Abstract
Patient: Female, 66
Final Diagnosis: Chorea • hyperglycemia • Basal Ganglia Syndrome (C-H-BG)
Symptoms: Hemibalism • hemichorea
Medication: —
Clinical Procedure: —
Specialty: Endocrinology and Metabolic
Objective:
Challenging differential diagnosis
Background:
Hemichorea-hemiballism (HCHB) is a spectrum of involuntary, continuous non-patterned movement involving 1 side of the body. Possible causes of HCHB include hemorrhagic or ischemic stroke, neoplasm, systemic lupus erythematosus, HHNK, Wilson’s disease, and thyrotoxicosis. This case illustrates the need to be aware of hyperglycemia as a cause of hemiballism/hemichorea, which is now referred to in the medical literature as C-H-BG (chorea, hyperglycemia, basal ganglia) syndrome.
Case Report:
A 66-year-old Hispanic woman presented to our care with hemiballism/hemichorea of the right arm and leg of 1 week duration. She had been admitted 3 months prior with toxic metabolic encephalopathy secondary to hyperosmolar hyperglycemic non-ketotic syndrome with a blood glucose level of 984 mg/dL. Her blood glucose level was normal but hemoglobin A1C was 12.2%. A brain MRI revealed an asymmetric T1 hyperintensity of the left putamen. This specific finding was compatible with hyperglycemia-induced hemichorea hemiballism syndrome. The hemiballism/hemichorea slowly improved over the course of the hospitalization with strict glycemic control. At the 3-month follow-up visit she had no involuntary movements of her extremities, and she had well controlled blood glucose levels and a hemoglobin A1C of 9.0.
Conclusions:
In a patient with normal glycemic levels but a history of uncontrolled diabetes, C-H-BG syndrome should be on the top of the differential list when the characteristic MRI findings of a hyperintensity in the basal ganglia are observed. This is a rare disease that deserves attention because it is reversible with correction of hyperglycemia. Thus, prompt recognition and treatment is essential to avoid adverse outcomes.
MeSH Keywords: Chorea; Basal Ganglia Diseases; Dyskinesias; Hyperglycinemia, Nonketotic
Background
Hyperosmolar hyperglycemic non-ketotic (HHNK) syndrome is a clinical syndrome of severe hyperglycemia, hyperosmolarity, and intracellular dehydration without ketoacidosis [1]. Chorea is characterized by involuntary random-appearing irregular movements that are not rhythmic or repetitive. Hemichorea is a hyperkinetic disorder involving 1 side of the body, which is caused by lesions of the contralateral striatum [1]. Ballism is defined as repetitive, constantly varying, large-amplitude involuntary movements of the proximal portion of the extremities. Hemiballism is characterized by involuntary flailing movements of the extremities on 1 side of the body. Hemiballism results from a lesion in the contralateral subthalamic nucleus and adjacent structures [1]. Hemichorea-hemiballism (HCHB) is a spectrum of involuntary, continuous non-patterned movement involving 1 side of the body. Hemiballism often evolves into hemichorea.
The causes of HCHB include hemorrhagic or ischemic stroke, neoplasm, systemic lupus erythematosus, HHNK, Wilson’s disease, and thyrotoxicosis [2]. Most cases have been described in individuals of Asian descent, females, and the elderly. The increased incidence in the Asian population may suggest an underlying genetic predisposition [3]. Female predisposition is likely related to postmenopausal estrogen-induced alterations of GABA or dopamine receptors [4]. HCHB has been described in patients with HHNK with longstanding uncontrolled diabetes. These cases illustrate the need to be aware of hyperglycemia as a cause of hemiballism/hemichorea, which is now referred to in the medical literature as C-H-BG (chorea, hyperglycemia, basal ganglia) syndrome. The precise cause of C-H-BG syndrome has not been determined.
The pathogenesis of C-H-BG syndrome is not fully understood but several theories have been suggested. Hyperglycemia impairs cerebral autoregulation, causing hypoperfusion and the activation of anaerobic metabolism and depletion of gammaaminobutyric acid (GABA) in basal ganglia neurons [5,6]. GABA is the main inhibitory neurotransmitter in the basal ganglia. GABA and acetate are depleted rapidly in non-ketotic hyperglycemia, causing a reduction in acetylcholine synthesis [6,7]. The hyperviscosity induced by hyperglycemia causes a disruption of the blood brain barrier and transient ischemia of vulnerable striatal neurons [7]. The synergistic effects of uncontrolled hyperglycemia and vascular insufficiency cause an incomplete transient dysfunction of the striatum, which eventually leads to hemichorea-hemiballism in these patients [8]. Histological findings in patients with C-H-BG syndrome have reported selective neuronal loss, gliosis, and reactive astrocytes, without evidence of hemorrhage or infarction at the striatal areas [8].
We report a unique case that highlights the presentation of hemiballism/hemichorea after significant hyperglycemia.
Case Report
A 66-year-old Hispanic woman presented to our care with continuous non-rhythmic involuntary movements in the right arm and leg one of 1 week duration. As explained by the patient, the involuntary movement initially started in her right leg then progressed to also involve the right arm and face. It had become so severe that it prevented her from performing her normal daily activities. However, these involuntary movements resolved during sleep. Three months prior to this admission, she was admitted for toxic metabolic encephalopathy secondary to hyperosmolar hyperglycemic non-ketotic syndrome due to medication non-compliance. Her blood glucose level on that admission was 984 mg/dL. Magnetic resonance imaging (MRI) of the brain on that admission did not reveal any acute pathology. Her past medical problems included type 2 diabetes mellitus (uncontrolled), hypertension, hyperlipidemia, and schizophrenia.
Physical examination findings were significant for right upper and lower extremity hemiballism and hemichorea-like movements. Finger-nose maneuver was normal on the left side but showed severe dysmetria on the right side. Her pupils were 3 mm bilaterally, equal, round, and reactive to light, with intact extraocular movements and no nystagmus. All cranial nerves were assessed and were normal on examination. No motor or sensory deficits to light, touch, pain, position sense, or vibration were noted. All deep tendon reflexes (DTR) such as biceps, knee tendon, and Babinski sign were normal. No deficits in muscle tone or strength (5/5 in all 4 extremities) or tremors were observed. Nuchal rigidity was observed but Kernig and Brudzinski signs were negative.
There were no significant findings on review of vital signs. The initial laboratory workup (Table 1) revealed renal insufficiency and microcytic anemia. The hemoglobin A1C was 12.2%, which correlates with an average serum glucose of 298 mg/dL (240–347 mg/dL). Computed tomography (CT) on admission revealed mild global cortical atrophy but no evidence of any acute intracranial pathology. Our differential diagnosis included neoplastic disorders (metastatic brain disease, brain tumor), Huntington’s disease, ischemic or hemorrhagic stroke, trauma, and drug toxicity (dopamine agonist or phenytoin). Stroke was excluded since there was no internal capsule involvement or hemiparesis. She had no other clinical manifestations or family history, and she was outside the usual age range for Huntington’s disease, had no recent traumatic injuries, and was not taking any anti-psychotic or anti-seizure medications.
Table 1.
Initial laboratory work-up.
| White blood cell count | 6.42×103 uL (4.5–11.0×103/UL) |
| Hemoglobin | 10.1 g/dL (12.0–15.0 g/dL) |
| Hematocrit | 32.0% (36.0–47.0%) |
| Platelet count | 231×103/uL (150–450×103/UL) |
| Sodium | 146 mmol/L (135–145 mmol/L) |
| Potassium | 4.5 mmol/L (3.5–5.1 mmol/L) |
| Chloride | 112 mmol/L (98–107 mmol/L) |
| CO2 | 22 mmol/L (21–32 mmol/L) |
| Serum glucose | 84 mg/dL (70–100 mg/dL) |
| BUN | 43 mg/dL (7–22 mg/dL) |
| Creatinine | 1.69 mg/dL (0.60–1.30 mg/dL) |
| Calcium | 8.8 mmol/L (8.5–10.1 mmol/L) |
| Albumin | 3.4 g/dL (3.4–5.0 g/dL) |
| Protein | 6.6 g/dL (6.4–8.2 g/dL) |
| AST | 16 IU/L (15–37 IU/L) |
| ALT | 19 IU/L (12–78 IU/L) |
| Alk. phosphatase | 66 IU/L (50–136 IU/L) |
| TSH | 4.41 MIU/L (0.35–5.0 MIU/L) |
The neurology consult service advised us to order an MRI of the brain, which revealed an asymmetric T1 hyperintensity of the left putamen without signal intensity changes. This specific finding is classic for hyperglycemia-induced hemichorea hemiballism syndrome (Figure 1). Our patient presented with a normal glucose level but had a longstanding history of uncontrolled diabetes, as noted by her elevated hemoglobin A1C. The onset usually occurs during an episode of non-ketotic hyperglycemia; however, it has also been reported to occur after a period of severe hyperglycemia.
Figure 1.
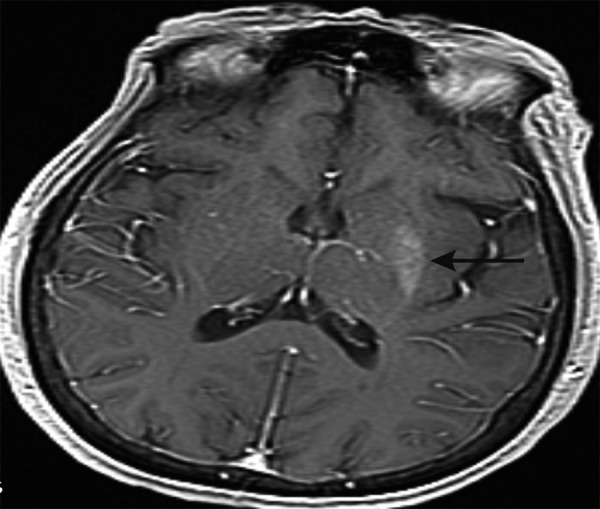
Brain MRI (sagittal): asymmetric T1 hyperintensity of the left putamen (black arrow).
Strict blood glucose control was initiated with glargine 20 units every night and scheduled dose of 3 units of insulin aspart before meals. The blood glucose levels were maintained from 80–120 mg/dL during hospitalization. The patient was initially placed on Haldol 0.5 mg twice daily, with no improvement after 2 days. Haldol was then increased to 1 mg twice daily, with slight improvement in these involuntary movements. On the 6th hospital day, the Haldol was discontinue and risperidone 1 mg twice daily was given for 1 day, then increased to 2 mg twice daily for the next day. By the 8th hospital day, the involuntary movements were significantly improved and she was able to perform physical therapy exercises. She was subsequently discharged to a rehabilitation facility on the 10th hospital day on risperidone and a strict glucose control regimen. During her 3-month follow-up visit, she had complete resolution of the involuntary movements of her extremities, well-controlled blood glucose levels, and a hemoglobin A1C of 9.0. The clinical improvement of her condition was correlated to better hyperglycemic control and physical rehabilitation upon discharge.
Discussion
Neuro-radiological imaging results are characteristic in patients with hemichorea-hemiballism non-ketotic hyperglycemia. Computed tomography (CT) demonstrates hyperattenuation in the striatum contralateral to the affected side. Magnetic resonance imagining (MRI) shows T1-weighted hyperintensities in striatum and globus pallidus, with restricted diffusion on diffusion-weighted imaging [9]. An abnormal signal may extend into the globus pallidus and up to the medial part of the cerebral peduncle in the midbrain along the striatonigral pathway [10]. Magnetic resonance spectroscopy may show low N-acetylaspartate to creatinine ratio and high choline-to-creatinine ratio and associated lactate peak [11]. Positron emission tomography (PET) has shown decreased glucose metabolism in the basal ganglia.
Several case reports have documented that the hemiballism/hemichorea can occur a few weeks after the blood glucose levels have been controlled and are actively being treated [12]. This suggests a delayed reaction to the severe hyperglycemia. Most patients with C-H-BG syndrome have a benign clinical course that can be managed medically. The onset of the disorder usually coincides with severe hyperglycemia and follows a temporal relationship between restoration of blood glycemic levels and improvement of the chorea [13]. The mainstay of treatment is aggressive glycemic control with either partial or complete resolution of hemichorea-hemiballism [14]. Clinical and radiological signs usually take about 6 months to resolve after the correction of hyperglycemia [14]. Case reports in the current medical literature have shown that most patients have dramatically recovered from their hyperkinesia after control of the hyperglycemia was achieved, showing that hemiballismhemichorea caused by hyperglycemia is a treatable disorder with a good prognosis [15].
Conclusions
In conclusion, although hemiballism-hemichorea is rarely caused by a dysfunction of glucose metabolism, we advise checking blood glucose whenever patients with this type of hyperkinesia are encountered. In a patient with normal glycemic levels but a history of uncontrolled diabetes, C-H-BG syndrome should be on the top of the differential list when the characteristic MRI findings of a hyperintensity in the basal ganglia are observed. This is a rare disease that deserves attention because it is reversible with correction of hyperglycemia. Thus, prompt recognition and treatment is essential to avoid adverse outcomes.
Footnotes
Disclosures
All participating authors in this study declare no financial, professional or personal conflicts.
No grant support was received for this case report.
All authors were involved in manuscript preparation and literature review.
References:
- 1.Qi X, Yan Y, Gao Y, Zheng Z, Chang Y. Hemichorea associated with non-ketotic hyperglycaemia: a case report. Diabetes Res Clin Pract. 2012;95(1):e1–e3. doi: 10.1016/j.diabres.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Padmanabhan S, Zagami AS, Poynten AM. A case of hemichorea-hemiballismus due to nonketotic hyperglycemia. Diabetes Care. 2013;36(4):e55–e56. doi: 10.2337/dc12-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal P. Hemichorea-hemiballism syndrome: a look through susceptibility weighted imaging. Ann Indian Acad Neurol. 2011;14(2):124–26. doi: 10.4103/0972-2327.82803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battisi C, Forte F, Rubenni E, et al. Two cases of hemichorea-hemiballism with nonketotic hyperglycemia: a new point of view. Neurol Sci. 2009;30(3):179–83. doi: 10.1007/s10072-009-0039-5. [DOI] [PubMed] [Google Scholar]
- 5.Nath J, Jambherkar K, Rao C, Armitano E. Radiological and pathological changes in hemiballism-hemichorea with striatal hyperintensity. J Magn Reson Imaging. 2006;23(4):564–68. doi: 10.1002/jmri.20548. [DOI] [PubMed] [Google Scholar]
- 6.Cheema H, Federman D, Kam A. Hemichorea-hemiballismus in non-ketotic hyperglycaemia. J Clin Neurosci. 2011;18(2):293–94. doi: 10.1016/j.jocn.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 7.Slabu H, Savedia-Cayabyab S, Senior P, Arnason T. Permanent haemichorea associated with transient hyperglycemia. BMJ Case Rep. 2011 doi: 10.1136/bcr.08.2011.4641. 2011. pii: bcr0820114641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JJ. Ipsilateral putamen hyperintensity on T1-weighted MRI in non-ketotic hyperglycemia with hemiballism-hemichorea: a case report. Parkinsonism Relat Disord. 2001;7(4):319–21. doi: 10.1016/s1353-8020(00)00072-9. [DOI] [PubMed] [Google Scholar]
- 9.Zaitout Z. CT and MRI findings in the basal ganglia in non-ketotic hyperglycaemia associated hemichorea and hemiballismus (HC-HB) Neuroradiology. 2012;54(10):1119–20. doi: 10.1007/s00234-012-1021-0. [DOI] [PubMed] [Google Scholar]
- 10.Mittal P. Hemichorea hemiballism syndrome: the first presentation of type 2 diabetes mellitus as a rare cause of chorea. Iran J Radiol. 2011;8(1):47–49. [PMC free article] [PubMed] [Google Scholar]
- 11.Narayanan S. Hyperglycemia-induced hemiballismus hemichorea: a case report and brief review of the literature. J Emerg Med. 2012;43(3):442–44. doi: 10.1016/j.jemermed.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Taboada GF, Lima GA, Castro JE, Liberato B. Dyskinesia associated with hyperglycemia and basal ganglia hyperintensity: report of a rare diabetic complication. Metab Brain Dis. 2013;28(1):107–10. doi: 10.1007/s11011-012-9357-z. [DOI] [PubMed] [Google Scholar]
- 13.Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002;200(1–2):57–62. doi: 10.1016/s0022-510x(02)00133-8. [DOI] [PubMed] [Google Scholar]
- 14.Hwang KJ, Hong IK, Ahn TB, et al. Cortical hemichorea-hemiballism. J Neurol. 2013;260(12):2986–92. doi: 10.1007/s00415-013-7096-7. [DOI] [PubMed] [Google Scholar]
- 15.Lin JJ, Chang MK. Hemiballism-hemichorea and non-ketotic hyperglycemia. J Neurol Neurosurg Psychiatry. 1994;57(6):748–50. doi: 10.1136/jnnp.57.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]


