Abstract
Oestrogen rapidly enhances fast excitatory postsynaptic potentials, facilitates long-term potentiation (LTP), and increases spine numbers. Each effect likely contributes to the steroid’s influence on cognition and memory. In this review, we will first describe a model for the substrates of LTP that includes an outline of synaptic events that occur during induction, expression, and consolidation. Briefly, critical signaling pathways involving the small GTPases RhoA and Rac/Cdc42 are activated by theta burst-induced calcium influx and initiate actin filament assembly via phosphorylation (inactivation) of cofilin. Reorganization of the actin cytoskeleton changes spine and synapse morphology, resulting in increased concentrations of AMPA receptors at stimulated contacts. We then use the synaptic model to develop a specific hypothesis about how oestrogen affects both baseline transmission and plasticity. Brief infusions of 17β-estradiol (E2) reversibly stimulate the RhoA, cofilin phosphorylation, and actin polymerization cascade of the LTP machinery; blocking this eliminates the steroid’s effects on transmission. We accordingly propose that E2 induces a weak form of LTP and thereby increases synaptic responses, a hypothesis that also accounts for how it markedly enhances theta burst induced potentiation. While E2’s effects on the cytoskeleton could be due to direct activation of small GTPases by oestrogen receptors on the synaptic membrane, the hormone also activates TrkB receptors for Brain-Derived Neurotrophic Factor (BDNF), a neurotrophin that engages the RhoA-cofilin sequence and promotes LTP. The latter observations raise the possibility that E2 produces its effects on synaptic physiology via transactivation of neighboring receptors that have prominent roles in the management of spine actin, synaptic physiology, and plasticity.
Keywords: estradiol, spines, RhoA, cofilin, pTrkB, LTP
I. Introduction
Steroids are profoundly powerful hormones that regulate a broad stroke of physiological and neurological processes. The gonadal hormone oestrogen, in particular, has received considerable attention in the past few decades for its potential role to improve certain forms of memory loss in aging women [1–4]. A new appreciation for oestrogen’s influence in the brain arises from both its genomic and non-genomic effects. Evidence for oestrogen receptors in the brain, particularly the hippocampus, emerged in the late 1980’s [5, 6]. Shortly thereafter a series of elegant papers by Gould, Woolley, and McEwen showed that over the course of several days oestrogen promoted the formation of new dendritic spines and excitatory synapses in the hippocampus [7–9]. At about the same time, discoveries into the rapid electrophysiological effects of oestrogen on synaptic plasticity in the hippocampus had been reported [10, 11] and are now confirmed by several independent laboratories [12–14]. More recent work has extended these findings by reporting oestrogen’s ability to enhance long-term potentiation (LTP), a well-established model of activity-dependent enhancement of synaptic efficacy thought to underlie learning and memory formation [15–20]. Given recent work showing that oestrogen can be locally produced in the hippocampus [21], and its rapid plasticity effects are likely mediated by oestrogen β-type receptors present in synaptic membranes [22], short-term latency effects of oestrogen on transmission and plasticity could be related to its acute influence on learning [23–26] and therefore underlie variations of memory performance associated with cyclic or age-related changes in oestrogen production.
Surprisingly little is known about the steps linking oestradiol receptors to the complex machinery that regulates synaptic strength and ultimately encoding of memories. However, the literature on peripheral actions provides a hint that the steroid regulates actin dynamics and thereby modifies the submembrane cytoskeleton in non-neuronal tissue [27]. There is potential relevance to its acute actions in adult brain because rapid, activity-driven changes to the spine cytoskeleton play an essential role in the production of LTP [28, 29]. Therefore, are the acute and rapid effects of oestrogen on cognition due to spine changes? Could presence of the steroid affect the second-long synaptic events leading to the encoding of new memories? Current research suggests it is likely to be the case. The following sections will first describe in some detail a currently accepted synaptic model of memory encoding and then describe new results showing how the synaptic model is affected by oestrogen. The final section will consider how oestrogen may be activating synaptic changes.
II. LTP as a memory model
The brain is a fascinating organ and one of its most remarkable features is its ability to store and retrieve information. Hebb in 1949 was the first to formulate a synaptic model describing that change in the strength of synaptic connections was the underlying mechanism for learning and memory. But supporting evidence for this model didn’t emerge until 1973, when Bliss and Lomo discovered that brief high-frequency stimulation produced long lasting changes in synaptic strength in the hippocampus, now termed LTP [30]. Subsequent discoveries showing that potentiation is vulnerable to disruption for several minutes after induction [31, 32] equipped LTP with additional properties of a memory-like substrate: synapse specificity, long-lasting stability, and a rapid consolidation process. The links to memory were further strengthened by evidence that LTP occurs during learning [33] and that agents which block the effect would cause memory loss [34]. LTP has now become the most important cellular correlate to study rapid cellular processes that contribute to the encoding and consolidation of memories.
One current synaptic model used to study learning-induced synaptic events examines three major phases of LTP; induction, expression, and consolidation. The induction of LTP occurs very rapidly (< 30s) and has two requirements; (1) the binding of glutamate to the NMDA receptor to dislodge Mg++ inside the channel and allow calcium to flow through, and (2) the amount of glutamate stimulation must be strong enough to cause sufficient membrane depolarization [35]. Blocking or enhancing NMDA receptor function will inhibit or enhance LTP, respectively. Studies showing that LTP requires the influx of dendritic calcium [36] led to the assumption that potentiation is expressed by post-synaptic changes [37]. While for many years there were contentious debates as to whether LTP was expressed pre- or postsynaptically [38–41], it is generally accepted now that postsynaptic up-regulation of AMPA receptor insertion and trafficking plays a major role in LTP expression [42, 43]. However, it was thought that up-regulation of AMPA receptor insertion and trafficking could only occur once activity-dependent forms of stimulation triggered reorganization of actin. Subsequent changes in spine morphology would then increase the size of the synapse and, thereby, accommodate the insertion of additional receptors [44]. Accordingly, results showing ultrastructural changes to the post-synaptic density [45, 46] confirmed the notion that increased numbers of receptors was a likely expression mechanism for LTP. These observations and hypotheses initiated several studies to determine whether synaptic activity would rapidly reorganize the spine actin cytoskeleton.
Pioneering studies investigating whether such an effect occurs in individual dendritic spines following the induction of LTP were successful in that they showed actin polymerization was crucial for stable LTP in vivo [47], in vitro work with slices [48], and cultured neurons [49]. Our laboratory extended these findings by developing a new slice in situ technique that could be used routinely in adult hippocampal slices and avoid the heavy background staining that often accompanies actin directed staining in culture. Phalloidin, a toxin that binds selectively to filamentous (F)-actin, was topically applied to slices following the end of an experiment; the slices were then fixed, sectioned, and mounted onto slides. Phalloidin labeling was photographed using epifluorescence microscopy and quantified using two dimensional edge detection software developed within the lab that counts puncta corresponding to size and dimensions of spines. Consistent with the above findings, we showed that naturalistic theta patterned stimulation produced a significant increase in the number of phalloidin-labeled spines within the activation zone [50].
Subsequent experiments confirmed that spine actin polymerization is also essential for LTP expression and consolidation in adult hippocampal tissue. Latrunculin A, at concentrations sufficient to block polymerization within spines [48], completely eliminated LTP stabilization [51]. In addition, post-theta stimulation treatments that block consolidation (e.g., adenosine) also disrupted polymerization and the expression of LTP [52]. Moreover, the actin effect had a threshold (number of theta bursts) comparable to that for inducing LTP and developed fast enough (~2 min) to participate in LTP consolidation [52]. Notably, various manipulations used to disrupt newly formed actin filaments became progressively less effective 10–15 min following LTP induction. Low frequency stimulation, for example, completely eliminated actin polymerization and LTP when applied 1 min, but not 15 min, after theta burst stimulation (TBS) [52], suggesting that actin polymerization following TBS is initially dynamic but then consolidates over time.
Actin remodeling takes place in the first 10–15 min following LTP induction, a time frame that corresponds to the consolidation stage of LTP [53]. The next task was to identify the signaling cascade that was activated during this limited time period. The development of dual immunofluorescence microscopy techniques for measuring the concentration of defined antigens within limited cellular compartments in adult tissue has made it possible to identify changes in actin-mediated pathways following TBS [44]. Cofilin, which promotes the disassembly of actin filaments, is inactivated by phosphorylation and encourages filament assembly [54], thus serves as an excellent marker for actin reorganization [47]. It was shown that naturalistic theta stimulation of the Schaffer collateral afferents of field CA1 stratum radiatum produced a marked increase in the number of phosphorylated (p)Cofilin positive spines in adult hippocampal tissue. A significant increase in pCofilin level was present at 2 min, but not 30 seconds, after stimulation and reached peak levels at about 7 min post-TBS, before declining toward baseline [44]. The significant change in pCofilin levels was accompanied with an increase in synapse size suggesting that TBS causes a rapid increase in the overall area of the synapse and thereby, allows for insertion of additional AMPA receptors via AMPA receptor trafficking.
But how does TBS cause AMPA receptor trafficking? Numerous studies have shown that CaMKII is the target of calcium influx following activity-dependent synaptic change and is believed by many to be the protein responsible for maintaining long term synaptic changes, also known as the “memory molecule” [55]. Recent studies have reported the initial steps occur in two phases. (1) Calcium-activated calmodulin causes activation of CaMKII through a translocation and autophosporylation event. These steps lead to CaMKII directed phosphorylation of the GluR1 subunit of the AMPA receptors [55]. (2) CaMKII phosphorylates stargazin, and in turn causes the insertion of AMPA receptors to the synaptic terminal which then increases channel conductance [56]. However, it is also possible that the role of CaMKII at the synapse is only temporary [57] and involves activation of Rho-GTPases, specifically RhoA and Cdc42. Both signaling proteins are critical for structural and functional plasticity [58] and have been shown to be involved in the consolidation of LTP [53]. Thus a current synaptic model for LTP would begin by an activity-dependent level of stimulation that increases calcium influx in dendritic spines, triggers the autophosphorylation of CaMKII and activates the RhoA or Rac/Cdc42 signaling pathways (Fig 1). On one hand, activity-dependent stimulation has been shown to enhance polymerized actin via the RhoA stimulated pathway which through its effector ROCK, triggers phosphorylation of cofilin. This event has been shown to last at least 15 min; time enough to allow for filament assembly [44]. On the other hand, filament elaboration and stabilization appears to be mediated by Rac/Cdc42 and its effector PAK [53]. Both pathways influence the activation and translocation of CaMKII to the postsynaptic density that then phosphorylates the AMPA GluR1 and encourages AMPAR trafficking to the synapse. Reorganization of the actin network modifies spine morphology and leads to increased synaptic current [44, 51–53, 59–61].
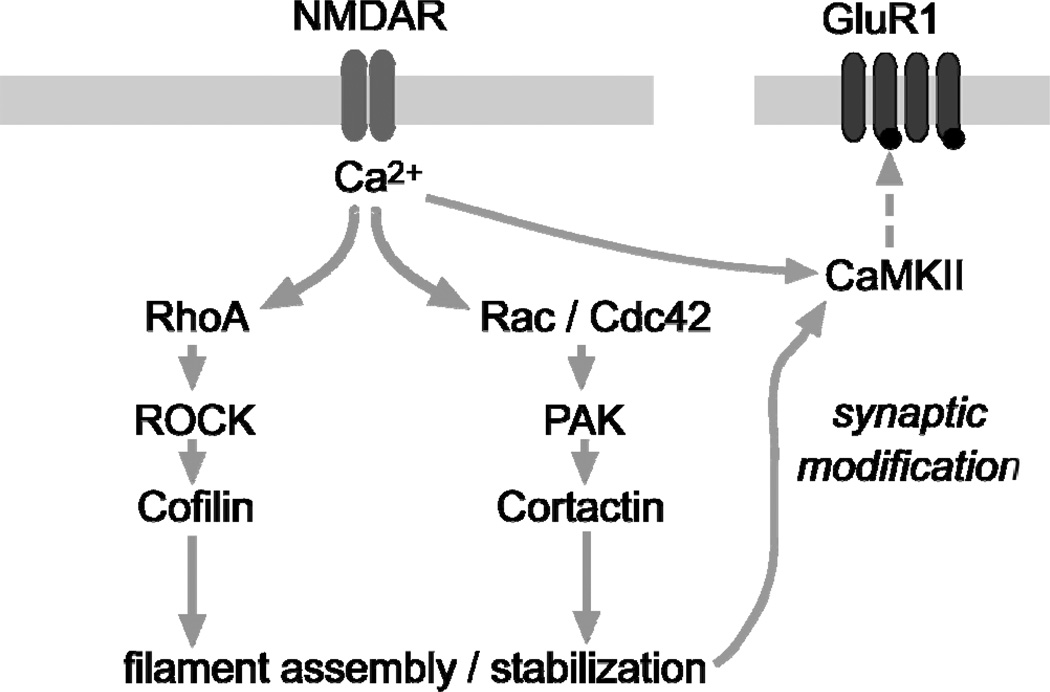
Figure 1.
Schematic of a proposed synaptic model showing signaling cascades that regulate actin dynamics during the production of LTP. It has been shown that chemically induced NMDA receptor-dependent LTP robustly activates RhoA and Rac [53], which is consistent with evidence that Rho-GTPases are activated by Ca2+ influx through NMDA receptors (blue bars) [106]. Activity-driven activation of the RhoA>ROCK>cofilin pathway leads to rapid filament assembly, while parallel activation of Rac/Cdc42>PAK>cortactin influences later stages of LTP stabilization. The activation of these two pathways leads to several activity-dependent changes in the synapse including activation and translocation of CaMKII to the postsynaptic density that will then phosphorylate AMPARs (red bars) and initiate receptor trafficking to the synapse. The latter events increase synaptic current through AMPAR’s, and thus, increase fEPSP size.
As mentioned previously several groups have shown that brief infusions of E2 rapidly increase synaptic transmission, alter synaptic connectivity, and enhance LTP. These short latency events raise the possibility that oestrogen, like other molecules such as BDNF and adenosine, are likely to be a part of a group of synaptic modulators [62, 63]. These molecules are released by naturalistic patterned afferent activity, bind to their receptors, but have no direct effects on membrane voltage. They instead act on the actin signaling cascade, modify the subsynaptic cytoskeleton, and thereby regulate synaptic plasticity. Given that oestrogen has been shown to be produced in hippocampal synapses [21] and has synaptic receptors in the hippocampus [22, 64–66], we tested if E2 could alter the spine cytoskeleton and initiate an actin signaling pathway as described above in adult hippocampal slices.
III. Rapid Effects of Oestrogen on LTP
In agreement with previous reports, it was found that baseline synaptic transmission increased rapidly during brief infusions of 17β-estradiol (E2) and a new oestrogen receptor s (ERβ) selective agonist, WAY200070 (WAY), but not during the infusion of the oestrogen receptor alpha (ERα) selective agonist, PPT, suggesting E2 actions were mediated through ERβ [17, 67]. Pinpointing the postsynaptic target of E2’s baseline effect was done by careful examination of E2’s effect on pharmacologically isolated postsynaptic receptor functions that mediate fast EPSP responses. The E2-induced increase in synaptic transmission was found to be selective to the AMPA receptor mediated response, not GABA or NMDA mediated receptor functions [17]. Together, the results are consistent with the idea that E2 may be enhancing transmission by facilitating the movement of AMPAR’s into the synapse [68, 69].
Previous studies have shown that E2 increases LTP, but did not address the question of whether E2’s influence was due to a reduction in stimulation threshold or an elevation in the normal LTP ceiling. Our studies [17] found that subthreshold levels of stimulation (2–3 theta bursts) in the presence of E2 or WAY produced significant increases in the magnitude of LTP compared to that elicited in control (vehicle) slices or those infused with PPT. Along these same lines, threshold levels of stimulation (5, 10 theta bursts) produced supranormal amounts of LTP in slices infused with E2 or WAY, but not PPT or vehicle-treated slices. These changes were not accompanied by changes in NMDA receptor function since there were no differences between groups in the area of theta burst responses and NMDA receptor-mediated burst size. These data suggest that naturally occurring theta pattern stimulation in combination with ERβ receptor activation reduces the threshold, and raise the ceiling, of LTP in the hippocampus and exerts its effects at stages following the induction of LTP.
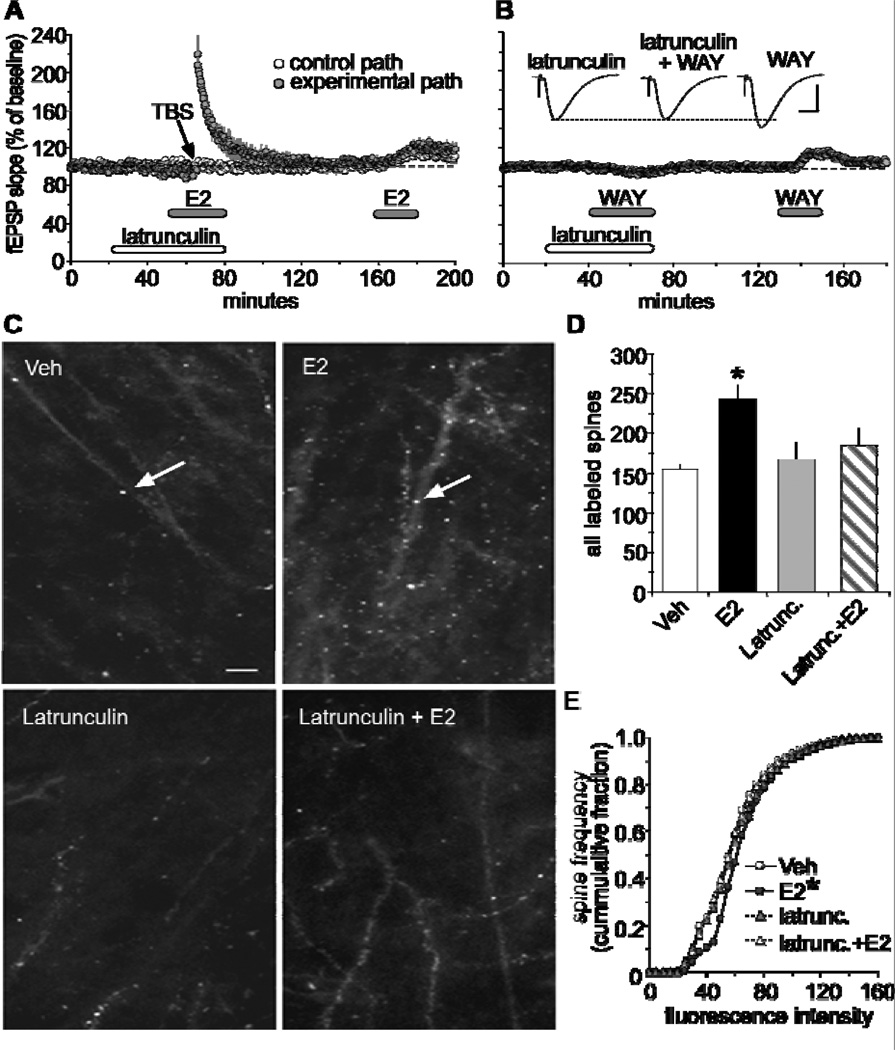
As noted above, rapid, activity-driven changes to the cytoskeletal network are essential for the production of stable LTP. The next question asked was whether the rapid effects of E2 on baseline transmission are triggering events leading to actin polymerization. Pretreating hippocampal slices with latrunculin A effectively eliminated E2’s effect on fEPSPs and failed to promote LTP in E2 treated slices [17]. The ERβ agonist, WAY, produced similar results (Fig 2A–B). These results strongly suggest that E2 triggers the same actin assembly process as used in the production of LTP. A direct measure of E2’s effect on actin assembly was then tested by applying fluorescence-tagged phalloidin to slices following a 20 min treatment of E2 [17]. Photomicrographs showed that a 20 min treatment with E2 increased the number of phalloidin-positive spines in area CA1 relative to control slices, and this effect was altogether absent in slices that were pretreated with latrunculin followed by E2 (Fig 2C). Blind quantitative analysis showed that E2 significantly increased the number of densely labeled F-actin in spines relative to controls indicating that E2 can rapidly activate the actin assembly pathway (Fig 2D–E).
Figure 2.
Estrogen promotes actin polymerization in CA1 dendritic spines. (A) Latrunculin (500nM) effectively blocked both E2-induced increases in synaptic transmission in the control pathway (white circle) and theta burst-induced LTP in the experimental pathway (dark circle). Following a 1hr washout period, fEPSP responses to E2 was restored. (B) Infusions of latrunculin blocked, in a reversible manner, the increase in baseline synaptic responses produced by the selective ERβ agonist, WAY200070 (WAY; 100nM). Inset, representative traces collected during treatments with latrunculin, WAY, and both. Scale 1mV/5ms. (C) Top two panels compare phalloidin labeling in CA1 stratum radiatum in slices treated with 1nM E2 or vehicle (Scale bar = 5µm). E2 treatment increases spine-like profiles (arrows) relative to vehicle. Bottom two panels illustrate the effect of latrunculin (500nM) alone and in combination with E2-induced baseline phalloidin labeling. (D) The group mean (±SEM) number of phalloidin-labeled spines in area CA1b was significantly greater in slices treated with E2 compared to vehicle controls (*p < 0.001), and that latrunculin blocks E2-induced increases in phalloidin labeling. (E) Cumulative frequency distribution of phalloidin-labeling intensities in CA1b in control versus E2-treated slices; E2 caused a significant rightward shift in the distribution curve relative to controls (*p < 0.001). This effect was blocked by latrunculin. Modified from [17].
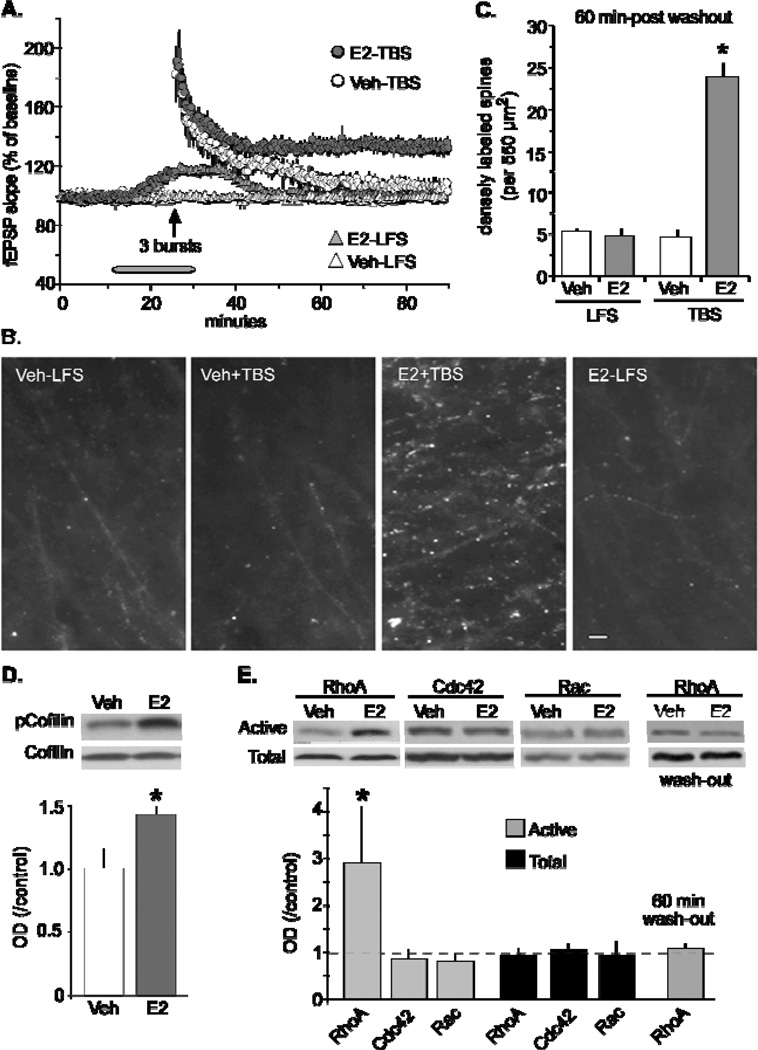
If E2 by itself causes a modest increase in actin filaments in spines, then it could potentially augment theta burst-induced polymerization needed for stable LTP [17]. The next set of experiments used a subthreshold level of stimulation to induce LTP (Fig 3A). As expected in control slices, 3 theta bursts produced a normal level of short-term potentiation that over time decayed back to baseline and the level of polymerized actin measured 60 min post-TBS was no different from control slices receiving baseline stimulation for the same period of time (Fig 3A–B). In slices that received a 20 min infusion of E2 we saw the expected increase in synaptic transmission that returned back to baseline levels during a 60 min washout and found no difference in the degree of polymerized actin relative to levels in control slices. In contrast, the delivery of 3 theta bursts following a 20 min treatment of E2 produced a non-decremental form of potentiation that remained above baseline 60 min after washout. Slices from this group also showed elevated levels of polymerized actin. Taken together, a brief exposure of E2 enhanced TBS-induced actin polymerization and LTP in adult hippocampal slices (Fig 3A–C).
Figure 3.
17β-estradial (E2) enhances theta burst-induced LTP and actin polymerization via RhoA signaling in hippocampal slices. (A) Infusions of 1 nM E2 (E2-LFS) caused a reversible increase in slope fEPSPs relative to control (vehicle) during single pulse stimulation in field CA1. The delivery of a subthreshold level of theta stimulation (upward arrow) in vehicle treated slices caused potentiation to decay back to baseline following a 60 min recording period. The same level of stimulation in the presence of E2 caused lasting potentiation. (B) At the end of the 60 min washout period, slices were processed for phalloidin labeling of field CA1 dendritic spines. Survey micrographs indicate slices that had received baseline test pulses (low-frequency stimulation; LFS) during E2 infusion (E2-LFS) did not show an increase in phalloidin positive spines above levels seen in slices treated with vehicle (Veh-LFS). In contrast, three theta bursts significantly enhanced the number of phalloidin-labeled puncta in slices infused with E2 (E2+TBS) relative to vehicle treated slices (Veh+TBS). Scale bar, 5µm. (C) The number of densely labeled spines was determined using an automatic counting program. Slices that received LFS only in the presence and absence of E2 had the same relative counts of densely labeled phalloidin after the 60 min washout period, while those that received theta bursts in the presence of E2 produced a significant increase in densely labeled spines relative to vehicle treated slices (p < 0.0001). (D) TopRepresentative Western blot images show both phosphorylated (p)-cofilin and total cofilin levels in hippocampal slices infused with 1nM E2 or vehicle. BottomOptical density measurements from blots confirm that a 20 min infusion of E2 significantly increased pCofilin relative to total cofilin (p < 0.05) in hippocampal slices from male rats. (E) TopA 20 min infusion of E2 selectively increased the activity of GTPase RhoA relative to total RhoA as assessed using the pull-down assay. BottomQuantitative analysis confirmed that E2 significantly increased activity levels of RhoA relative to related GTPases Cdc42 and Rac (p < 0.05). Following a 60 min washout of E2, activated RhoA levels were not significantly different from vehicle-treated slices. Modified from [17].
With the above findings in hand, biochemical investigations were initiated to identify the actin-signaling cascade activated by E2 [17]. Experiments were first designed to target the protein responsible for altering the state of polymerized actin, as mentioned above, the constitutively active protein, cofilin. Using the same concentration that significantly enhanced fEPSP responses in area CA1, E2 was shown to significantly increase pCofilin in adult hippocampal slices (Fig 3D). This effect explains how E2 influences actin polymerization; phosphorylation of cofilin inactivates the protein and encourages actin filament assembly. Additional studies explored which of the upstream regulators of cofilin are triggered by E2. Using GTP-ase pull-down assays, it was found that E2 caused a notable increase in activated RhoA, with little to no change in Rac/Cdc42 activity (Fig 3E). Furthermore, the increase in activated RhoA returned to normal levels following a 60min washout. RhoA exerts its effects by activating downstream, ROCK. Past studies have shown that inhibitors of ROCK block cofilin phosphorylation and prevent the stabilization of LTP [53]. The ROCK inhibitor, H1151, also prevented E2-induced increases in synaptic transmission without affecting baseline fEPSPs. Collectively, the results strongly suggest that brief infusions of E2 produce a weak form of potentiation, and activates the RhoA>ROCK>LIM kinase>cofilin>actin assembly pathway previously implicated in the production of LTP [53]. The hormone’s weak form of potentiation that readily washes out may be due to its inability to activate the Rac>PAK (p21-activated kinase) sequence as indicated by others to be responsible for stabilizing newly formed filaments and LTP in adult hippocampal slices [53].
III. Transactivation of Membrane Receptors
How does oestrogen trigger the activation of RhoA? Because there are no selective antagonists for extra-nuclear membrane receptors ERα or ERβ, it is difficult to answer this question. However, there are clues in the literature that suggest the hormone may be binding to an extra-nuclear G-protein-coupled oestrogen receptor, GPR30 [70, 71]. This receptor is expressed in important areas of the brain responsible for spatial learning and memory including the hippocampus [72, 73] and has most recently been identified at the post-synaptic density in dendritic spines of field CA1 [74]. It was also mentioned that treatment with a GPR30 agonist, G-1, increased PSD95 expression in the female rat hippocampus [75], however, the effect was observed 2 days later. Therefore, it is unlikely that the rapid effects of oestrogen, as described above, are due to activation of this novel receptor. Additional studies using the G-1 agonist, and selective GPR30 antagonist G-15 [76], are needed to confirm this point.
Another possible route in which oestrogen may elicit its rapid effects is by transactivating nearby membrane receptors [64]. It has been proposed that prolonged treatments with E2 transactivate tropomyosin-related kinase B (TrkB) receptors for brain-derived neurotrophic factor (BDNF) and promote spine growth [77]. An ‘acute’ version of this hypothesis is plausible given that BDNF is known to stimulate Rho GTPase signaling [78] including activation of RhoA [79]. Furthermore, there is a large body of literature implicating E2 as a mediator of BDNF-induced function [65]. For example, Scharfman and colleagues [80] found that increases in excitatory transmission that occur during proestrus are prevented by the kinase inhibitor, K252A, which blocks Trk signaling. These findings are consistent with a hypothesis that E2 causes rapid activation of TrkB that then engages the RhoA-initiated signaling pathway and, in turn, leads to enhanced fEPSPs.
The first part of the above hypothesis was tested using dual immunofluorescence microscopy to determine the number of synapses labeled with postsynaptic density marker PSD95 and colocalized with activated TrkB [81]. We found that E2 caused a significant increase in the number of field CA1 synapses associated with intense staining of activated TrkB, as assessed 10 min after the initial onset of E2-induced increase in synaptic responses. Therefore, it appears that either E2 transactivates TrkB or facilitates the TrkB response to BDNF. On the other hand, it is possible that E2 releases BDNF [80, 82, 83] which then stimulates TrkB receptors. Additional studies are needed to determine whether the absence of these oestrogen effects occur in BDNF-knockout mice.
Another membrane receptor that is intimately associated with initiating actin signaling and the stabilization of LTP are the adhesion receptors belonging to the β1 integrin family [52, 84–88]. These receptors are expressed at high levels throughout the hippocampus [89], in particular to field CA1, and upon ligand binding they have been shown to facilitate synaptic transmission in adult hippocampal slices by increasing phosphorylation of GluR1 and CaMKII activity [60]. Most recently we have reported that synaptic integrins, and integrin signaling, are activated by naturalistic theta pattern stimulation [90], and that this form of stimulation results in LTP-related actin polymerization [52]. The notion that acute applications of E2 could trigger integrin signaling is also supported by a recent study investigating nongenomic effects of E2 in human platelet cells. Brief hormone applications caused a rapid and transient increase in activation of integrin α (IIb) β3 via tyrosine kinases Src and Pyk2 [91]. These results prompted us to begin investigating whether E2 can rapidly activate the integrin β1 receptor in hippocampal (most prominent integrin receptor subtype in field CA1) slices. If positive results are achieved, then it is possible that multiple transactivation routes for rapid, E2-driven synaptic change can occur. A discussion of the possible routes taken by E2 is described in detail in an earlier report [81] and leads to an important question of whether the rapid effects observed following hormone infusion reflects the true nature of circulating hormone or is it due to locally produced and released E2 in the brain. There is convincing evidence showing that axon terminals within the hippocampus of both male and female rats synthesize E2 and have the ability to release it [21, 92]. It would be of interest to determine whether naturalistic patterned stimulation, such as TBS, could evoke the release of E2 from axon terminals in the hippocampus. It is particularly important in light of recent reports showing “sex-specific” hormones elicit significant functions in subjects of the opposite sex [93–95]. Since steroid hormones play a critical role in neural circuit development and subsequent behaviors, and investigations of oestrogenic effects in male rats has been largely neglected, additional work investigating gender differences in the degree of E2 release would provide a better understanding of E2’s rapid actions in the brain. Research in this area would help build a unified hypothesis of E2’s actions as a synaptic modulator in the hippocampus and perhaps direct novel sex-specific therapies to relieve neurological or mental health disorders including Alzheimer’s disease, depression, schizophrenia, and post-traumatic stress disorder.
IV. Summary
The present review first describes a current view of a LTP model for memory that proposes activity-dependent activation of actin signaling and the reorganization of the spine cytoskeleton are necessary for consolidation of LTP, hence the encoding of memories. It is initiated by a naturalistic theta pattern of stimulation found to occur in the brain during learning [96, 97]. Theta burst-induced LTP increases potentiation by activating NMDA receptors to allow calcium influx into the dendritic spine that then leads to the expression and consolidation of LTP. The latter two stages involve the activation of RhoA and Rac/Cdc42 actin signaling pathways that work in concert to induce cytoskeletal reorganization. As a result, an increase in synapse size allows for a greater number of phophorylated AMPA receptors to be inserted into the synapse and enhances synaptic current.
Next, we described results of how the current synaptic model is affected by E2 in hippocampal slices from adult male rats. Brief infusions of E2 rapidly enhanced synaptic transmission in a reversible manner and lowered the threshold for inducing stable potentiation. The E2 effect in the male hippocampus was selective in that it did not influence inhibitory transmission or NMDAR-mediated responses. However, more recent studies using ovariectomized rats show that E2 influences a wider range of synaptic effects in a sex-specific manner suggesting that gender differences may exist in how the hippocampus responds to the steroid [98, 99]. It was also shown that short infusions of E2 increased actin polymerization in dendritic spines. This process appears to be responsible for E2-induced increases in fEPSPs because latrunculin A, a toxin that prevents filament assembly, completely blocked E2 effect on transmission. While several studies have shown that E2 causes the formation of dendritic spines in less than an hour [100–102], future work should include live-imaging studies examining whether spines form or change shape during the period when the hormone increases synaptic transmission and if there are possible gender differences.
In search for the origin of E2’s influence on synaptic transmission, a series of biochemical studies revealed that E2 engages the RhoA signaling pathway in adult hippocampal slices. Short infusions caused a notable increase in the activity of RhoA without influencing the parallel Rac/Cdc42 pathway and were accompanied with a large increase in phosphorylated cofilin, a well-established link for allowing growth of actin filaments [103, 104]. Other studies using dissociated neurons have shown that E2 activates the Ras-Family GTPase Rap and its effector Erk1/2 [105]. This Rap-driven response is critical for E2-induced rapid spine growth in cortical neurons and would be interesting to determine if comparable results were obtained in adult hippocampus slices.
Lastly, a recent review [81] explored the possibility that the hormone’s influence on RhoA may include the transactivation of neighboring membrane receptors [77] associated with activating this actin pathway [79]. E2 induced an increase in synaptic TrkB receptor phosphorylation 10 min after the initial rise in the fEPSP [81]. However, transactivation of the TrkB receptor is not the only possible mechanism by which E2 may be carrying out its effects. Adhesion receptors belonging to the integrin family have shown to regulate the cytoskeleton [52] and enhance glutamatergic transmission ([60]. Continuing work in these areas will likely provide a better understanding of the degree to which brief activation of oestrogen signaling pathways at synapses can be used to relieve neurological and mental health disorders associated with abnormal levels of the hormone.
Acknowledgements
We would like to thank Drs Gary Lynch and Christine Gall for their collaboration on projects described here. These studies were supported by National Institute of Neurological Disorders and Stroke grant NS045260; National Institute for Mental Health grant MH082042; and National Science Foundation grant 1146708.
References
- 1.Devi G, Hahn K, Massimi S, Zhivotovskaya E. Prevalence of memory loss complaints and other symptoms associated with the menopause transition: a community survey. Gender medicine. 2005;2:255–264. doi: 10.1016/s1550-8579(05)80055-5. [DOI] [PubMed] [Google Scholar]
- 2.Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Hormones and behavior. 2010;58:929–935. doi: 10.1016/j.yhbeh.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–1857. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wegesin DJ, Stern Y. Effects of hormone replacement therapy and aging on cognition: evidence for executive dysfunction. Neuropsychology, development, and cognition. Section B, Aging, neuropsychology and cognition. 2007;14:301–328. doi: 10.1080/13825580600802893. [DOI] [PubMed] [Google Scholar]
- 5.Loy R, Gerlach JL, McEwen BS. Autoradiographic localization of estradiol-binding neurons in the rat hippocampal formation and entorhinal cortex. Brain Res. 1988;467:245–251. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- 6.Maggi A, Susanna L, Bettini E, Mantero G, Zucchi I. Hippocampus: a target for estrogen action in mammalian brain. Mol Endocrinol. 1989;3:1165–1170. doi: 10.1210/mend-3-7-1165. [DOI] [PubMed] [Google Scholar]
- 7.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong M, Moss RL. Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17 beta-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Res. 1991;543:148–152. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]
- 11.Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrer HF, Araque A, Buno W. Estradiol regulates the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. J Neurosci. 2003;23:6338–6344. doi: 10.1523/JNEUROSCI.23-15-06338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fugger HN, Kumar A, Lubahn DB, Korach KS, Foster TC. Examination of estradiol effects on the rapid estradiol mediated increase in hippocampal synaptic transmission in estrogen receptor alpha knockout mice. Neuroscience letters. 2001;309:207–209. doi: 10.1016/s0304-3940(01)02083-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim MT, Soussou W, Gholmieh G, Ahuja A, Tanguay A, Berger TW, Brinton RD. 17beta-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience. 2006;141:391–406. doi: 10.1016/j.neuroscience.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 15.Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 17.Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharrow KM, Kumar A, Foster TC. Calcineurin as a potential contributor in estradiol regulation of hippocampal synaptic function. Neuroscience. 2002;113:89–97. doi: 10.1016/s0306-4522(02)00151-3. [DOI] [PubMed] [Google Scholar]
- 19.Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 23.Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 25.Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Giretti MS, Fu XD, De Rosa G, Sarotto I, Baldacci C, Garibaldi S, Mannella P, Biglia N, Sismondi P, Genazzani AR, Simoncini T. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS ONE. 2008;3:e2238. doi: 10.1371/journal.pone.0002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch G, Rex CS, Gall CM. LTP consolidation: substrates, explanatory power, and functional significance. Neuropharmacology. 2007;52:12–23. doi: 10.1016/j.neuropharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of physiology. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrionuevo G, Schottler F, Lynch G. The effects of repetitive low frequency stimulation on control and "potentiated" synaptic responses in the hippocampus. Life sciences. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- 32.Staubli U, Chun D. Factors regulating the reversibility of long-term potentiation. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roman F, Staubli U, Lynch G. Evidence for synaptic potentiation in a cortical network during learning. Brain Res. 1987;418:221–226. doi: 10.1016/0006-8993(87)90089-8. [DOI] [PubMed] [Google Scholar]
- 34.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 35.Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. The Journal of physiology. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 37.Lynch G, Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- 38.Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- 39.Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990;346:177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- 40.Nicoll RA. Expression mechanisms underlying long-term potentiation: a postsynaptic view. Philos Trans R Soc Lond B Biol Sci. 2003;358:721–726. doi: 10.1098/rstb.2002.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Wang XB, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11388–11393. doi: 10.1073/pnas.0802978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Wang XB, Frerking M, Zhou Q. Spine expansion and stabilization associated with long-term potentiation. J Neurosci. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris KM, Fiala JC, Ostroff L. Structural changes at dendritic spine synapses during long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:745–748. doi: 10.1098/rstb.2002.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annual review of neuroscience. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 47.Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 48.Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 50.Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta stimulation polymerizes actin in dendritic spines of hippocampus. J Neurosci. 2005;25:2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kramar EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009 doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annual review of cell and developmental biology. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 55.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF. Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci. 2011;14:727–735. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Kramar EA, Chen LY, Babayan AH, Andres AL, Gall CM, Lynch G, Baram TZ. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol Psychiatry. doi: 10.1038/mp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kramar EA, Bernard JA, Gall CM, Lynch G. Integrins modulate fast excitatory transmission at hippocampal synapses. J Biol Chem. 2003;278:10722–10730. doi: 10.1074/jbc.M210225200. [DOI] [PubMed] [Google Scholar]
- 61.Kwak S, Matus A. Denervation induces long-lasting changes in the distribution of microtubule proteins in hippocampal neurons. Journal of neurocytology. 1988;17:189–195. doi: 10.1007/BF01674206. [DOI] [PubMed] [Google Scholar]
- 62.Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.zur Nedden S, Hawley S, Pentland N, Hardie DG, Doney AS, Frenguelli BG. Intracellular ATP influences synaptic plasticity in area CA1 of rat hippocampus via metabolism to adenosine and activity-dependent activation of adenosine A1 receptors. J Neurosci. 2011;31:6221–6234. doi: 10.1523/JNEUROSCI.4039-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tabatadze N, Smejkalova T, Woolley CS. Distribution and posttranslational modification of synaptic ERalpha in the adult female rat hippocampus. Endocrinology. 2013;154:819–830. doi: 10.1210/en.2012-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srivastava DP, Woolfrey KM, Liu F, Brandon NJ, Penzes P. Estrogen receptor ss activity modulates synaptic signaling and structure. J Neurosci. 2010;30:13454–13460. doi: 10.1523/JNEUROSCI.3264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 69.Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, Thompson R, Baudry M. 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21936–21941. doi: 10.1073/pnas.0912558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 71.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annual review of physiology. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 72.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. The Journal of endocrinology. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 73.Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2011;1379:53–60. doi: 10.1016/j.brainres.2010.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akama KT, Thompson LI, Milner TA, McEwen BS. Post-synaptic Density-95 (PSD-95) Binding Capacity of G-protein-coupled Receptor 30 (GPR30), an Estrogen Receptor That Can Be Identified in Hippocampal Dendritic Spines. J Biol Chem. 2013;288:6438–6450. doi: 10.1074/jbc.M112.412478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behavioral neuroscience. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nature chemical biology. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamauchi J, Chan JR, Shooter EM. Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8774–8779. doi: 10.1073/pnas.0402795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kramar EA, Babayan AH, Gall CM, Lynch G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krizsan-Agbas D, Pedchenko T, Hasan W, Smith PG. Oestrogen regulates sympathetic neurite outgrowth by modulating brain derived neurotrophic factor synthesis and release by the rodent uterus. Eur J Neurosci. 2003;18:2760–2768. doi: 10.1111/j.1460-9568.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- 83.Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–120. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 84.Bahr BA, Staubli U, Xiao P, Chun D, Ji ZX, Esteban ET, Lynch G. Arg-Gly-Asp-Ser-selective adhesion and the stabilization of long-term potentiation: pharmacological studies and the characterization of a candidate matrix receptor. J Neurosci. 1997;17:1320–1329. doi: 10.1523/JNEUROSCI.17-04-01320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan CS, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J Neurosci. 2003;23:7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chun D, Gall CM, Bi X, Lynch G. Evidence that integrins contribute to multiple stages in the consolidation of long term potentiation in rat hippocampus. Neuroscience. 2001;105:815–829. doi: 10.1016/s0306-4522(01)00173-7. [DOI] [PubMed] [Google Scholar]
- 87.Staubli U, Vanderklish P, Lynch G. An inhibitor of integrin receptors blocks long-term potentiation. Behavioral and neural biology. 1990;53:1–5. doi: 10.1016/0163-1047(90)90712-f. [DOI] [PubMed] [Google Scholar]
- 88.Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26:1813–1822. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci. 1999;19:1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Babayan A, Kramar EA, Barrett R, Jafari M, Jakob J, Chen L, Rex CS, Lauterborn JC, Wood M, Gall CM, Lynch G. Integrin Dynamics Produce a Delayed Stage of LTP and Memory Consolidation. J Neurosci. doi: 10.1523/JNEUROSCI.2024-12.2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moro L, Reineri S, Piranda D, Pietrapiana D, Lova P, Bertoni A, Graziani A, Defilippi P, Canobbio I, Torti M, Sinigaglia F. Nongenomic effects of 17beta-estradiol in human platelets: potentiation of thrombin-induced aggregation through estrogen receptor beta and Src kinase. Blood. 2005;105:115–121. doi: 10.1182/blood-2003-11-3840. [DOI] [PubMed] [Google Scholar]
- 92.Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- 93.Gagnidze K, Pfaff DW. Sex on the brain. Cell. 2009;139:19–21. doi: 10.1016/j.cell.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 94.Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meyer K, Korz V. Age dependent differences in the regulation of hippocampal steroid hormones and receptor genes: relations to motivation and cognition in male rats. Hormones and behavior. 2013;63:376–384. doi: 10.1016/j.yhbeh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 96.Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmidt B, Hinman JR, Jacobson TK, Szkudlarek E, Argraves M, Escabi MA, Markus EJ. Dissociation between Dorsal and Ventral Hippocampal Theta Oscillations during Decision-Making. J Neurosci. 2013;33:6212–6224. doi: 10.1523/JNEUROSCI.2915-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang GZ, Woolley CS. Estradiol Acutely Suppresses Inhibition in the Hippocampus through a Sex-Specific Endocannabinoid and mGluR-Dependent Mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 102.Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gungabissoon RA, Bamburg JR. Regulation of growth cone actin dynamics by ADF/cofilin. J Histochem Cytochem. 2003;51:411–420. doi: 10.1177/002215540305100402. [DOI] [PubMed] [Google Scholar]
- 104.Sarmiere PD, Bamburg JR. Regulation of the neuronal actin cytoskeleton by ADF/cofilin. J Neurobiol. 2004;58:103–117. doi: 10.1002/neu.10267. [DOI] [PubMed] [Google Scholar]
- 105.Srivastava DP, Penzes P. Rapid estradiol modulation of neuronal connectivity and its implications for disease. Frontiers in endocrinology. 2011;2:77. doi: 10.3389/fendo.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Semenova MM, Maki-Hokkonen AM, Cao J, Komarovski V, Forsberg KM, Koistinaho M, Coffey ET, Courtney MJ. Rho mediates calcium-dependent activation of p38alpha and subsequent excitotoxic cell death. Nat Neurosci. 2007;10:436–443. doi: 10.1038/nn1869. [DOI] [PubMed] [Google Scholar]