds was examined using real time PCR. Several significant differences in cytokine expression were observed among the three strains. Wounds from CD4 deficient mice showed increased expression of several different cytokines. When compared to wounds from wild type and CD8 deficient mice, wounds from CD4 deficient mice expressed significantly higher levels of IL-1β and IL-6 at multiple time points (p<0.001, Fig. 3A, B). At unique time points, wounds from CD4 deficient mice also showed higher expression of IL-17, IFN-γ, and CXCL-1 than wounds from wild type and CD8 deficient mice (p<0.001, Fig. 3C, D, and E). Wounds from both wild type and CD4 deficient mice showed higher levels of TNF-α than wounds of CD8 deficient at days 1 and 3 (p<0.001 and 0.05 respectively, Fig. 3F). However, at day 5, TNF-α expression was highest in the wounds of CD8 deficient mice (p<0.05, Fig. 3F). Wounds from wild type and CD4 deficient mice also expressed more CCL-2 than those of CD8 deficient mice (p<0.05, Fig. 3G). Levels of TGF-β1 expression were similar in all three strains (Fig. 3H). Finally, three Th2 cytokines including IL-4, IL-10, and IL-13) were examined. Wounds from CD8 deficient and wild type mice expressed significantly higher levels of IL-4 than CD4 deficient mice at days 1 and 3 (p<0.001 and 0.05 respectively, Fig. 3I). IL-4 levels in wounds of CD8 deficient mice remained higher than CD4 deficient and wild type mice from days 10 to 21 (p<0.05, Fig. 3I). Wounds from each strain exhibited different and variable patterns of IL-10 expression (Fig 3J), and baseline levels of IL-10 were significantly lower in CD4 deficient and CD8 deficient mice than in wild type mice. IL-10 expression in CD4 deficient mice was also lower than wild type at day 1 (p<0.05, Fig. 3J). IL-13 expression patterns were also examined, and no significant changes in IL-13 levels were observed among the strains at any time point (Fig. 3K).
Figure 3.
CD4 or CD8 lymphocyte deficiency changes the profiles of cytokine expression in the wounds. mRNA expression of cytokines was determined by real-time PCR. A) IL-1β, * p<0.05 compared to wild type; # p<0.001 compared to wild type and CD8 deficient mice. B) IL-6, #p<0.001 compared to wild type and CD8 deficient, *p<0.05 compared to CD8 deficient. C) IL-17 and D) IFN-γ, #p<0.01 compared to wild type and CD8 deficient. E) CXCL-1, # p<0.001 compared to wild type and CD8 deficient, * p<0.05 compared to CD8 deficient, **p<0.05 compared to wild type and CD4 deficient. F) TNF-α, # p<0.001 compared to wild type and CD4 deficient, * p<0.05 compared to wild type and CD4 deficient. G) CCL-2, * p<0.05 compared to wild type and CD4 deficient. H) TGF-β1. I) IL-4, *p<0.01 and **p<0.05 compared to wild type and CD4 deficient mice. #p<0.05 compared to CD4 deficient mice. J) IL-10, *p<0.05, #p<0.001 compared to CD4 or CD8 deficient; **p<0.05 compared to CD8 deficient. K) IL-13. N=3 in each group and each time point; a two-way ANOVA followed by a Tukey’s multiple comparisons test was used. Error bars = standard deviation.
The absence of CD4 and CD8 lymphocytes does not influence wound breaking strength, collagen content or angiogenesis
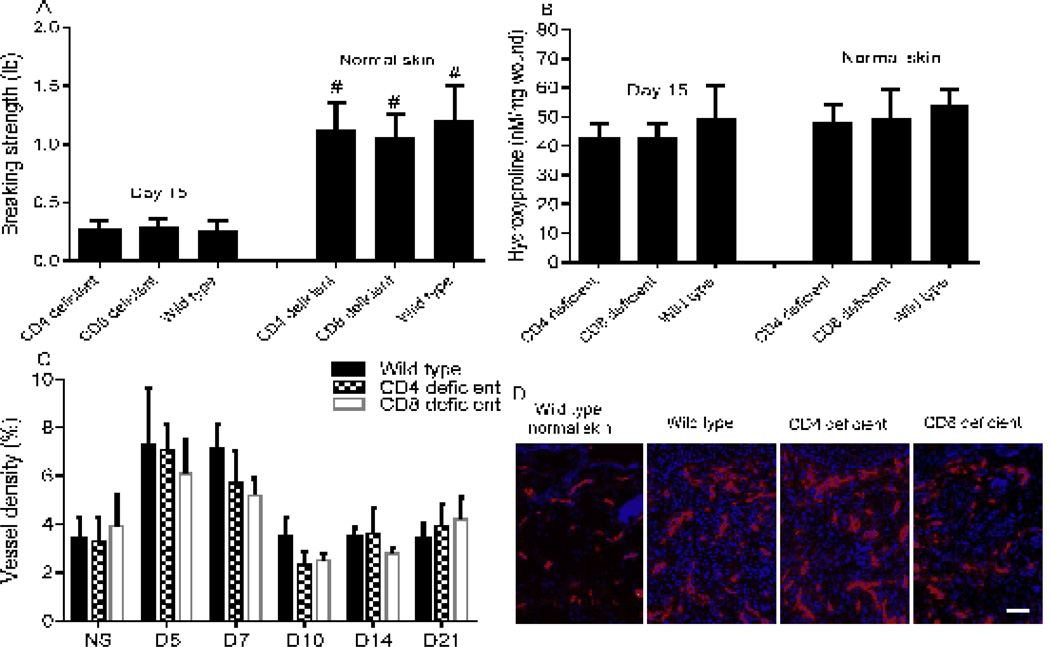
The breaking strength of both healed wounds and normal skin from wild type, CD4 and CD8 deficient mice was examined using a tensiometer. The breaking strength of normal skin was similar in all three groups of mice (Fig. 4A). The breaking strength of day 15 wounds was also similar in all three groups, and in all cases was approximately 20% of the breaking strength of normal skin (p<0.01, Fig. 4A). To assess collagen content, wound hydroxyproline levels were examined. No significant differences in hydroxyproline content were observed among the three groups nor between day 15 wounds and normal skin (Fig 4B). The influence of the absence of CD4+ or CD8+ cells on wound vascularity was also examined. In all groups, the highest vessel density (6–7%) was seen at day 5, with slightly decreased vascularity at day 7 (Fig. 4C&D). From day 10, the regression of capillaries became evident in all groups as vascularity returned to the levels of normal skin. Together, the results demonstrate that the absence of CD4+ and CD8+ cells does not alter wound breaking strength, collagen content, or angiogenesis.
Figure 4.
CD4 or CD8 lymphocyte deficiency does not change wound breaking strength, collagen content or angiogenesis. A) Breaking strength of normal skin and 8 mm wounds at day 15. N=5 in each group; # p<0.01 compared to their day 15 wounds, t test. B) Hydroxyproline concentrations in normal skin and 3 mm wounds at day 15. C) Vessel density expressed as the percent CD31 positive area in 3 mm wound beds. D) Representative photos of day 5 wound sections stained with anti-CD31 antibody. Red: CD31+ endothelial cells, blue: DAPI stained nuclei. Scale Bar: 100µm. Error bars = standard deviation.
Discussion
Consistent with previous reports (8, 17), the current study shows that CD4 and CD8 lymphocytes progressively infiltrate into skin wounds. The majority of CD4 and CD8 lymphocytes migrate into wounds only after neutrophils and macrophages, and are present at time points when wounds are almost or completely re-epithelialized. The time course of lymphocytic infiltration might suggest that lymphocytes do not play an important role in the process of wound healing. However, the total depletion of T cells using Thy1.2 antibodies before injury has been shown to cause a significant impairment of wound breaking strength and collagen deposition (9, 10) as well as significantly delayed wound closure (23).
While the global depletion of T cells using anti-Thy1.2 has a negative effect, studies using specific depletion of CD4+ and CD8+ cells have yielded different results. Surprisingly, the combined depletion of CD4+ and CD8+ cells (via anti-CD4 and anti-CD8 antibodies) has been shown to result in improved wound-breaking strength and collagen levels (10). These results suggest that a small population of cells that are positive for Thy 1.2 but negative for CD4 and CD8 antigens that are important to wound healing. One candidate population is the unique population of T lymphocytes termed dendritic epidermal T cells (DETC, γδ-T cells), a cell type that is strictly limited in their distribution to murine epidermis. Wound repair has been shown to be significantly impaired in DETC deficient mice (24), probably because DETC are an important source of key growth factors such as fibroblast growth factor-7, -10 and insulin like growth factor-1(24, 25). Since DETC express Thy1, but not CD4 and CD8 (26, 27), the impaired wound healing effects observed in Thy1.2 depleted mice may result primarily from the depletion of DETC rather than CD4 or CD8 lymphocytes. Another consideration in the impaired wound healing of Thy1.2 depleted mice is the effect on the populations of other Thy1-expressing cells including, neurons, endothelial cells, fibroblasts, keratinocytes, and stem cells (28, 29). In support of this idea, a recent study has demonstrated that Thy1 siRNA treatment inhibits fibroblast migration and delays wound closure in a mouse model (30). Thus the use of Thy1 as an exclusive maker for T lymphocytes in wounds needs further consideration.
The current study was undertaken to resolve the variety of conflicting results that have been produced in studies that have examined wound healing following specific CD4+ cell or CD8+ cell depletion. Studies of CD4 depleted mice or rats have demonstrated both no difference in wound healing and impaired healing in the experimental animals (11, 12). Studies of CD8-specific depletion have generally shown enhanced wound healing in experimental groups, suggesting that CD8 cells have an inhibitory effect on wound healing in terms of collagen deposition and wound breaking strength.
In this study, we utilized CD4 and CD8 lymphocyte deficient mice to examine the role of each cell type on wound repair; these strains represent a resource that was not available when the original antibody depletion studies were performed. Our results show that the absence of CD4+ cells causes delayed infiltration of CD8 cells in the wounds and up-regulated IL-1β, IL-6, IL-17, CXCL-1, and IFN-γ expression and down-regulated IL-4 and IL-10. The neutrophil chemoattractant, CXCL-1 was upregulated in wounds of CD4 deficient mice, a finding that may explain the observed elevated infiltration of neutrophils. Interestingly, our studies suggest that CD4 and CD8 lymphocytes play somewhat opposite roles in regulating the inflammatory response that occurs following injury including the expression of inflammatory cytokines and infiltration of inflammatory cells. CD8 cell deficiency resulted in a significant decrease in neutrophil infiltration into wounds. However, this decrease failed to affect wound healing. This result is not surprising, since prior studies suggest that neutrophils are not essential to healing (31). In contrast, several studies demonstrate that macrophages are critical inflammatory cells for prompt and appropriate wound healing (32, 33). Despite the presumed role of macrophages, our data shows that a significant decrease of macrophages in CD8 deficient mice did not lead to retarded repair. This result may indicate that the decrease in macrophages in the wounds of CD8 deficient mice did not reach a degree that could change the healing outcome. Taken together, our study demonstrates that normal skin wounds exhibit a progressive infiltration of CD4+ and CD8+ lymphocytes, and that both subsets express regulatory and/or inflammatory cytokines. Our studies also strongly suggest that while these subsets of T cells influence wound inflammation, this influence is not critical to healing outcomes. The molecular and cellular changes observed in the absence of CD4+ or CD8+ cells, although significant, do not appear to alter the course of healing. This finding may speak to the redundancy and variety of compensatory mechanisms that are available to support the wound healing process. The possibility remains that either CD4 or CD8 lymphocytes may become critically important under situations in which wound healing is stressed such in ischemic or infected wounds.
One important consideration to the interpretation of our results is that CD4 lymphocytes are not a homogenous population. Several subsets have been identified such as Th1, Th2, Th9, Th17, and regulatory T cells. These subsets have different patterns of cytokine production, antigen presentation cells, transcription factors, and phenotypic and functional markers and play distinct roles in immune response (34–36). The roles of these individual subsets were not examined in our study and may differentially influence skin wound healing, particularly in pathological wound repair. Another potential caveat to our results is our use of a mouse model. The murine immune system does reflect human biology in many respects and mice have been extensively used for immunologic studies. Moreover, a recent study suggests that there are only about 300 genes that are truly unique to one species or the other (37). However, there are many known differences in immune systems of mice and humans (38, 39). Importantly, recent genomic studies suggest that inflammatory responses in mouse models generally correlate quite poorly with human responses (40). While few would argue that murine studies continue to provide valuable and relevant information, caution remains warranted in directly extrapolating experimental findings in mice to human conditions.
Supplementary Material
Acknowledgments
LC, NDM, and YZ performed the experiment. LC and LAD designed the study. LC analyzed the data. LC and LAD wrote the paper. We are grateful to Dr. Shujuan Guo for her technical assistance and to Dr. Wendy Cerny and Ariel Johnson for critical reading of this manuscript.
Founding sources: This publication was supported by NIH Grants R01GM50875, the Schour Scholars Fund (NDM), and the College of Dentistry, University of Illinois at Chicago. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, Schour Scholars Fund, or University of Illinois at Chicago.
Footnotes
Conflict of interests
The authors have declared no conflicting interests.
References
- 1.Guo S, Dipietro LA. Factors affecting wound healing. Journal of dental research. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. American journal of surgery. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 3.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. The Journal of investigative dermatology. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 4.Postlethwaite AE, Snyderman R, Kang AH. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. The Journal of experimental medicine. 1976;144:1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RL, Ziff M. Lymphokine stimulation of collagen accumulation. The Journal of clinical investigation. 1976;58:240–252. doi: 10.1172/JCI108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahl SM, Wahl LM, McCarthy JB. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. Journal of immunology (Baltimore, Md: 1950) 1978;121:942–946. [PubMed] [Google Scholar]
- 7.Postlethwaite AE, Smith GN, Mainardi CL, Seyer JM, Kang AH. Lymphocyte modulation of fibroblast function in vitro: stimulation and inhibition of collagen production by different effector molecules. Journal of immunology (Baltimore, Md: 1950) 1984;132:2470–2477. [PubMed] [Google Scholar]
- 8.Fishel RS, Barbul A, Beschorner WE, Wasserkrug HL, Efron G. Lymphocyte participation in wound healing. Morphologic assessment using monoclonal antibodies. Ann Surg. 1987;206:25–29. doi: 10.1097/00000658-198707000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson JM, Barbul A, Breslin RJ, Wasserkrug HL, Efron G. Significance of T-lymphocytes in wound healing. Surgery. 1987;102:300–305. [PubMed] [Google Scholar]
- 10.Efron JE, Frankel HL, Lazarou SA, Wasserkrug HL, Barbul A. Wound healing and T-lymphocytes. The Journal of surgical research. 1990;48:460–463. doi: 10.1016/0022-4804(90)90013-r. [DOI] [PubMed] [Google Scholar]
- 11.Barbul A, Breslin RJ, Woodyard JP, Wasserkrug HL, Efron G. The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann Surg. 1989;209:479–483. doi: 10.1097/00000658-198904000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis PA, Corless DJ, Aspinall R, Wastell C. Effect of CD4(+) and CD8(+) cell depletion on wound healing. The British journal of surgery. 2001;88:298–304. doi: 10.1046/j.1365-2168.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 13.Barbul A, Shawe T, Rotter SM, Efron JE, Wasserkrug HL, Badawy SB. Wound healing in nude mice: a study on the regulatory role of lymphocytes in fibroplasia. Surgery. 1989;105:764–769. [PubMed] [Google Scholar]
- 14.Chircop MP, Yu Y, Berney CR, Yang JL, Crowe PJ, Walsh WR. Wound healing and growth factor expression in T lymphocyte deficiency. ANZ J Surg. 2002;72:491–495. doi: 10.1046/j.1445-2197.2002.02424.x. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Saeki H, Tsunemi Y, Shibata S, Tamaki K, Sato S. Thymus and activation-regulated chemokine (TARC)/CC chemokine ligand (CCL) 17 accelerates wound healing by enhancing fibroblast migration. Experimental dermatology. 2011;20:669–674. doi: 10.1111/j.1600-0625.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- 16.Boyce DE, Jones WD, Ruge F, Harding KG, Moore K. The role of lymphocytes in human dermal wound healing. The British journal of dermatology. 2000;143:59–65. doi: 10.1046/j.1365-2133.2000.03591.x. [DOI] [PubMed] [Google Scholar]
- 17.Martin CW, Muir IF. The role of lymphocytes in wound healing. Br J Plast Surg. 1990;43:655–662. doi: 10.1016/0007-1226(90)90185-3. [DOI] [PubMed] [Google Scholar]
- 18.Wilson L, Fathke C, Isik F. Tissue dispersion and flow cytometry for the cellular analysis of wound healing. BioTechniques. 2002;32:548–551. doi: 10.2144/02323st07. [DOI] [PubMed] [Google Scholar]
- 19.Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods (San Diego, Calif) 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- 20.Ranzer MJ, Chen L, DiPietro LA. Fibroblast function and wound breaking strength is impaired by acute ethanol intoxication. Alcoholism, clinical and experimental research. 2011;35:83–90. doi: 10.1111/j.1530-0277.2010.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahemtulla A, Fung-Leung WP, Schilham MW, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 22.Fung-Leung WP, Schilham MW, Rahemtulla A, et al. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 23.Maeda S, Fujimoto M, Matsushita T, Hamaguchi Y, Takehara K, Hasegawa M. Inducible costimulator (ICOS) and ICOS ligand signaling has pivotal roles in skin wound healing via cytokine production. The American journal of pathology. 2011;179:2360–2369. doi: 10.1016/j.ajpath.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jameson J, Ugarte K, Chen N, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 25.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 26.Bergstresser PR, Sullivan S, Streilein JW, Tigelaar RE. Origin and function of Thy-1+ dendritic epidermal cells in mice. The Journal of investigative dermatology. 1985;85:85s–90s. doi: 10.1111/1523-1747.ep12275516. [DOI] [PubMed] [Google Scholar]
- 27.Bergstresser PR, Tigelaar RE, Streilein JW. Thy-1 antigen-bearing dendritic cells in murine epidermis are derived from bone marrow precursors. The Journal of investigative dermatology. 1984;83:83–87. doi: 10.1111/1523-1747.ep12262587. [DOI] [PubMed] [Google Scholar]
- 28.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 29.Craig W, Kay R, Cutler RL, Lansdorp PM. Expression of Thy-1 on human hematopoietic progenitor cells. The Journal of experimental medicine. 1993;177:1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MJ, Shin JO, Jung HS. Thy-1 knockdown retards wound repair in mouse skin. Journal of dermatological science. 2012 doi: 10.1016/j.jdermsci.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thrombosis and haemostasis. 2004;92:275–280. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- 32.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert reviews in molecular medicine. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. The American journal of pathology. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akdis M, Palomares O, van de Veen W, van Splunter M, Akdis CA. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. The Journal of allergy and clinical immunology. 2012;129:1438–1449. doi: 10.1016/j.jaci.2012.05.003. quiz 1450-1431. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan MH. Th9 cells: differentiation and disease. Immunological reviews. 2013;252:104–115. doi: 10.1111/imr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zygmunt B, Veldhoen M. T helper cell differentiation more than just cytokines. Advances in immunology. 2011;109:159–196. doi: 10.1016/B978-0-12-387664-5.00005-4. [DOI] [PubMed] [Google Scholar]
- 37.Waterston RH, Lindblad-Toh K, Birney E, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 38.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. Journal of immunology (Baltimore, Md: 1950) 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 39.Elbe A, Foster CA, Stingl G. T-cell receptor alpha beta and gamma delta T cells in rat and human skin--are they equivalent? Seminars in immunology. 1996;8:341–349. doi: 10.1006/smim.1996.0045. [DOI] [PubMed] [Google Scholar]
- 40.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.